Melatonin-Mediated Molecular Responses in Plants: Enhancing Stress Tolerance and Mitigating Environmental Challenges in Cereal Crop Production
Abstract
1. Introduction
2. Effects of Melatonin on Stress Tolerance in Cereal Crops
2.1. Maize
2.2. Wheat
2.3. Barley
2.4. Rice
2.5. Millet
2.6. Sorghum
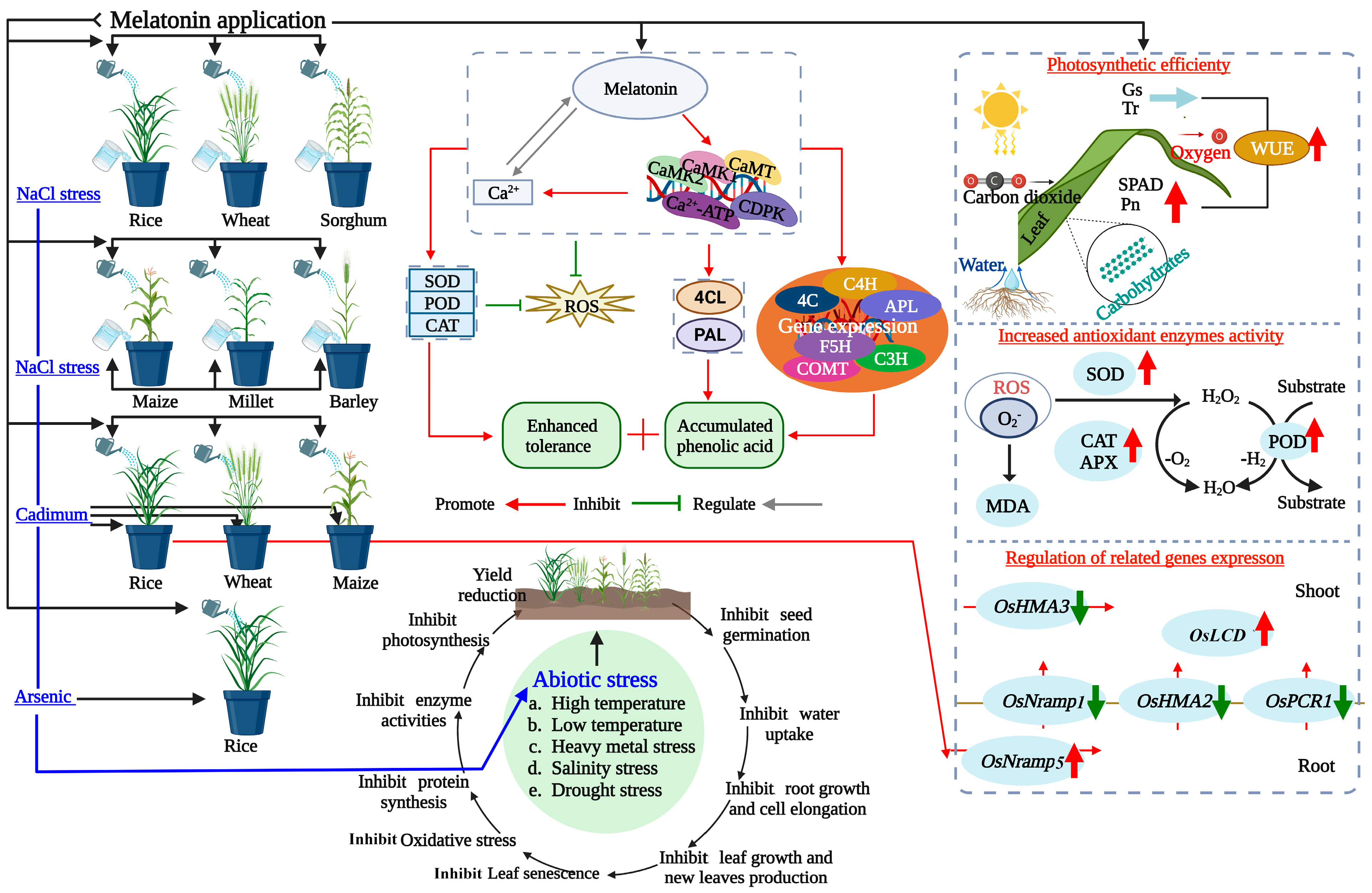
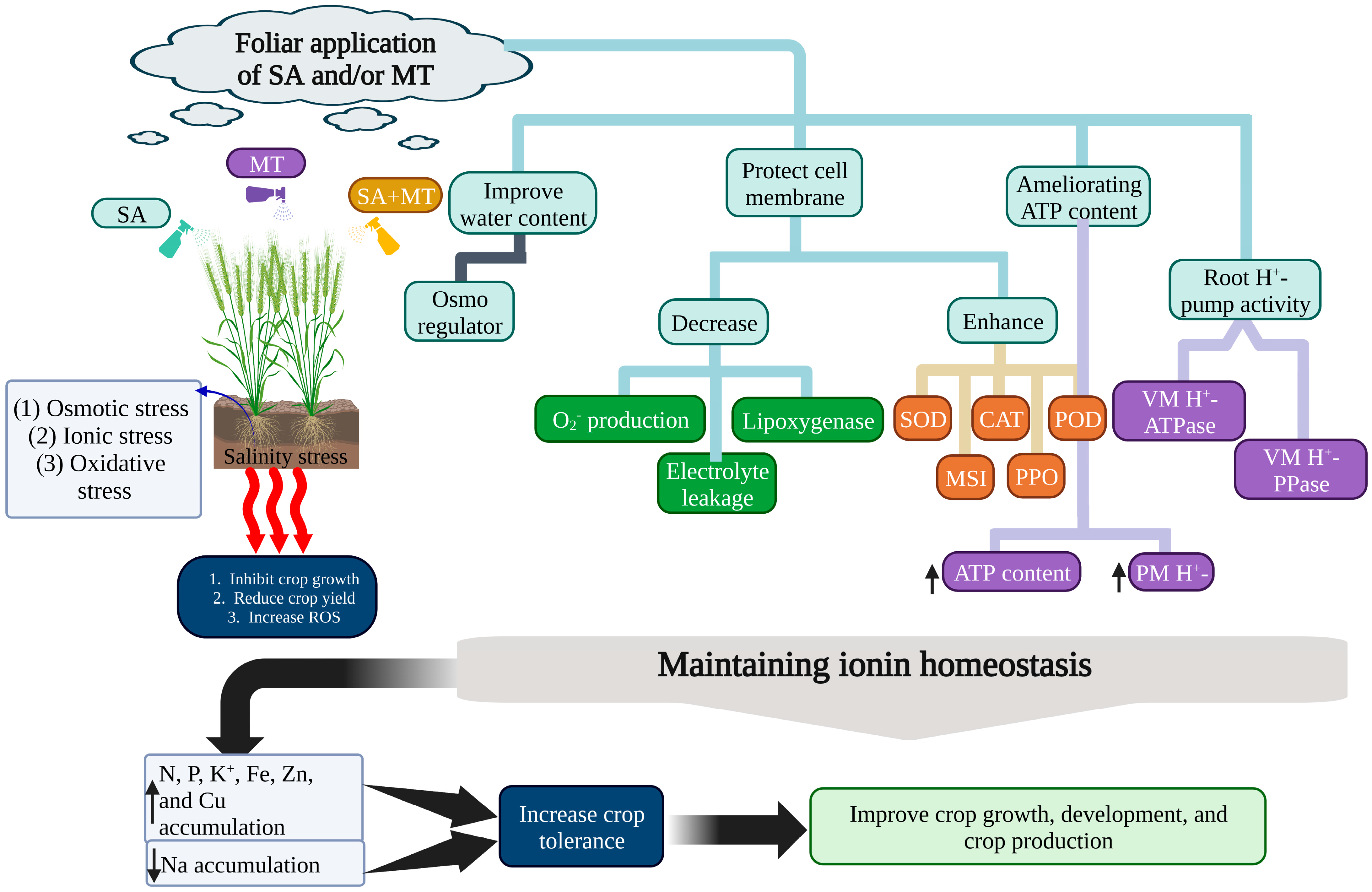
3. Melatonin: Boosting Resilience in Cereal Crops
3.1. Molecular Insights into Melatonin-Mediated Plant Stress Tolerance
3.2. Melatonin’s Role in Enhancing Photosynthesis, Nutrient Uptake, and Seed Development
4. Enhancing Crop Resilience and Sustainability in Agriculture
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, X.; Gao, W.; Cui, B.; Abd El-Aty, A. Structure and genetic regulation of starch formation in sorghum (Sorghum bicolor (L.) Moench) endosperm: A review. Int. J. Biol. Macromol. 2023, 239, 124315. [Google Scholar] [CrossRef]
- Miao, H.; Li, D.; Wang, J.; Sun, Y.; Liu, L.; Liu, R.; Li, H. Effects of melatonin on the growth and yield of wheat under drought condition. Agric. Res. Arid Areas 2020, 38, 161–167+191. [Google Scholar]
- Kaul, J.; Jain, K.; Olakh, D. An overview on role of yellow maize in food, feed and nutrition security. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 3037–3048. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, L.; Gao, Y.; Yang, Q.; Dong, K.; Liu, T.; Feng, B. Comparative analysis of drought-responsive physiological and transcriptome in broomcorn millet (Panicum miliaceum L.) genotypes with contrasting drought tolerance. Ind. Crops Prod. 2022, 177, 114498. [Google Scholar] [CrossRef]
- Ostmeyer, T.J.; Bahuguna, R.N.; Kirkham, M.; Bean, S.; Jagadish, S. Enhancing sorghum yield through efficient use of nitrogen–challenges and opportunities. Front. Plant Sci. 2022, 13, 845443. [Google Scholar] [CrossRef]
- Muhammad, I.; Khan, A.; Mustafa, A.-Z.; Elshikh, M.S.; Shen, W. Elucidating the modulatory effect of melatonin on enzyme activity and oxidative stress in wheat: A global meta-analysis. Physiol. Plant. 2024, 176, e14294. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Rai, P.K.; Song, H.; Kim, K.-H. Nanoparticles modulate heavy-metal and arsenic stress in food crops: Hormesis for food security/safety and public health. Sci. Total Environ. 2023, 902, 166064. [Google Scholar] [CrossRef] [PubMed]
- Vancov, T.; McIntosh, S. Alkali pretreatment of cereal crop residues for second-generation biofuels. Energy Fuels 2011, 25, 2754–2763. [Google Scholar] [CrossRef]
- Onyeonagu, C.; Njoku, O. Crop residues and agro-industrial by-products used in traditional sheep and goat production in rural communities of Markudi LGA. Agro-Science 2010, 9, 161–169. [Google Scholar] [CrossRef]
