Current Fertility Preservation Steps in Young Women Suffering from Cancer and Future Perspectives
Abstract
1. Introduction
2. Anatomy and Physiology of Ovarian Tissue
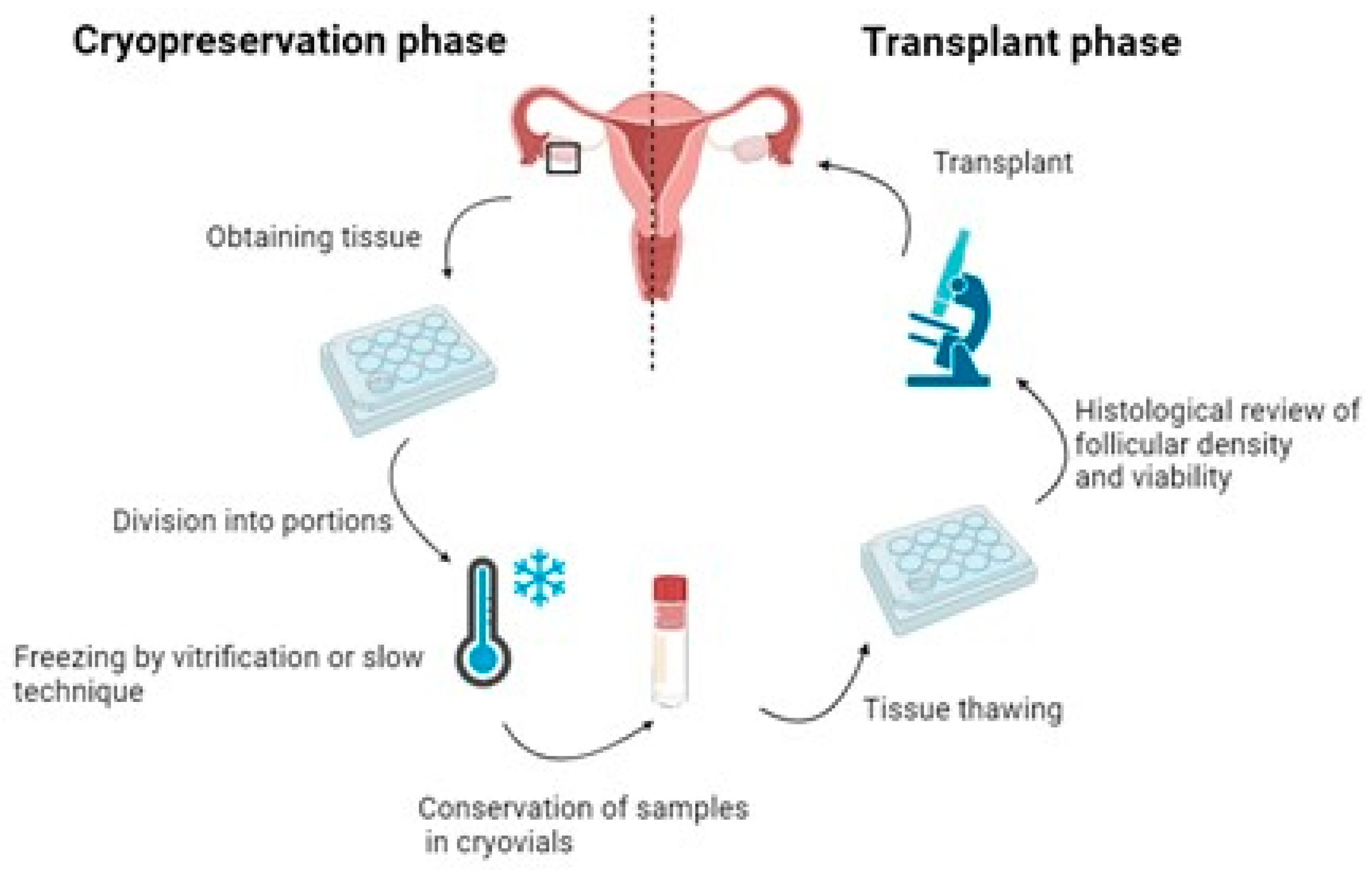
3. Steps in the Current Fertility Preservation Protocols
3.1. Ovarian Tissue Extraction
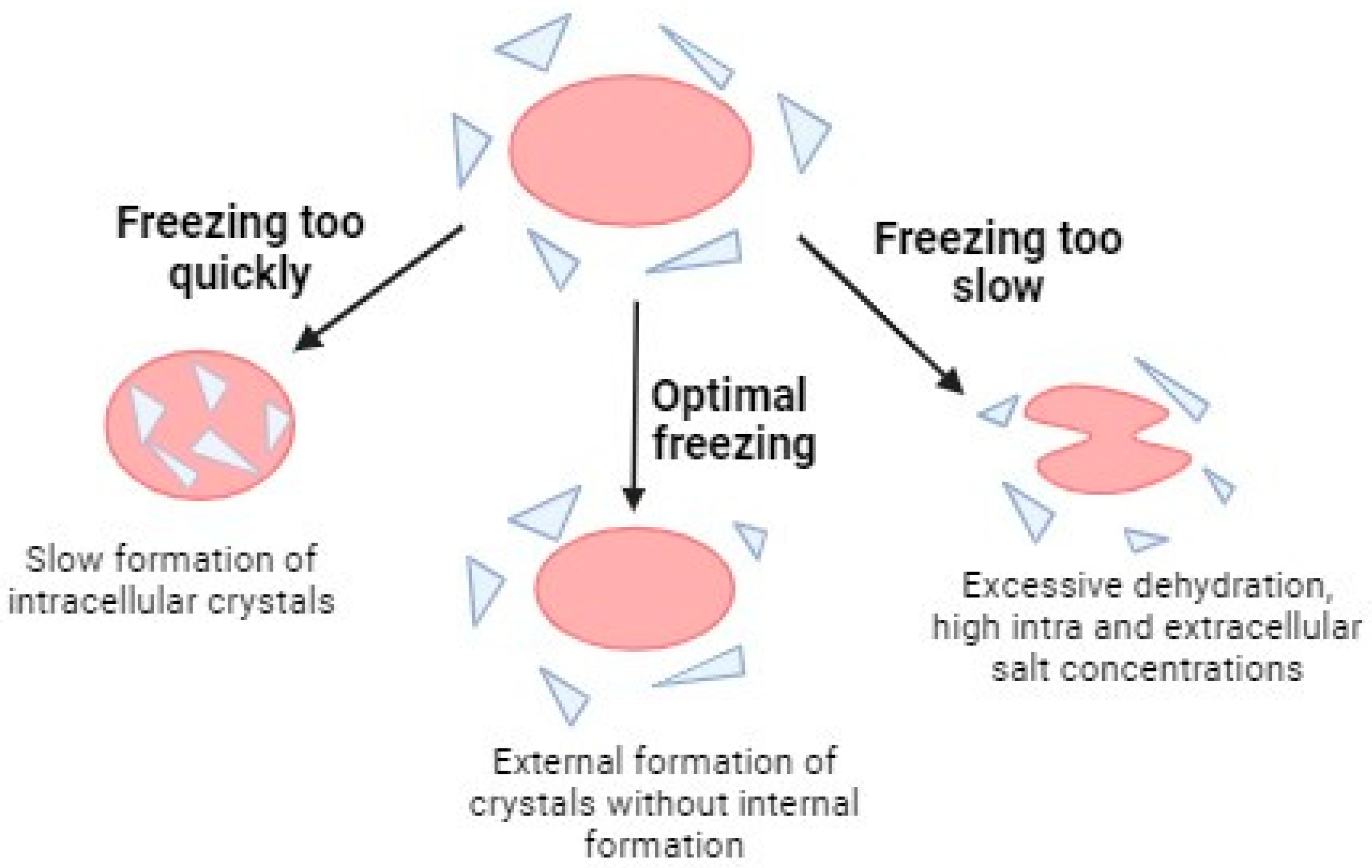
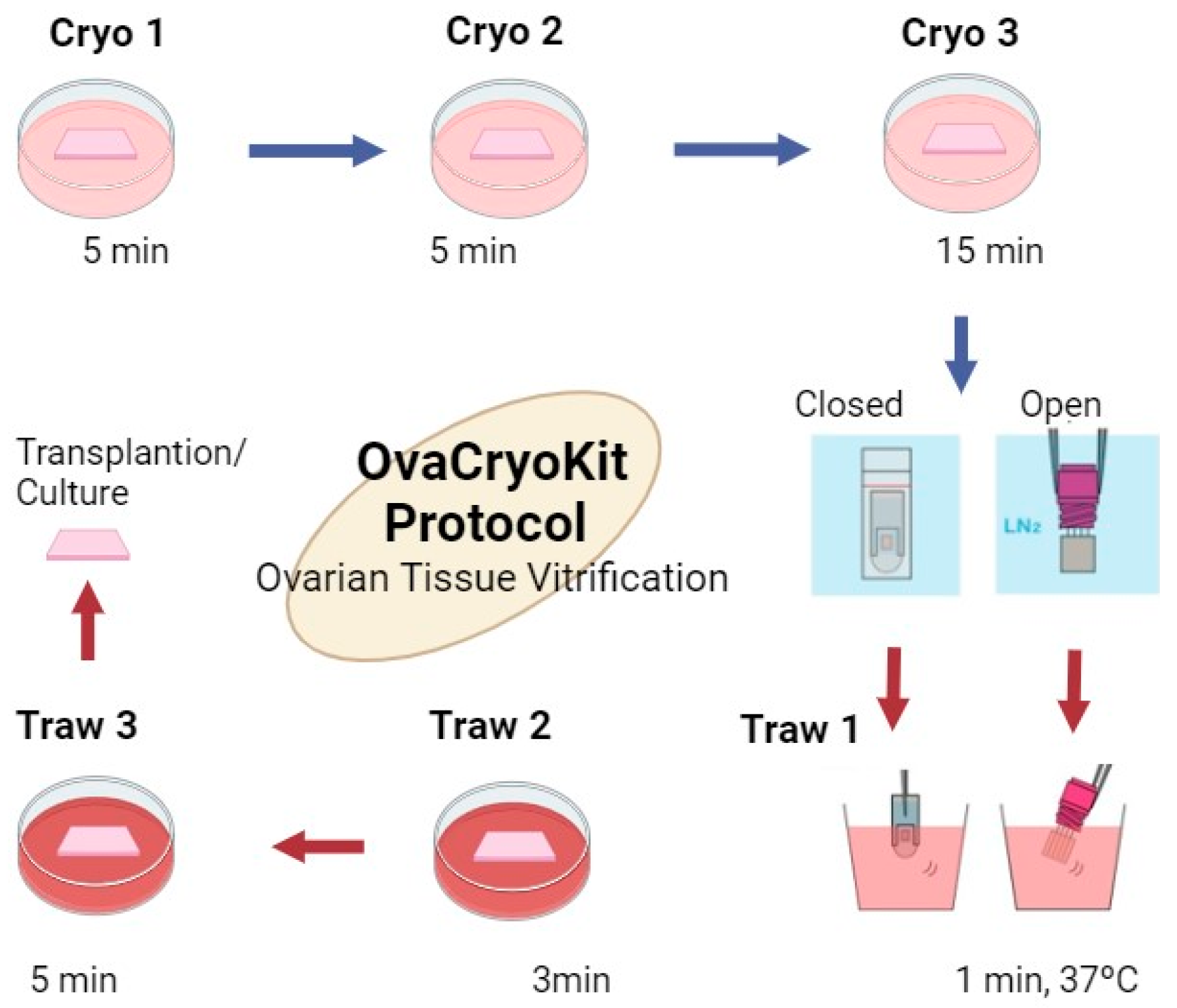
3.2. Freezing Methods
3.3. Ovarian Tissue Thawing and Transplantation
4. Risks Associated with Ovarian Tissue Transplant
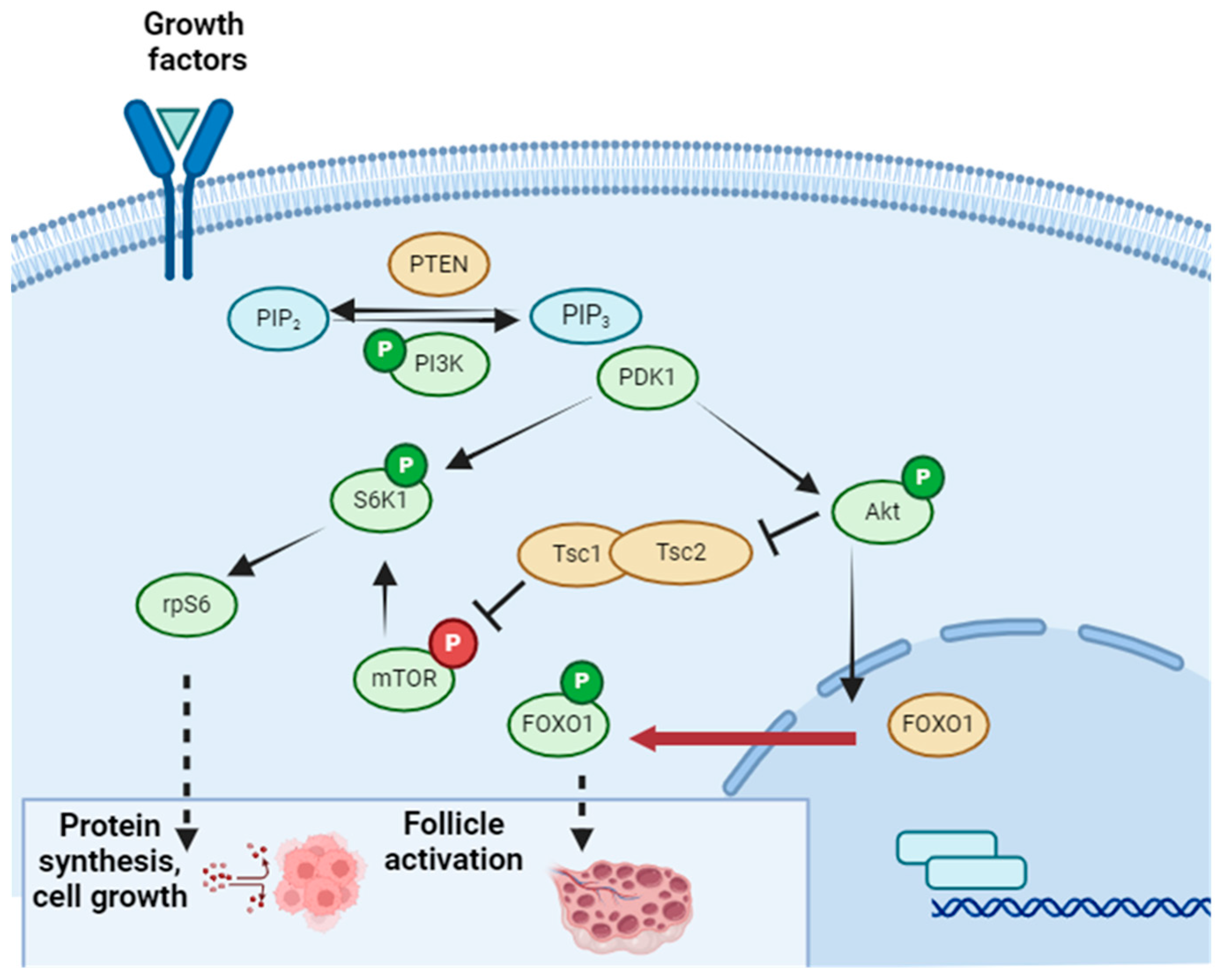
5. In Vitro Oogenesis from Stem Cells and In Vitro Follicle Growth Protocol for Avoiding Transplant
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Pesce, R.; Marconi, M.; Vélez, C.; Marconi, G.; Glujovsky, D.; Baronio, M.; Coscia, A. Preservación de la fertilidad. Reproducción 2017, 32, 34–39. [Google Scholar]
- World Health Organization [Internet]. 2021. Available online: https://www.who.int/es (accessed on 12 December 2022).
- Spears, N.; Lopes, F.; Stefansdottir, A.; Rossi, V.; De Felici, M.; Anderson, R.A.; Klinger, F.G. Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update 2019, 25, 673–693. [Google Scholar] [CrossRef] [PubMed]
- Sheshpari, S.; Shahnazi, M.; Mobarak, H.; Ahmadian, S.; Bedate, A.M.; Nariman-Saleh-Fam, Z.; Nouri, M.; Rahbarghazi, R.; Mahdipour, M. Ovarian function and reproductive outcome after ovarian tissue transplantation: A systematic review. J. Transl. Med. 2019, 17, 396. [Google Scholar] [CrossRef] [PubMed]
- Batiza Resendiz, V.A.; Aguilar Melgar, A.; Luna Rojas, R.M.; Pérez-Peña, E.; Gutiérrez Gutiérrez, A.; Ruvalcaba Castrellón, L.A.; Salazar López-Ortiz, C.; Michel Vergara, J.A.; Shaw Dulin, R.J.; Barquet Muñoz, S.A.; et al. Preservación de la fertilidad: Opinión de un grupo de expertos. Ginecol. Obs. Mex. 2020, 88, 767–805. [Google Scholar]
