Imbalance between Actin Isoforms Contributes to Tumour Progression in Taxol-Resistant Triple-Negative Breast Cancer Cells
Abstract
1. Introduction
2. Results
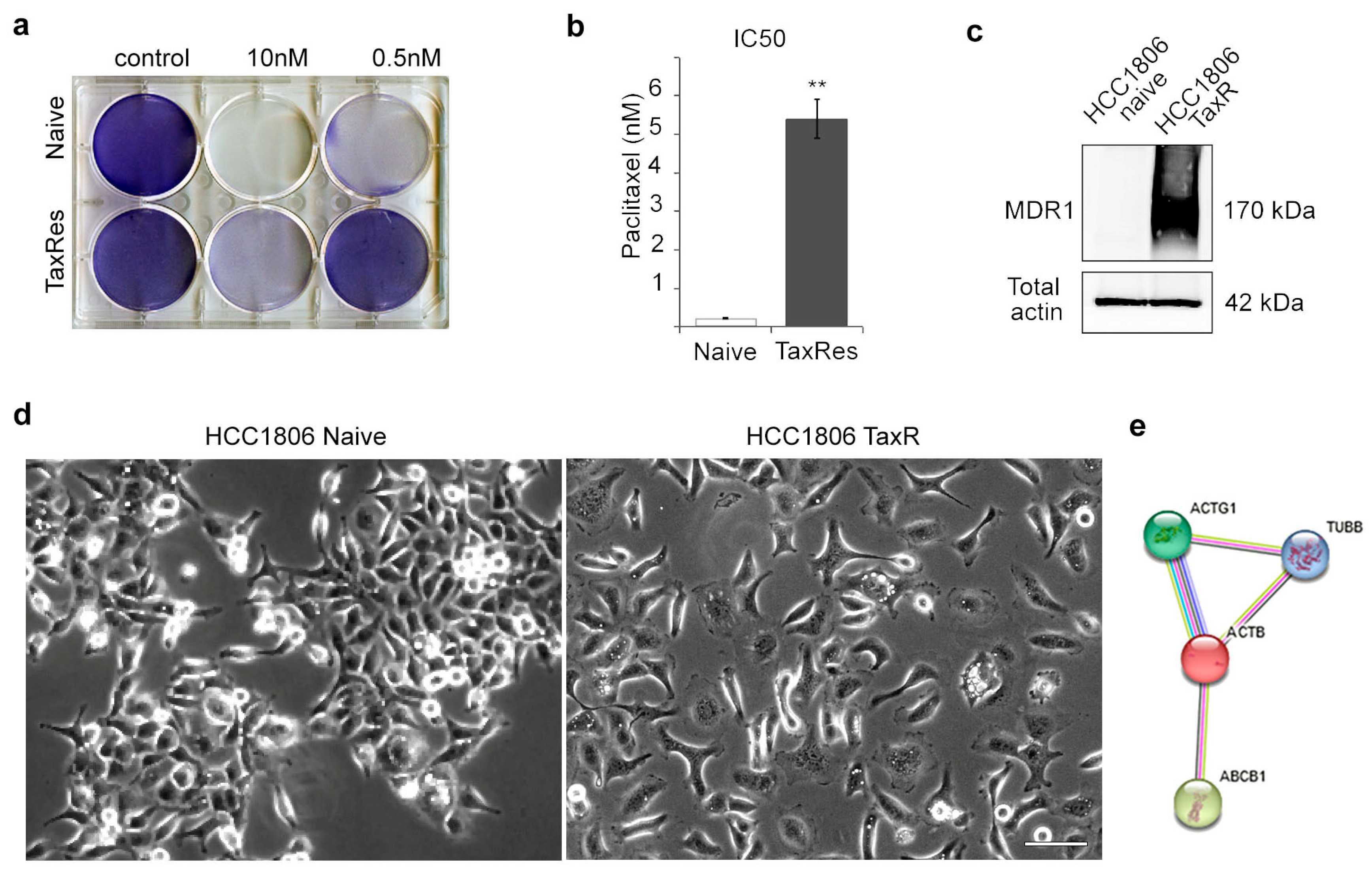
2.1. Characterisation of the Taxol-Resistant HCC1806 Cell Line
2.2. Proliferative and Invasive Features of Naïve and Taxol-Resistant TNBC Cells
- Consistent with the level of resistance (Figure 1a), we observed high proliferative activity of HCC1806-TaxR cells cultured in the presence of taxol (paclitaxel, PTX 0.1 and 1 μM), thereby revealing loss of sensitivity of these cancer cells to this particular chemotherapeutic agent. Moreover, proliferative activity of HCC1806-TaxR cells cultured in the presence of taxol was even higher when compared with non-treated HCC1806-TaxR (Figure 2a). As expected, the proliferative activity of taxol-treated naïve HCC1806 cells was significantly reduced, as shown in Figure 2a. Real-time monitoring of cellular proliferation capacity by the iCELLigence system showed that the growth of HCC1806-TaxR cells was significantly lower than that of parental HCC1806 cells (Figure 2b) (104 initial cells/well, 96 h of incubation). By day 7 of incubation, the number of viable HCC1806-TaxR cells was 5-fold lower compared with parental cells, according to phase contrast microscopy (Figure 2c);
- HCC1806-TaxR cells had an increased ability to form colonies under adhesive conditions (104 cells/dish, 14 days of incubation) compared to parental cells (Figure 2d,e);
- The data from the Matrigel migration/experimental invasion assay (250 × 103 cells/well, 12 h of incubation, 8 µm filter pore size) demonstrated that HCC1806-TaxR cells exhibited a more motile and invasive phenotype than HCC1806-naïve cells (Figure 2f,g).

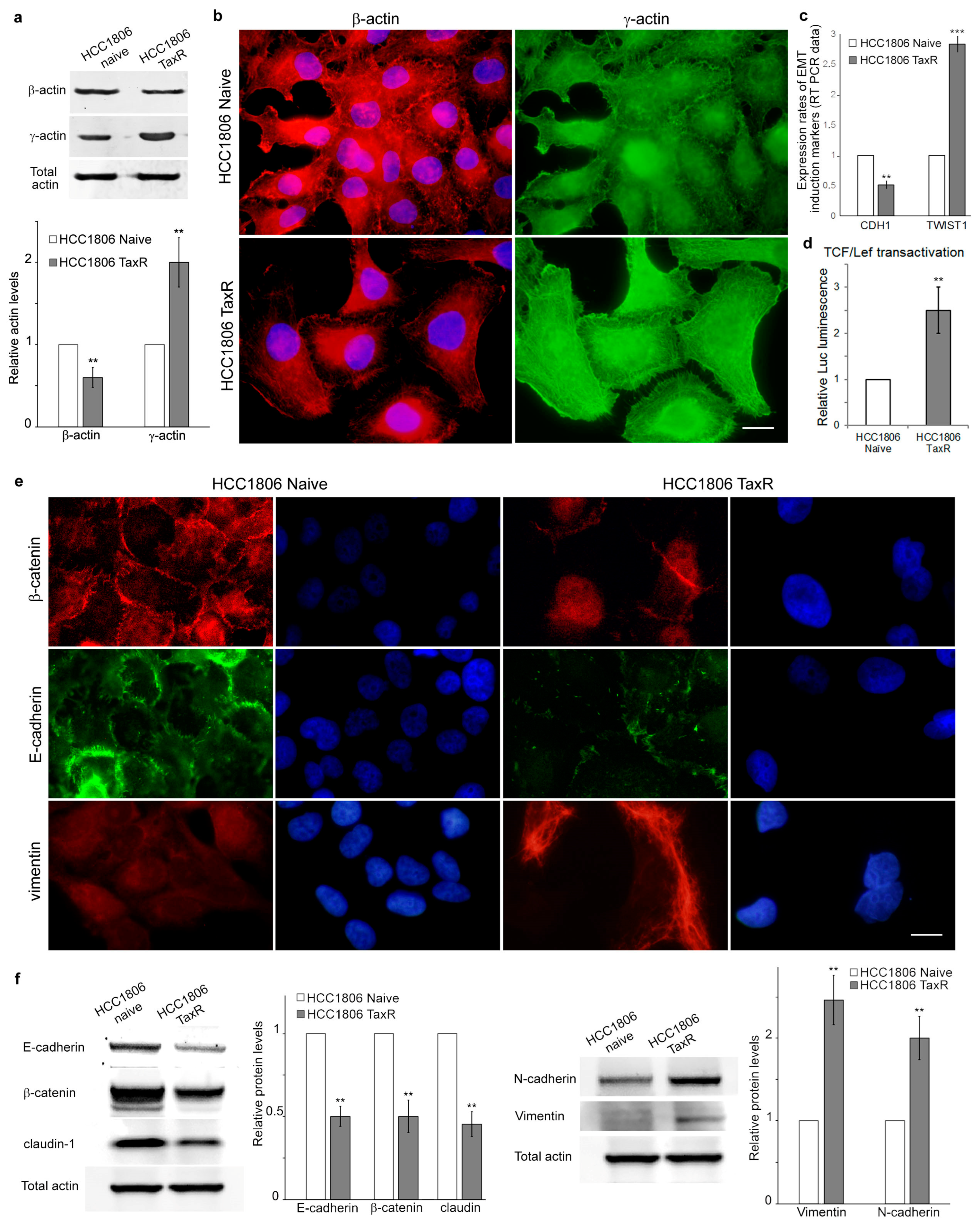
2.3. Reorganisation of the Cytoskeleton and the Intercellular Junctions in the Naïve and Taxol-Resistant HCC1806 Cells
