Novel Fe3O4 Nanoparticles with Bioactive Glass–Naproxen Coating: Synthesis, Characterization, and In Vitro Evaluation of Bioactivity
Abstract
1. Introduction
- (i)
- Magnetic nanoparticles can provide mechanical strength and the ability to heat up in an alternating current magnetic field.
- (ii)
- The greater surface of bioactive glass nanoparticles presents an incomparable and promising feature similar to the biological apatite. Nanoparticles improve cellular adhesion, enhance osteoblast proliferation and differentiation, and increase biomineralization for implants [42].
- (iii)
- An anti-inflammatory drug can favor bone regeneration by avoiding severe inflammation in the cancer-treated area [43].
2. Results and Discussion
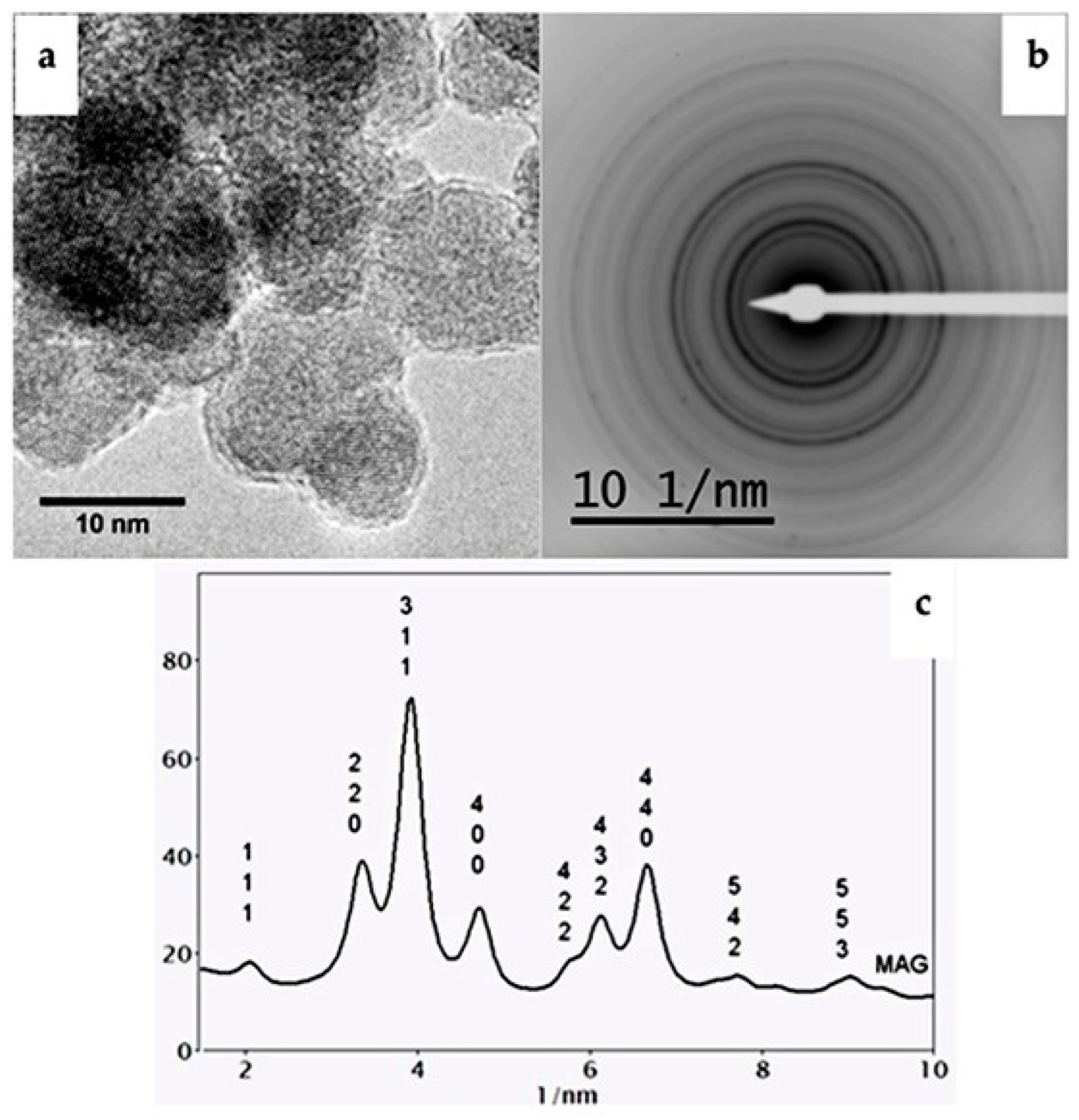
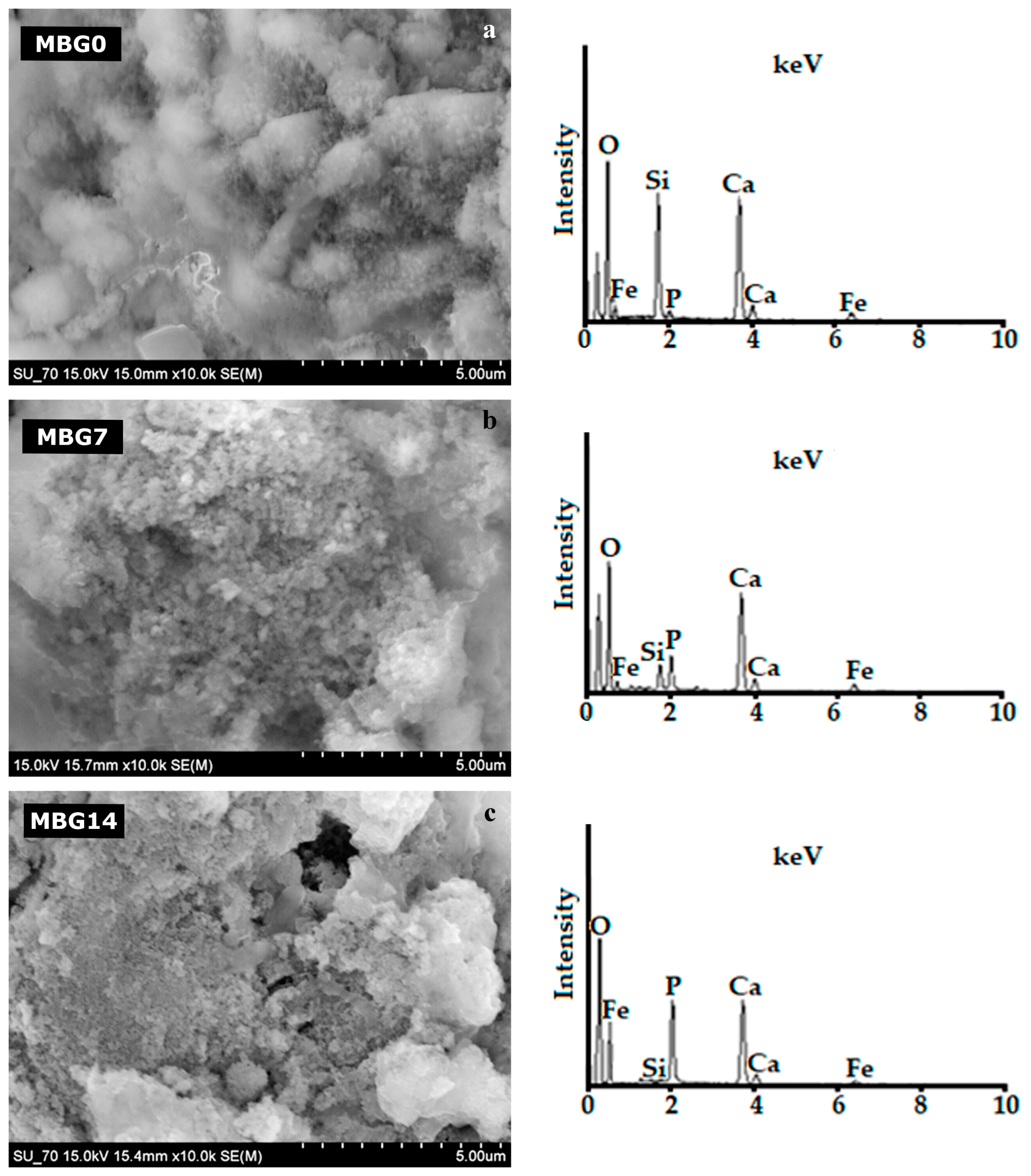
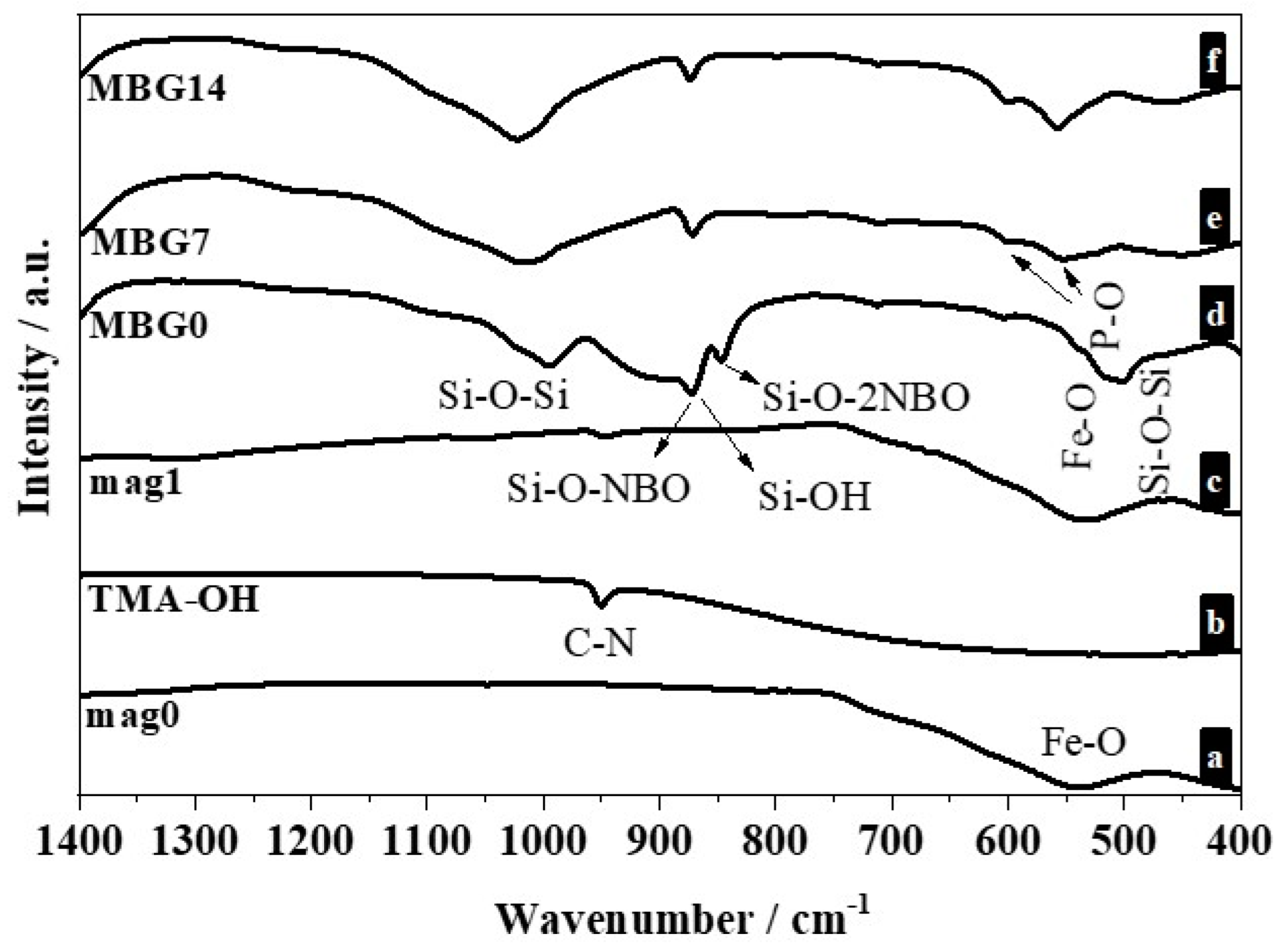
2.1. Characterization of the Synthesized Magnetic Nanoparticles
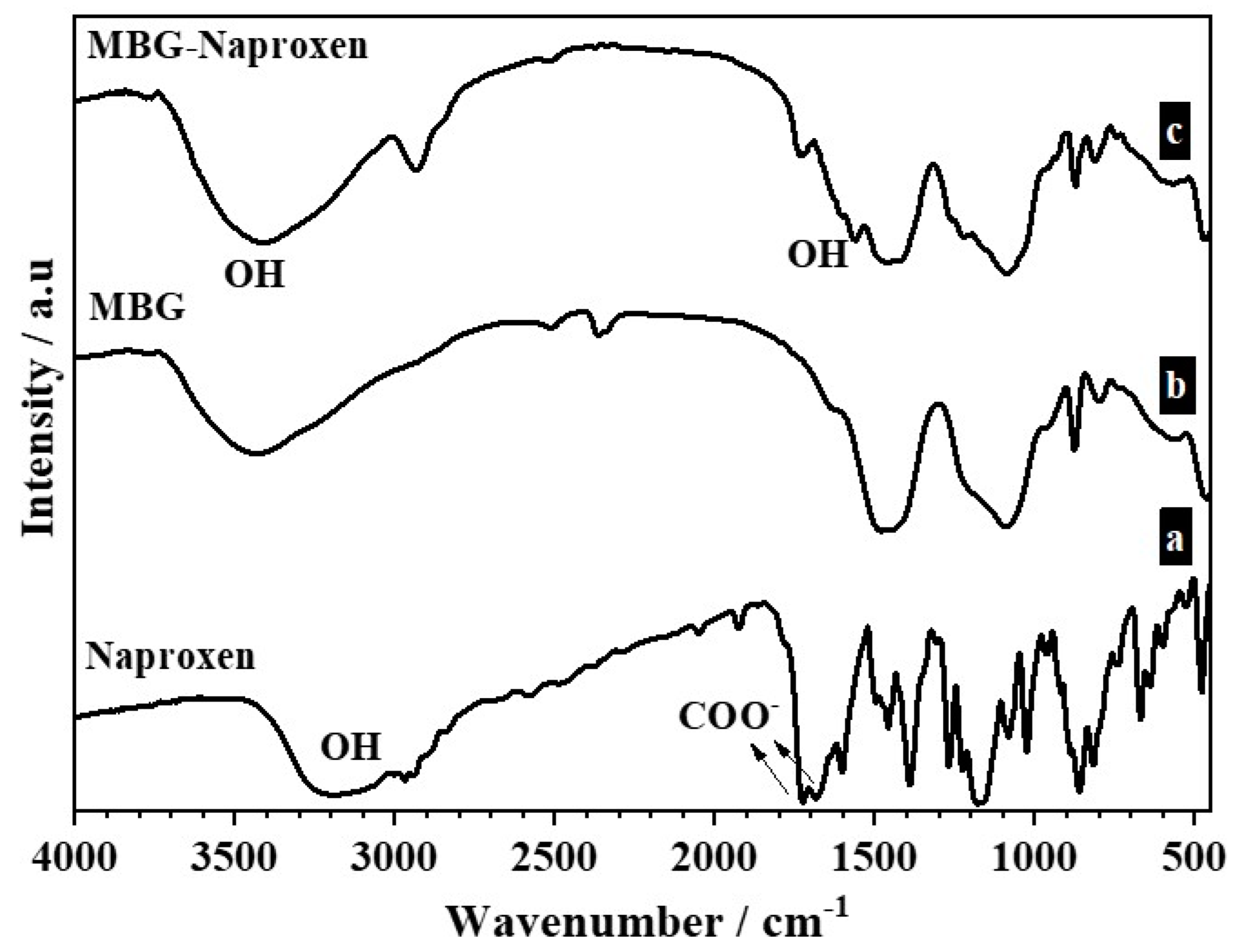
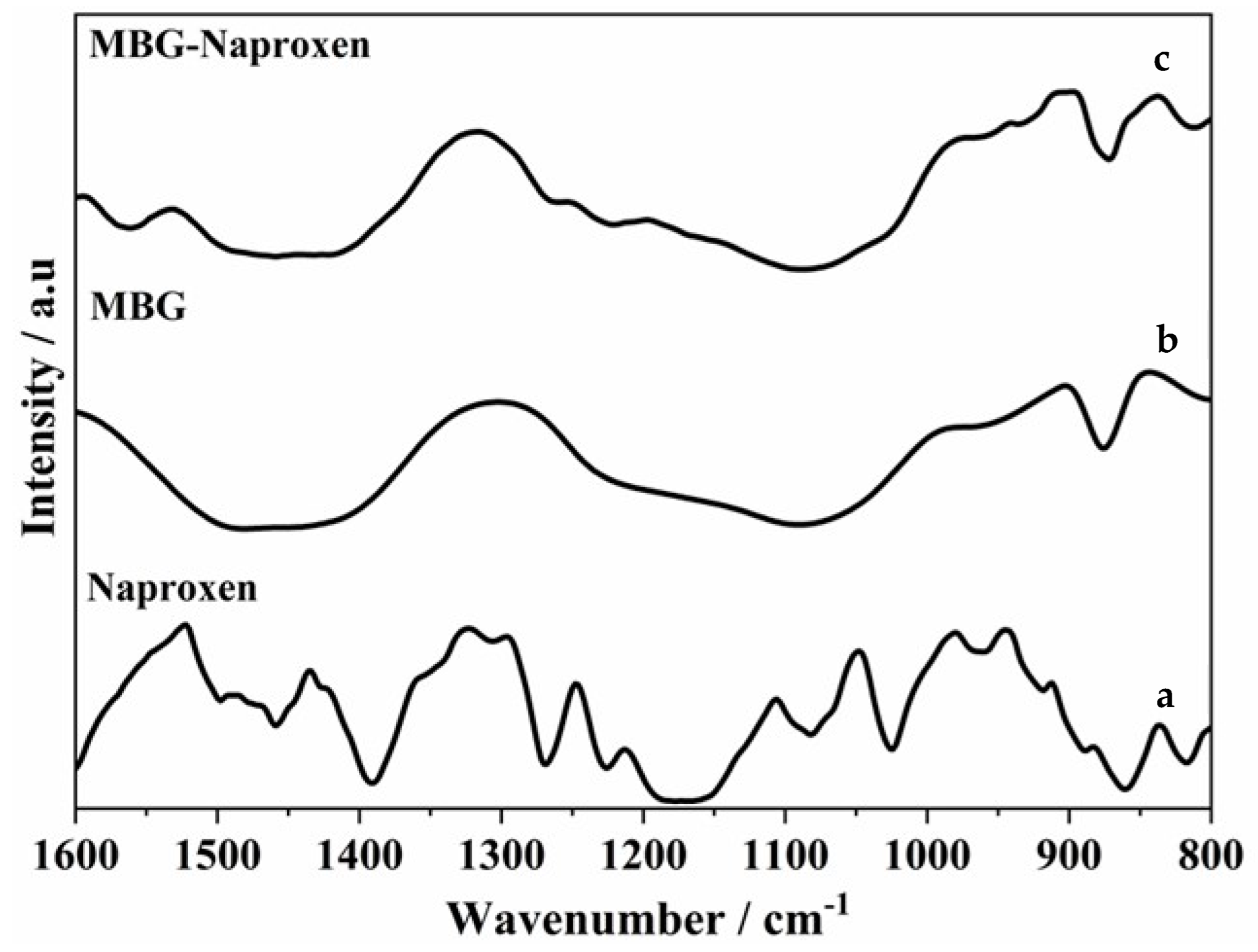
2.2. Drug Loading Study
2.3. Biological Assays
2.3.1. Cell Viability Assay with RAW 264.7 Cells, MC3T3-E1, and Saos-2
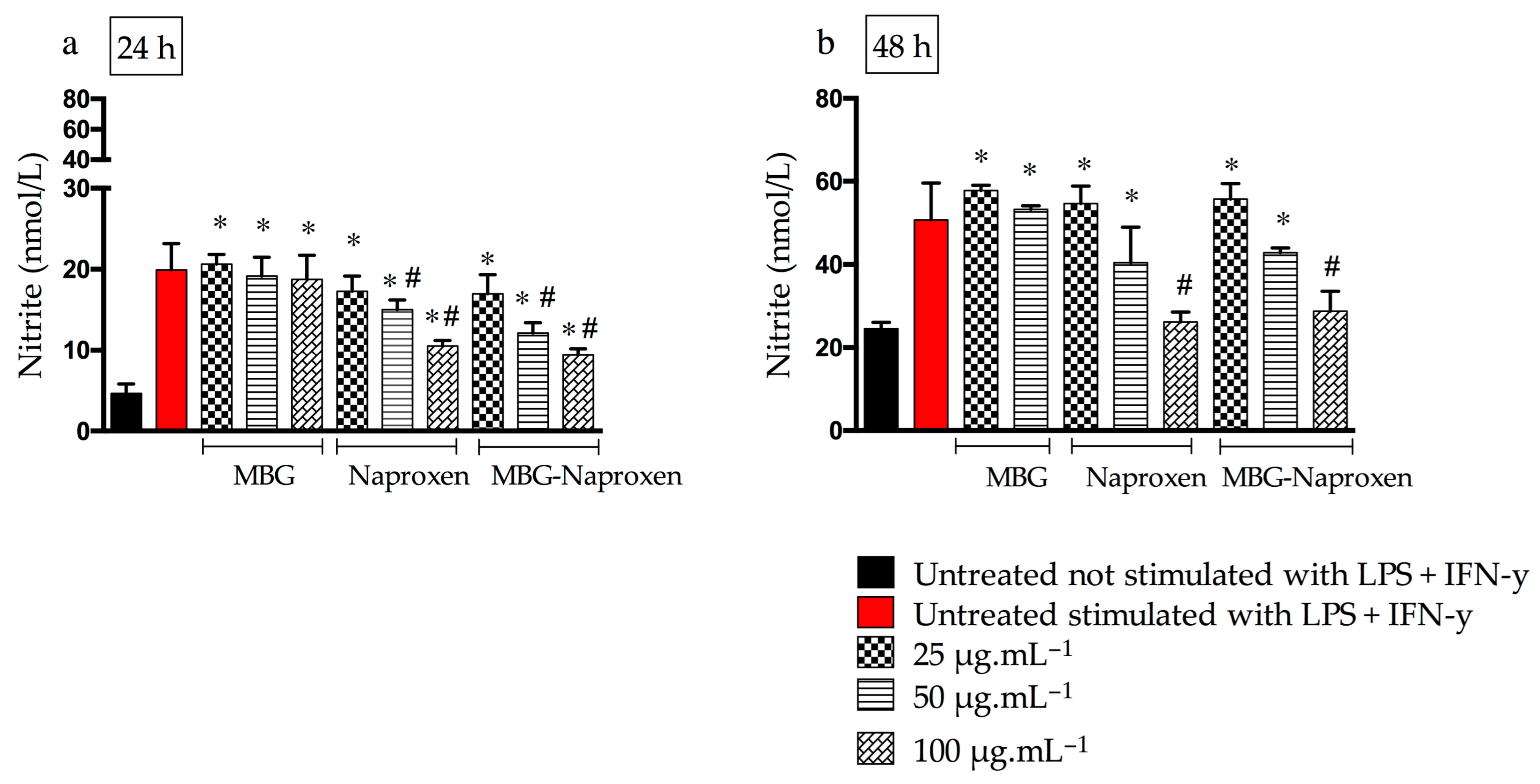
2.3.2. Nitric Oxide
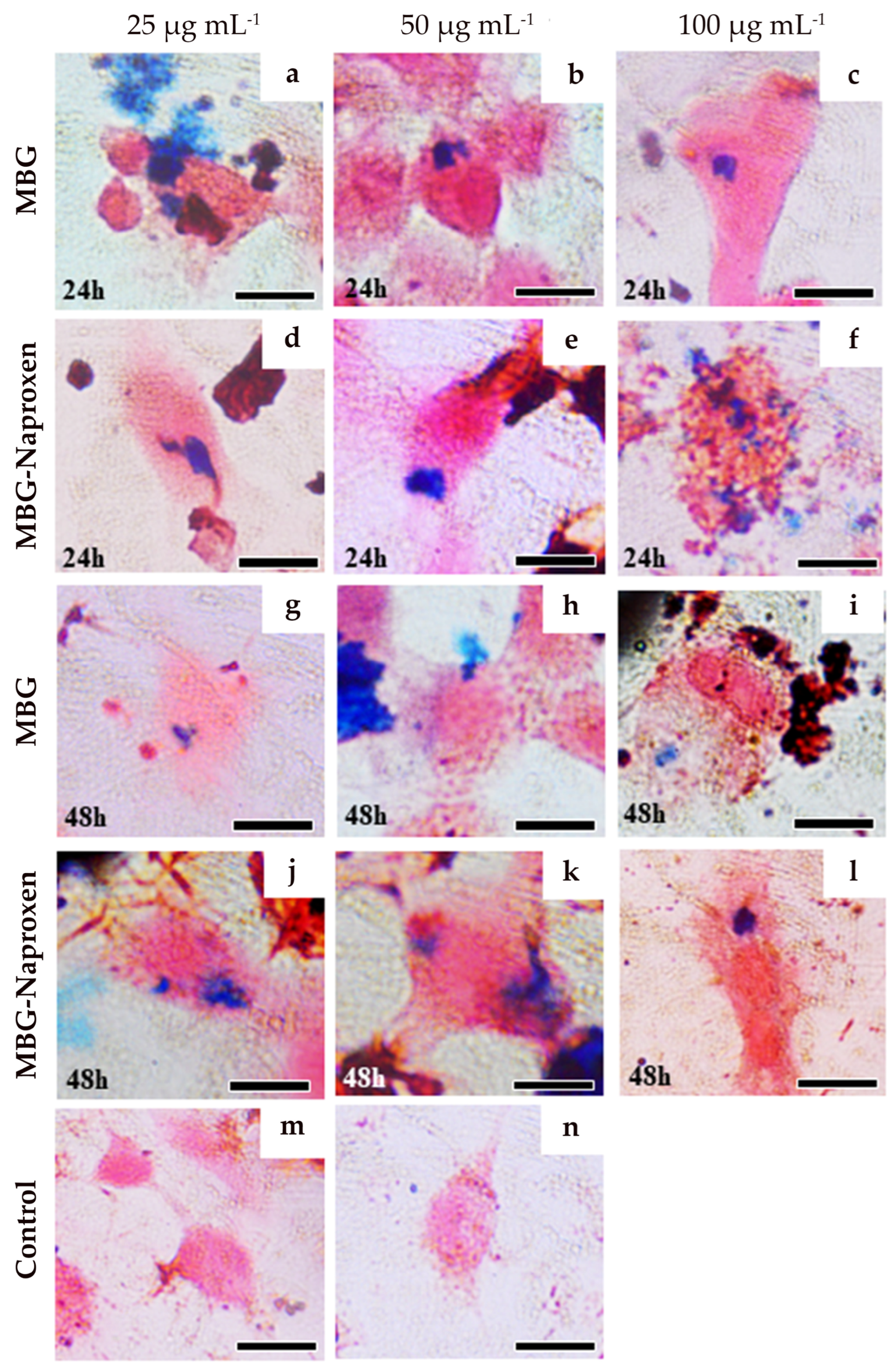
2.3.3. Prussian Blue Staining for Intracellular Iron Detection
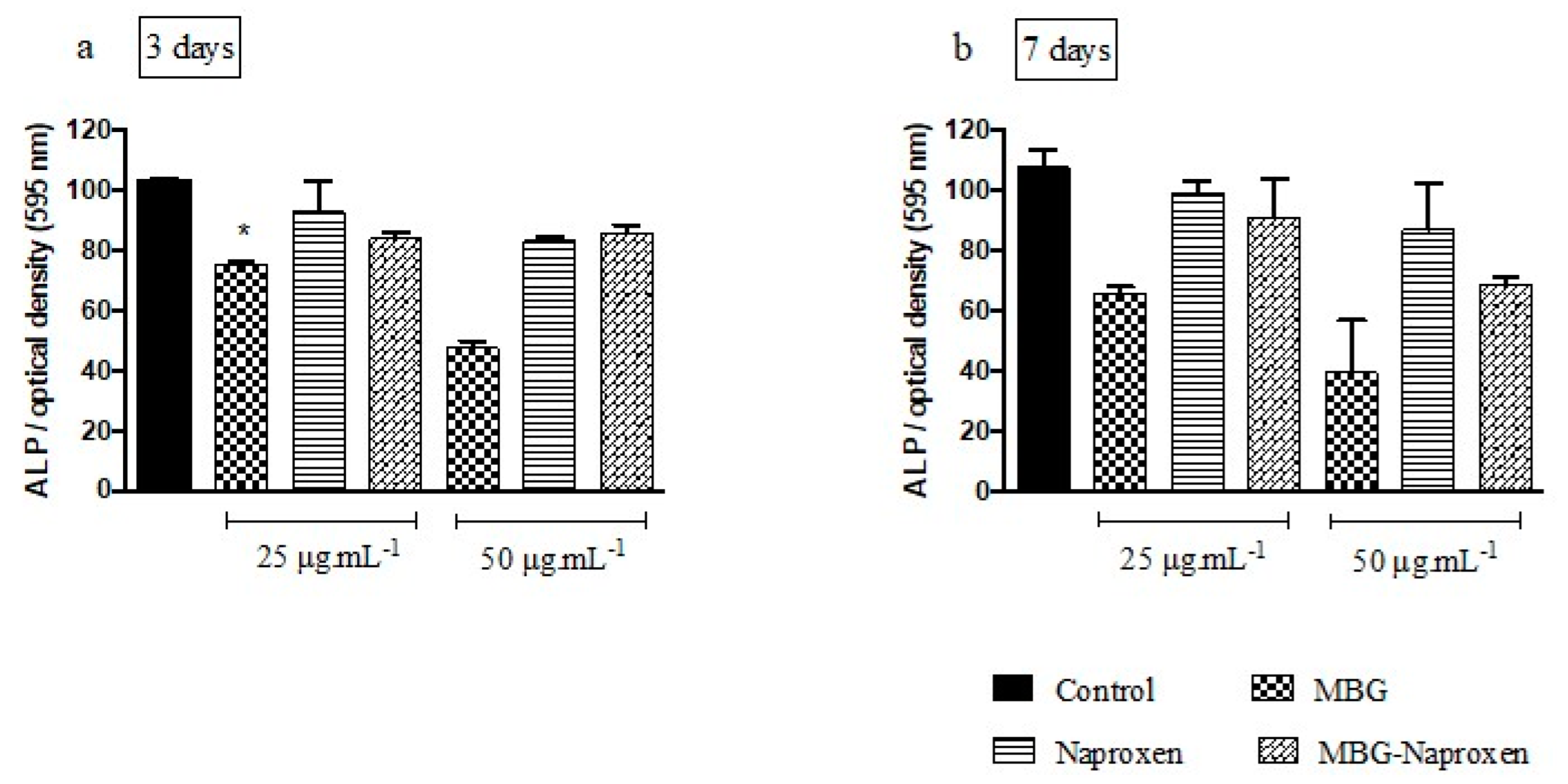
2.3.4. Alkaline Phosphatase Activity with MC3T3-E1 Cells
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Iron Oxide Nanoparticles (mag0 and mag-1 Samples)
3.3. Coating the Magnetic Nanoparticles with Bioactive Glass (MBG Sample)
3.4. Preparation of Drug Loaded MBG (MBG-Naproxen Sample)
3.5. Assessment of In Vitro Bioactivity
3.6. Characterization
3.7. Biological Assays—In Vitro Studies
3.7.1. Sample Preparation
3.7.2. RAW 264.7 Cells Culture
3.7.3. MC3T3-E1 Cells Culture
3.7.4. Saos-2 Cells Culture
3.7.5. Cell Viability in RAW 264.7
3.7.6. Cell Viability in MC3T3-E1
3.7.7. Cell Viability in Saos-2
3.7.8. Alkaline Phosphatase Activity
3.7.9. Prussian Blue Staining for Intracellular Iron Detection
3.7.10. Nitric Oxide
3.7.11. Statistical Analysis
4. Final Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BG | Bioactive glass |
| M | Magnetic |
| MBG | Sample mag1 coated with glass |
| IL-1β | Interleukin 1β |
| NSAID | Nonsteroidal anti-inflammatory drugs |
| HRTEM | High-resolution transmission electron microscopy |
| SAD or SAED | Selected area electron diffraction |
| TEM | Transmission electron microscopy |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive X-ray spectrometry |
