Structure and Properties of Polylactide Composites with TiO2–Lignin Hybrid Fillers
Abstract
:1. Introduction
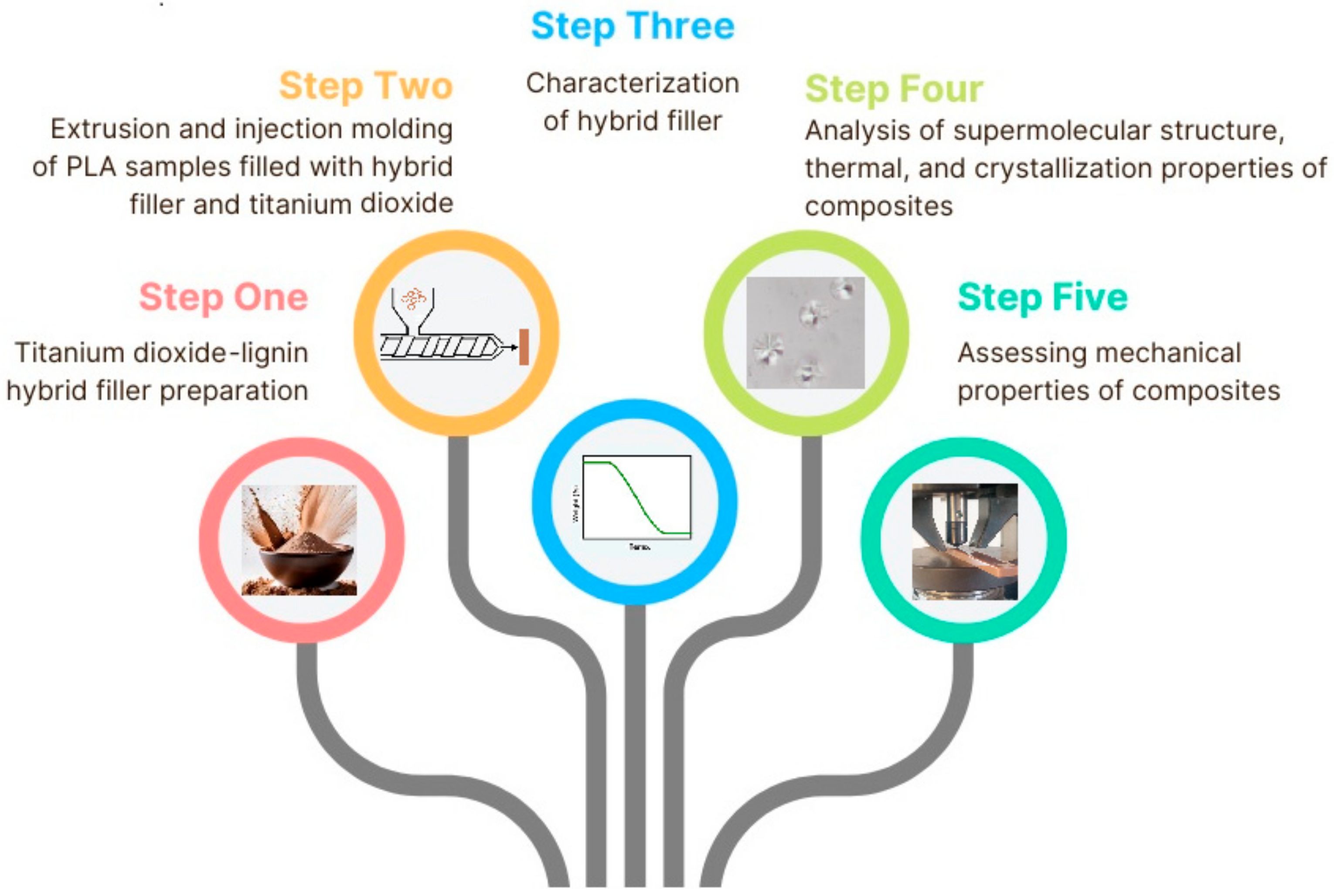
2. Results and Discussion
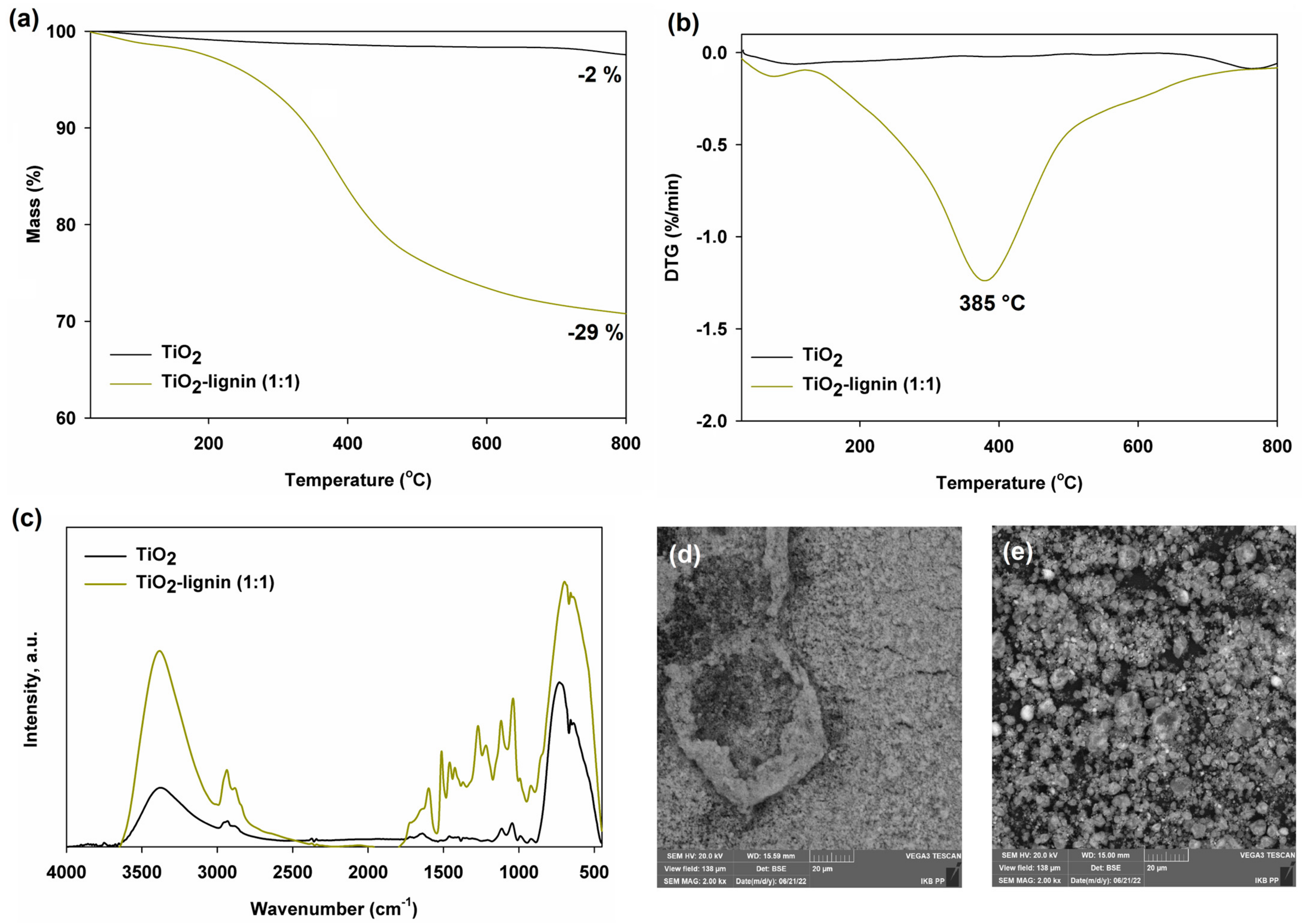
2.1. Characteristics of Fillers
2.2. Supermolecular Structure
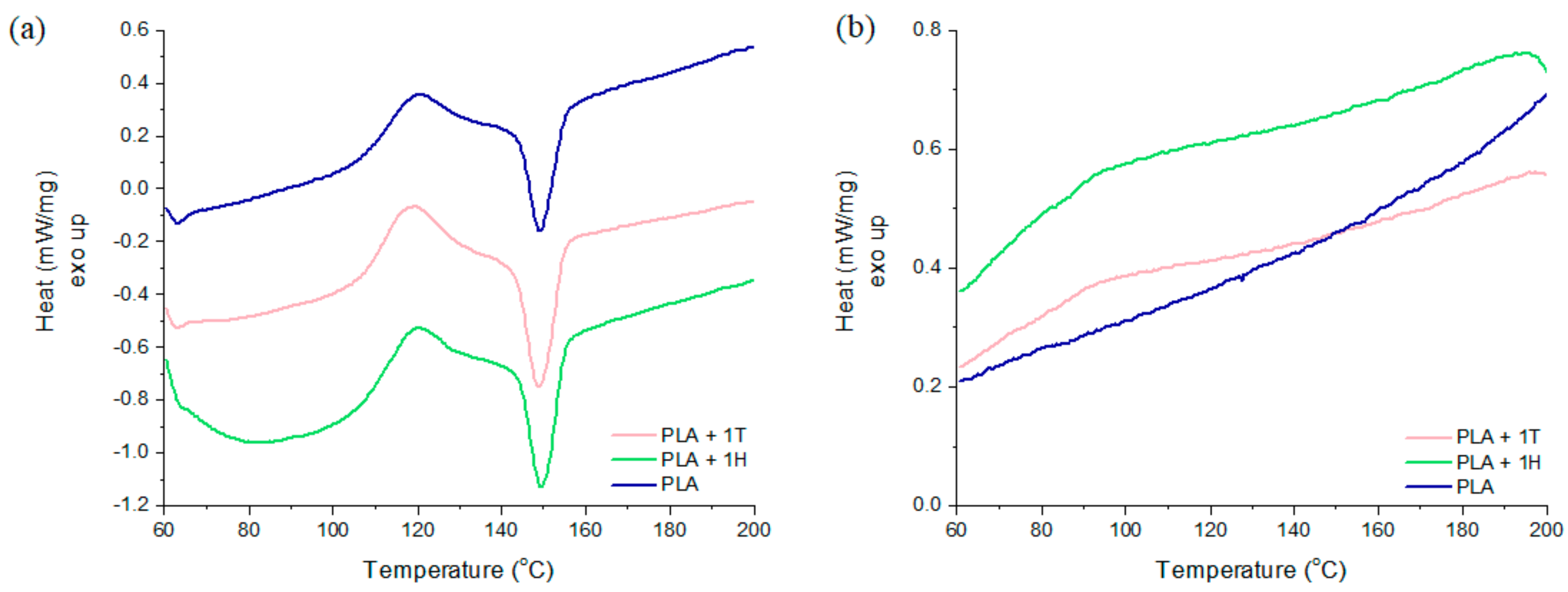
2.3. Crystallization Studies
2.4. Mechanical Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of TiO2–Lignin Hybrid
3.3. Characteristics of Fillers
3.3.1. Fourier Transform Infrared Spectroscopy
3.3.2. Thermogravimetric Analysis
3.3.3. Dispersive and Morphological Properties
3.3.4. Elemental Analysis
3.3.5. Electrophoretic Mobility
3.4. Preparation of Composites
3.5. Characteristics of Composites
3.5.1. X-ray Diffraction
3.5.2. Differential Scanning Calorimetry
3.5.3. Polarized Light Microscopy
3.5.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cajnko, M.M.; Oblak, J.; Grilc, M.; Likozar, B. Enzymatic bioconversion process of lignin: Mechanisms, reactions and kinetics. Bioresour. Technol. 2021, 340, 125655. [Google Scholar] [CrossRef]
- Sosa, F.H.B.; Bjelić, A.; Coutinho, J.A.P.; Costa, M.C.; Likozar, B.; Jasiukaitytė-Grojzdek, E.; Grilc, M.; da Costa Lopes, A.M. Conversion of Organosolv and Kraft lignins into value-added compounds assisted by an acidic deep eutectic solvent. Sustain. Energy Fuels 2022, 6, 4800–4815. [Google Scholar] [CrossRef]
- Ročnik, T.; Likozar, B.; Jasiukaitytė-Grojzdek, E.; Grilc, M. Catalytic lignin valorisation by depolymerisation, hydrogenation, demethylation and hydrodeoxygenation: Mechanism, chemical reaction kinetics and transport phenomena. Chem. Eng. J. 2022, 448, 137309. [Google Scholar] [CrossRef]
- Khan, A.; Nair, V.; Colmenares, J.C.; Gläser, R. Lignin-Based Composite Materials for Photocatalysis and Photovoltaics. Top. Curr. Chem. 2018, 376, 20. [Google Scholar] [CrossRef]
