Amphetamine Exposure during Embryogenesis Alters Expression and Function of Tyrosine Hydroxylase and the Vesicular Monoamine Transporter in Adult C. elegans
Abstract
1. Introduction
2. Results
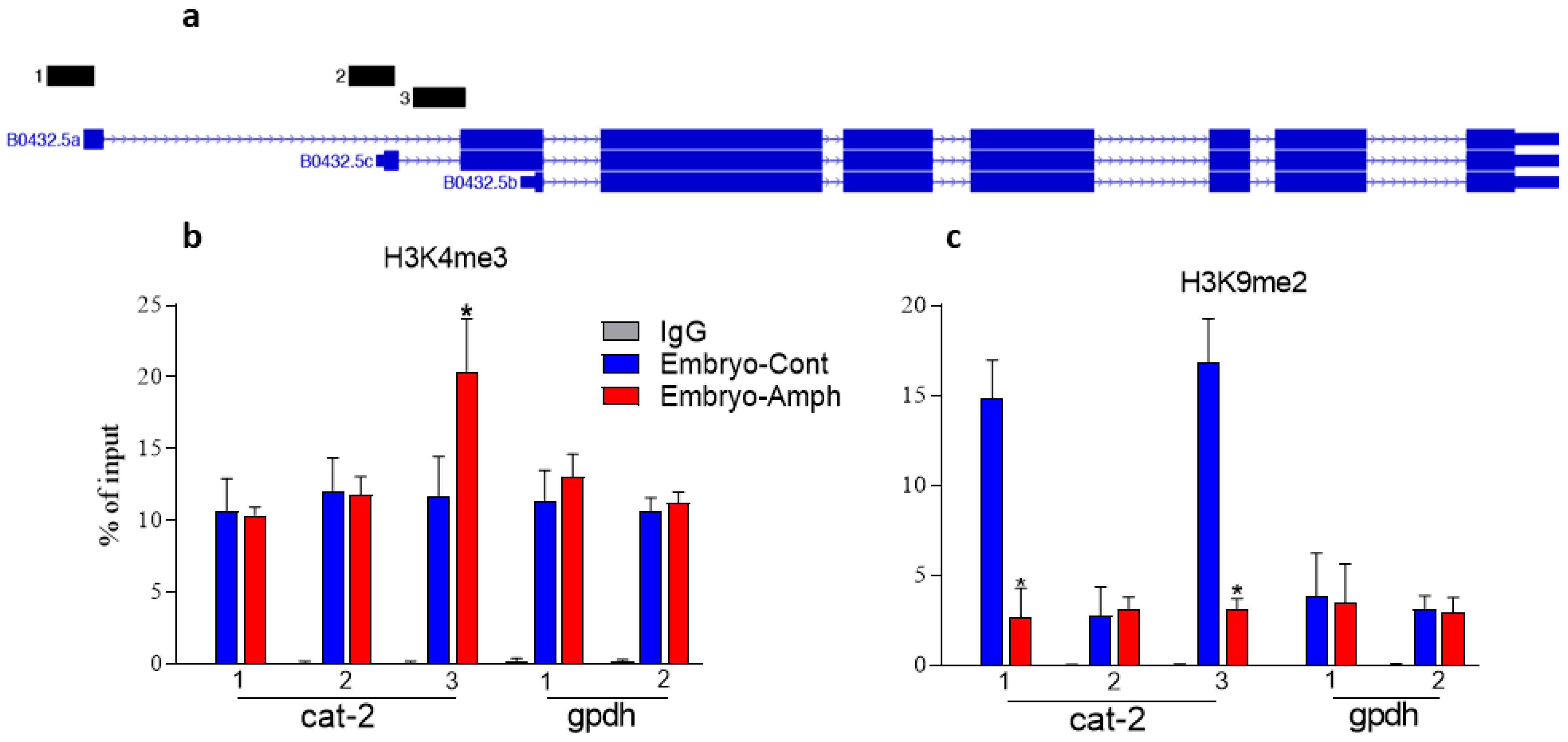
2.1. Exposure to Amphetamine during Embryogenesis Alters Histone Marks in the C. elegans Tyrosine Hydroxylase Cat-2 Gene
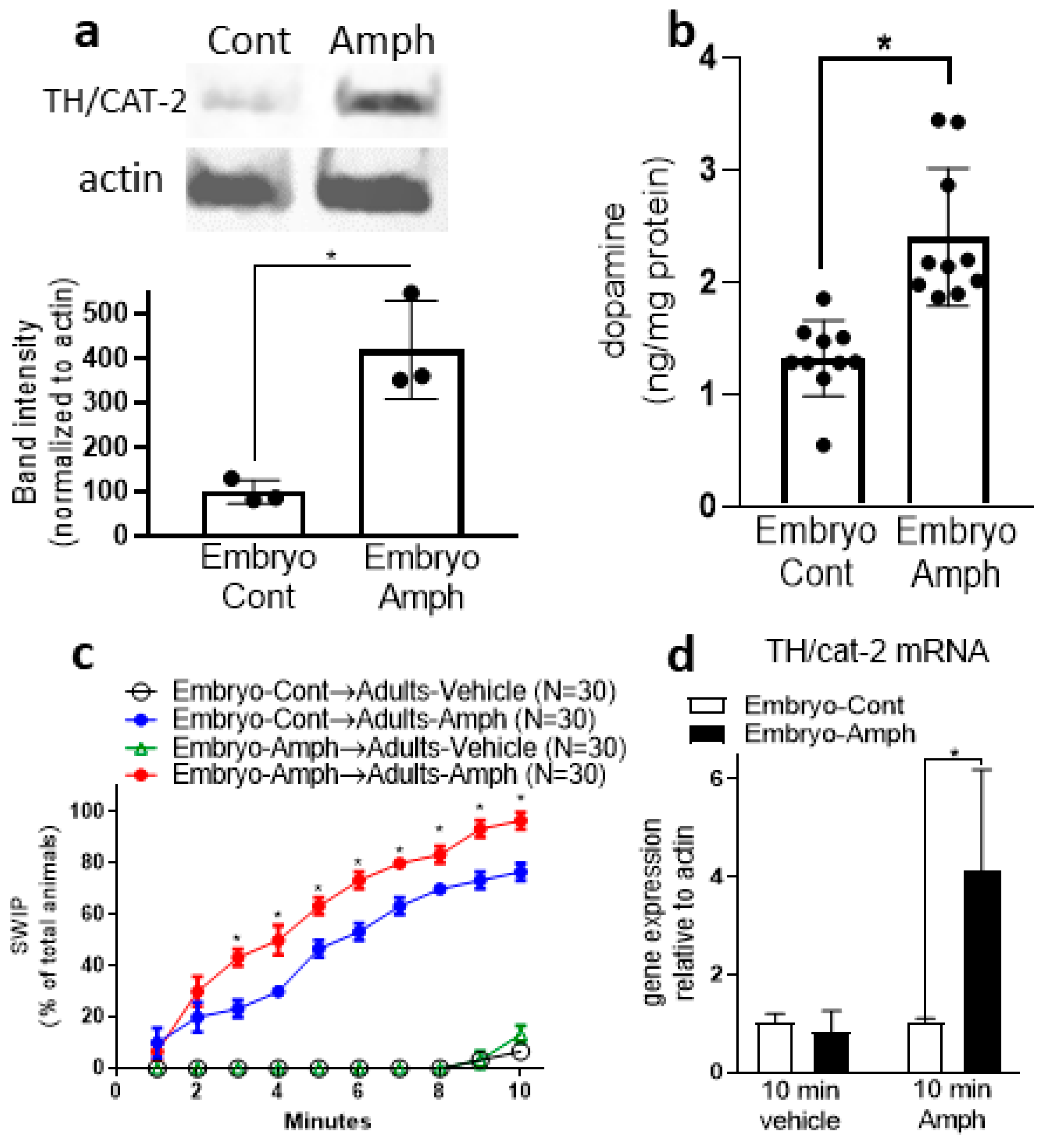
2.2. Exposure to Amphetamine during Embryogenesis Upregulates Protein Expression of CAT-2/TH in Adult Animals
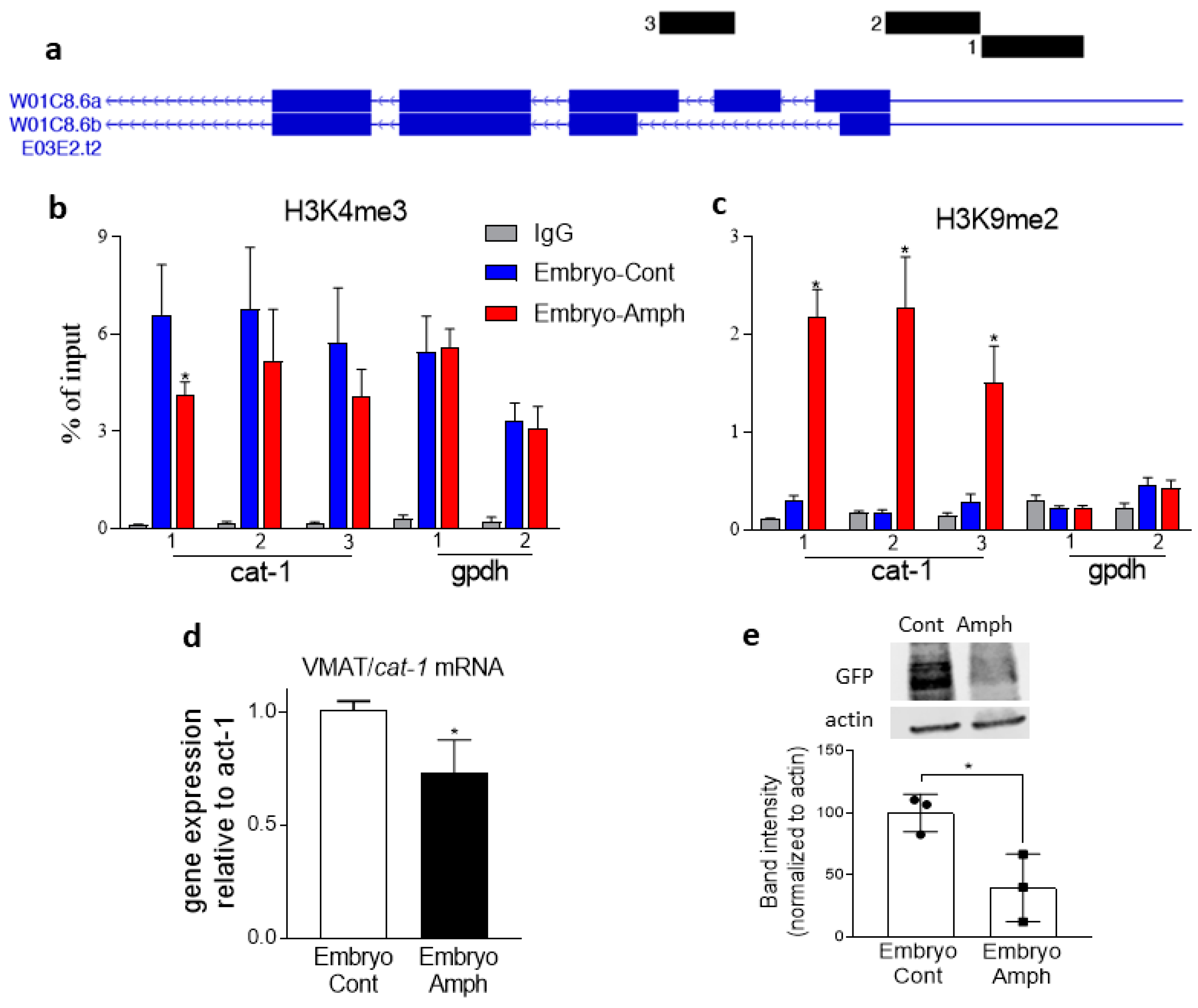
2.3. Exposure to Amphetamine during Embryogenesis Alters Histone Marks and Expression of the VMAT2 Homolog Cat-1 Gene in Adult C. elegans
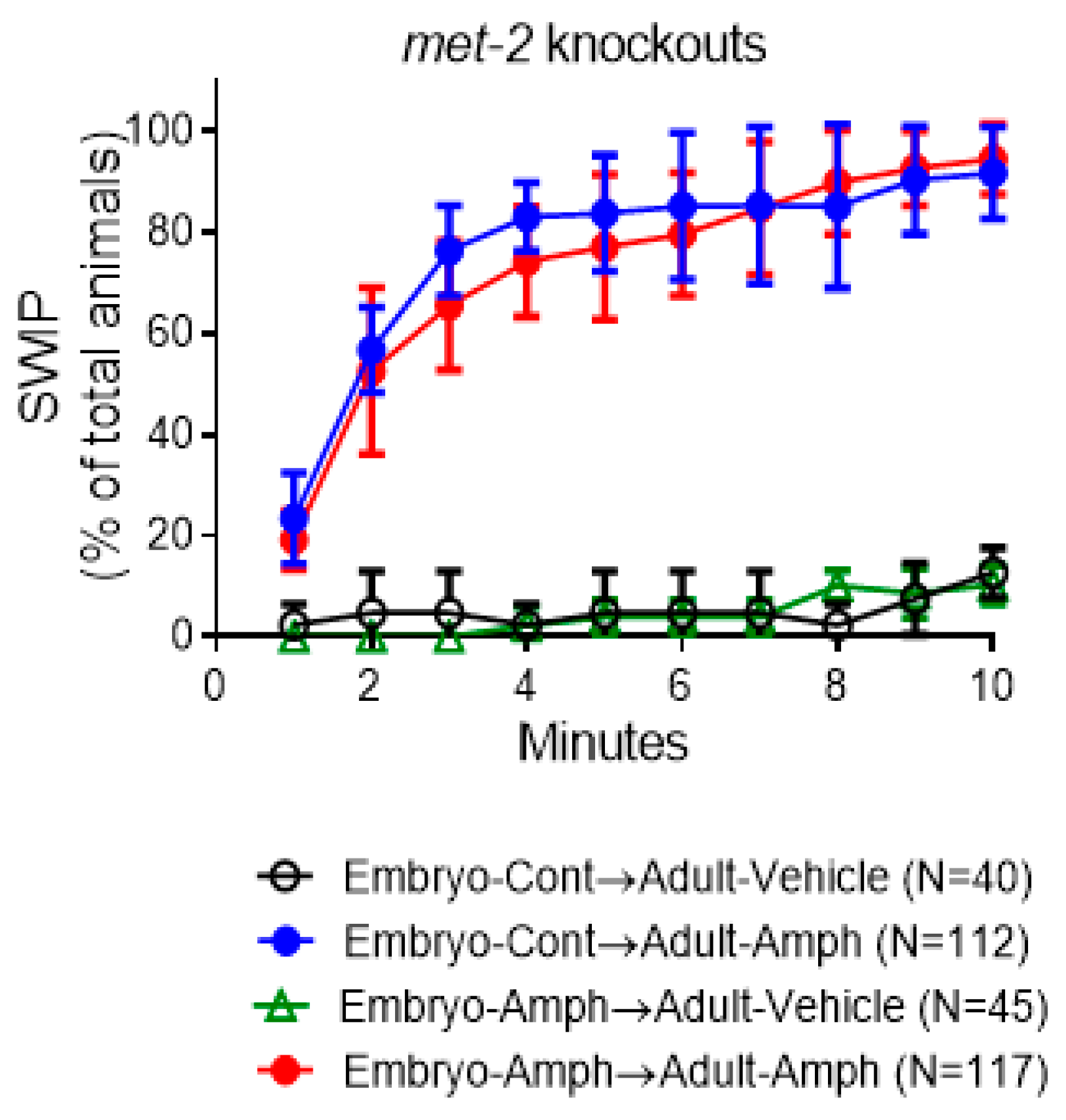
2.4. The Behavioral Effects Caused by Embryonic Amphetamine Exposure Are in Part Blocked by Genetic Inhibition of MET-2, the C. elegans Homolog of the Human Histone 3 Lysine 9 Methyltransferase SETDB1
3. Discussion
4. Materials and Methods
4.1. Worm Husbandry and Strains
4.2. Embryonic Exposure to Amphetamine
4.3. Swimming-Induced Paralysis (SWIP) Assays
4.4. Chromatin Immunoprecipitation (ChIP) Assays
4.5. Quantitative Reverse Transcript PCR (RT-qPCR)
4.6. Western Blot
4.7. Dopamine ELISA Assay
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sulzer, D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron Rev. 2011, 69, 628–649. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Sonders, M.S.; Poulsen, N.W.; Galli, A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog. Neurobiol. 2005, 75, 406–433. [Google Scholar] [CrossRef] [PubMed]