- Leiro, F.O.; Bianchi, R. Preservación de la fertilidad. Rev. Argent. De Coloproctología 2021, 32, 56–57. [Google Scholar]
- Bedoschi, G.; Navarro, P.A.; Oktay, K. Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Future Oncol. 2016, 12, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Wallace, W.H.B.; Telfer, E.E. Ovarian tissue cryopreservation for fertility preservation: Clinical and research perspectives. Hum. Reprod. Open 2017, 2017, hox001. [Google Scholar] [CrossRef]
- Chen, J.; Han, Y.; Shi, W.; Yan, X.; Shi, Y.; Yang, Y.; Gao, H.; Li, Y. Ovarian tissue bank for fertility preservation and anti-menopause hormone replacement. Front. Endocrinol. 2022, 13, 950297. [Google Scholar] [CrossRef]
- Poirot, C.; Brugieres, L.; Yakouben, K.; Prades-Borio, M.; Marzouk, F.; de Lambert, G.; Pacquement, H.; Bernaudin, F.; Neven, B.; Paye-Jaouen, A.; et al. Ovarian tissue cryopreservation for fertility preservation in 418 girls and adolescents up to 15 years of age facing highly gonadotoxic treatment. Twenty years of experience at a single center. Acta Obstet. Et Gynecol. Scand. 2019, 98, 630–637. [Google Scholar] [CrossRef]
- Marin, L.; Bedoschi, G.; Kawahara, T.; Oktay, K.H. History, Evolution and Current State of Ovarian Tissue Auto-Transplantation with Cryopreserved Tissue: A Successful Translational Research Journey from 1999 to 2020. Reprod. Sci. 2020, 27, 955–962. [Google Scholar] [CrossRef]
- van der Perk, M.M.; van der Kooi, A.-L.L.; Bos, A.M.; Broer, S.L.; Veening, M.A.; van Leeuwen, J.; van Santen, H.M.; van Dorp, W.; Heuvel-Eibrink, M.M.v.D. Oncofertility Perspectives for Girls with Cancer. J. Pediatr. Adolesc. Gynecol. 2022, 35, 523–526. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, J.R. Ovarian Cryopreservation and Transplantation. Fertil. Preserv. 2021, 243–259. [Google Scholar] [CrossRef]
