Targeting the High-Density Lipoprotein Proteome for the Treatment of Post-Acute Sequelae of SARS-CoV-2
Abstract
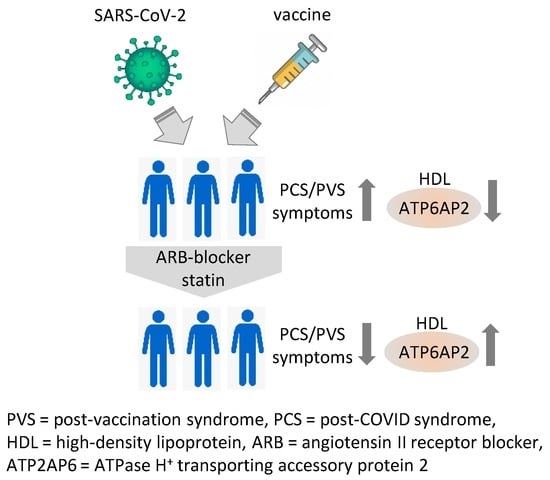
1. Introduction
2. Results
2.1. Study Population
2.2. PVS and PCS Patients Display Severe Fatigue Characteristics at Baseline
2.3. Impact of Medical Treatment on the Clinical Presentation
2.4. The HDL Proteome Is Altered in PVS/PCS Patients Compared to Healthy Controls
2.5. Treatment Alters the HDL Proteome in Patients
2.6. The HDL Proteome of Patients after Treatment Reduced Expression of Endothelial Inflammatory Markers
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Population
4.2. Isolation of High-Density Lipoprotein
4.3. SDS-Gel Electrophoresis of Isolated HDL Proteins
4.4. Cell Viability Assay
4.5. Cell Culture and Stimulation Experiments
4.6. Real-Time PCR
4.7. Mass Spectrometry
4.8. Bioinformatic Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO COVID-19 Dashboard. Available online: https://covid19.who.int/ (accessed on 9 February 2024).
- WHO. Coronavirus disease (COVID-19): Post COVID-19 condition. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition (accessed on 9 February 2024).
- WHO. COVID-19 Vaccination WHO SARS-CoV-2 Vaccination. Available online: https://covid19.who.int/?mapFilter=vaccinations (accessed on 9 February 2024).
- Kaulen, L.D.; Doubrovinskaia, S.; Mooshage, C.; Jordan, B.; Purrucker, J.; Haubner, C.; Seliger, C.; Lorenz, H.; Nagel, S.; Wildemann, B.; et al. Neurological Autoimmune Diseases following Vaccinations against SARS-CoV-2: A Case Series. Eur. J. Neurol. 2022, 29, 555–563. [Google Scholar] [CrossRef] [PubMed]
- EMA. SARS-CoV-2 Vaccine Safety. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines/safety-covid-19-vaccines (accessed on 28 January 2024).
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Golla, R.; Vuyyuru, S.; Kante, B.; Kumar, P.; Mathew, D.T.; Makharia, G.; Kedia, S.; Ahuja, V. Long-Term Gastrointestinal Sequelae Following COVID-19: A Prospective Follow-up Cohort Study. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022, 21, 789–796.e1. [Google Scholar] [CrossRef] [PubMed]
- Camera, M.; Brambilla, M.; Canzano, P.; Becchetti, A.; Conti, M.; Agostoni, P.G.; Pengo, M.; Tortorici, E.; Mancini, M.E.; Andreini, D.; et al. Long COVID-19 Syndrome: Association of Cardiopulmonary Impairment with a Persistent Platelet Activation. Eur. Heart J. 2022, 43, ehac544.3038. [Google Scholar] [CrossRef]
- Iwasaki, A.; Putrino, D. Why We Need a Deeper Understanding of the Pathophysiology of Long COVID. Lancet Infect. Dis. 2023, 23, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Guimarães Sousa, S.; Kleiton de Sousa, A.; Maria Carvalho Pereira, C.; Sofia Miranda Loiola Araújo, A.; de Aguiar Magalhães, D.; Vieira de Brito, T.; dos Reis Barbosa, A.L. SARS-CoV-2 Infection Causes Intestinal Cell Damage: Role of Interferon’s Imbalance. Cytokine 2022, 152, 155826. [Google Scholar] [CrossRef] [PubMed]
- Schieffer, E.; Schieffer, B. The Race for ACE: Targeting Angiotensin-Converting Enzymes (ACE) in SARS-CoV-2 Infection. J. Renin Angiotensin Aldosterone Syst. 2022, 2022, 2549063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.-J.; Xie, J.; Liu, Y.-M.; Zhao, Y.-C.; Huang, X.; Lin, L.; et al. Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality among Patients with Hypertension Hospitalized With COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef]