| ATR-FTIR | Attenuated total reflection Fourier transform infrared |
| XRD | X-ray powder diffraction |
| mag0 | Iron oxide nanoparticles |
| mag1 | Iron oxide nanoparticles coated with tetramethylammonium hydroxide |
| MBG0 | Sample mag1 coated with glass before being soaked in SBF |
| MBG7 | Sample MBG0 after being soaked in SBF for 7 days |
| MBG14 | Sample MBG0 after being soaked in SBF for 14 days |
| MBG-naproxen | MBG sample loaded with naproxen |
| SBF | Simulated body fluid |
| TMAOH | Tetramethylammonium hydroxide |
| IR | Infrared |
| NBOs | Of non-bridging oxygens |
| The powder diffraction file | |
| ICDD | International Centre for Diffraction Data |
| IL-6 | Interleukin-6 |
| SD | Standard deviation |
| DMSO | Dimethylsulfoxide |
| NO | Nitric oxide |
| LPS | Lipopolysaccharide |
| IFN-γ | Interferon γ |
| ALP | Alkaline phosphatase activity |
| PVA | Polyvinyl alcohol |
| TEOS | Tetraethyl orthosilicate |
| RPMI-1640 | Roswell Park Memorial Institute 1640 medium |
| α-MEM | Alpha minimum essential medium |
| FBS | Fetal bovine serum |
| MTT method | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| BCIP | Bromo-4-chloro-3-indolyl phosphate kit |
| NBT | Nitro-blue tetrazolium salt |
| LPS | Lipopolysaccharide |
| SDS | Sodium dodecyl sulfate |
| RT | Room temperature |
| PBS | Phosphate-buffered saline |
| ATCC | American Type Culture Collection |
| BCRJ | Banco de Células do Rio de Janeiro |
References
- Arcos, D.; Real, R.P.d.; Vallet-Regi, M. A novel bioactive and magnetic biphasic material. Biomaterials 2002, 23, 2151–2158. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.E.; Hench, L.L. An investigation of bioactive glass powders by sol–gel proccesing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef]
- Perez-Pariente, J.; Balas, F.; Vallet-Reg, M. Surface and chemical study of SiO2 • P2O5 • CaO • (MgO) bioactive glasses. Chem. Mater. 2000, 12, 750–755. [Google Scholar] [CrossRef]
- Vallet-Regı, M.; Arcos, D.; Perez-Pariente, J. Evolution of porosity during in vitro hydroxycarbonate apatite growth in sol–gel glasses. J. Biomed. Mater. Res. 2000, 51, 23–28. [Google Scholar] [CrossRef]
- Mao, C.; Chen, X.; Hu, Q.; Miao, G.; Lin, C. Acute toxicity and in vivo biodistribution of monodispersed mesoporous bioactive glass spheres in intravenously exposed mice. Mater. Sci. Eng. C 2016, 58, 682–691. [Google Scholar] [CrossRef]
- Plotner, A.N.; Mostow, E.N. A review of bioactive materials and chronic wounds. Cutis 2010, 85, 259–266. [Google Scholar]