- Gosden, R.G.; Robert, T.; Morris, M.D. Appreciation of an enlightened surgeon and pioneer of ovarian transplantation. Fertil. Steril. 2010, 94, 1960–1963. [Google Scholar] [CrossRef]
- Baird, D.T.; Webb, R.; Campbell, B.K.; Harkness, L.M.; Gosden, R.G. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 °C. Endocrinology 1999, 140, 462–471. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Demylle, D.; Jadoul, P.; Pirard, C.; Squifflet, J.; Martinez-Madrid, B.; Van Langendonckt, A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Obs. Gynecol. 2005, 105, 214. [Google Scholar] [CrossRef]
- Ernst, E.; Kjærsgaard, M.; Birkebæk, N.H.; Clausen, N.; Andersen, C.Y. Stimulation of puberty in a girl with chemo- and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur. J. Cancer 2013, 49, 911–914. [Google Scholar] [CrossRef]
- Fuentealba, I. Quistes ováricos en recién nacidas, niñas y adolescentes: Aspectos ultrasonográficos. Rev. Chil. De Radiol. 2006, 12, 15–20. [Google Scholar] [CrossRef]
- Fan, Y.; Flanagan, C.L.; Brunette, M.A.; Jones, A.S.; Baker, B.M.; Silber, S.J.; Shikanov, A. Fresh and cryopreserved ovarian tissue from deceased young donors yields viable follicles. F S Sci. 2021, 2, 248–258. [Google Scholar] [CrossRef]
- Christianson, M.S.; Lukish, D.A.; McCarter, R.; Pryor, H.; Lukish, J.R. Ovarian tissue cryopreservation in young females with cancer and its impact on ovarian follicle density. J. Pediatr. Surg. 2021, 56, 2354–2359. [Google Scholar] [CrossRef]
- Ruiz-Hoyos, B.M. Evaluación de la reserva ovárica: Pasado, presente y futuro. Rev. Med. 2020, 28, 77–88. [Google Scholar] [CrossRef]
- Clark, R.A.; Mostoufi-Moab, S.; Yasui, Y.; Vu, N.K.; Sklar, C.A.; Motan, T.; Brooke, R.; Gibson, T.; Oeffinger, K.C.; Howell, R.; et al. Predicting acute ovarian failure in female survivors of childhood cancer: A cohort study in the Childhood Cancer Survivor Study (CCSS) and the St Jude Lifetime Cohort (SJLIFE). Lancet Oncol. 