- Castruita, J.A.S.; Schneider, U.V.; Mollerup, S.; Leineweber, T.D.; Weis, N.; Bukh, J.; Pedersen, M.S.; Westh, H. SARS-CoV-2 Spike mRNA Vaccine Sequences Circulate in Blood up to 28 Days after COVID-19 Vaccination. APMIS 2023, 131, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Minz, M.M.; Bansal, M.; Kasliwal, R.R. Statins and SARS-CoV-2 Disease: Current Concepts and Possible Benefits. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 2063–2067. [Google Scholar] [CrossRef]
- Tan, W.Y.T.; Young, B.E.; Lye, D.C.; Chew, D.E.K.; Dalan, R. Statin Use Is Associated with Lower Disease Severity in COVID-19 Infection. Sci. Rep. 2020, 10, 17458. [Google Scholar] [CrossRef]
- Guntur, V.P.; Nemkov, T.; de Boer, E.; Mohning, M.P.; Baraghoshi, D.; Cendali, F.I.; San-Millán, I.; Petrache, I.; D’Alessandro, A. Signatures of Mitochondrial Dysfunction and Impaired Fatty Acid Metabolism in Plasma of Patients with Post-Acute Sequelae of COVID-19 (PASC). Metabolites 2022, 12, 1026. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Xie, Y.; Al-Aly, Z. Risks and Burdens of Incident Dyslipidaemia in Long COVID: A Cohort Study. Lancet Diabetes Endocrinol. 2023, 11, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Berber, E.; Sumbria, D.; Kokkaya, S. A Metabolic Blueprint of COVID-19 and Long-Term Vaccine Efficacy. Drug Metab. Pers. Ther. 2023, 38, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and Atherosclerotic Cardiovascular Disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Kluck, G.E.G.; Yoo, J.-A.; Sakarya, E.H.; Trigatti, B.L. Good Cholesterol Gone Bad? HDL and COVID-19. Int. J. Mol. Sci. 2021, 22, 10182. [Google Scholar] [CrossRef] [PubMed]
- Vaisar, T.; Tang, C.; Babenko, I.; Hutchins, P.; Wimberger, J.; Suffredini, A.F.; Heinecke, J.W. Inflammatory Remodeling of the HDL Proteome Impairs Cholesterol Efflux Capacity. J. Lipid Res. 2015, 56, 1519–1530. [Google Scholar] [CrossRef]
- von Eckardstein, A.; Nofer, J.-R.; Assmann, G. High Density Lipoproteins and Arteriosclerosis: Role of Cholesterol Efflux and Reverse Cholesterol Transport. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 13–27. [Google Scholar] [CrossRef]
- Green, P.S.; Vaisar, T.; Pennathur, S.; Kulstad, J.J.; Moore, A.B.; Marcovina, S.; Brunzell, J.; Knopp, R.H.; Zhao, X.-Q.; Heinecke, J.W. Combined Statin and Niacin Therapy Remodels the High-Density Lipoprotein Proteome. Circulation 2008, 118, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.M.; McKenzie, B.; Kemeh, G.; Sampson, M.; Perl, S.; Young, N.S.; Fessler, M.B.; Remaley, A.T. Rosuvastatin Alters the Proteome of High Density Lipoproteins: Generation of Alpha-1-Antitrypsin Enriched Particles with Anti-Inflammatory Properties. Mol. Cell. Proteom. 2015, 14, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial Dysfunction and Altered Endothelial Biomarkers in Patients with Post-COVID-19 Syndrome and Chronic Fatigue Syndrome (ME/CFS). J. Transl. Med. 2022, 20, 138. [Google Scholar] [CrossRef] [PubMed]
- University of Cincinnaty. HDL-Proteom. Available online: https://homepages.uc.edu/~davidswm/HDLproteome.html (accessed on 28 January 2024).