- Devi, S.; Gupta, C.; Jat, S.L.; Parmar, M. Crop residue recycling for economic and environmental sustainability: The case of India. Open Agric. 2017, 2, 486–494. [Google Scholar] [CrossRef]
- Yonar, A.; Yonar, H.; Mishra, P.; Kumari, B.; Abotaleb, M.; Badr, A. Modeling and forecasting of wheat of South Asian region countries and role in food security. Adv. Comput. Intell. 2021, 1, 11. [Google Scholar] [CrossRef]
- Subudhi, P.K. Molecular Research in Rice. Int. J. Mol. Sci. 2023, 24, 10063. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xie, C.; Bao, Y.; Liu, F.; Wang, H.; Wang, Y. Oat: Current state and challenges in plant-based food applications. Trends Food Sci. Technol. 2023, 134, 56–71. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Mosaad, I.S.M.; Al-Ghamdi, A.A.; Abbasi, A.M.; Zhou, X.-B. Melatonin Application Alleviates Stress-Induced Photosynthetic Inhibition and Oxidative Damage by Regulating Antioxidant Defense System of Maize: A Meta-Analysis. Antioxidants 2022, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Kosakivska, I.V.; Vedenicheva, N.P.; Babenko, L.M.; Voytenko, L.V.; Romanenko, K.O.; Vasyuk, V.A. Exogenous phytohormones in the regulation of growth and development of cereals under abiotic stresses. Mol. Biol. Rep. 2022, 49, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Qin, C.; Qin, Y.; Du, M.; Begum, N.; Lian, H. Acetylcholine Alleviates Salt Stress in Zea mays L. by Promoting Seed Germination and Regulating Phytohormone Level and Antioxidant Capacity. J. Plant Growth Regul. 2023, 43, 341–352. [Google Scholar] [CrossRef]
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and oxidative stress: A review. Med. Sci. Monit. 2004, 10, 141–147. [Google Scholar]
- Iwaniuk, P.; Konecki, R.; Kaczynski, P.; Rysbekova, A.; Lozowicka, B. Influence of seven levels of chemical/biostimulator protection on amino acid profile and yield traits in wheat. Crop J. 2022, 10, 1198–1206. [Google Scholar] [CrossRef]
- Weller, S.; Culbreath, A.; Gianessi, L.; Godfrey, L.; Jachetta, J.; Norsworthy, J.; Palumbo, J.; Madsen, J. The Contributions of Pesticides to Pest Management in Meeting the Global Need for Food Production by 2050; Council for Agricultural Science and Technology (CAST): Ames, IA, USA, 2014; pp. 1–28. [Google Scholar]
- Wang, J.; Zhang, L.; Tao, N.; Wang, X.; Deng, S.; Li, M.; Zu, Y.; Xu, C. Small Peptides Isolated from Enzymatic Hydrolyzate of Pneumatophorus japonicus Bone Promote Sleep by Regulating Circadian Rhythms. Foods 2023, 12, 464. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Li, L.; Sun, Q.; Wang, Q.; Huang, H.; Tong, Z.; Zhang, J. Melatonin-mediated development and abiotic stress tolerance in plants. Front. Plant Sci. 2023, 14, 1100827. [Google Scholar] [CrossRef]