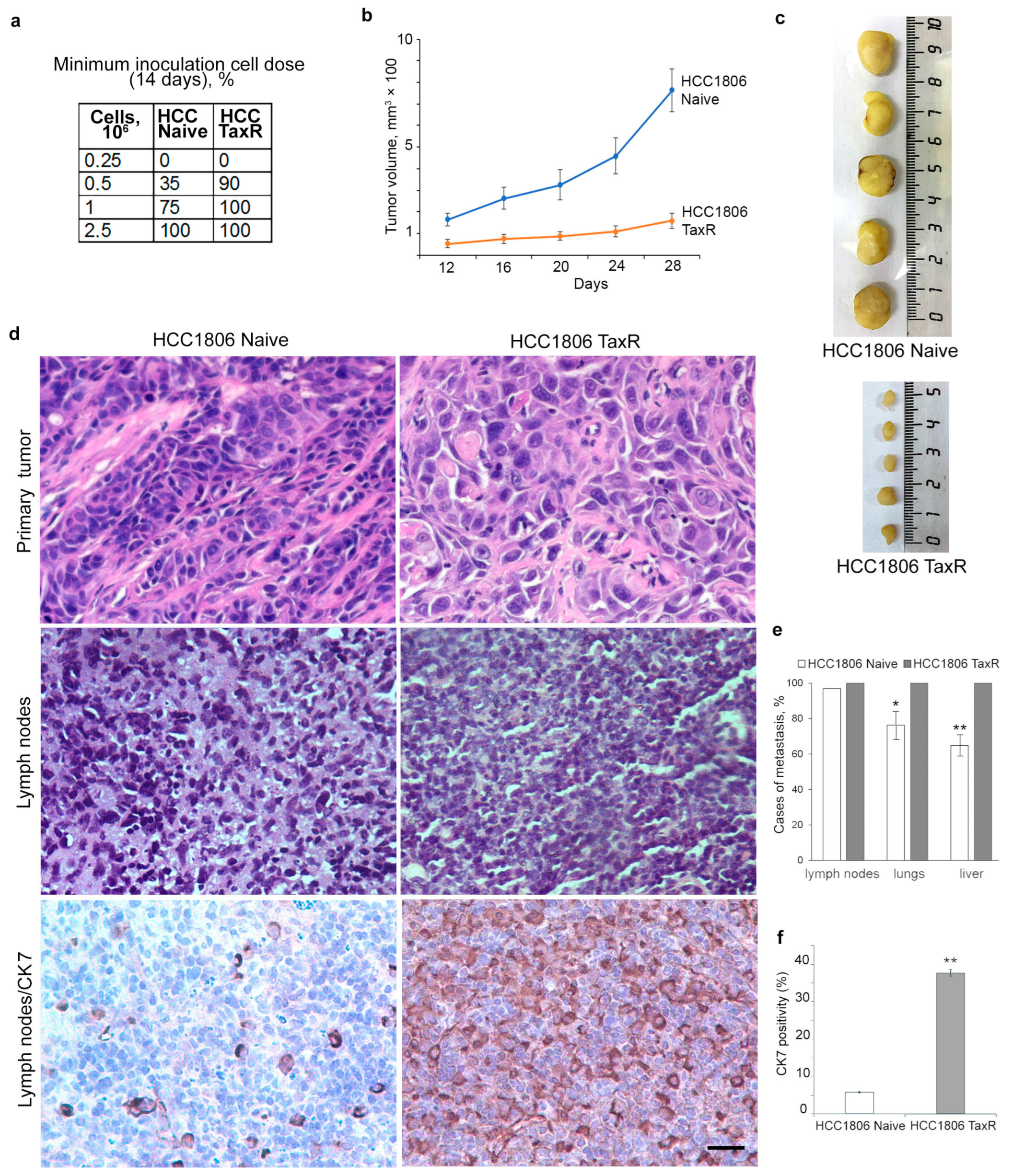
2.4. Tumour Growth of HCC1806-Naïve and HCC1806-TaxR Cells In Vivo
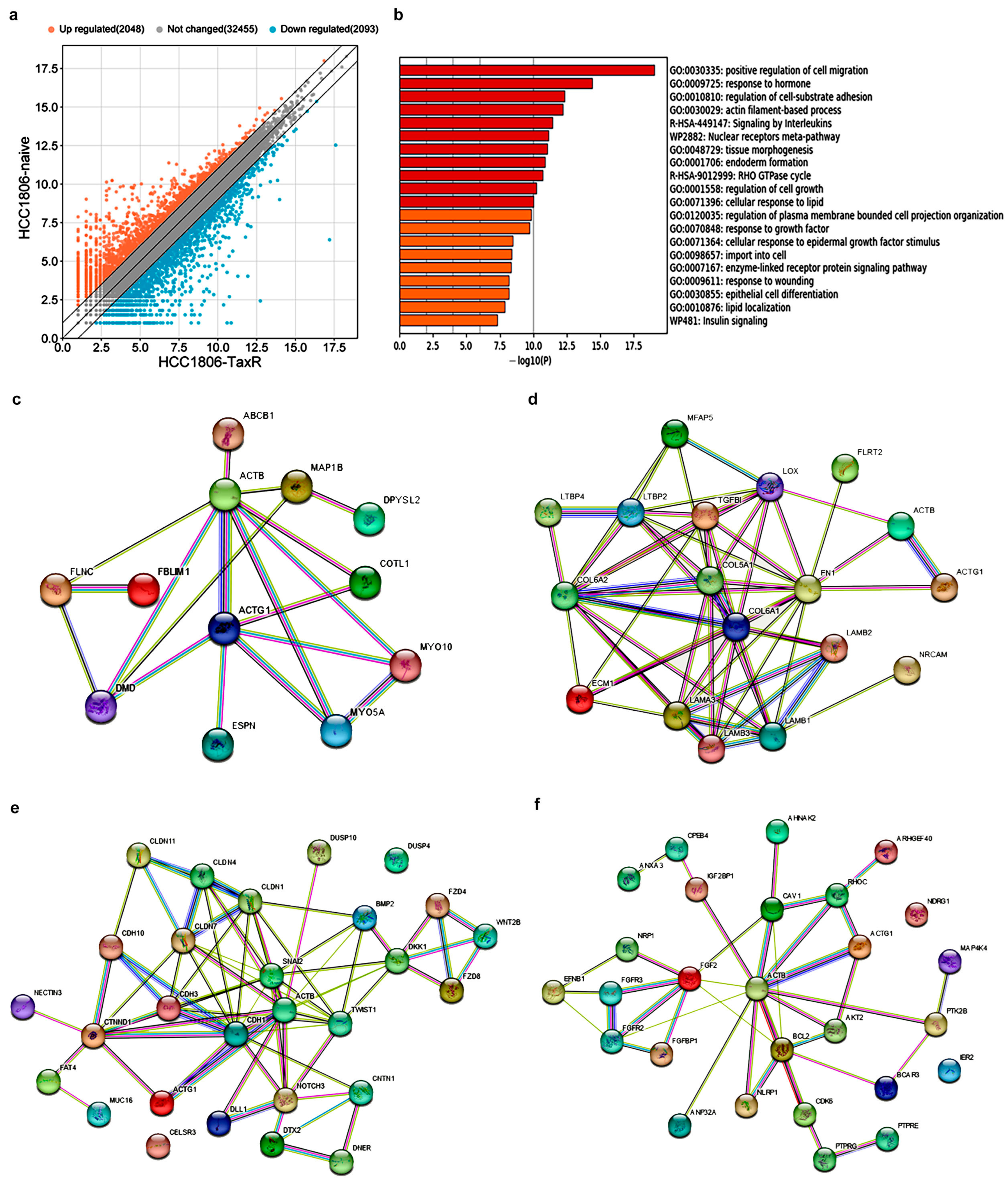
2.5. Transcriptome Analysis and Identification of Differentially Expressed Genes Potentially Involved in the Development of the Taxol Resistance Phenotype of the HCC1806 Subline
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cytotoxicity Assay/Cellular Survival MTS-Based Assay
4.3. Real-Time Monitoring of Cell Proliferation
4.4. Cell Proliferation Rate
4.5. Colony Formation Assay
4.6. Boyden Chamber Cell Migration/Experimental Invasion Assay
4.7. Immunofluorescence Microscopy
4.8. Western Blotting
4.9. Antibodies
4.10. RNA Sequencing and Transcriptome Analysis
4.11. Luciferase Assay
4.12. BALB/c Athymic Mice Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, M.; Li, Z.; Shen, G.; Wang, T.; Li, J.; Wang, M.; Liu, Z.; Zhao, F.; Ren, D.; Zhao, J. Efficacy and safety of first-line treatment for metastatic triple-negative breast cancer: A network meta-analysis. Cancer Pathog. Ther. 2024, 2, 81–90. [Google Scholar] [CrossRef]
- Krause, W. Resistance to anti-tubulin agents: From vinca alkaloids to epothilones. Cancer Drug Resist. 2019, 2, 82–106. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer—How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Tsodikov, A.; Bauer, K.R.; Parise, C.A.; Caggiano, V. The role of human epidermal growth factor receptor 2 in the survival of women with estrogen and progesterone receptor-negative, invasive breast cancer: The California Cancer Registry, 1999–2004. Cancer 2008, 112, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Dugina, V.; Shagieva, G.; Novikova, M.; Lavrushkina, S.; Sokova, O.; Kireev, I.; Kopnin, P. Impaired Expression of Cytoplasmic Actins Leads to Chromosomal Instability of MDA-MB-231 Basal-Like Mammary Gland Cancer Cell Line. Molecules 2021, 26, 2151. [Google Scholar] [CrossRef] [PubMed]