- Visco, V.; Vitale, C.; Rispoli, A.; Izzo, C.; Virtuoso, N.; Ferruzzi, G.J.; Santopietro, M.; Melfi, A.; Rusciano, M.R.; Maglio, A.; et al. Post-COVID-19 Syndrome: Involvement and Interactions between Respiratory, Cardiovascular and Nervous Systems. J. Clin. Med. 2022, 11, 524. [Google Scholar] [CrossRef]
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.; Wildi, K.; Li Bassi, G.; Pardo, C.A.; Choi, A.; Cho, S.-M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 2023, 12, 816. [Google Scholar] [CrossRef]
- Begue, F.; Tanaka, S.; Mouktadi, Z.; Rondeau, P.; Veeren, B.; Diotel, N.; Tran-Dinh, A.; Robert, T.; Vélia, E.; Mavingui, P.; et al. Altered High-Density Lipoprotein Composition and Functions during Severe COVID-19. Sci. Rep. 2021, 11, 2291. [Google Scholar] [CrossRef]
- Schmitz, G.; Grandl, M. The Molecular Mechanisms of HDL and Associated Vesicular Trafficking Mechanisms to Mediate Cellular Lipid Homeostasis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1718–1722. [Google Scholar] [CrossRef]
- Wei, C.; Wan, L.; Yan, Q.; Wang, X.; Zhang, J.; Yang, X.; Zhang, Y.; Fan, C.; Li, D.; Deng, Y.; et al. HDL-Scavenger Receptor B Type 1 Facilitates SARS-CoV-2 Entry. Nat. Metab. 2020, 2, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- NIH. Myosin Light Chain 6. Available online: https://www.ncbi.nlm.nih.gov/gene/4637 (accessed on 28 January 2024).
- Robichaud, S.; Fairman, G.; Vijithakumar, V.; Mak, E.; Cook, D.P.; Pelletier, A.R.; Huard, S.; Vanderhyden, B.C.; Figeys, D.; Lavallée-Adam, M.; et al. Identification of Novel Lipid Droplet Factors That Regulate Lipophagy and Cholesterol Efflux in Macrophage Foam Cells. Autophagy 2021, 17, 3671–3689. [Google Scholar] [CrossRef]
- Yoshinari, M.; Nishibata, Y.; Masuda, S.; Nakazawa, D.; Tomaru, U.; Arimura, Y.; Amano, K.; Yuzawa, Y.; Sada, K.-E.; Atsumi, T.; et al. Low Disease Activity of Microscopic Polyangiitis in Patients with Anti-Myosin Light Chain 6 Antibody That Disrupts Actin Rearrangement Necessary for Neutrophil Extracellular Trap Formation. Arthritis Res. Ther. 2022, 24, 274. [Google Scholar] [CrossRef]
- Sykes, R.A.; Neves, K.B.; Alves-Lopes, R.; Caputo, I.; Fallon, K.; Jamieson, N.B.; Kamdar, A.; Legrini, A.; Leslie, H.; McIntosh, A.; et al. Vascular Mechanisms of Post-COVID-19 Conditions: Rho-Kinase Is a Novel Target for Therapy. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Nohria, A.; Prsic, A.; Liu, P.-Y.; Okamoto, R.; Creager, M.A.; Selwyn, A.; Liao, J.K.; Ganz, P. Statins Inhibit Rho Kinase Activity in Patients with Atherosclerosis. Atherosclerosis 2009, 205, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Kronstein-Wiedemann, R.; Stadtmüller, M.; Traikov, S.; Georgi, M.; Teichert, M.; Yosef, H.; Wallenborn, J.; Karl, A.; Schütze, K.; Wagner, M.; et al. SARS-CoV-2 Infects Red Blood Cell Progenitors and Dysregulates Hemoglobin and Iron Metabolism. Stem Cell Rev. Rep. 2022, 18, 1809–1821. [Google Scholar] [CrossRef] [PubMed]