- Li, H.; Chang, J. Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect. Acta Biomater. 2013, 9, 6981–6991. [Google Scholar] [CrossRef]
- Lewandowska-Lancucka, J.; Fiejdasz, S.; Rodzik, L.; Koziel, M.; Nowakowska, M. Bioactive hydrogel-nanosilica hybrid materials: A potential injectable scaffold for bone tissue engineering. Biomed. Mater. 2015, 10, 015020. [Google Scholar] [CrossRef]
- Su, L.; Li, Y.; Liu, Y.; Ma, R.; Liu, Y.; Huang, F.; An, Y.; Ren, Y.; van der Mei, H.C.; Busscher, H.J.; et al. Antifungal-inbuilt metal–organic-frameworks eradicate Candida albicans biofilms. Adv. Funct. Mater. 2020, 30, 2000537. [Google Scholar] [CrossRef]
- Song, N.; Zhou, Z.; Song, Y.; Li, M.; Yu, X.; Hu, B.; Yu, Z. In situ oxidation-regulated self-assembly of peptides into transformable scaffolds for cascade therapy. Nano Today 2021, 38, 101198. [Google Scholar] [CrossRef]
- Iovene, A.; Zhao, Y.; Wang, S.; Amoako, K. Bioactive polymeric materials for the advancement of regenerative medicine. J. Funct. Biomater. 2021, 12, 14. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Pan, Z.; Liu, Y. Advanced bioactive nanomaterials for biomedical applications. Exploration 2021, 1, 20210089. [Google Scholar] [CrossRef]
- Andrade, A.L.; Souza, D.M.; Vasconcellos, W.A.; Ferreira, R.V.; Domingues, R.Z. Tetracycline and/or hydrocortisone incorporation and release by bioactive glasses compounds. J. Non-Cryst. Solids 2009, 355, 811–816. [Google Scholar] [CrossRef]
- Andrade, A.L.; Manzi, D.; Domingues, R.Z. Tetracycline and propolis incorporation and release by bioactive glassy compounds. J. Non-Cryst. Solids 2006, 352, 3502–3507. [Google Scholar] [CrossRef]
- Andrade, A.L.; Militani, I.A.; de Almeida, K.J.; Belchior, J.C.; dos Reis, S.C.; Costa e Silva, R.M.F.; Domingues, R.Z. Theoretical and experimental studies of the controlled release of tetracycline incorporated into bioactive glasses. AAPS PharmSciTech. 2018, 19, 1287–1296. [Google Scholar] [CrossRef]
- Gong, D.; Celi, N.; Zhang, D.; Cai, J. Magnetic biohybrid microrobot multimers based on chlorella cells for enhanced targeted drug delivery. ACS Appl. Mater. Interfaces 2022, 14, 6320–6330. [Google Scholar] [CrossRef]
- Montazerian, M.; Zanotto, E.D. History and trends of bioactive glass-ceramics. J. Biomed. Mater. Res. A 2016, 104, 1231–1249. [Google Scholar] [CrossRef]
- Dimitriadis, K.; Tulyaganov, D.U.; Agathopoulos, S. Development of novel alumina-containing bioactive glass-ceramics in the CaO-MgO-SiO2 system as candidates for dental implant applications. J. Eur. Ceram. Soc. 2021, 41, 929–940. [Google Scholar] [CrossRef]
