Antenna-Biased Odorant Receptor PstrOR17 Mediates Attraction of Phyllotreta striolata to (S)-Cis-Verbenol and (−)-Verbenone
Abstract
1. Introduction
2. Results
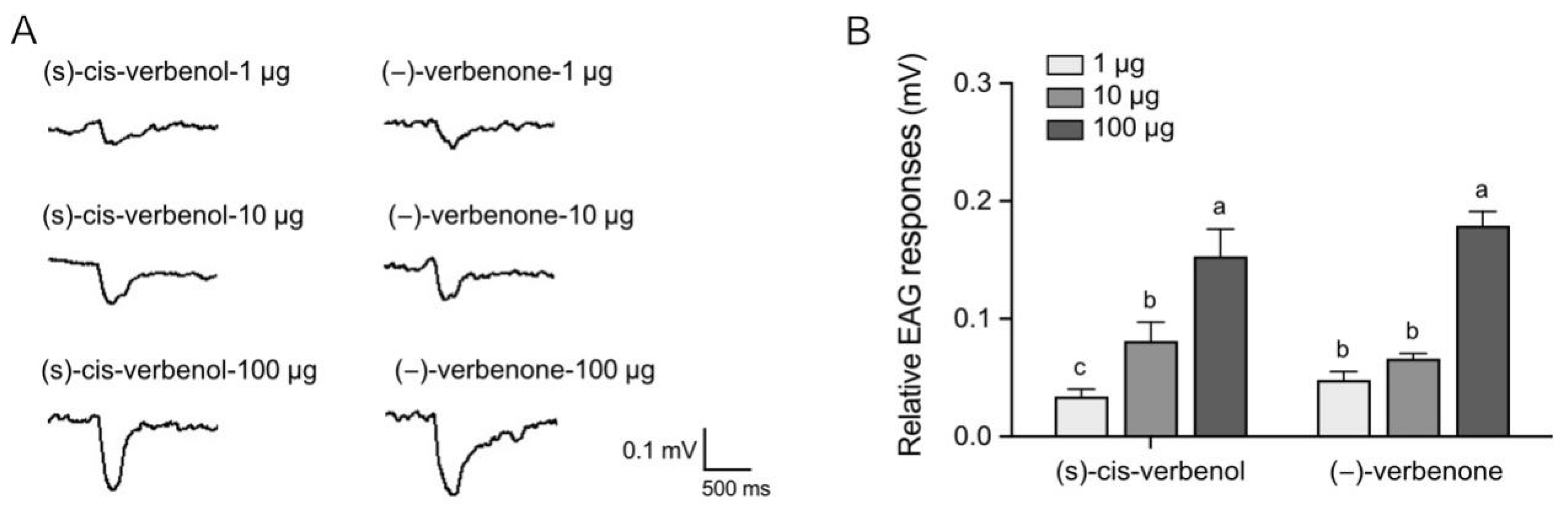
2.1. (−)-Verbenone and (S)-Cis-Verbenol Elicit Behavioral and Electrophysiological Responses in P. striolata Adults
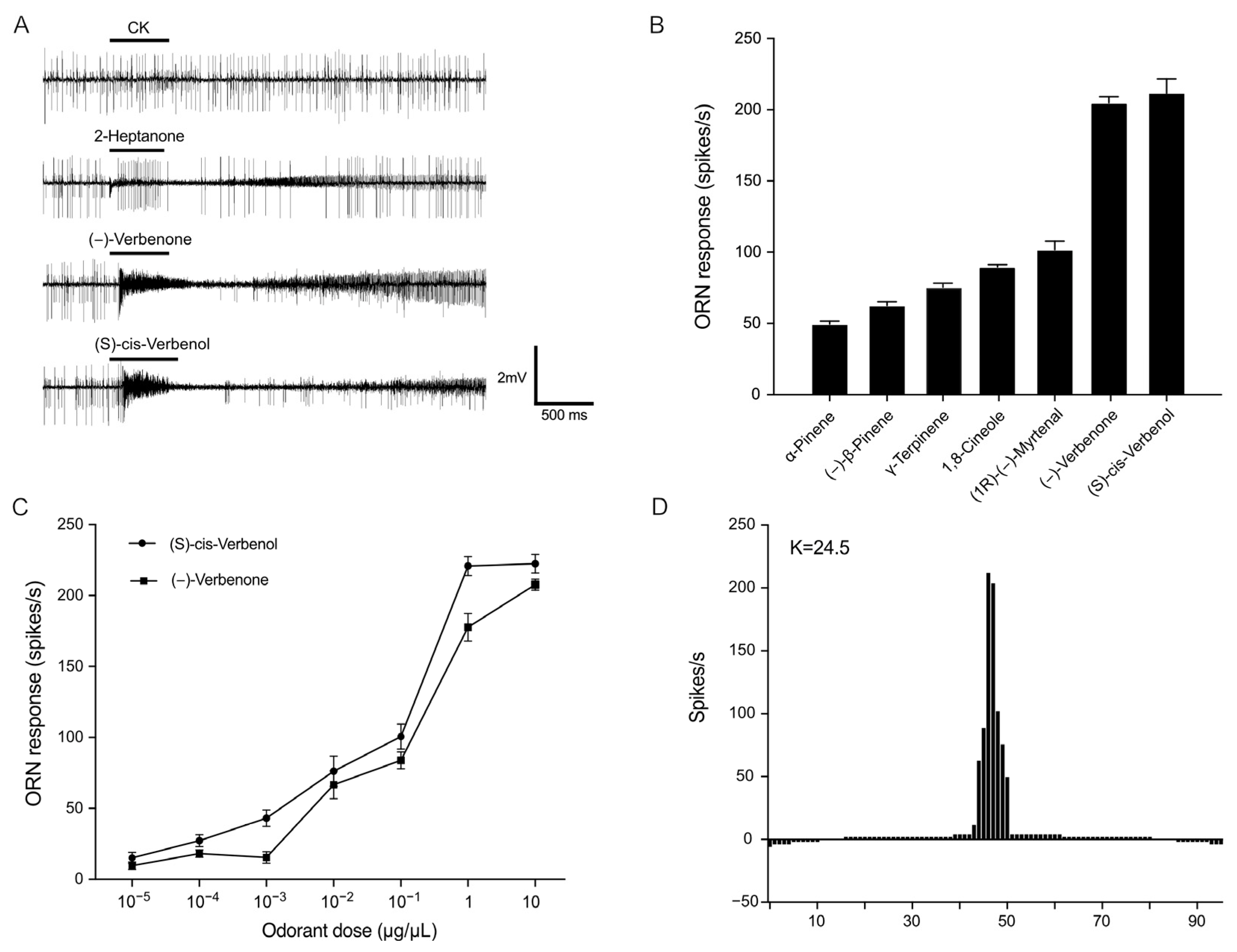
2.2. PstrOR17 Narrowly Tuned to (−)-Verbenone and (S)-Cis-Verbenol
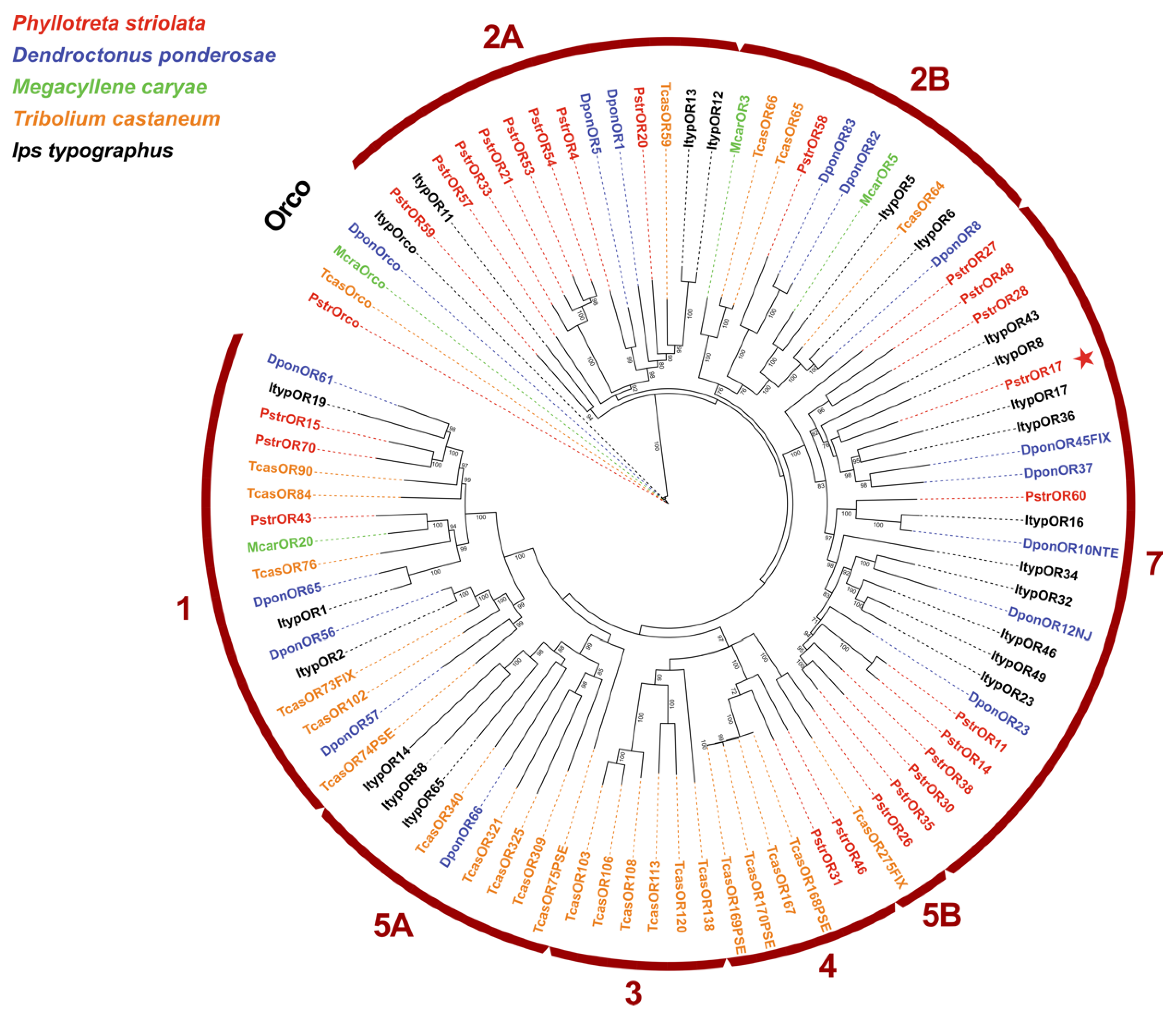
2.3. PstrOR17 Sequence Analysis
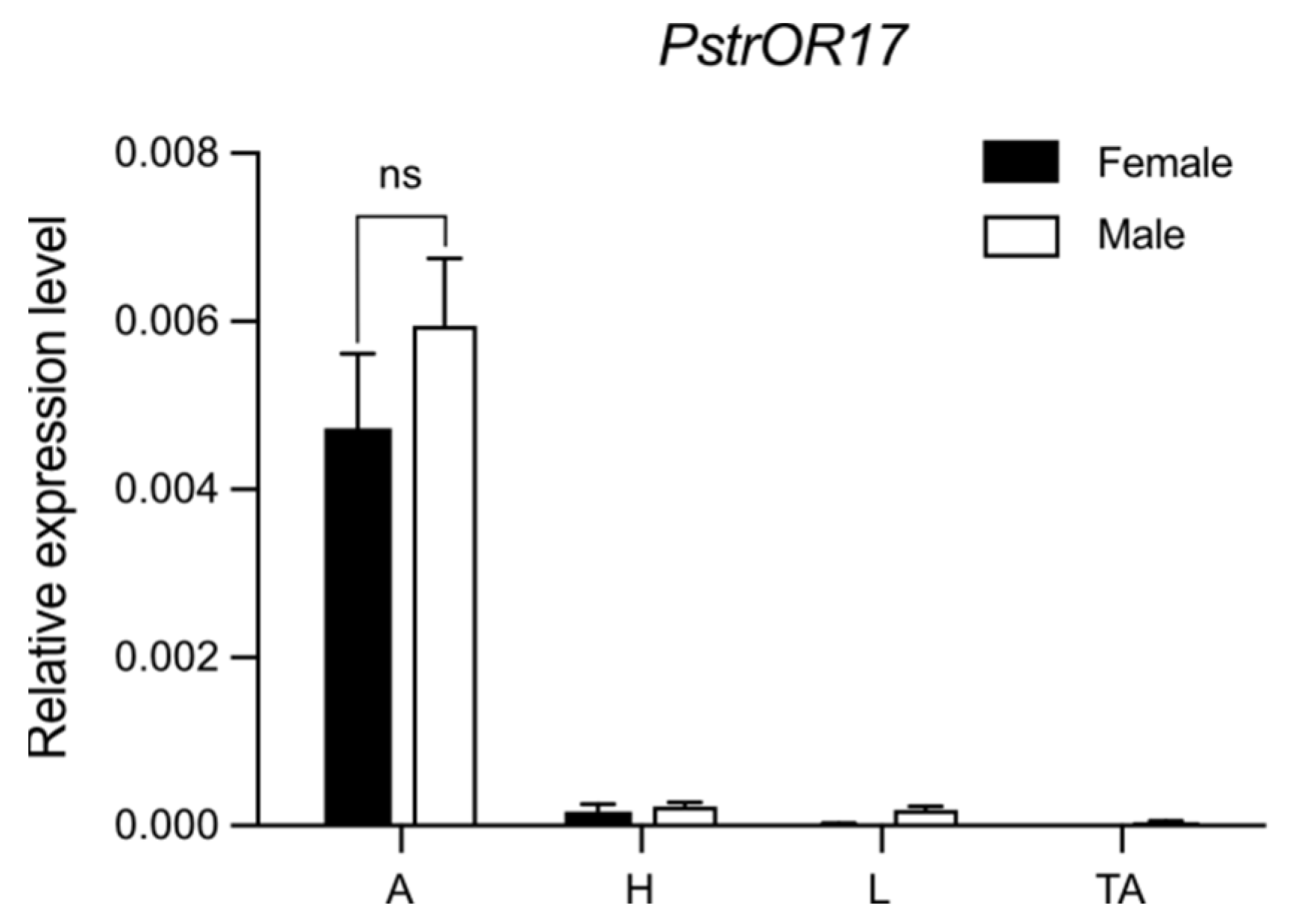
2.4. PstrOR17 Was Highly Expressed in Antennae
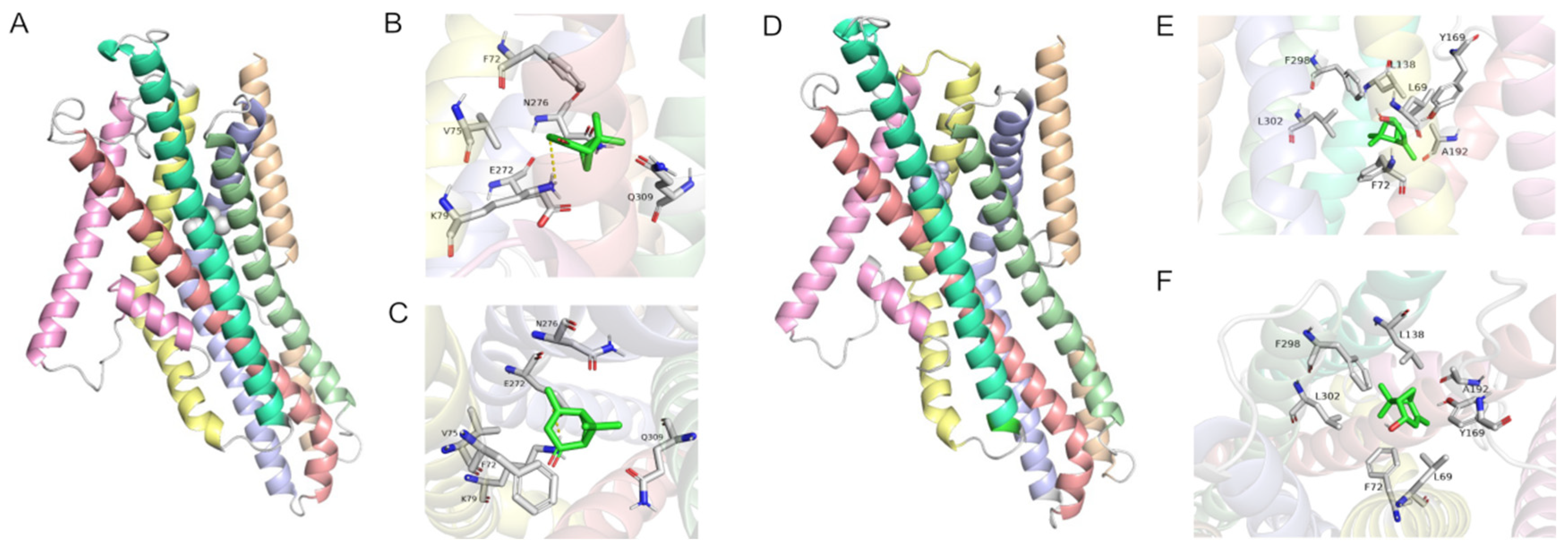
2.5. Modeling and Molecular Docking of PstrOR17
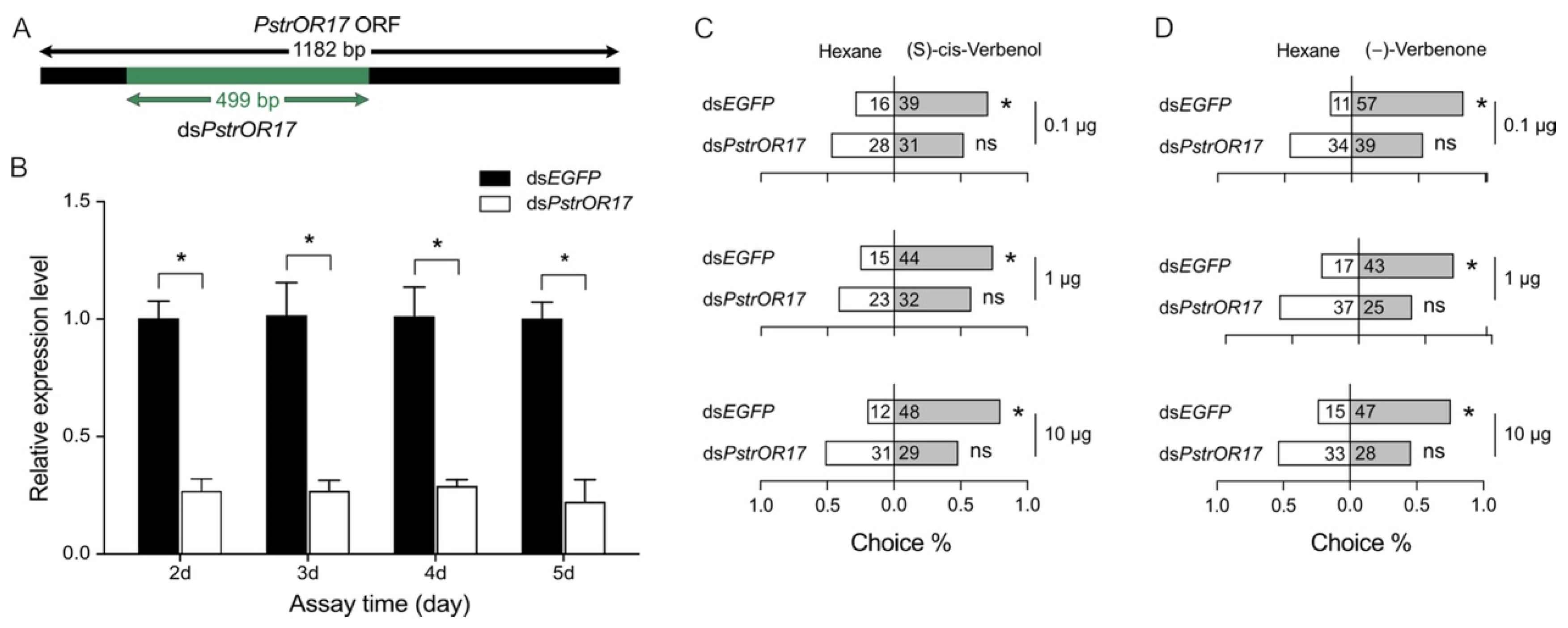
2.6. Knockdown of PstrOR17 Attenuates Behavioral Responses to (−)-Verbenone and (S)-Cis-Verbenol
3. Discussion
4. Materials and Methods
4.1. Insect Rearing
4.2. Compounds
4.3. Behavioral Assays
4.4. Electroantennogram
4.5. RNA Extraction and cDNA Synthesis
4.6. Generation of UAS Line Constructs
4.7. Single Sensillum Recording
4.8. Real-Time Quantitative PCR
4.9. Sequence and Phylogenetic Analysis
4.10. Homology Modeling and Molecular Docking
4.11. RNA Interference
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soroka, J.; Grenkow, L.; Otani, J.; Gavloski, J.; Olfert, O. Flea beetle (Coleoptera: Chrysomelidae) species in canola (Brassicaceae) on the northern Great Plains of North America. Can. Entomol. 2018, 150, 100–115. [Google Scholar] [CrossRef]
- Zu, Z.H.; Yan, C.J. Identification and analysis of the complete mitochondrial genome of Phyllotreta striolata (Coleoptera, Chrysomelidae). Mitochondrial DNA Part B Resour. 2019, 4, 2150–2151. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, G.M.; Zheng, Y.L.; Wei, S.J. Lack of genetic structure among populations of striped flea beetle Phyllotreta striolata (Coleoptera: Chrysomelidae) across southern China. Front. Ecol. Evol. 2022, 9, 775114. [Google Scholar] [CrossRef]