2020, 21, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Capecce, E.; Pelanda, M.; Dicugno, M.; de Sampaio, E.G.; Buongiorno, G.; Corazza, N.; Peñaloza, M.; Sequera, A.M.; Ruibal, G. La hormona antimülleriana como marcador de función ovárica. Rev. Argent. De Endocrinol. Y Metab. 2016, 53, 106–113. [Google Scholar] [CrossRef]
- Kim, S.S. Assessment of long term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J. Assist. Reprod. Genet. 2012, 29, 1153. [Google Scholar] [CrossRef][Green Version]
- Andersen, C.Y.; Mamsen, L.S.; Kristensen, S.G. Fertility preservation: Freezing of ovarian tissue and clinical opportunities. Reproduction 2019, 158, F27–F34. [Google Scholar] [CrossRef] [PubMed]
- Lukish, J.R. Laparoscopic assisted extracorporeal ovarian harvest: A novel technique to optimize ovarian tissue for cryopreservation in young females with cancer. J. Pediatr. Surg. 2021, 56, 626–628. [Google Scholar] [CrossRef]
- Del-Pozo-Lerida, S.; Salvador, C.; Martinez-Soler, F.; Tortosa, A.; Perucho, M.; Gimenez-Bonafe, P. Preservation of fertility in patients with cancer (Review) [Internet]. Oncol. Rep. 2019, 41, 2607–2614. [Google Scholar] [PubMed]
- Lantsberg, D.; Farhi, A.; Zaslavsky-Paltiel, I.; Silverman, B.G.; Lerner-Geva, L.; Orvieto, R. Deliveries following fertility preservation by ovarian tissue cryopreservation without autotransplantation—What should be expected? J. Assist. Reprod. Genet. 2019, 36, 335–340. [Google Scholar] [CrossRef]
- Lee, S.; Ozkavukcu, S.; Ku, S.Y. Current and Future Perspectives for Improving Ovarian Tissue Cryopreservation and Transplantation Outcomes for Cancer Patients. Reprod. Sci. 2021, 28, 1746–1758. [Google Scholar] [CrossRef]
- Hossay, C.; Donnez, J.; Dolmans, M.M. Whole Ovary Cryopreservation and Transplantation: A Systematic Review of Challenges and Research Developments in Animal Experiments and Humans. J. Clin. Med. 2020, 9, 3196. [Google Scholar] [CrossRef]