- Andrade, A.L.; Fabris, J.D.; Domingues, R.Z.; Pereira, M.C. Current status of magnetite-based core@shell structures for diagnosis and therapy in oncology. Curr. Pharm. Des. 2015, 21, 5417–5433. [Google Scholar] [CrossRef]
- Andrade, A.L.; Fabris, J.D.; Pereira, M.C.; Domingues, R.Z.; Ardisson, J.D. Preparation of composite with silica-coated nanoparticles of iron oxide spinels for applications based on magnetically induced hyperthermia. Hyperfine Interact. 2013, 218, 71–82. [Google Scholar] [CrossRef]
- Miola, M.; Pakzad, Y.; Banijamali, S.; Kargozar, S.; Vitale-Brovarone, C.; Yazdanpanah, A.; Bretcanu, O.; Ramedani, A.; Verne, E.; Mozafari, M. Glass-ceramics for cancer treatment: So close, or yet so far? Acta Biomater. 2019, 83, 55–70. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses entering the mainstream. Drug Discov. Today 2018, 23, 1700–1704. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, S.B.; Kim, Y.U.; Kim, K.N.; Choi, S.Y.; Lee, K.H.; Shim, I.B.; Kim, C.S. Effect of ferrite thermoseeds on destruction of carcinoma cells under alternating magnetic field. J. Mater. Sci. 2003, 38, 4221–4233. [Google Scholar] [CrossRef]
- Ebisawa, Y.; Miyaji, F.; Kokubo, T.; Ohura, K.; Nakamura, T. Bioactivity of ferrimagnetic glass-ceramics in the system FeO-Fe2O3-CaO-SiO2. Biomoterials 1997, 18, 1277–1284. [Google Scholar] [CrossRef]
- Datta, N.R.; Puric, E.; Klingbiel, D.; Gomez, S.; Bodis, S. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: A systematic review and meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1073–1087. [Google Scholar] [CrossRef]
- Datta, N.R.; Rogers, S.; Ordonez, S.G.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int. J. Hyperth. 2015, 32, 31–40. [Google Scholar] [CrossRef]
- Bian, W.; Wang, Y.; Pan, Z.; Chen, N.; Li, X.; Wong, W.L.; Liu, X.; He, Y.; Zhang, K.; Lu, Y.J. Review of functionalized nanomaterials for photothermal therapy of cancers. ACS Appl. Nano Mater. 2021, 4, 11353–11385. [Google Scholar] [CrossRef]
- Rismanchian, M.; Khodaeian, N.; Bahramian, L.; Fathi, M.; Sadeghi-Aliabadi, H. In-vitro comparison of cytotoxicity of two bioactive glasses in micropowder and nanopowder forms. Iran. J. Pharm. Res. 2013, 12, 437–443. [Google Scholar]
- Xue, Y.; Zhang, Z.; Niu, W.; Chen, M.; Wang, M.; Guo, Y.; Mao, C.; Lin, C.; Lei, B. Enhanced physiological stability and long-term toxicity/biodegradation in vitro/in vivo of monodispersed glycerolphosphate-functionalized bioactive glass nanoparticles. Part. Part. Syst. Charact. 2019, 36, 1800507. [Google Scholar] [CrossRef]