- Freyberg, Z.; Sonders, M.S.; Aguilar, J.I.; Hiranita, T.; Karam, C.S.; Flores, J.; Pizzo, A.B.; Zhang, Y.; Farino, Z.J.; Chen, A.; et al. Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nat. Commun. 2016, 7, 10652. [Google Scholar] [CrossRef] [PubMed]
- Mukda, S.; Vimolratana, O.; Govitrapong, P. Melatonin attenuates the amphetamine-induced decrease in vesicular monoamine transporter-2 expression in postnatal rat striatum. Neurosci. Lett. 2011, 488, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Lapin, I.P.; Rogawski, M.A. Effects of D1 and D2 dopamine receptor antagonists and catecholamine depleting agents on the locomotor stimulation induced by dizocilpine in mice. Behav. Brain Res. 1995, 70, 145–151. [Google Scholar] [CrossRef]
- Janenaite, E.; Vengeliene, V.; Bespalov, A.; Behl, B. Potential role of tyrosine hydroxylase in the loss of psychostimulant effect of amphetamine under conditions of impaired dopamine transporter activity. Behav. Brain Res. 2017, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; He, Z.; Shen, X.; Roman, R.J.; Ma, T. Differential action of methamphetamine on tyrosine hydroxylase and dopamine transport in the nigrostriatal pathway of μ-opioid receptor knockout mice. Int. J. Neurosci. 2012, 122, 305–313. [Google Scholar] [CrossRef]
- Good, R.L.; Liang, L.P.; Patel, M.; Radcliffe, R.A. Mouse strain- and age-dependent effects of binge methamphetamine on dopaminergic signaling. Neurotoxicology 2011, 32, 751–759. [Google Scholar] [CrossRef]