- Bhowal, B.; Bhattacharjee, A.; Goswami, K.; Sanan-Mishra, N.; Singla-Pareek, S.L.; Kaur, C.; Sopory, S. Serotonin and Melatonin Biosynthesis in Plants: Genome-Wide Identification of the Genes and Their Expression Reveal a Conserved Role in Stress and Development. Int. J. Mol. Sci. 2021, 22, 11034. [Google Scholar] [CrossRef]
- Lei, K.; Sun, S.; Zhong, K.; Li, S.; Hu, H.; Sun, C.; Zheng, Q.; Tian, Z.; Dai, T.; Sun, J. Seed soaking with melatonin promotes seed germination under chromium stress via enhancing reserve mobilization and antioxidant metabolism in wheat. Ecotoxicol. Environ. Saf. 2021, 220, 112241. [Google Scholar] [CrossRef]
- Ma, S.; Gai, P.; Geng, B.; Wang, Y.; Ullah, N.; Zhang, W.; Zhang, H.; Fan, Y.; Huang, Z. Exogenous Melatonin Improves Waterlogging Tolerance in Wheat through Promoting Antioxidant Enzymatic Activity and Carbon Assimilation. Agronomy 2022, 12, 2876. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Hu, Q.; Zhang, X.; Jiang, J.; Zhang, Y.; Zhang, Z. Melatonin biosynthesis and signal transduction in plants in response to environmental conditions. J. Exp. Bot. 2022, 73, 5818–5827. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.; Hernández-Ruiz, J. Melatonin: Synthesis from tryptophan and its role in higher plant. In Amino Acids in Higher Plants; CAB International: Wallingford, UK, 2015; pp. 390–435. [Google Scholar]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef]
- Qiao, Y.; Ren, J.; Yin, L.; Liu, Y.; Deng, X.; Liu, P.; Wang, S. Exogenous melatonin alleviates PEG-induced short-term water deficiency in maize by increasing hydraulic conductance. BMC Plant Biol. 2020, 20, 218. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Huang, Z.; Li, S.; Ashraf, U.; Yang, W.; Liu, H.; Xu, D.; Li, W.; Mo, Z. Melatonin and nitrogen applications modulate early growth and related physio-biochemical attributes in maize under Cd stress. J. Soil Sci. Plant Nutr. 2021, 21, 978–990. [Google Scholar] [CrossRef]
- Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous Melatonin Improves Salt Tolerance by Mitigating Osmotic, Ion, and Oxidative Stresses in Maize Seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Malik, Z.; Afzal, S.; Dawood, M.; Abbasi, G.H.; Khan, M.I.; Kamran, M.; Zhran, M.; Hayat, M.T.; Aslam, M.N.; Rafay, M. Exogenous melatonin mitigates chromium toxicity in maize seedlings by modulating antioxidant system and suppresses chromium uptake and oxidative stress. Environ. Geochem. Health 2022, 44, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Mubarik, M.S.; Sharif, R.; Habib, M.; Jabeen, W.; Zhang, C.; Chen, H.; Chen, Z.H.; Siddique, K.H.; Zhuang, W. Developing drought-smart, ready-to-grow future crops. Plant Genome 2023, 16, e20279. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Munsif, F.; Mihoub, A.; Jamal, A.; Saeed, M.F.; Babar, S.; Fawad, M.; Zia, A. Beneficial Effect of Melatonin on Growth and Chlorophyll Content in Wheat (Triticum aestivum L.) Grown Under Salt Stress Conditions. Gesunde Pflanz. 2022, 74, 997–1009. [Google Scholar] [CrossRef]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331. [Google Scholar] [CrossRef] [PubMed]
- Dradrach, A.; Iqbal, M.; Lewinska, K.; Jedroszka, N.; Gull, e.F.; Rana, M.A.K.; Tanzeem-ul-Haq, H.S. Effects of Soil Application of Chitosan and Foliar Melatonin on Growth, Photosynthesis, and Heavy Metals Accumulation in Wheat Growing on Wastewater Polluted Soil. Sustainability 2022, 14, 8293. [Google Scholar] [CrossRef]
- Ou, C.; Cheng, W.; Wang, Z.; Yao, X.; Yang, S. Exogenous melatonin enhances Cd stress tolerance in Platycladus orientalis seedlings by improving mineral nutrient uptake and oxidative stress. Ecotoxicol. Environ. Saf. 2023, 252, 114619. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O.J.; Back, K. Molecular Regulation of Antioxidant Melatonin Biosynthesis by Brassinosteroid Acting as an Endogenous Elicitor of Melatonin Induction in Rice Seedlings. Antioxidants 2022, 11, 918. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, Y.; Zia-ur-Rehman, M.; Hussain, S.M.; Qayyum, M.F.; Rizwan, M.; Alharby, H.F.; Alabdallah, N.M.; Alharbi, B.M.; Ali, S. Combined effects of zinc oxide nanoparticles and melatonin on wheat growth, chlorophyll contents, cadmium (Cd) and zinc uptake under Cd stress. Sci. Total Environ. 2023, 864, 161061. [Google Scholar] [CrossRef]
- Karami-Mohajeri, S.; Abdollahi, M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: A systematic review. Hum. Exp. Toxicol. 2011, 30, 1119–1140. [Google Scholar] [CrossRef]
- Asghari, M.H.; Moloudizargari, M.; Bahadar, H.; Abdollahi, M. A review of the protective effect of melatonin in pesticide-induced toxicity. Expert Opin. Drug Metab. Toxicol. 2017, 13, 545–554. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The influence of humic acids and nitrophenols on metabolic compounds and pesticide behavior in wheat under biotic stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S.; Kumar, D.; Sharma, S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Giraldo Acosta, M.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Melatonin as a possible natural safener in crops. Plants 2022, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic insights on melatonin-mediated drought stress mitigation in plants. Physiol. Plant. 2021, 172, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qin, H.; Zhang, B.; Mao, W.; Lou, L.; Shen, C.; Mao, J.; Lin, Q. Development of melatonin nano-delivery systems to reduce cadmium accumulation in rice (Oryza sativa L.) seedlings: Insights from photosynthetic efficiency, antioxidative response and gene expression. Environ. Exp. Bot. 2022, 196, 104822. [Google Scholar] [CrossRef]