- Zhou, G.; Groth, T. Host responses to biomaterials and anti-inflammatory design-a brief review. Macromol. Biosci. 2018, 18, 1800112. [Google Scholar] [CrossRef]
- Yu, T.; Tutwiler, V.J.; Spiller, K. Biomaterials in Regenerative Medicine and the Immune System; Springer International Publishing: Cham, Switzerland, 2015; pp. 17–34. [Google Scholar]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef]
- Patrono, C. Cardiovascular effects of nonsteroidal antiinflammatory drugs. Curr. Cardiol. Rep. 2016, 18, 25. [Google Scholar] [CrossRef]
- Adibkia, K.; Javadzadeh, Y.; Dastmalchi, S.; Mohammadi, G.; Niri, F.K.; Alaei-Beirami, M. Naproxen–eudragit® RS100 nanoparticles: Preparation and physicochemical characterization. Colloids Surf. B 2011, 83, 155–159. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Rostamkalaei, S.S.; Asadi, M.; Asare-Addo, K.; Nokhodchi, A. The design of naproxen solid lipid nanoparticles to target skin layers. Colloids Surf. B 2016, 145, 626–633. [Google Scholar] [CrossRef]
- Espinosa-Cano, E.; Huerta-Madronal, M.; Camara-Sanchez, P.; Seras-Franzoso, J.; Schwartz, S., Jr.; Abasolo, I.; Roman, J.S.; Aguilar, M.R. Hyaluronic acid (HA)-coated naproxen-nanoparticles selectively target breast cancer stem cells through COX-independent pathways. Mater. Sci. Eng. C 2021, 124, 112024. [Google Scholar] [CrossRef]
- Vrana, N.E.; Erdemli, O.; Francius, G.; Fahs, A.; Rabineau, M.; Debry, C.; Tezcaner, A.; Keskin, D.; Lavalle, P. Double entrapment of growth factors by nanoparticles loaded into polyelectrolyte multilayer films. J. Mater. Chem. B 2014, 2, 999–1008. [Google Scholar] [CrossRef]
- Wewers, M.; Czyz, S.; Finke, J.H.; John, E.; Van Eerdenbrugh, B.; Juhnke, M.; Bunjes, H.; Kwade, A. Influence of formulation parameters on redispersibility of naproxen nanoparticles from granules produced in a fluidized bed process. Pharmaceutics 2020, 12, 363. [Google Scholar] [CrossRef]
- Razzaq, A.; Qureshi, I.Z. Naproxen sodium nanoparticles are less toxic and gastroprotective agents than the conventional NSAID drug naproxen sodium in Balb/c mice. Toxicol. Appl. Pharmacol. 2022, 452, 116192. [Google Scholar] [CrossRef]
- Gu, B.; Cai, J.; Peng, G.; Zhou, H.; Zhang, W.; Zhang, D.; Gong, D. Metal organic framework-loaded biohybrid magnetic microrobots for enhanced antibacterial treatment. Colloids Surf. A 2024, 685, 133295. [Google Scholar] [CrossRef]