- Wang, B.; Shi, T.; Zhang, Y.; Chen, C.; Li, Q.; Fan, Y. Lignin-based highly sensitive flexible pressure sensor for wearable electronics. J. Mater. Chem. C 2018, 6, 6423–6428. [Google Scholar] [CrossRef]
- Yao, H.; Wang, Y.; Liu, J.; Xu, M.; Ma, P.; Ji, J.; You, Z. Review on Applications of Lignin in Pavement Engineering: A Recent Survey. Front. Mater. 2022, 8, 803524. [Google Scholar] [CrossRef]
- Ma, C.; Kim, T.-H.; Liu, K.; Ma, M.-G.; Choi, S.-E.; Si, C. Multifunctional Lignin-Based Composite Materials for Emerging Applications. Front. Bioeng. Biotechnol. 2021, 9, 708976. [Google Scholar] [CrossRef]
- Makri, S.P.; Xanthopoulou, E.; Valera, M.A.; Mangas, A.; Marra, G.; Ruiz, V.; Koltsakidis, S.; Tzetzis, D.; Zoikis Karathanasis, A.; Deligkiozi, I.; et al. Poly(Lactic Acid) Composites with Lignin and Nanolignin Synthesized by In Situ Reactive Processing. Polymers 2023, 15, 2386. [Google Scholar] [CrossRef]
- Arjhan, A.; Wacharawichanant, S.; Opaprakasit, P. Influence of Lignin Content on Morphology and Properties of Poly (Lactic Acid)/Lignin Composite Films. Key Eng. Mater. 2022, 914, 155–161. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Wang, Q.; Ji, X.; Yang, G.; Chen, J.; Fatehi, P. Strong, ductile and biodegradable polylactic acid/lignin-containing cellulose nanofibril composites with improved thermal and barrier properties. Ind. Crops Prod. 2021, 171, 113898. [Google Scholar] [CrossRef]
- Esakkimuthu, E.S.; DeVallance, D.; Pylypchuk, I.; Moreno, A.; Sipponen, M.H. Multifunctional lignin-poly (lactic acid) biocomposites for packaging applications. Front. Bioeng. Biotechnol. 2022, 10, 1025076. [Google Scholar] [CrossRef] [PubMed]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, T.-Q.; Shi, Q.; Sun, R.-C. Application of Lignin in Thermoplastic Materials. In Green Chemistry and Chemical Engineering; Han, B., Wu, T., Eds.; Springer: New York, NY, USA, 2019; pp. 405–426. [Google Scholar]