- Bubenikova-Valesova, V.; Kacer, P.; Syslova, K.; Rambousek, L.; Janovsky, M.; Schutova, B.; Hruba, L.; Slamberova, R. Prenatal methamphetamine exposure affects the mesolimbic dopaminergic system and behavior in adult offspring. Int. J. Dev. Neurosci. 2009, 27, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Nasif, F.J.; Cuadra, G.R.; Ramirez, O.A. Permanent alteration of central noradrenergic system by prenatally administered amphetamine. Brain Res. Dev. Brain Res. 1999, 112, 181–188. [Google Scholar] [CrossRef]
- Madsen, K.B.; Robakis, T.K.; Liu, X.; Momen, N.; Larsson, H.; Dreier, J.W.; Kildegaard, H.; Groth, J.B.; Newcorn, J.H.; Thomsen, P.H.; et al. In utero exposure to ADHD medication and long-term offspring outcomes. Mol. Psychiatry 2023, 28, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Ke, T.; Ambigapathy, G.; Ton, T.; Dhasarathy, A.; Carvelli, L. Long-Lasting Epigenetic Changes in the Dopamine Transporter in Adult Animals Exposed to Amphetamine during Embryogenesis: Investigating Behavioral Effects. Int. J. Mol. Sci. 2023, 24, 13092. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.E.; Fon, E.A.; Hastings, T.G.; Edwards, R.H.; Sulzer, D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J. Neurosci. 2002, 22, 8951–8960. [Google Scholar] [CrossRef] [PubMed]
- Heusner, C.L.; Hnasko, T.S.; Szczypka, M.S.; Liu, Y.; During, M.J.; Palmiter, R.D. Viral restoration of dopamine to the nucleus accumbens is sufficient to induce a locomotor response to amphetamine. Brain Res. 2003, 980, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Carvelli, L.; Matthies, D.S.; Galli, A. Molecular Mechanisms of Amphetamine Actions in Caenorhabditis elegans. Mol. Pharmacol. 2010, 78, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.L.; Jayanthi, S. Epigenetics of addiction. Neurochem. Int. 2021, 147, 105069. [Google Scholar] [CrossRef]