- Zheng, K.; Kang, J.; Rutkowski, B.; Gaweda, M.; Zhang, J.; Wang, Y.; Founier, N.; Sitarz, M.; Taccardi, N.; Boccaccini, A.R. Toward highly dispersed mesoporous bioactive glass nanoparticles with high Cu concentration using Cu/ascorbic acid complex as precursor. Front. Chem. 2019, 7, 497. [Google Scholar] [CrossRef]
- Tavakoli, M.; Bateni, E.; Rismanchian, M.; Fathi, M.; Doostmohammadi, A.; Rabiei, A.; Sadeghi, H.; Etebari, M.; Mirian, M. Genotoxicity effects of nano bioactive glass and Novabone bioglass on gingival fibroblasts using single cell gel electrophoresis (comet assay): An in vitro study. Dent. Res. J. 2012, 9, 314–320. [Google Scholar]
- Borges, R.; Pelosine, A.M.; de Souza, A.C.S.; Machado, J., Jr.; Justo, G.Z.; Gamarra, L.F.; Marchi, J. Bioactive glasses as carriers of cancer-targeted drugs: Challenges and opportunities in bone cancer treatment. Materials 2022, 15, 9082. [Google Scholar] [CrossRef]
- Andrade, A.L.; Cavalcante, L.C.D.; Fabris, J.D.; Pereira, M.C.; Fernandez-Outon, L.E.; Pedersoli, D.C.; Ardisson, J.D.; Domingues, R.Z.; Ferreira, J.M.F. Magnetically induced heating by iron oxide nanoparticles dispersed in liquids of different viscosities. Ceram. Int. 2020, 46, 21496–21504. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Waldron, R.D. Infrared Spectra of Ferrites. Phys. Rev. 1955, 99, 1727–1735. [Google Scholar] [CrossRef]
- Ouasri, A.; Rhandour, A.; Dhamelincourt, M.C.; Dhamelincourt, P.; Mazzah, A. Vibrational study of (CH3)4NSbCl6 and [(CH3)4N]2SiF6. Spectrochim. Acta 2002, 58, 2779–2788. [Google Scholar] [CrossRef]
- Posset, U.; Locklin, E.; Thull, R.; Kiefer, W. Vibrational spectroscopic study of tetracalcium phosphate in pure polycrystalline form and as a constituent of a self-setting bone cement. J. Biomed. Mater. Res. 1998, 40, 640–645. [Google Scholar] [CrossRef]
- Serra, J.; Gonzalez, P.; Liste, S.; Serra, C.; Chiussi, S.; Leon, B.; Perez-Amor, M.; Ylanen, H.O.; Hupa, M. FTIR and XPS studies of bioactive silica based glasses. J. Non-Cryst. Solids 2003, 332, 20–27. [Google Scholar] [CrossRef]
- Du, G.H.; Liu, Z.L.; Xia, X.; Chu, Q.; Zhang, S.M. Characterization and application of Fe3O4/SiO2 nanocomposites. J. Sol.-Gel. Sci. Techn. 2006, 39, 285–291. [Google Scholar] [CrossRef]
- Serra, J.; Gonzalez, P.; Liste, S.; Chiussi, S.; Leon, B.; Perez-Amor, M.; Ylanen, H.O.; Hupa, M. Influence of the non-brindging oxygen groups on the bioactivity of silicate glasses. J. Mater. Sci.: Mater. Med. 2002, 13, 1221–1225. [Google Scholar] [CrossRef]
- Arcos, D.; Greenspan, D.C.; Vallet-Regi, M. A new quantitative method to evaluate the in vitro bioactivity of melt and sol-gel-derived silicate glasses. J. Biomed. Mater. Res.-Part A 2003, 65, 344–351. [Google Scholar] [CrossRef]