- Zhu, Y.; Buonocore, G.; Lavorgna, M. Photocatalytic activity of PLA/TiO2 nanocomposites and TiO2-active multilayered hybrid coatings. Ital. J. Food Sci. 2012, 24, 102–106. [Google Scholar]
- Man, C.; Zhang, C.; Liu, Y.; Wang, W.; Ren, W.; Jiang, L.; Reisdorffer, F.; Nguyen, T.P.; Dan, Y. Poly (lactic acid)/titanium dioxide composites: Preparation and performance under ultraviolet irradiation. Polym. Degrad. Stab. 2012, 97, 856–862. [Google Scholar] [CrossRef]
- Nomai, J.; Suksut, B.; Schlarb, A.K. Crystallization behavior of poly (lactic acid)/titanium dioxide nanocomposites. KMUTNB Int. J. Appl. Sci. Technol. 2015, 8, 251–258. [Google Scholar] [CrossRef]
- Song, T.; Liu, M.; Tian, J.; Wang, S.; Li, Q. Effect of PLA/TiO2/Lg filler competition and synergy on crystallization behavior, mechanics and functionality of composite foaming materials. Polymer 2023, 271, 125797. [Google Scholar] [CrossRef]
- Deghiche, A.; Haddaoui, N.; Zerriouh, A.; Fenni, S.E.; Cavallo, D.; Erto, A.; Benguerba, Y. Effect of the stearic acid-modified TiO2 on PLA nanocomposites: Morphological and thermal properties at the microscopic scale. J. Environ. Chem. Eng. 2021, 9, 106541. [Google Scholar] [CrossRef]
- Grząbka-Zasadzińska, A.; Klapiszewski, Ł.; Bula, K.; Jesionowski, T.; Borysiak, S. Supermolecular structure and nucleation ability of polylactide-based composites with silica/lignin hybrid fillers. J. Therm. Anal. Calorim. 2016, 126, 263–275. [Google Scholar] [CrossRef]
- Grząbka-Zasadzińska, A.; Klapiszewski, Ł.; Borysiak, S.; Jesionowski, T. Thermal and Mechanical Properties of Silica–Lignin/Polylactide Composites Subjected to Biodegradation. Materials 2018, 11, 2257. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353. [Google Scholar]