- Jambhekar, A.; Dhall, A.; Shi, Y. Roles and regulation of histone methylation in animal development. Nat. Rev. Mol. Cell Biol. 2019, 20, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Valles, A.; Vaissière, T.; Griggs, E.M.; Mikaelsson, M.A.; Takács, I.F.; Young, E.J.; Rumbaugh, G.; Miller, C.A. Methamphetamine-associated memory is regulated by a writer and an eraser of permissive histone methylation. Biol. Psychiatry 2014, 76, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wilkinson, M.; Liu, X.; Purushothaman, I.; Ferguson, D.; Vialou, V.; Maze, I.; Shao, N.; Kennedy, P.; Koo, J.; et al. Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens. Genome Biol. 2014, 15, R65. [Google Scholar] [CrossRef]
- Santos-Rosa, H.; Schneider, R.; Bannister, A.J.; Sherriff, J.; Bernstein, B.E.; Emre, N.C.T.; Schreiber, S.L.; Mellor, J. Active genes are tri-methylated at K4 of histone H3. Nature 2002, 419, 407–411. [Google Scholar] [CrossRef]
- Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.-Y.; Schones, D.E.; Wang, Z.; Wei, G.; Chepelev, I.; Zhao, K. High-resolution profiling of histone methylations in the human genome. Cell 2007, 129, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Taracha, E.; Czarna, M.; Turzyńska, D.; Maciejak, P. Amphetamine-induced prolonged disturbances in tissue levels of dopamine and serotonin in the rat brain. Pharmacol. Rep. 2023, 75, 596–608. [Google Scholar] [CrossRef]