- Bertran, C.A.; Bueno, O.V.M. Combustion Synthesis of 58S Bioglass Using Sol-Gel Self-Propagating Combustion Method. Key Eng. Mater. 2014, 631, 36–42. [Google Scholar] [CrossRef]
- Li, Y.; Coughlan, A.; Laffir, F.R.; Pradhan, D.; Mellott, N.P.; Wren, A.W. Investigating the mechanical durability of bioactive glasses as a function of structure, solubility and incubation time. J. Non-Cryst. Solids 2013, 380, 25–34. [Google Scholar] [CrossRef]
- Elliott, J.C. Studies in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 1994; pp. 1–389. [Google Scholar]
- Pappas, G.S.; Liatsi, P.; Kartsonakis, I.A.; Danilidis, I.; Kordas, G. Synthesis and characterization of new SiO2–CaO hollow nanospheres by sol–gel method: Bioactivity of the new system. J. Non-Cryst. Solids 2008, 354, 755–760. [Google Scholar] [CrossRef]
- Paudel, A.; den Mooter, G.V. Influence of solvent composition on the miscibility and physical stability of naproxen/PVP K 25 solid dispersions prepared by cosolvent spray-drying. Pharm. Res. 2012, 29, 251–270. [Google Scholar] [CrossRef]
- dos Santos, V.M.R.; Novack, K.M.; de Sousa, D.V.M.; Oliveira, S.R.; Donnici, C.L. Síntese e caracterização de novos copolímeros fosforilados. Polim. Cienc. Tecnol. 2015, 25, 19–24. [Google Scholar] [CrossRef]
- Silveira, B.M.; Novack, K.M.; Marcondes, H.C.; dos Santos, V.M.R. Preparation, characterization, and biological activity against artemia salina of news copolymer PMMA-g-PEG derivatives incorporated with fluconazole. Macromol. Symp. 2018, 378, 1700066. [Google Scholar] [CrossRef]
- Allo, B.A.; Rizkalla, A.S.; Mequanint, K. Synthesis and electrospinning of ε-polycaprolactone-bioactive glass hybrid biomaterials via a sol-gel process. Langmuir 2010, 26, 18340–18348. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; Gonzalez, P.; Leon, B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Painter, P.C.; Park, Y.; Coleman, M.M. Thermodynamics of hydrogen bonding in polymer blends. 1. The application of association models. Macromolecules 1989, 22, 570–579. [Google Scholar] [CrossRef]
- Sousa, L.R.D.; Amparo, T.R.; de Souza, G.H.B.; Ferraz, A.T.; Fonseca, K.S.; de Azevedo, A.S.; do Nascimento, A.M.; Andrade, A.L.; Seibert, J.B.; Valverde, T.M.; et al. Anti-Trypanosoma cruzi potential of vestitol isolated from lyophilized red propolis. Molecules 2023, 28, 7812. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. International Organisation for Standardization: Geneva, Switzerland, 2009.