- Brockman, R.; Kuesel, R.; Archer, K.; O’Hearn, K.; Wilson, N.; Scott, D.; Williams, M.; Bessin, R.; Gonthier, D. The impact of plant essential oils and fine mesh row covers on flea beetle (Chrysomelidae) management in brassicaceous greens production. Insects 2020, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Briar, S.S.; Antwi, F.; Shrestha, G.; Sharma, A.; Reddy, G.V.P. Potential biopesticides for crucifer flea beetle, Phyllotreta cruciferae (Coleoptera: Chrysomelidae) management under dryland canola production in Montana. Phytoparasitica 2018, 46, 247–254. [Google Scholar] [CrossRef]
- Zimmer, C.T.; Muller, A.; Heimbach, U.; Nauen, R. Target-site resistance to pyrethroid insecticides in German populations of the cabbage stem flea beetle, Psylliodes chrysocephala L. (Coleoptera: Chrysomelidae). Pest. Biochem. Physiol. 2014, 108, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.; Alford, A.M.; Kuhar, T.P. Flea beetle (Coleoptera: Chrysomelidae) populations, effects of feeding injury, and efficacy of insecticide treatments on eggplant and cabbage in southwest Virginia. J. Econ. Entomol. 2020, 113, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, R.J.; Zilkowski, B.W.; Cosse, A.A.; Schnupf, U.; Vermillion, K.; Momany, F.A. Male-specific sesquiterpenes from Phyllotreta flea beetles. J. Nat. Prod. 2011, 74, 585–595. [Google Scholar] [CrossRef]
- Beran, F.; Jimenez-Aleman, G.H.; Lin, M.Y.; Hsu, Y.C.; Mewis, I.; Srinivasan, R.; Ulrichs, C.; Boland, W.; Hansson, B.S.; Reinecke, A. The aggregation pheromone of Phyllotreta striolata (Coleoptera: Chrysomelidae) revisited. J. Chem. Ecol. 2016, 42, 748–755. [Google Scholar] [CrossRef]