- Carvelli, L.; Blakely, R.D.; DeFelice, L.J. Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proc. Natl. Acad. Sci. USA 2008, 105, 14192–14197. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.W.; Hardie, S.L.; Jessen, T.N.; Carvelli, L.; Matthies, D.S.; Blakely, R.D. Vigorous Motor Activity in Caenorhabditis elegans Requires Efficient Clearance of Dopamine Mediated by Synaptic Localization of the Dopamine Transporter DAT-1. J. Neurosci. 2007, 27, 14216–14227. [Google Scholar] [CrossRef]
- Nago-Iwashita, Y.; Moriya, Y.; Hara, S.; Ogawa, R.; Aida, R.; Miyajima, K.; Shimura, T.; Muramatsu, S.-I.; Ide, S.; Ikeda, K.; et al. Overexpression of tyrosine hydroxylase in dopaminergic neurons increased sensitivity to methamphetamine. Neurochem. Int. 2023, 164, 105491. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, L.M.; Sullivan, P.; Dunn, A.R.; Bermejo, M.K.; Fu, R.; Masoud, S.T.; Gregersen, E.; Urs, N.M.; Nazari, R.; Jensen, P.H.; et al. Enhanced tyrosine hydroxylase activity induces oxidative stress, causes accumulation of autotoxic catecholamine metabolites, and augments amphetamine effects in vivo. J. Neurochem. 2021, 158, 960–979. [Google Scholar] [CrossRef] [PubMed]
- Lohoff, F.W.; Carr, G.V.; Brookshire, B.; Ferraro, T.N.; Lucki, I. Deletion of the vesicular monoamine transporter 1 (vmat1/slc18a1) gene affects dopamine signaling. Brain Res. 2019, 1712, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Padeken, J.; Methot, S.P.; Gasser, S.M. Establishment of H3K9-methylated heterochromatin and its functions in tissue differentiation and maintenance. Nat. Rev. Mol. Cell Biol. 2022, 23, 623–640. [Google Scholar] [CrossRef]
- Husmann, D.; Gozani, O. Histone lysine methyltransferases in biology and disease. Nat. Struct. Mol. Biol. 2019, 26, 880–889. [Google Scholar] [CrossRef]