- Li, Y.; Kroger, M.; Liu, W.K. Endocytosis of PEGylated nanoparticles accompanied by structural and free energy changes of the grafted polyethylene glycol. Biomaterials 2014, 35, 8467–8478. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef]
- Shimizu, C.; Kubo, M.; Takano, K.; Takano, A.; Kijima, H.; Saji, H.; Katsuyama, I.; Sasano, H.; Koike, T. Interleukin-6 (IL-6) producing phaeochromocytoma: Direct IL-6 suppression by non-steroidal anti-inflammatory drugs. Clin. Endocrinol. 2001, 54, 405–410. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Yuan, X.; Xie, J.; Leong, D.T. The influence of lysosomal stability of silver nanomaterials on their toxicity to human cells. Biomaterials 2014, 35, 6707–6715. [Google Scholar] [CrossRef]
- Su, J.Y.; Chen, S.H.; Chen, Y.P.; Chen, W.C. Evaluation of magnetic nanoparticle- labeled chondrocytes cultivated on a type II collagen-chitosan/poly(lactic-co-glycolic) acid biphasic scaffold. Int. J. Mol. Sci. 2017, 18, 87. [Google Scholar] [CrossRef]
- Wen, J.; Li, H.; Dai, H.; Hua, S.; Long, X.; Li, H.; Ivanovski, S.; Xu, C. Intra-articular nanoparticles based therapies for osteoarthritis and rheumatoid arthritis management. Mater. Today Bio 2023, 19, 100597. [Google Scholar] [CrossRef]
- Marchandet, L.; Lallier, M.; Charrier, C.; Baudhuin, M.; Ory, B.; Lamoureux, F. Mechanisms of resistance to conventional therapies for osteosarcoma. Cancers 2021, 13, 683. [Google Scholar] [CrossRef]
- Endo-Munoz, L.; Cumming, A.; Sommerville, S.; Dickinson, I.; Saunders, N.A. Osteosarcoma is characterised by reduced expression of markers of osteoclastogenesis and antigen presentation compared with normal bone. Br. J. Cancer 2010, 103, 73–81. [Google Scholar] [CrossRef]
- Queiroz, S.L.; Batista, A.A. Biological functions of nitric oxide. Quím. Nova 1999, 22, 584–590. [Google Scholar] [CrossRef]
- Syahida, A.; Israf, D.A.; Permana, D.; Lajis, N.H.; Khozirah, S.; Afiza, A.W.; Khaizurin, T.A.; Somchit, M.N.; Sulaiman, M.R.; Nasaruddin, A.A. Atrovirinone inhibits pro-inflammatory mediator release from murine macrophages and human whole blood. Immunol. Cell Biol. 2006, 84, 250–258. [Google Scholar] [CrossRef]
- Abramson, S.B.; Amin, A.R.; Clancy, R.M.; Attur, M. The role of nitric oxide in tissue destruction. Best Pract. Res. Clin. Rheumatol. 2001, 15, 831–845. [Google Scholar] [CrossRef]
- Hawkey, C.J. COX-1 and COX-2 inhibitors. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 801–820. [Google Scholar] [CrossRef]
- Kang, J.S.; Jeon, Y.J.; Kim, H.M.; Han, S.H.; Yang, K.H. Inhibition of inducible nitric-oxide synthase expression by silymarin in lipopolysaccharide-stimulated macrophages. J. Pharmacol. Exp. Ther. 2002, 302, 138–144. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Dai, J.; Wang, X.; Shen, S.G. Overexpression of Dlx2 enhances osteogenic differentiation of BMSCs and MC3T3-E1 cells via direct upregulation of Osteocalcin and Alp. Int. J. Oral Sci. 2019, 11, 12. [Google Scholar] [CrossRef]
- Hart, N.H.; Newton, R.U.; Tan, J.; Rantalainen, T.; Chivers, P.; Siafarikas, A.; Nimphius, S. Biological basis of bone strength: Anatomy, physiology and measurement. J. Musculoskelet. Neuronal Interact. 2020, 20, 347–371. [Google Scholar]
- Lopes, D.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275. [Google Scholar] [CrossRef]
- Valverde, T.M.; Castro, E.G.; Cardoso, M.H.S.; Martins-Junior, P.A.; Souza, L.M.O.; Silva, P.P.; Ladeira, L.O.; Kitten, G.T. A novel 3D bone-mimetic scaffold composed of collagen/MTA/MWCNT modulates cell migration and osteogenesis. Life Sci. 2016, 162, 115–124. [Google Scholar] [CrossRef]
- Dantas, P.C.L.; Martins-Junior, P.A.; Coutinho, D.C.O.; Andrade, V.B.; Valverde, T.M.; Avila, E.S.; Almeida, T.C.S.; Queiroz-Junior, C.M.; Sa, M.A.; Góes, A.M.; et al. Nanohybrid composed of graphene oxide functionalized with sodium hyaluronate accelerates bone healing in the tibia of rats. Mater. Sci. Eng. C 2021, 123, 111961. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.; Guo, Z.; Zhang, G.; Zhang, H. Iron oxide nanoparticles combined with static magnetic fields in bone remodeling. Cells 2022, 11, 3298. [Google Scholar] [CrossRef]
- Ha, S.W.; Weitzmann, M.N.; Beck, G.R., Jr. Bioactive silica nanoparticles promote osteoblast differentiation through stimulation of autophagy and direct association with LC3 and p62. ACS Nano 2014, 8, 5898–5910. [Google Scholar] [CrossRef]
- Queiroz, P.M.; Barrioni, B.; Valverde, T.M.; Goes, A.M.; Gomes, D.A.; Pereira, M.M. Study of the manganese and calcium synergetic influence on mesoporous bioactive glass characteristic. J. Non-Cryst. Solids 2023, 622, 122656. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; D’Alessandro, T.; Sandri, M.; Russo, A.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Magnetic hydroxyapatite bone substitutes to enhance tissue regeneration: Evaluation in vitro using osteoblast-like cells and in vivo in a bone defect. PLoS ONE 2012, 7, e38710. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Yan, X.Z.; Yang, W.; Yang, F.; Kersten-Niessen, M.; Jansen, J.A.; Both, S.K. Effects of continuous passaging on mineralization of MC3T3-E1 cells with improved osteogenic culture protocol. Tissue Eng. Part C Methods 2014, 20, 198–204. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 16, 55–263. [Google Scholar] [CrossRef] [PubMed]
- Bitonto, V.; Garello, F.; Scherberich, A.; Filippi, M. Prussian blue staining to visualize iron oxide nanoparticles. Methods Mol. Biol. 2023, 2566, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, F.S.; Lopez, J.L.; Lacerda, S.M.S.N.; Procopio, M.S.; Figueiredo, A.F.A.; Martins, E.M.N.; Guimaraes, P.P.G.; Ladeira, L.O.; Kitten, G.T.; Dias, F.F.; et al. Biotechnological approach to induce human fibroblast apoptosis using superparamagnetic iron oxide nanoparticles. J. Inorg. Biochem. 2020, 206, 111017. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Gloglowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and (15 N)-nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valverde, T.M.; dos Santos, V.M.R.; Viana, P.I.M.; Costa, G.M.J.; de Goes, A.M.; Sousa, L.R.D.; Xavier, V.F.; Vieira, P.M.d.A.; de Lima Silva, D.; Domingues, R.Z.; et al. Novel Fe3O4 Nanoparticles with Bioactive Glass–Naproxen Coating: Synthesis, Characterization, and In Vitro Evaluation of Bioactivity. Int. J. Mol. Sci. 2024, 25, 4270. https://doi.org/10.3390/ijms25084270
Valverde TM, dos Santos VMR, Viana PIM, Costa GMJ, de Goes AM, Sousa LRD, Xavier VF, Vieira PMdA, de Lima Silva D, Domingues RZ, et al. Novel Fe3O4 Nanoparticles with Bioactive Glass–Naproxen Coating: Synthesis, Characterization, and In Vitro Evaluation of Bioactivity. International Journal of Molecular Sciences. 2024; 25(8):4270. https://doi.org/10.3390/ijms25084270
Chicago/Turabian StyleValverde, Thalita Marcolan, Viviane Martins Rebello dos Santos, Pedro Igor Macário Viana, Guilherme Mattos Jardim Costa, Alfredo Miranda de Goes, Lucas Resende Dutra Sousa, Viviane Flores Xavier, Paula Melo de Abreu Vieira, Daniel de Lima Silva, Rosana Zacarias Domingues, and et al. 2024. "Novel Fe3O4 Nanoparticles with Bioactive Glass–Naproxen Coating: Synthesis, Characterization, and In Vitro Evaluation of Bioactivity" International Journal of Molecular Sciences 25, no. 8: 4270. https://doi.org/10.3390/ijms25084270
APA StyleValverde, T. M., dos Santos, V. M. R., Viana, P. I. M., Costa, G. M. J., de Goes, A. M., Sousa, L. R. D., Xavier, V. F., Vieira, P. M. d. A., de Lima Silva, D., Domingues, R. Z., Ferreira, J. M. d. F., & Andrade, Â. L. (2024). Novel Fe3O4 Nanoparticles with Bioactive Glass–Naproxen Coating: Synthesis, Characterization, and In Vitro Evaluation of Bioactivity. International Journal of Molecular Sciences, 25(8), 4270. https://doi.org/10.3390/ijms25084270