- Liu, Q.; Wang, S.; Zheng, Y.; Luo, Z.; Cen, K. Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2008, 82, 170–177. [Google Scholar] [CrossRef]
- Erdem, B.; Hunsicker, R.A.; Simmons, G.W.; Sudol, E.D.; Dimonie, V.L.; El-Aasser, M.S. XPS and FTIR Surface Characterization of TiO2 Particles Used in Polymer Encapsulation. Langmuir 2001, 17, 2664–2669. [Google Scholar] [CrossRef]
- Klapiszewska, I.; Parus, A.; Ławniczak, Ł.; Jesionowski, T.; Klapiszewski, Ł.; Ślosarczyk, A. Production of antibacterial cement composites containing ZnO/lignin and ZnO–SiO2/lignin hybrid admixtures. Cem. Concr. Compos. 2021, 124, 104250. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Grząbka-Zasadzińska, A.; Borysiak, S.; Jesionowski, T. Preparation and characterization of polypropylene composites reinforced by functional ZnO/lignin hybrid materials. Polym. Test. 2019, 79, 106058. [Google Scholar] [CrossRef]
- Wysokowski, M.; Klapiszewski, Ł.; Moszyński, D.; Bartczak, P.; Szatkowski, T.; Majchrzak, I.; Siwińska-Stefańska, K.; Bazhenov, V.V.; Jesionowski, T. Modification of chitin with kraft lignin and development of new biosorbents for removal of cadmium (II) and nickel (II) ions. Mar. Drugs 2014, 12, 2245–2268. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Nowacka, M.; Milczarek, G.; Jesionowski, T. Physicochemical and electrokinetic properties of silica/lignin biocomposites. Carbohydr. Polym. 2013, 94, 345–355. [Google Scholar] [CrossRef]
- Jędrzejczak, P.; Parus, A.; Balicki, S.; Kornaus, K.; Janczarek, M.; Wilk, K.A.; Jesionowski, T.; Ślosarczyk, A.; Klapiszewski, Ł. The influence of various forms of titanium dioxide on the performance of resultant cement composites with photocatalytic and antibacterial functions. Mater. Res. Bull. 2023, 160, 112139. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.; Cab, C.; De Coss, R.d.