- Tollsten, L.; Bergström, G. Headspace volatiles of whole plants and macerated plant parts of Brassica and Sinapis. Phytochemistry 1988, 27, 4013–4018. [Google Scholar] [CrossRef]
- Cetin, H.; Gudek, M. Effect of Essential oil from the leaves of rosemary used in the control of Callosobruchus maculatus (F.) on the hydration coefficient, cookability, taste and color of the edible chickpea. J. Essent. Oil Bear. Plants. 2020, 23, 301–310. [Google Scholar] [CrossRef]
- Frühbrodt, T.; Schebeck, M.; Andersson, M.N.; Holighaus, G.; Kreuzwieser, J.; Burzlaff, T.; Delb, H.; Biedermann, P.H.W. Verbenone—The universal bark beetle repellent? Its origin, effects, and ecological roles. J. Pest Sci. 2023, 97, 35–71. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhang, J.; Yan, Q.; Miao, C.L.; Han, W.K.; Hou, W.; Yang, K.; Hansson, B.S.; Peng, Y.C.; Guo, J.M.; et al. The molecular basis of host selection in a crucifer-specialized moth. Curr. Biol. 2020, 30, 4476–4482. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-Induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bisch-Knaden, S.; Fandino, R.A.; Yan, S.W.; Obiero, G.F.; Grosse-Wilde, E.; Hansson, B.S.; Knaden, M. The olfactory coreceptor IR8a governs larval feces-mediated competition avoidance in a hawkmoth. Proc. Natl. Acad. Sci. USA 2019, 116, 21828–21833. [Google Scholar] [CrossRef] [PubMed]
- Li, R.T.; Huang, L.Q.; Dong, J.F.; Wang, C.Z. A moth odorant receptor highly expressed in the ovipositor is involved in detecting host-plant volatiles. eLife 2020, 9, e53706. [Google Scholar] [CrossRef] [PubMed]
- Hallem, E.A.; Ho, M.G.; Carlson, J.R. The molecular basis of odor coding in the drosophila antenna. Cell 2004, 117, 965–979. [Google Scholar] [CrossRef] [PubMed]