- Parihar, A.; Kumar, A.; Panda, U.; Khan, R.; DSParihar, R. Khan. Criopreservación: Una descripción general completa, desafíos y perspectivas futuras. Adv. Biol. 2023, 7, 2200285. [Google Scholar]
- Whaley, D.; Damyar, K.; Witek, R.P.; Mendoza, A.; Alexander, M.; Lakey, J.R.T. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transpl. 2021, 30, 0963689721999617. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Shimada, M. Pretreatment of ovaries with collagenase before vitrification keeps the ovarian reserve by maintaining cell-cell adhesion integrity in ovarian follicles. Sci. Rep. 2020, 10, 6841. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.D.; Soares, M.M.; de Cássia Antonino, D.; Júnior, J.M.; Mohallem, R.F.F.; Rodrigues, A.P.R.; Figueiredo, J.R.; Beletti, M.E.; Jacomini, J.O.; Alves, B.G.; et al. Positiveeffect of resveratrol against preantral follicles degenerationafter ovarian tissue vitrification. Theriogenology 2018, 114, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.C.; Liu, X.C.; Yang, S.H.; Song, L.L.; Zhou, S.J.; Deng, S.L.; Tian, L.; Cheng, L.Y. Melatonin Inhibits Oxidative Stress and Apoptosis in Cryopreserved Ovarian Tissues via Nrf2/HO-1 Signaling Pathway. Front. Mol. Biosci. 2020, 29, 163. [Google Scholar] [CrossRef] [PubMed]
- Luz, H.K.M.; Santos, R.R.; Wanderley, L.S.; Faustino, L.R.; Silva, C.M.G.; Carvalho, A.A.; Campello, C.C.; Santos, F.W.; Figueiredo, J.R.; Rodrigues, A.P.R. Catalase preventslipid peroxidation and enhances survival of caprine preantralfollicles cryopreserved in a 1, 2-propanediol-freezing medium. Biopreserv. Biobank 2012, 10, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.; Mbemya, G.T.; Guerreiro, D.D.; Brito, D.C.C.; Donfack, N.J.; Morais, M.L.G.S.; Rodrigues, G.Q.; Bruno, J.B.; Rocha, R.M.P.; Alves, B.G.; et