- Dugina, V.; Alieva, I.; Khromova, N.; Kireev, I.; Gunning, P.W.; Kopnin, P. Interaction of microtubules with the actin cytoskeleton via cross-talk of EB1-containing +TIPs and γ-actin in epithelial cells. Oncotarget 2016, 7, 72699–72715. [Google Scholar] [CrossRef]
- Po’uha, S.; Honore, S.; Braguer, D.; Kavallaris, M. Partial depletion of gamma-actin suppresses microtubule dynamics. Cytoskeleton 2013, 70, 148–160. [Google Scholar] [CrossRef]
- Boichuk, S.; Galembikova, A.; Sitenkov, A.; Khusnutdinov, R.; Dunaev, P.; Valeeva, E.; Usolova, N. Establishment and characterization of a triple negative basal-like breast cancer cell line with multi-drug resistance. Oncol. Lett. 2017, 14, 5039–5045. [Google Scholar] [CrossRef]
- Dugina, V.; Khromova, N.; Rybko, V.; Blizniukov, O.; Shagieva, G.; Chaponnier, C.; Kopnin, B.; Kopnin, P. Tumor promotion by γ and suppression by β non-muscle actin isoforms. Oncotarget 2015, 6, 14556–14571. [Google Scholar] [CrossRef]
- Baranwal, S.; Naydenov, N.G.; Harris, G.; Dugina, V.; Morgan, K.G.; Chaponnier, C.; Ivanov, A.I. Nonredundant roles of cytoplasmic β- and γ-actin isoforms in regulation of epithelial apical junctions. Mol. Biol. Cell 2012, 23, 3542–3553. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discov. 2019, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; Mcphail, A.T. Plant Antitumor Agents.VI.The Isolation and Structure of Taxol, a Novel Antileukemic and Antitumor Agent from Taxus brevifolia2. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Dan, V.M.; Raveendran, R.S.; Baby, S. Resistance to Intervention: Paclitaxel in Breast Cancer. Mini Rev. Med. Chem. 2021, 21, 1237–1268. [Google Scholar] [CrossRef] [PubMed]