- Towbin, B.D.; González-Aguilera, C.; Sack, R.; Gaidatzis, D.; Kalck, V.; Meister, P.; Askjaer, P.; Gasser, S.M. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell 2012, 150, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Loyola, A.; Bonaldi, T.; Roche, D.; Imhof, A.; Almouzni, G. PTMs on H3 variants before chromatin assembly potentiate their final epigenetic state. Mol. Cell 2006, 24, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Loyola, A.; Tagami, H.; Bonaldi, T.; Roche, D.; Quivy, J.P.; Imhof, A.; Nakatani, Y.; Dent, S.Y.R.; Almouzni, G. The HP1alpha-CAF1-SetDB1-containing complex provides H3K9me1 for Suv39-mediated K9me3 in pericentric heterochromatin. EMBO Rep. 2009, 10, 769–775. [Google Scholar] [CrossRef] [PubMed]
- McLeod, J.; Sinal, C.J.; Perrot-Sinal, T.S. Evidence for non-genomic transmission of ecological information via maternal behavior in female rats. Genes. Brain Behav. 2007, 6, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.G.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- Schreiber, M.A.; McIntire, S.L. A Caenorhabditis elegans p38 MAP kinase pathway mutant protects from dopamine, methamphetamine, and MDMA toxicity. Neurosci. Lett. 2011, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, J.; Aryal, D.K.; Wetsel, W.C.; Nass, R.; Benovic, J.L. G protein-coupled receptor kinase-2 (GRK-2) regulates serotonin metabolism through the monoamine oxidase AMX-2 in Caenorhabditis elegans. J. Biol. Chem. 2017, 292, 5943–5956. [Google Scholar] [CrossRef] [PubMed]
- Engleman, E.A.; Katner, S.N.; Neal-Beliveau, B.S. Caenorhabditis elegans as a Model to Study the Molecular and Genetic Mechanisms of Drug Addiction. Prog. Mol. Biol. Transl. Sci. 2016, 137, 229–252. [Google Scholar] [PubMed]