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, F.A.; Valle Iulianelli, G.C.; Bruno Tavares, M.I. Development and properties evaluation of bio-based PLA/PLGA blend films reinforced with microcrystalline cellulose and organophilic silica. Polym. Eng. Sci. 2017, 57, 464–472. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly (lactic acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Ur Rehman, Z. Review of Recent Advances in Polylactic Acid/TiO2 Composites. Materials 2019, 12, 3659. [Google Scholar] [CrossRef] [PubMed]
- Saiprasit, P.; Schlarb, A. The effect of the compounding procedure on the morphology and mechanical properties of PLA/PBAT-based nanocomposites. Int. Polym. Process. 2021, 36, 219–227. [Google Scholar] [CrossRef]
- Looijmans, S.F.S.P.; Spanjaards, M.M.A.; Puskar, L.; Cavallo, D.; Anderson, P.D.; van Breemen, L.C.A. Synergy of Fiber Surface Chemistry and Flow: Multi-Phase Transcrystallization in Fiber-Reinforced Thermoplastics. Polymers 2022, 14, 4850. [Google Scholar] [CrossRef] [PubMed]
- Haubruge, H.G.; Daussin, R.; Jonas, A.M.; Legras, R.; Wittmann, J.C.; Lotz, B. Epitaxial Nucleation of Poly(ethylene terephthalate) by Talc: Structure at the Lattice and Lamellar Scales. Macromolecules 2003, 36, 4452–4456. [Google Scholar] [CrossRef]
- Ke, T.; Sun, X. Melting behavior and crystallization kinetics of starch and poly(lactic acid) composites. J. Appl. Polym. Sci. 2003, 89, 1203–1210. [Google Scholar] [CrossRef]
- Spiridon, I.; Tanase, C.E. Design, characterization and preliminary biological evaluation of new lignin-PLA biocomposites. Int. J. Biol. Macromol. 2018, 114, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.F.; Akil, H.M.; Ahmad, Z.A. Particle size—Dependent on the static and dynamic compression properties of polypropylene/silica composites. Mater. Des. 2013, 45, 539–547. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Inoue, Y. Thermal and mechanical properties of biodegradable blends of poly(L-lactic acid) and lignin. Polym. Int. 2003, 52, 949–955. [Google Scholar] [CrossRef]