- del Mármol, J.; Yedlin, M.A.; Ruta, V. The structural basis of odorant recognition in insect olfactory receptors. Nature 2021, 597, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Jafari, S.; Pask, G.; Zhou, X.F.; Reinberg, D.; Desplan, C. Evolution, developmental expression and function of odorant receptors in insects. J. Exp. Biol. 2020, 223, 208215. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Amrein, H.; Morozov, P.S.; Rzhetsky, A.; Axel, R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 1999, 96, 725–736. [Google Scholar] [CrossRef]
- Li, Z.; Capoduro, R.; Bastin-Heline, L.; Zhang, S.; Sun, D.; Lucas, P.; Dabir-Moghaddam, D.; Francois, M.C.; Liu, Y.; Wang, G.; et al. A tale of two copies: Evolutionary trajectories of moth pheromone receptors. Proc. Natl. Acad. Sci. USA 2023, 120, e2221166120. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, J.K.; Roberts, R.E.; Sonntag, Y.; Hou, X.Q.; Grosse-Wilde, E.; Machara, A.; Zhang, D.D.; Hansson, B.S.; Johanson, U.; Lofstedt, C.; et al. Putative ligand binding sites of two functionally characterized bark beetle odorant receptors. BMC Biol. 2021, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.E.; Biswas, T.; Yuvaraj, J.K.; Grosse-Wilde, E.; Powell, D.; Hansson, B.S.; Lofstedt, C.; Andersson, M.N. Odorant receptor orthologues in conifer-feeding beetles display conserved responses to ecologically relevant odours. Mol. Ecol. 2022, 31, 3693–3707. [Google Scholar] [CrossRef] [PubMed]
- Chahda, J.S.; Soni, N.; Sun, J.S.; Ebrahim, S.A.M.; Weiss, B.L.; Carlson, J.R. The molecular and cellular basis of olfactory response to tsetse fly attractants. PLoS Genet. 2019, 15, e1008005. [Google Scholar] [CrossRef]
- Gonzalez, F.; Witzgall, P.; Walker, W.B. Protocol for heterologous expression of insect odourant receptors in Drosophila. Front. Ecol. Evol. 2016, 4, 24. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; He, K.; Wang, G.R. Comparison of research methods for functional characterization of insect olfactory receptors. Sci. Rep. 2016, 6, 32806. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.B.; Du, L.X.; Chen, Q.Y.; Feng, Y.L.; Zhang, J.; Zhang, X.X.; Tian, K.; Cao, S.; Huang, T.Y.; Jacquin-Joly, E.; et al. Odorant receptors for detecting flowering plant cues are functionally conserved across moths and butterflies. Mol. Biol. Evol. 2021, 38, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Mo, B.T.; Li, G.C.; Li, Z.L.; Huang, L.Q.; Sun, Y.L.; Dong, J.F.; Smith, D.P.; Wang, C.Z. Sex pheromone communication in an insect parasitoid, Campoletis chlorideae Uchida. Proc. Natl. Acad. Sci. USA 2022, 119, e2215442119. [Google Scholar] [CrossRef]
- Sun, Y.L.; Dong, J.F.; Ning, C.; Ding, P.P.; Huang, L.Q.; Sun, J.G.; Wang, C.Z. An odorant receptor mediates the attractiveness of cis-jasmone to Campoletis chlorideae, the endoparasitoid of Helicoverpa armigera. Insect Mol. Biol. 2019, 28, 23–34. [Google Scholar] [CrossRef]
- Shan, S.; Song, X.; Khashaveh, A.; Wang, S.N.; Lu, Z.Y.; Dhiloo, K.H.; Li, R.J.; Zhang, Y.J. A female-biased odorant receptor tuned to the lepidopteran sex pheromone in parasitoid Microplitis mediator guiding habitat of host insects. J. Adv. Res. 2023, 43, 1–12. [Google Scholar] [CrossRef]
- Antony, B.; Johny, J.; Montagne, N.; Jacquin-Joly, E.; Capoduro, R.; Cali, K.; Persaud, K.; Al-Saleh, M.A.; Pain, A. Pheromone receptor of the globally invasive quarantine pest of the palm tree, the red palm weevil (Rhynchophorus ferrugineus). Mol. Ecol. 2021, 30, 2025–2039. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Yi, J.K.; Li, Y.S.; Liu, J.N.; Wang, J.; Xi, J.H. Three host plant volatiles, hexanal, lauric acid, and tetradecane, are detected by an antenna-biased expressed odorant receptor 27 in the dark black chafer Holotrichia parallela. J. Agric. Food Chem. 2020, 68, 7316–7323. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wang, X.; Zhao, S.W.; Fang, K.; Wang, Z.; Liu, J.A.; Xi, J.H.; Wang, S.; Zhang, J.H. Response of odorant receptors with phenylacetaldehyde and the effects on the behavior of the rice water weevil (Lissorhoptrus oryzophilus). J. Agric. Food Chem. 2023, 71, 6541–6551. [Google Scholar] [CrossRef]
- Mitchell, R.F.; Hughes, D.T.; Luetje, C.W.; Millar, J.G.; Soriano-Agaton, F.; Hanks, L.M.; Robertson, H.M. Sequencing and characterizing odorant receptors of the cerambycid beetle Megacyllene caryae. Insect Biochem. Mol. Biol. 2012, 42, 499–505. [Google Scholar] [CrossRef]
- Xie, J.; Liu, T.; Yi, C.; Liu, X.; Tang, H.; Sun, Y.; Shi, W.; Khashaveh, A.; Zhang, Y. Antenna-biased odorant receptor HvarOR25 in Hippodamia variegata tuned to allelochemicals from hosts and habitat involved in perceiving preys. J. Agric. Food Chem. 2022, 70, 1090–1100. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Bin, S.Y.; He, H.L.; Wang, Z.B.; Li, M.; Lin, J.T. Differential expression analysis of chemoreception genes in the striped slea beetle Phyllotreta striolata using a transcriptomic approach. PLoS ONE 2016, 11, e0153067. [Google Scholar]
- Mitchell, R.F.; Schneider, T.M.; Schwartz, A.M.; Andersson, M.N.; McKenna, D.D. The diversity and evolution of odorant receptors in beetles (Coleoptera). Insect Mol. Biol. 2020, 29, 77–91. [Google Scholar] [CrossRef]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell. Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef]
- Fan, Y.N.; Zhang, C.Z.; Qin, Y.; Yin, X.H.; Dong, X.Y.; Desneux, N.; Zhou, H.X. Monitoring the methyl eugenol response and non-responsiveness mechanisms in oriental fruit fly Bactrocera dorsalis in China. Insects 2022, 13, 1004. [Google Scholar] [CrossRef] [PubMed]