- Sakouhi, L.; Kadri, O.; Werghi, S.; Massoud, M.B.; Kharbech, O.; Murata, Y.; Chaoui, A. Seed pretreatment with melatonin confers cadmium tolerance to chickpea seedlings through cellular redox homeostasis and antioxidant gene expression improvement. Environ. Sci. Pollut. Res. 2023, 30, 73612–73627. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Lu, X.; Xu, L.; Zhang, L.; Zhang, W.; Han, J.; Tong, T.; Zhang, X.; Xue, D. Effects of exogenous melatonin on physiology and waxy genes expression in barley under drought stress. Plant Physiol. J. 2020, 56, 1073–1080. [Google Scholar]
- Lu, X.; Min, W.; Shi, Y.; Tian, L.; Li, P.; Ma, T.; Zhang, Y.; Luo, C. Exogenous melatonin alleviates alkaline stress by removing reactive oxygen species and promoting antioxidant defence in rice seedlings. Front. Plant Sci. 2022, 13, 849553. [Google Scholar] [CrossRef]
- Liu, L.; Li, D.; Ma, Y.; Shen, H.; Zhao, S.; Wang, Y. Combined Application of Arbuscular Mycorrhizal Fungi and Exogenous Melatonin Alleviates Drought Stress and Improves Plant Growth in Tobacco Seedlings. J. Plant Growth Regul. 2021, 40, 1074–1087. [Google Scholar] [CrossRef]
- Wei, H.; He, W.; Kuang, Y.; Wang, Z.; Wang, Y.; Hu, W.; Tang, M.; Chen, H. Arbuscular mycorrhizal symbiosis and melatonin synergistically suppress heat-induced leaf senescence involves in abscisic acid, gibberellin, and cytokinin-mediated pathways in perennial ryegrass. Environ. Exp. Bot. 2023, 213, 105436. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Explicating the cross-talks between nanoparticles, signaling pathways and nutrient homeostasis during environmental stresses and xenobiotic toxicity for sustainable cultivation of cereals. Chemosphere 2022, 286, 131827. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A.; Corpas, F.J.; López-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: New York, NY, USA, 2018; pp. 1–26. [Google Scholar] [CrossRef]
- Rodziewicz, P.; Swarcewicz, B.; Chmielewska, K.; Wojakowska, A.; Stobiecki, M. Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiol. Plant. 2014, 36, 1–19. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Sun, H.; Wu, J.; Liu, L.; Wang, J.; Wang, B.; Wang, Q.; Sun, Z.; Li, D. Melatonin Increases Drought Resistance through Regulating the Fine Root and Root Hair Morphology of Wheat Revealed with RhizoPot. Agronomy 2023, 13, 1881. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Guo, D.; Wang, T.; Tian, Y.; Ma, C.; Liu, X.; Wang, C.; Zheng, X. Melatonin enhances KCl salinity tolerance by maintaining K+ homeostasis in Malus hupehensis. Plant Biotechnol. J. 2023, 21, 2273–2290. [Google Scholar] [CrossRef]