- Shakoor Shar, A.; Wang, N.; Chen, T.; Zhao, X.; Weng, Y. Development of PLA/Lignin Bio-Composites Compatibilized by Ethylene Glycol Diglycidyl Ether and Poly (ethylene glycol) Diglycidyl Ether. Polymers 2023, 15, 4049. [Google Scholar] [CrossRef]
- Yang, W.; Dominici, F.; Fortunati, E.; Kenny, J.M.; Puglia, D. Effect of lignin nanoparticles and masterbatch procedures on the final properties of glycidyl methacrylate-g-poly (lactic acid) films before and after accelerated UV weathering. Ind. Crops Prod. 2015, 77, 833–844. [Google Scholar] [CrossRef]
- Zhu, J.; Xue, L.; Wei, W.; Mu, C.; Jiang, M.; Zhou, Z. Modification of lignin with silane coupling agent to improve the interface of poly (L-lactic) acid/lignin composites. BioResources 2015, 10, 4315–4325. [Google Scholar] [CrossRef]
- Flores, A.; Ania, F.; Baltá-Calleja, F.J. From the glassy state to ordered polymer structures: A microhardness study. Polymer 2009, 50, 729–746. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, M.; Qu, Z.; Mi, J.; Wang, X.; Deng, Y. Thermal and Rheological Properties of Poly(lactic acid)/Low-Density Polyethylene Blends and Their Supercritical CO2 Foaming Behavior. J. Polym. Environ. 2018, 26, 3564–3573. [Google Scholar] [CrossRef]
- PN-EN ISO 527-1; Differential Scanning Calorimetry (DSC)—Part 3: Determination of Temperature and Enthalpy of Melting and Crystallization. ISO: Warsaw, Poland, 2018.
- PN-EN ISO 178:2019-06; Plastics—Determination of Flexural Properties. ISO: Warsaw, Poland, 2019.
- PN-EN ISO 179:2023-11; Plastics—Charpy Impact Testing—Part 1: Non-Instrumental Impact Testing. ISO: Warsaw, Poland, 2023.
- PN-EN ISO 2039-1:2004; Plastics—Determination of Hardness—Part 1: Ball Indentation Method. ISO: Warsaw, Poland, 2004.