- Berrieman, H.K.; Lind, M.J.; Cawkwell, L. Do β-tubulin mutations have a role in resistance to chemotherapy? Lancet Oncol. 2004, 5, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Roninson, I.B. The role of the MDR1 (P-glycoprotein) gene in multidrug resistance in vitro and in vivo. Biochem. Pharmacol. 1992, 43, 95–102. [Google Scholar] [CrossRef]
- Yang, C.P.H.; Horwitz, S.B. Taxol®: The First Microtubule Stabilizing Agent. Int. J. Mol. Sci. 2017, 18, 1733. [Google Scholar] [CrossRef]
- Martello, L.; Verdier-Pinard, P.; Shen, H.; He, L.; Research, K.T.-C. Elevated levels of microtubule destabilizing factors in a Taxol-resistant/dependent A549 cell line with an α-tubulin mutation. Cancer Res. 2003, 63, 1207–1213. [Google Scholar]
- Wiesen, K.M.; Xia, S.; Yang, C.P.H.; Horwitz, S.B. Wild-type class I beta-tubulin sensitizes Taxol-resistant breast adenocarcinoma cells harboring a beta-tubulin mutation. Cancer Lett. 2007, 257, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Weinberg, R.A. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Kordias, D.; Kostara, C.E.; Papadaki, S.; Verigos, J.; Bairaktari, E.; Magklara, A. Omics Analysis of Chemoresistant Triple Negative Breast Cancer Cells Reveals Novel Metabolic Vulnerabilities. Cells 2022, 11, 2719. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Vagner, S.; Robert, C. Persistent Cancer Cells: The Deadly Survivors. Cell 2020, 183, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Gil-Henn, H.; Patsialou, A.; Wang, Y.; Warren, M.S.; Condeelis, J.S.; Koleske, A.J. Arg/Abl2 promotes invasion and attenuates proliferation of breast cancer in vivo. Oncogene 2013, 32, 2622–2630. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Wang, W.; Wyckoff, J.B.; Condeelis, J.S. Breast Cancer Cells Isolated by Chemotaxis from Primary Tumors Show Increased Survival and Resistance to Chemotherapy. Cancer Res. 2004, 64, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Kavallaris, M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 2010, 10, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Chew, Y.M.; Cross, R.A. Taxol acts differently on different tubulin isotypes. Commun. Biol. 2023, 6, 1–15. [Google Scholar] [CrossRef]
- Kavallaris, M.; Kuo, D.Y.S.; Burkhart, C.A.; Regl, D.L.; Norris, M.D.; Haber, M.; Horwitz, S.B.; Kuo, D.Y.; Burkhart, C.A.; Lee Regl, D.; et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J. Clin. Investig. 1997, 100, 1282–1293. [Google Scholar] [CrossRef]
- Albrethsen, J.; Angeletti, R.H.; Horwitz, S.B.; Yang, C.P.H. Proteomics of cancer cell lines resistant to microtubule stabilizing agents. Mol. Cancer Ther. 2014, 13, 260. [Google Scholar] [CrossRef] [PubMed]
- Mozzetti, S.; Ferlini, C.; Concolino, P.; Filippetti, F.; Raspaglio, G.; Prislei, S.; Gallo, D.; Martinelli, E.; Ranelletti, F.O.; Ferrandina, G.; et al. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin. Cancer Res. 2005, 11, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.H.; Yap, E.H.; Xiao, H.; Fiser, A.; Horwitz, S.B. 2-(m-Azidobenzoyl)taxol binds differentially to distinct β-tubulin isotypes. Proc. Natl. Acad. Sci. USA 2016, 113, 11294–11299. [Google Scholar] [CrossRef] [PubMed]
- Magnani, M.; Ortuso, F.; Soro, S.; Alcaro, S.; Tramontano, A.; Botta, M. The βI/βIII-tubulin isoforms and their complexes with antimitotic agents: Docking and molecular dynamics studies. FEBS J. 2006, 273, 3301–3310. [Google Scholar] [CrossRef] [PubMed]
- Ferlini, C.; Cicchillitti, L.; Raspaglio, G.; Bartollino, S.; Cimitan, S.; Bertucci, C.; Mozzetti, S.; Gallo, D.; Persico, M.; Fattorusso, C.; et al. Paclitaxel directly binds to Bcl-2 and functionally mimics activity of Nur77. Cancer Res. 2009, 69, 6906–6914. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sparano, J.A.; Fineberg, S.; Stead, L.; Sunkara, J.; Horwitz, S.B.; McDaid, H.M. High Expression of Class III β-Tubulin Predicts Good Response to Neoadjuvant Taxane and Doxorubicin/Cyclophosphamide-Based Chemotherapy in Estrogen Receptor–Negative Breast Cancer. Clin. Breast Cancer 2013, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Po’uha, S.T.; Kavallaris, M. Gamma-actin is involved in regulating centrosome function and mitotic progression in cancer cells. Cell Cycle 2015, 14, 3908–3919. [Google Scholar] [CrossRef]
- Verrills, N.M.; Liem, N.L.; Liaw, T.Y.E.; Hood, B.D.; Lock, R.B.; Kavallaris, M. Proteomic analysis reveals a novel role for the actin cytoskeleton in vincristine resistant childhood leukemia—An in vivo study. Proteomics 2006, 6, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Dugina, V.; Shagieva, G.; Khromova, N.; Kopnin, P. Divergent impact of actin isoforms on cell cycle regulation. Cell Cycle 2018, 17, 2610–2621. [Google Scholar] [CrossRef]
- Shagieva, G.S.; Alieva, I.B.; Chaponnier, C.; Dugina, V.B. Divergent Impact of Actin Isoforms on Division of Epithelial Cells. Nat. Cell Biol. 2020, 85, 1072–1081. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Lechuga, S.; Marino-Melendez, A.; Naydenov, N.G. Unique and redundant functions of cytoplasmic actins and nonmuscle myosin II isoforms at epithelial junctions. Ann. N. Y. Acad. Sci. 2022, 1515, 61. [Google Scholar] [CrossRef] [PubMed]
- Alberico, E.O.; Zhu, Z.C.; Wu, Y.F.O.; Gardner, M.K.; Kovar, D.R.; Goodson, H.V. Interactions between the Microtubule Binding Protein EB1 and F-Actin. J. Mol. Biol. 2016, 428, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Patsialou, A.; Bravo-Cordero, J.J.; Wang, Y.; Entenberg, D.; Liu, H.; Clarke, M.; Condeelis, J.S. Intravital multiphoton imaging reveals multicellular streaming as a crucial component of in vivo cell migration in human breast tumors. Intravital 2013, 2, e25294. [Google Scholar] [CrossRef] [PubMed]