- Jiang, M.; Ye, F.; Liu, F.; Brestic, M.; Li, X. Rhizosphere melatonin application reprograms nitrogen-cycling related microorganisms to modulate low temperature response in barley. Front. Plant Sci. 2022, 13, 998861. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Rizwan, M.; Nawaz, A.; Irshad, S.; Manoharadas, S. Exogenously applied melatonin enhanced chromium tolerance in pepper by up-regulating the photosynthetic apparatus and antioxidant machinery. Sci. Hortic. 2024, 323, 112468. [Google Scholar] [CrossRef]
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin Improves Drought Resistance in Maize Seedlings by Enhancing the Antioxidant System and Regulating Abscisic Acid Metabolism to Maintain Stomatal Opening Under PEG-Induced Drought. J. Plant Biol. 2021, 64, 299–312. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Khan, A.; Muhammad, N.; Ullah, S.; Adnan, M.; Ali, S.; Liang, Q.P.; et al. Melatonin-priming enhances maize seedling drought tolerance by regulating the antioxidant defense system. Plant Physiol. 2023, 191, 2301–2315. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Li, G.; Cui, Z.; Yang, F.; Jiang, X.; Diallo, L.; Kong, F. Seed Priming with Melatonin Improves the Seed Germination of Waxy Maize under Chilling Stress via Promoting the Antioxidant System and Starch Metabolism. Sci. Rep. 2019, 9, 15044. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chen, Y.-E.; Zhao, Y.-Q.; Ding, C.-B.; Liao, J.-Q.; Hu, C.; Zhou, L.-J.; Zhang, Z.-W.; Yuan, S.; Yuan, M. Exogenous Melatonin Alleviates Oxidative Damages and Protects Photosystem II in Maize Seedlings Under Drought Stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Cui, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Su, W.; Javed, T.; El-Serehy, H.A.; Jia, Z.; et al. Exogenous Application of Melatonin Induces Tolerance to Salt Stress by Improving the Photosynthetic Efficiency and Antioxidant Defense System of Maize Seedling. J. Plant Growth Regul. 2021, 40, 1270–1283. [Google Scholar] [CrossRef]