- Rapozzi, V.; Juarranz, A.; Habib, A.; Ihan, A.; Strgar, R. Is Haem the Real Target of COVID-19? Photodiagn. Photodyn. Ther. 2021, 35, 102381. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, G.C.; Souza-Silva, F.; Sacramento, C.Q.; Trugilho, M.R.O.; Valente, R.H.; Napoleão-Pêgo, P.; Dias, S.S.G.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Carels, N.; et al. SARS-CoV-2 Proteins Bind to Hemoglobin and Its Metabolites. Int. J. Mol. Sci. 2021, 22, 9035. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.W.; Stamler, J.S.; Piantadosi, C.A. Hemoglobin, Nitric Oxide and Molecular Mechanisms of Hypoxic Vasodilation. Trends Mol. Med. 2009, 15, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Grau, M.; Ibershoff, L.; Zacher, J.; Bros, J.; Tomschi, F.; Diebold, K.F.; Predel, H.; Bloch, W. Even Patients with Mild COVID-19 Symptoms after SARS-CoV-2 Infection Show Prolonged Altered Red Blood Cell Morphology and Rheological Parameters. J. Cell. Mol. Med. 2022, 26, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Stafforini, D.M.; McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M. Platelet-Activating Factor Acetylhydrolases. J. Biol. Chem. 1997, 272, 17895–17898. [Google Scholar] [CrossRef] [PubMed]
- Tjoelker, L.W.; Stafforini, D.M. Platelet-Activating Factor Acetylhydrolases in Health and Disease. Biochim. Biophys. Acta 2000, 1488, 102–123. [Google Scholar] [CrossRef]
- Palur Ramakrishnan, A.V.K.; Varghese, T.P.; Vanapalli, S.; Nair, N.K.; Mingate, M.D. Platelet Activating Factor: A Potential Biomarker in Acute Coronary Syndrome? Cardiovasc. Ther. 2017, 35, 64–70. [Google Scholar] [CrossRef]
- Kim, G.R.; Zhao, T.; Kee, H.J.; Kee, S.-J.; Jeong, M.H. MicroRNA-212-5p and Its Target PAFAH1B2 Suppress Vascular Proliferation and Contraction via the Downregulation of RhoA. PLoS ONE 2021, 16, e0249146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pu, Z.; Wang, G.; Li, Y.; Wang, Y.; Li, N.; Peng, F. FAM3C: An Emerging Biomarker and Potential Therapeutic Target for Cancer. Biomark. Med. 2021, 15, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, W.; Wang, J.; Meng, Y.; Guan, Y.; Yang, J. FAM3 Gene Family: A Promising Therapeutical Target for NAFLD and Type 2 Diabetes. Metabolism 2018, 81, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Watanabe, N.; Akatsu, H.; Nishimura, M. Neuronal Expression of ILEI/FAM3C and Its Reduction in Alzheimer’s Disease. Neuroscience 2016, 330, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Nakano, M.; Mitsuishi, Y.; Hara, N.; Mano, T.; Iwata, A.; Murayama, S.; Suzuki, T.; Ikeuchi, T.; Nishimura, M. Transcriptional Downregulation of FAM3C/ILEI in the Alzheimer’s Brain. Hum. Mol. Genet. 2021, 31, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Guida, M.C.; Hermle, T.; Graham, L.A.; Hauser, V.; Ryan, M.; Stevens, T.H.; Simons, M. ATP6AP2 Functions as a V-ATPase Assembly Factor in the Endoplasmic Reticulum. Mol. Biol. Cell 2018, 29, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N.; Peters, J. Functions of the (pro)Renin Receptor (Atp6ap2) at Molecular and System Levels: Pathological Implications in Hypertension, Renal and Brain Development, Inflammation, and Fibrosis. Pharmacol. Res. 2021, 173, 105922. [Google Scholar] [CrossRef] [PubMed]
- Prestes, E.B.; Bruno, J.C.P.; Travassos, L.H.; Carneiro, L.A.M. The Unfolded Protein Response and Autophagy on the Crossroads of Coronaviruses Infections. Front. Cell. Infect. Microbiol. 2021, 11, 668034. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.-A.; Sesterheim, P.; Wartchow, K.M.; Bobermin, L.D.; Leipnitz, G.; Quincozes-Santos, A. Why Antidiabetic Drugs Are Potentially Neuroprotective during the Sars-CoV-2 Pandemic: The Focus on Astroglial UPR and Calcium-Binding Proteins. Front. Cell. Neurosci. 2022, 16, 905218. [Google Scholar] [CrossRef]