- Conchou, L.; Lucas, P.; Meslin, C.; Proffit, M.; Staudt, M.; Ronou, M. Insect odorscapes: From plant volatiles to natural olfactory scenes. Front. Physiol. 2019, 10, e00972. [Google Scholar] [CrossRef] [PubMed]
- Keeling, C.I.; Tittiger, C.; MacLean, M.; Blomquist, G.J. 4—Pheromone production in bark beetles. In Insect Pheromone Biochemistry and Molecular Biology, 2nd ed.; Blomquist, G.J., Vogt, R.G., Eds.; Academic Press: London, UK, 2021; pp. 123–162. [Google Scholar]
- Shepherd, W.P.; Sullivan, B.T. Southern pine beetle, Dendroctonus frontalis, antennal and behavioral responses to nonhost leaf and bark volatiles. J. Chem. Ecol. 2013, 39, 481–493. [Google Scholar] [CrossRef]
- Szmigielski, R.; Cieslak, M.; Rudzinski, K.J.; Maciejewska, B. Identification of volatiles from Pinus silvestris attractive for Monochamus galloprovincialis using a SPME-GC/MS platform. Environ. Sci. Pollut. Res. 2012, 19, 2860–2869. [Google Scholar] [CrossRef][Green Version]
- Branco, S.; Mateus, E.P.; da Silva, M.D.R.G.; Mendes, D.; Pereira, M.M.A.; Schutz, S.; Paiva, M.R. Olfactory responses of Anaphes nitens (Hymenoptera, Mymaridae) to host and habitat cues. J. Appl. Entomol. 2021, 145, 675–687. [Google Scholar] [CrossRef]
- Branco, S.; Mateus, E.P.; da Silva, M.D.R.G.; Mendes, D.; Pereira, M.M.A.; Schutz, S.; Paiva, M.R. Identification of pheromone candidates for the eucalyptus weevil, Gonipterus platensis (Coleoptera, Curculionidae). J. Appl. Entomol. 2020, 144, 41–53. [Google Scholar] [CrossRef]
- Abdullah, Z.S.; Greenfield, B.P.J.; Ficken, K.J.; Taylor, J.W.D.; Wood, M.; Butt, T.M. A new attractant for monitoring western flower thrips, Frankliniella occidentalis in protected crops. Springerplus 2015, 4, 89. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, H.B.; Yu, J.L.; Pan, D.; Tao, Y.; Lei, Q.; Chen, Y.; Liu, Z.; Wang, J.J. Two odorant receptors regulate 1-octen-3-ol induced oviposition behavior in the oriental fruit fly. Commun. Biol. 2023, 6, 176. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.Q.; Wang, G.R.; Liu, F.; Liu, Y. An odorant receptor of the green mirid bug, Apolygus lucorum, tuned to linalool. Insect Biochem. Mol. Biol. 2022, 144, 103764. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Chen, P.T.; Xu, Z.Y.; Qian, J.L.; Zhu, G.N.; Jin, Y.F.; Chen, B.S.; Chen, M.L. A potential link between aromatics-induced oviposition repellency behaviors and specific odorant receptor of Aedes albopictus. Pest Manag. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Chen, P.; Yan, R.; Chen, G.; Qian, J.; Zhu, G.; Chen, M.; Guo, Y. Antenna-Biased Odorant Receptor PstrOR17 Mediates Attraction of Phyllotreta striolata to (S)-Cis-Verbenol and (−)-Verbenone. Int. J. Mol. Sci. 2024, 25, 4362. https://doi.org/10.3390/ijms25084362
Xu Z, Chen P, Yan R, Chen G, Qian J, Zhu G, Chen M, Guo Y. Antenna-Biased Odorant Receptor PstrOR17 Mediates Attraction of Phyllotreta striolata to (S)-Cis-Verbenol and (−)-Verbenone. International Journal of Molecular Sciences. 2024; 25(8):4362. https://doi.org/10.3390/ijms25084362
Chicago/Turabian StyleXu, Zhanyi, Peitong Chen, Ru Yan, Guoxing Chen, Jiali Qian, Guonian Zhu, Mengli Chen, and Yirong Guo. 2024. "Antenna-Biased Odorant Receptor PstrOR17 Mediates Attraction of Phyllotreta striolata to (S)-Cis-Verbenol and (−)-Verbenone" International Journal of Molecular Sciences 25, no. 8: 4362. https://doi.org/10.3390/ijms25084362
APA StyleXu, Z., Chen, P., Yan, R., Chen, G., Qian, J., Zhu, G., Chen, M., & Guo, Y. (2024). Antenna-Biased Odorant Receptor PstrOR17 Mediates Attraction of Phyllotreta striolata to (S)-Cis-Verbenol and (−)-Verbenone. International Journal of Molecular Sciences, 25(8), 4362. https://doi.org/10.3390/ijms25084362