- Chen, Y.-E.; Mao, J.-J.; Sun, L.-Q.; Huang, B.; Ding, C.-B.; Gu, Y.; Liao, J.-Q.; Hu, C.; Zhang, Z.-W.; Yuan, S.; et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Okant, M.; Kaya, C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Res. 2019, 26, 11864–11874. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Li, H.J.; Zhao, C.F.; Xue, J.Q.; Zhang, R.H. Exogenous Melatonin Improves Drought Tolerance in Maize Seedlings by Regulating Photosynthesis and the Ascorbate-Glutathione Cycle. Russ. J. Plant Physiol. 2020, 67, 809–821. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, H.; Wang, J.; Wang, Y.; Zhang, R. Melatonin Enhances Drought Tolerance by Regulating Leaf Stomatal Behavior, Carbon and Nitrogen Metabolism, and Related Gene Expression in Maize Plants. Front. Plant Sci. 2021, 12, 779382. [Google Scholar] [CrossRef]
- Alharby, H.F.; Fahad, S. Melatonin application enhances biochar efficiency for drought tolerance in maize varieties: Modifications in physio-biochemical machinery. Agron. J. 2020, 112, 2826–2847. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Khan, M.N.; Ali, H.M.; Siddiqui, M.H.; Al-Huqail, A.A.; AlZuaibr, F.M.; Al-Muwayhi, M.A.; Marraiki, N.; Al-Humaid, L.A. Exogenous melatonin mitigates boron toxicity in wheat. Ecotoxicol. Environ. Saf. 2020, 201, 110822. [Google Scholar] [CrossRef] [PubMed]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin Suppressed the Heat Stress-Induced Damage in Wheat Seedlings by Modulating the Antioxidant Machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B.; Shawky, B.T. Synergistic Effects of Salicylic Acid and Melatonin on Modulating Ion Homeostasis in Salt-Stressed Wheat (Triticum aestivum L.) Plants by Enhancing Root H+-Pump Activity. Plants 2022, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Sun, F.; Gao, X.; Xie, K.; Zhang, C.; Liu, S.; Xi, Y. Proteomic analysis of melatonin-mediated osmotic tolerance by improving energy metabolism and autophagy in wheat (Triticum aestivum L.). Planta 2018, 248, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Yin, H.; Liu, W.; Hu, X.; Li, D.; Lan, C.; Gao, L.; He, Z.; Cui, F.; et al. The pathway of melatonin biosynthesis in common wheat (Triticum aestivum L.). J. Pineal Res. 2022, 74, e12841. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, J.; Ma, Y.; Huang, M.; Qiu, T.; Bian, H.; Han, N.; Wang, J. Function, Mechanism, and Application of Plant Melatonin: An Update with a Focus on the Cereal Crop, Barley (Hordeum vulgare L.). Antioxidants 2022, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernandez-Ruiz, J. Chemical stress by different agents affects the melatonin content of barley roots. J. Pineal Res. 2009, 46, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernandez-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-l.; Xi, Q.-q.; Wei, X.-y.; Xu, L.; Wang, Q.-q.; Fu, J.-y.; Ling, C.; Zuo, Y.; Zhao, Y.; He, H.-y.; et al. Rhythmical redox homeostasis can be restored by exogenous melatonin in hulless barley (Hordeum vulgare L.var. nudum) under cold stress. Environ. Exp. Bot. 2022, 194, 104756. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, Y.; He, H.; Xi, Q.; Fu, J.; Zhao, Y. Exogenous melatonin improves growth in hulless barley seedlings under cold stress by influencing the expression rhythms of circadian clock genes. Peerj 2021, 9, e10740. [Google Scholar] [CrossRef]