- Cannata Serio, M.; Rujano, M.A.; Simons, M. Mutations in ATP6AP2 Cause Autophagic Liver Disease in Humans. Autophagy 2018, 14, 1088–1089. [Google Scholar] [CrossRef]
- Rujano, M.A.; Cannata Serio, M.; Panasyuk, G.; Péanne, R.; Reunert, J.; Rymen, D.; Hauser, V.; Park, J.H.; Freisinger, P.; Souche, E.; et al. Mutations in the X-Linked ATP6AP2 Cause a Glycosylation Disorder with Autophagic Defects. J. Exp. Med. 2017, 214, 3707–3729. [Google Scholar] [CrossRef] [PubMed]
- Korvatska, O.; Strand, N.S.; Berndt, J.D.; Strovas, T.; Chen, D.-H.; Leverenz, J.B.; Kiianitsa, K.; Mata, I.F.; Karakoc, E.; Greenup, J.L.; et al. Altered Splicing of ATP6AP2 Causes X-Linked Parkinsonism with Spasticity (XPDS). Hum. Mol. Genet. 2013, 22, 3259–3268. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.; Speth, R.C.; Trivedi, M. Renin-Angiotensin System Gene Expression and Neurodegenerative Diseases. J. Renin-Angiotensin Aldosterone Syst. 2016, 17, 1470320316666750. [Google Scholar] [CrossRef] [PubMed]
- Bracke, A.; Halbach, O.V.B. Roles and Functions of Atp6ap2 in the Brain. Neural Regen. Res. 2018, 13, 2038. [Google Scholar] [CrossRef] [PubMed]
- Wendling, O.; Champy, M.-F.; Jaubert, S.; Pavlovic, G.; Dubos, A.; Lindner, L.; Jacobs, H.; Mark, M.; Combe, R.; Da Cruz, I.G.; et al. Atp6ap2 Ablation in Adult Mice Impairs Viability through Multiple Organ Deficiencies. Sci. Rep. 2017, 7, 9618. [Google Scholar] [CrossRef] [PubMed]
- Yang, T. Soluble (Pro)Renin Receptor in Hypertension. Nephron 2023, 147, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, N.; Stuart, D.; Peterson, C.S.; Hu, C.; Wheatley, W.; Min Cho, J.; Symons, J.D.; Kohan, D.E. Loss of Soluble (Pro)Renin Receptor Attenuates Angiotensin-II Induced Hypertension and Renal Injury. Circ. Res. 2021, 129, 50–62. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, F.; Liu, X.; Hu, J.; Su, J.; Lu, X.; Lu, A.; Cho, J.M.; Symons, J.D.; Zou, C.-J.; et al. Soluble (pro)Renin Receptor Induces Endothelial Dysfunction and Hypertension in Mice with Diet-Induced Obesity via Activation of Angiotensin II Type 1 Receptor. Clin. Sci. 2021, 135, 793–810. [Google Scholar] [CrossRef]
- Daniloski, Z.; Jordan, T.X.; Wessels, H.-H.; Hoagland, D.A.; Kasela, S.; Legut, M.; Maniatis, S.; Mimitou, E.P.; Lu, L.; Geller, E.; et al. Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells. Cell 2021, 184, 92–105.e16. [Google Scholar] [CrossRef]
- Bader, M.; Ganten, D. Regulation of Renin: New Evidence from Cultured Cells and Genetically Modified Mice. J. Mol. Med. 2000, 78, 130–139. [Google Scholar] [CrossRef]
- Bernardo, A.; Malara, M.; Bertuccini, L.; De Nuccio, C.; Visentin, S.; Minghetti, L. The Antihypertensive Drug Telmisartan Protects Oligodendrocytes from Cholesterol Accumulation and Promotes Differentiation by a PPAR-γ-Mediated Mechanism. Int. J. Mol. Sci. 2021, 22, 9434. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; Greenberg, S.M. Cerebral Amyloid Angiopathy: A Systematic Review. J. Clin. Neurol. 2011, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, L.; Palmer, K.; Calandri, I.; Guekht, A.; Beghi, E.; Carroll, W.; Frontera, J.; García-Azorín, D.; Westenberg, E.; Winkler, A.S.; et al. Changes in Cognitive Functioning after COVID-19: A Systematic Review and Meta-Analysis. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 18, 1047–1066. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Boutajangout, A.; Masurkar, A.V.; Betensky, R.A.; Ge, Y.; Vedvyas, A.; Debure, L.; Moreira, A.; Lewis, A.; Huang, J.; et al. Comparison of Serum Neurodegenerative Biomarkers among Hospitalized COVID-19 Patients versus Non-COVID Subjects with Normal Cognition, Mild Cognitive Impairment, or Alzheimer’s Dementia. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 18, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, N.; Araya, R.; Kamikubo, Y.; Kaneshiro, N.; Imaoka, R.; Jin, H.; Kashiyama, T.; Hashimoto, Y.; Kurosawa, M.; Uehara, T.; et al. TMEM30A Is a Candidate Interacting Partner for the β-Carboxyl-Terminal Fragment of Amyloid-β Precursor Protein in Endosomes. PLoS ONE 2018, 13, e0200988. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Byrnes, C.; Lee, Y.T.; Tuymetova, G.; Duffy, H.B.D.; Bakir, J.Y.; Pettit, S.N.; Angina, J.; Springer, D.A.; Allende, M.L.; et al. SARS-CoV-2 ORF3a Expression in Brain Disrupts the Autophagy–Lysosomal Pathway, Impairs Sphingolipid Homeostasis, and Drives Neuropathogenesis. FASEB J. 2023, 37, e22919. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.N.; Houlihan, P.R.; Matamala, E.; Cabezas-Bratesco, D.; Lee, G.Y.; Cristofori-Armstrong, B.; Dilan, T.L.; Sanchez-Martinez, S.; Matthies, D.; Yan, R.; et al. The SARS-CoV-2 Accessory Protein Orf3a Is Not an Ion Channel, but Does Interact with Trafficking Proteins. eLife 2023, 12, e84477. [Google Scholar] [CrossRef] [PubMed]
- Ziff, O.J.; Ashton, N.J.; Mehta, P.R.; Brown, R.; Athauda, D.; Heaney, J.; Heslegrave, A.J.; Benedet, A.L.; Blennow, K.; Checkley, A.M.; et al. Amyloid Processing in COVID-19-associated Neurological Syndromes. J. Neurochem. 2022, 161, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; et al. A Prospective Observational Study of Post-COVID-19 Chronic Fatigue Syndrome Following the First Pandemic Wave in Germany and Biomarkers Associated with Symptom Severity. Nat. Commun. 2022, 13, 5104. [Google Scholar] [CrossRef]
- Bell, D.S. The Doctor’s Guide to Chronic Fatigue Syndrome; Addison-Wesley, Publishing Company: Reading, MA, USA, 1993. [Google Scholar]
- Morfeld, M.; Bullinger, M.; Nantke, J.; Brähler, E. The version 2.0 of the SF-36 Health Survey: Results of a population-representative study. Soz. Praventivmed. 2005, 50, 292–300. [Google Scholar] [CrossRef]
- Michielsen, H.J.; De Vries, J.; Van Heck, G.L. Psychometric Qualities of a Brief Self-Rated Fatigue Measure: The Fatigue Assessment Scale. J. Psychosom. Res. 2003, 54, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Chalder, T. Measuring Fatigue in Clinical and Community Settings. J. Psychosom. Res. 2010, 69, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Cotler, J.; Holtzman, C.; Dudun, C.; Jason, L.A. A Brief Questionnaire to Assess Post-Exertional Malaise. Diagnostics 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Demichev, V.; Messner, C.B.; Vernardis, S.I.; Lilley, K.S.; Ralser, M. DIA-NN: Neural Networks and Interference Correction Enable Deep Proteome Coverage in High Throughput. Nat. Methods 2020, 17, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Aditya, B. Generifying and Intuifying Cross-Platform Omics Analysis. R Package Version 1.8.0. 2023. Available online: https://github.com/bhagwataditya/autonomics (accessed on 9 February 2024).