| Sample | Dispersion Properties | Elemental Content (%) | pH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | |||||||
| Particle Size Distribution (nm) | Polydispersity Index (PdI) | N | C | H | S | Zeta Potential (mV) | |||||
| TiO2 | 91–531 | 0.198 | - | 0.1 | 0.4 | - | 20.1 | −7.2 | −20.4 | −29.6 | −35.1 |
| TiO2–lignin (1:1) | 106–1110 | 0.686 | 0.3 | 30.1 | 4.3 | 1.2 | 0.1 | −29.5 | −34.8 | −35.9 | −40.1 |
| Sample | Tg (°C) | Tcc (°C) | Tm (°C) | Xc (%) |
|---|---|---|---|---|
| PLA | 63.6 | 120.2 | 149.3 | 1 |
| PLA + 1T | 62.6 | 119.0 | 149.0 | 2 |
| PLA + 3T | 61.9 | 120.6 | 148.1 | 7 |
| PLA + 5T | 61.2 | nd | 150.9 | 8 |
| PLA + 1H | 63.3 | 120.2 | 149.1 | 20 |
| PLA + 3H | 62.0 | 119.4 | 148.1 | 2 |
| PLA + 5H | 62.2 | 119.6 | 148.6 | 1 |
| Sample | Uniaxial Stretching | Impact Strength | Bending | Hardness | ||
|---|---|---|---|---|---|---|
| YM (MPa) | σM (MPa) | EB (%) | Re (kJ/m2) | σM (MPa) | [MPa] | |
| PLA | 2290 ± 68 | 65.6 ± 0.6 | 6.0 ± 0.9 | 2.7 ± 0.5 | 40.2 ± 0.5 | 46.6 ± 3.1 |
| PLA + 1T | 2220 ± 21 | 62.7 ± 0.5 | 9.1 ± 0.9 | 2.5 ± 0.3 | 40.6 ± 0.4 | 50.9 ± 1.2 |
| PLA + 3T | 2300 ± 12 | 61.4 ± 0.8 | 7.8 ± 0.9 | 2.3 ± 0.2 | 40.5 ± 0.6 | 50.1 ± 2.5 |
| PLA + 5T | 2222 ± 65 | 57.9 ± 0.9 | 8.3 ± 0.7 | 2.4 ± 0.3 | 39.4 ± 0.6 | 49.5 ± 3.3 |
| PLA + 1H | 2270 ± 26 | 63.1 ± 0.3 | 8.1 ± 0.7 | 2.3 ± 0.1 | 42.0 ± 0.1 | 48.9 ± 1.3 |
| PLA + 3H | 2320 ± 27 | 61.5 ± 0.7 | 6.6 ± 1.2 | 2.6 ± 0.4 | 42.6 ± 0.4 | 44.1 ± 2.6 |
| PLA + 5H | 2360 ± 7 | 59.3 ± 0.4 | 4.8 ± 0.7 | 2.5 ± 0.3 | 42.7 ± 0.5 | 45.0 ± 3.0 |
| Polymer Matrix | Filler Type | Filler Content (%) | Sample Name |
|---|---|---|---|
| PLA | - | 0 | PLA |
| TiO2 | 1 | PLA + 1T | |
| 3 | PLA + 3T | ||
| 5 | PLA + 5T | ||
| TiO2–lignin | 1 | PLA + 1H | |
| 3 | PLA + 3H | ||
| 5 | PLA + 5H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grząbka-Zasadzińska, A.; Piątek, A.; Klapiszewski, Ł.; Borysiak, S. Structure and Properties of Polylactide Composites with TiO2–Lignin Hybrid Fillers. Int. J. Mol. Sci. 2024, 25, 4398. https://doi.org/10.3390/ijms25084398
Grząbka-Zasadzińska A, Piątek A, Klapiszewski Ł, Borysiak S. Structure and Properties of Polylactide Composites with TiO2–Lignin Hybrid Fillers. International Journal of Molecular Sciences. 2024; 25(8):4398. https://doi.org/10.3390/ijms25084398
Chicago/Turabian StyleGrząbka-Zasadzińska, Aleksandra, Agata Piątek, Łukasz Klapiszewski, and Sławomir Borysiak. 2024. "Structure and Properties of Polylactide Composites with TiO2–Lignin Hybrid Fillers" International Journal of Molecular Sciences 25, no. 8: 4398. https://doi.org/10.3390/ijms25084398