- Philippar, U.; Roussos, E.T.; Oser, M.; Yamaguchi, H.; Kim, H.D.; Giampieri, S.; Wang, Y.; Goswami, S.; Wyckoff, J.B.; Lauffenburger, D.A.; et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev. Cell 2008, 15, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Roussos, E.T.; Balsamo, M.; Alford, S.K.; Wyckoff, J.B.; Gligorijevic, B.; Wang, Y.; Pozzuto, M.; Stobezki, R.; Goswami, S.; Segall, J.E.; et al. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J. Cell Sci. 2011, 124, 2120. [Google Scholar] [CrossRef] [PubMed]
- Gertler, F.; Condeelis, J. Metastasis: Tumor cells becoming MENAcing. Trends Cell Biol. 2011, 21, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, G.S.; Goswami, S.; Jones, J.G.; Oktay, M.H.; Condeelis, J.S. Signatures of breast cancer metastasis at a glance. J. Cell Sci. 2016, 129, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, G.S.; Condeelis, J.S.; Oktay, M.H. Chemotherapy-Induced Metastasis: Molecular Mechanisms, Clinical Manifestations, Therapeutic Interventions. Cancer Res. 2019, 79, 4567. [Google Scholar] [CrossRef] [PubMed]
- Volk-Draper, L.; Hall, K.; Griggs, C.; Rajput, S.; Kohio, P.; DeNardo, D.; Ran, S. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res. 2014, 74, 5421. [Google Scholar] [CrossRef]
- Ran, S. The role of TLR4 in chemotherapy-driven metastasis. Cancer Res. 2015, 75, 2405. [Google Scholar] [CrossRef]
- Keklikoglou, I.; Cianciaruso, C.; Güç, E.; Squadrito, M.L.; Spring, L.M.; Tazzyman, S.; Lambein, L.; Poissonnier, A.; Ferraro, G.B.; Baer, C.; et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat. Cell Biol. 2019, 21, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Gingis-Velitski, S.; Loven, D.; Benayoun, L.; Munster, M.; Bril, R.; Voloshin, T.; Alishekevitz, D.; Bertolini, F.; Shaked, Y. Host response to short-term, single-agent chemotherapy induces matrix metalloproteinase-9 expression and accelerates metastasis in mice. Cancer Res. 2011, 71, 6986–6996. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M. My battle with cancer. Part 1. Oncoscience 2024, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dugina, V.; Vasileva, M.; Khromova, N.; Vinokurova, S.; Shagieva, G.; Mikheeva, E.; Galembikova, A.; Dunaev, P.; Kudlay, D.; Boichuk, S.; et al. Imbalance between Actin Isoforms Contributes to Tumour Progression in Taxol-Resistant Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2024, 25, 4530. https://doi.org/10.3390/ijms25084530
Dugina V, Vasileva M, Khromova N, Vinokurova S, Shagieva G, Mikheeva E, Galembikova A, Dunaev P, Kudlay D, Boichuk S, et al. Imbalance between Actin Isoforms Contributes to Tumour Progression in Taxol-Resistant Triple-Negative Breast Cancer Cells. International Journal of Molecular Sciences. 2024; 25(8):4530. https://doi.org/10.3390/ijms25084530
Chicago/Turabian StyleDugina, Vera, Maria Vasileva, Natalia Khromova, Svetlana Vinokurova, Galina Shagieva, Ekaterina Mikheeva, Aigul Galembikova, Pavel Dunaev, Dmitry Kudlay, Sergei Boichuk, and et al. 2024. "Imbalance between Actin Isoforms Contributes to Tumour Progression in Taxol-Resistant Triple-Negative Breast Cancer Cells" International Journal of Molecular Sciences 25, no. 8: 4530. https://doi.org/10.3390/ijms25084530
APA StyleDugina, V., Vasileva, M., Khromova, N., Vinokurova, S., Shagieva, G., Mikheeva, E., Galembikova, A., Dunaev, P., Kudlay, D., Boichuk, S., & Kopnin, P. (2024). Imbalance between Actin Isoforms Contributes to Tumour Progression in Taxol-Resistant Triple-Negative Breast Cancer Cells. International Journal of Molecular Sciences, 25(8), 4530. https://doi.org/10.3390/ijms25084530