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]




| Antigen-Status | Control | Patients | ||
|---|---|---|---|---|
| S+/N− | S+/N+ | PVS (S+/N−) | PCS (S+/N+) | |
| n | 8 | 8 | 8 | 8 |
| sex (female/male) | 5/3 | 8/0 | 7/1 | 6/2 |
| age (years) | 35 (22–51) | 29 (24–34) | 32 (25–51) | 36 (25–48) |
| BMI (kg/m2) | 23.4 (20.2–36.5) | 24.9 (18.8–29.4) | 21.9 (16.9–27.2) | 23.4 (19.4–27.7) |
| Antigen-Status | PVS Patients | PCS Patients | ||||
|---|---|---|---|---|---|---|
| S+/N− (n = 8) | S+/N− (n = 8) | p | S+/N+ (n = 7) | S+/N+ (n = 7) | p | |
| Therapy | before | after | before | after | ||
| Bell disability scale | 35 (20–50) | 50 (30–90) | 0.012 | 60 (40–70) | 80 (50–90) | 0.004 |
| Chalder fatigue scale (CFQ) | 28 (15–33) | 17.5 (12–30) | 0.003 | 25 (15–31) | 19 (12–23) | 0.0004 |
| fatigue assessment scale (FAS) | 45 (27–50) | 34.5 (19–48) | 0.090 | 40 (28–43) | 27 (23–39) | 0.046 |
| work ability index | 13.5 (11.5–24.5) | 17.5 (11.5–22) | 0.432 | 21.0 (14–40) | 29.0 (20–41.5) | 0.120 |
| post-exertional malaise (PEM) | 7 | 6 | 6 | 3 | ||
| short-form health survey (SF-36) subgroups | ||||||
| physical functioning | 47.5 (0–80) | 60.0 (20–90) | 0.056 | 30.0 (15–95) | 40.0 (30–100) | 0.128 |
| physical role functioning | 0 (0) | 0 (0–50) | 0.197 | 0 (0–50) | 0 (0–75) | 0.140 |
| bodily pain | 27.0 (12–100) | 36.5 (0–100) | 0.373 | 22.0 (0–100) | 52.0 (11–100) | 0.036 |
| general health perceptions | 20.0 (10–30) | 17.5 (5–67) | 0.224 | 20.0 (5–30) | 30.0 (5–55) | 0.018 |
| physical sum scale | 29.9 (9.8–36.5) | 32.9 (18.1–46.0) | 0.0016 | 27.4 (21.0–46.2) | 33.4 (24.4–47.0) | 0.019 |
| vitality | 7.5 (0–55) | 15.0 (5–65) | 0.197 | 5.0 (0–20) | 15.0 (0–50) | 0.049 |
| social role functioning | 12.5 (0–50) | 31.3 (0–100) | 0.214 | 12.5 (0–75) | 37.5 (12.5–87.5) | 0.0009 |
| emotional role functioning | 83.3 (0–100) | 100 (0–100) | 0.401 | 33.3 (0–100) | 33.3 (0–100) | 0.568 |
| mental health | 40.0 (24–76) | 62.0 (8–100) | 0.414 | 44.0 (0–64) | 64.0 (0–80) | 0.052 |
| mental sum scale | 34.9 (17.3–52.0) | 48.2 (15.2–50.7) | 0.215 | 29.4 (18.6–44.4) | 39.0 (13.0–49.0) | 0.292 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grote, K.; Schaefer, A.-C.; Soufi, M.; Ruppert, V.; Linne, U.; Mukund Bhagwat, A.; Szymanski, W.; Graumann, J.; Gercke, Y.; Aldudak, S.; et al. Targeting the High-Density Lipoprotein Proteome for the Treatment of Post-Acute Sequelae of SARS-CoV-2. Int. J. Mol. Sci. 2024, 25, 4522. https://doi.org/10.3390/ijms25084522
Grote K, Schaefer A-C, Soufi M, Ruppert V, Linne U, Mukund Bhagwat A, Szymanski W, Graumann J, Gercke Y, Aldudak S, et al. Targeting the High-Density Lipoprotein Proteome for the Treatment of Post-Acute Sequelae of SARS-CoV-2. International Journal of Molecular Sciences. 2024; 25(8):4522. https://doi.org/10.3390/ijms25084522
Chicago/Turabian StyleGrote, Karsten, Ann-Christin Schaefer, Muhidien Soufi, Volker Ruppert, Uwe Linne, Aditya Mukund Bhagwat, Witold Szymanski, Johannes Graumann, Yana Gercke, Sümeya Aldudak, and et al. 2024. "Targeting the High-Density Lipoprotein Proteome for the Treatment of Post-Acute Sequelae of SARS-CoV-2" International Journal of Molecular Sciences 25, no. 8: 4522. https://doi.org/10.3390/ijms25084522
APA StyleGrote, K., Schaefer, A.-C., Soufi, M., Ruppert, V., Linne, U., Mukund Bhagwat, A., Szymanski, W., Graumann, J., Gercke, Y., Aldudak, S., Hilfiker-Kleiner, D., Schieffer, E., & Schieffer, B. (2024). Targeting the High-Density Lipoprotein Proteome for the Treatment of Post-Acute Sequelae of SARS-CoV-2. International Journal of Molecular Sciences, 25(8), 4522. https://doi.org/10.3390/ijms25084522