al. Effect of catalaseor alpha lipoic acid supplementation in the vitrification solution of ovine ovarian tissue. Biopreserv. Biobank 2018, 16, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Lierman, S.; Bus, A.; Andries, S.; Trias, E.; Bols, P.E.J.; Tilleman, K. Passive slow freezing is an efficacious and cost-effective alternative to controlled slow freezing for ovarian tissue cryopreservation. Cryobiology 2021, 100, 164–172. [Google Scholar] [CrossRef]
- Gosden, R.G. General principles of cryopreservation. Methods Mol. Biol. 2014, 1154, 261–268. [Google Scholar] [PubMed]
- Murray, K.A.; Gibson, M.I. Chemical approaches to cryopreservation. Nat. Rev. Chem. 2022, 6, 579–593. [Google Scholar] [CrossRef]
- Gook, D.A. Human ovarian tissue slow freezing. Methods Mol. Biol. 2017, 1568, 161–176. [Google Scholar]
- Herraiz, S.; Novella-Maestre, E.; Rodríguez, B.; Díaz, C.; Sánchez-Serrano, M.; Mirabet, V.; Pellicer, A. Improving ovarian tissue cryopreservation for oncologic patients: Slow freezing versus vitrification, effect of different procedures and devices. Fertil. Steril. 2014, 101, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, S.; Cervelló, I. New insights for fertility preservation by ovarian tissue cryopreservation and transplantation in pediatric cancer patients. Fertil. Steril. 2020, 114, 1191. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.S.; Kim, E.J.; Youm, H.W.; Kim, S.K.; Lee, J.R.; Suh, C.S.; Kim, S.H. Improvement in ovarian tissue quality with supplementation of antifreeze protein during warming of vitrified mouse ovarian tissue. Yonsei Med. J. 2018, 59, 331–336. [Google Scholar] [CrossRef] [PubMed]
- KITAZATOCORPORATION Ova Cryo Kit- Ovarian Tissue Vitrification Kit. 2021. 2021. Available online: https://kitazato-ivf.com/vitrification/ovarian-tissue-vitrification/ (accessed on 6 March 2024).