- Cruz-Muros, I.; Afonso-Oramas, D.; Abreu, P.; Rodríguez, M.; González, M.C.; González-Hernández, T. Deglycosylation and subcellular redistribution of VMAT2 in the mesostriatal system during normal aging. Neurobiol. Aging 2008, 29, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, T.; Poquette, K.E.; Amro Gazze, S.L.; Carvelli, L. Amphetamine Exposure during Embryogenesis Alters Expression and Function of Tyrosine Hydroxylase and the Vesicular Monoamine Transporter in Adult C. elegans. Int. J. Mol. Sci. 2024, 25, 4219. https://doi.org/10.3390/ijms25084219
Ke T, Poquette KE, Amro Gazze SL, Carvelli L. Amphetamine Exposure during Embryogenesis Alters Expression and Function of Tyrosine Hydroxylase and the Vesicular Monoamine Transporter in Adult C. elegans. International Journal of Molecular Sciences. 2024; 25(8):4219. https://doi.org/10.3390/ijms25084219
Chicago/Turabian StyleKe, Tao, Katie E. Poquette, Sophia L. Amro Gazze, and Lucia Carvelli. 2024. "Amphetamine Exposure during Embryogenesis Alters Expression and Function of Tyrosine Hydroxylase and the Vesicular Monoamine Transporter in Adult C. elegans" International Journal of Molecular Sciences 25, no. 8: 4219. https://doi.org/10.3390/ijms25084219
APA StyleKe, T., Poquette, K. E., Amro Gazze, S. L., & Carvelli, L. (2024). Amphetamine Exposure during Embryogenesis Alters Expression and Function of Tyrosine Hydroxylase and the Vesicular Monoamine Transporter in Adult C. elegans. International Journal of Molecular Sciences, 25(8), 4219. https://doi.org/10.3390/ijms25084219