- Shi, Q.; Xie, Y.; Wang, Y.; Li, S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 8538. [Google Scholar] [CrossRef] [PubMed]
- Kometas, M.; Christman, G.M.; Kramer, J.; Rhoton-Vlasak, A. Methods of Ovarian Tissue Cryopreservation: Is Vitrification Superior to Slow Freezing? Ovarian Tissue Freezing Methods. Reprod. Sci. 2021, 28, 3291–3302. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yoshioka, N.; Takae, S.; Sugishita, Y.; Tamura, M.; Hashimoto, S.; Morimoto, Y.; Kawamura, K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum. Reprod. 2015, 30, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, R.; Vicenti, R.; Magnani, V.; Paradisi, R.; Lima, M.; De Meis, L.; Rossi, S.; Raimondo, D.; Casadio, P.; Venturoli, S.; et al. Ovarian tissue cryopreservation and transplantation: 20 years experience in Bologna University. Front. Endocrinol. 2022, 13, 1035109. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; Donnez, J.; Cacciottola, L. Fertility Preservation: The Challenge of Freezing and Transplanting Ovarian Tissue. Trends Mol. Med. 2021, 27, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; von Wolff, M.; Poirot, C.; Diaz-Garcia, C.; Cacciottola, L.; Boissel, N.; Liebenthron, J.; Pellicer, A.; Donnez, J.; Andersen, C.Y. Transplantation of cryopreserved ovarian tissue in a series of 285 women: A review of five leading European centers. Fertil. Steril. 2021, 115, 1102–1115. [Google Scholar] [CrossRef]
- Masciangelo, R.; Hossay, C.; Chiti, M.C.; Manavella, D.D.; Amorim, C.A.; Donnez, J.; Dolmans, M.M. Role of the PI3K and Hippo pathways in follicle activation after grafting of human ovarian tissue. J. Assist. Reprod. Genet. 2020, 37, 101–108. [Google Scholar] [CrossRef]
- Gu, R.; Ge, N.; Huang, B.; Fu, J.; Zhang, Y.; Wang, N.; Xu, Y.; Li, L.; Peng, X.; Zou, Y.; et al. Impacts of vitrification on the transcriptome of human ovarian tissue in patients with gynecological cancer. Front. Genet. 2023, 17, 1114650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, W.; Todorov, P.; Pei, C.; Isachenko, E.; Rahimi, G.; Mallmann, P.; Nawroth, F.; Isachenko, V. RNA Transcripts in Human Ovarian Cells: Two-Time Cryopreservation Does Not Affect Developmental Potential. Int. J. Mol. Sci. 2023, 24, 6880. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Ryu, K.J.; Lee, S.; Kim, T. Changes in Telomere Length and Senescence Markers During Human Ovarian Tissue Cryopreservation. Sci. Rep. 2021, 11, 2238. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nakamura, H.; Tabata, Y.; Fujimori, Y.; Kumasawa, K.; Kimura, T. Effect of sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogels on frozen-thawed human ovarian tissue in a xenograft model. J. Obstet. Gynaecol. Res. 2018, 44, 1947–1955. [Google Scholar] [CrossRef]
- Cacciottola, L.; Nguyen, T.Y.T.; Chiti, M.C.; Camboni, A.; Amorim, C.A.; Donnez, J.; Dolmans, M.M. Long-term advantages of ovarian reserve maintenance and follicle development using adipose tissue-derived stem cells in ovarian tissue transplantation. J. Clin. Med. 2020, 9, 2980. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, R.; Heytens, E.; Oktay, K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS ONE 2011, 6, e19475. [Google Scholar] [CrossRef] [PubMed]
- Grosbois, J.; Devos, M.; Demeestere, I. Implications of Nonphysiological Ovarian Primordial Follicle Activation for Fertility Preservation. Endocr. Rev. 2020, 41, 847–872. [Google Scholar] [CrossRef]
- Oktay, K.; Bedoschi, G.; Pacheco, F.; Turan, V.; Emirdar, V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am. J. Obs. Gynecol. 2016, 214, 94. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Marinescu, C.; Saussoy, P.; Van Langendonckt, A.; Amorim, C.; Donnez, J. Reimplantation of cryopreserved ovarian tissue in patients with acute lymphoblastic leukemia is potentially hazardous. Blood 2010, 116, 2908–2914. [Google Scholar] [CrossRef]
- Meirow, D.; Hardan, I.; Dor, J.; Fridman, E.; Elizur, S.; Ra’anani, H.; Slyusarevsky, E.; Amariglio, N.; Schiff, E.; Rechavi, G.; et al. Search for evidence of disease and contamination of malignant cells in stored ovarian tissue from hematologic cancer patients. Hum. Reprod. 2008, 23, 1007–1013. [Google Scholar] [CrossRef]
- Soares, M.; Saussoy, P.; Maskens, M.; Reul, H.; Amorim, C.A.; Donnez, J.; Dolmans, M.M. Elimination of malignant cells from cryopreserved ovarian tissue is possible in leukemia patients. HemaSphere 2017, 1, e5. [Google Scholar]
- Shapira, M.; Raanani, H.; Barshack, I.; Amariglio, N.; Derech-Haim, S.; Marciano, M.N.; Schiff, E.; Orvieto, R.; Meirow, D. First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertil. Steril. 2018, 109, 48–53. [Google Scholar] [CrossRef]
- Hikabe, O.; Hamazaki, N.; Nagamatsu, G.; Obata, Y.; Hirao, Y.; Hamada, N.; Shimamoto, S.; Imamura, T.; Nakashima, K.; Saitou, M.; et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016, 539, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Morohaku, K.; Tanimoto, R.; Sasaki, K.; Kawahara-Miki, R.; Kono, T.; Hayashi, K.; Hirao, Y.; Obata, Y. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc. Natl. Acad. Sci. USA 2016, 113, 9021–9026. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, M.; Yuan, Y.; Wang, X.; Fu, R.; Wan, H.; Xie, M.; Liu, M.; Guo, X.; Zheng, Y.; et al. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell 2016, 18, 330–340. [Google Scholar] [CrossRef]
- Panula, S.; Medrano, J.V.; Kee, K.; Bergström, R.; Nguyen, H.N.; Byers, B.; Wilson, K.D.; Wu, J.C.; Simon, C.; Hovatta, O.; et al. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum. Mol. Genet. 2011, 20, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Easley, C.A., 4th; Phillips, B.T.; McGuire, M.M.; Barringer, J.M.; Valli, H.; Hermann, B.P.; Simerly, C.R.; Rajkovic, A.; Miki, T.; Orwig, K.E.; et al. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell. Rep. 2012, 2, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhu, L.; Jin, L. In vitro culture of human follicle including activation, growth, and maturation: A review of research progress. Front. Endocrinol. 2020, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Telfer, E.E.; McLaughlin, M. In vitro development of ovarian follicles. Semin. Reprod. Med. 2011, 29, 15–23. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, J.; Romero, M.M.; Smith, K.N.; Shea, L.D.; Woodruff, T.K. In vitro follicle growth supports meiotic maturation of human oocytes. Sci. Rep. 2015, 5, 17323. [Google Scholar] [CrossRef]
- Marin, D.; Yang, M.; Wang, T. In vitro growth of human ovarian follicles for fertility preservation. Reprod. Med. Dev. 2018, 2, 230–236. [Google Scholar] [CrossRef]
- Telfer, E.E. Future developments: In vitro growth (IVG) of human ovarian follicles. Acta Obstet. Gynecol. Scand. 2019, 98, 653–658. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marco, A.; Gargallo, M.; Ciriza, J.; Shikanov, A.; Baquedano, L.; García Pérez-Llantada, J.; Malo, C. Current Fertility Preservation Steps in Young Women Suffering from Cancer and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 4360. https://doi.org/10.3390/ijms25084360
Marco A, Gargallo M, Ciriza J, Shikanov A, Baquedano L, García Pérez-Llantada J, Malo C. Current Fertility Preservation Steps in Young Women Suffering from Cancer and Future Perspectives. International Journal of Molecular Sciences. 2024; 25(8):4360. https://doi.org/10.3390/ijms25084360
Chicago/Turabian StyleMarco, Alicia, Marta Gargallo, Jesús Ciriza, Ariella Shikanov, Laura Baquedano, Javier García Pérez-Llantada, and Clara Malo. 2024. "Current Fertility Preservation Steps in Young Women Suffering from Cancer and Future Perspectives" International Journal of Molecular Sciences 25, no. 8: 4360. https://doi.org/10.3390/ijms25084360
APA StyleMarco, A., Gargallo, M., Ciriza, J., Shikanov, A., Baquedano, L., García Pérez-Llantada, J., & Malo, C. (2024). Current Fertility Preservation Steps in Young Women Suffering from Cancer and Future Perspectives. International Journal of Molecular Sciences, 25(8), 4360. https://doi.org/10.3390/ijms25084360