- Tian, X.; He, X.; Xu, J.; Yang, Z.; Fang, W.; Yin, Y. Mechanism of calcium in melatonin enhancement of functional substance-phenolic acid in germinated hulless barley. Rsc Adv. 2022, 12, 29214–29222. [Google Scholar] [CrossRef] [PubMed]
- Danilova, E.D.; Zlobin, I.E.; Kuznetsov, V.V.; Efimova, M.V. Exogenic Melatonin Reduces the Toxic Effect of Polymetallic Stress on Barley Plants. Dokl. Biochem. Biophys. 2021, 499, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Barman, D.; Ghimire, O.P.; Chinnusamy, V.; Kumar, R.R.; Arora, A. Amelioration of heat stress during reproductive stage in rice by melatonin. Indian J. Agric. Sci. 2019, 89, 91–96. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, J.; Sun, X.; Zhu, Y.; Li, Q.; Zhang, L.; Zhao, D.; Huang, L.; Zhang, C.; Liu, Q. Exogenous melatonin improves the quality performance of rice under high temperature during grain filling. Agronomy 2022, 12, 949. [Google Scholar] [CrossRef]
- Barman, D.; Kumar, R.; Ghimire, O.P.; Ramesh, R.; Gupta, S.; Nagar, S.; Pal, M.; Dalal, M.; Chinnusamy, V.; Arora, A. Melatonin induces acclimation to heat stress and pollen viability by enhancing antioxidative defense in rice (Oryza sativa L.). Environ. Exp. Bot. 2024, 220, 105693. [Google Scholar] [CrossRef]
- Chen, X.; Laborda, P.; Liu, F. Exogenous Melatonin Enhances Rice Plant Resistance Against Xanthomonas oryzae pv. oryzae. Plant Dis. 2020, 104, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, H.; Wang, N.; Liu, Y.; Hu, H. Effects of exogenous melatonin on the growth of rice seedlings under As stress. Chin. J. Ecol. 2018, 37, 1738–1743. [Google Scholar]
- Jan, R.; Asif, S.; Asaf, S.; Du, X.-X.; Park, J.-R.; Nari, K.; Bhatta, D.; Lee, I.-j.; Kim, K.-M. Melatonin alleviates arsenic (As) toxicity in rice plants via modulating antioxidant defense system and secondary metabolites and reducing oxidative stress. Environ. Pollut. 2023, 318, 120868. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wei, H.; Ding, Y.; Li, W.; Liu, Z.; Chen, L.; Tang, S.; Ding, C.; Jiang, Y.; Li, G. Melatonin regulates antioxidant strategy in response to continuous salt stress in rice seedlings. Plant Physiol. Biochem. 2021, 165, 239–250. [Google Scholar] [CrossRef]
- Sher, A.; Hassan, M.U.; Sattar, A.; Ul-Allah, S.; Ijaz, M.; Hayyat, Z.; Bibi, Y.; Hussain, M.; Qayyum, A. Exogenous application of melatonin alleviates the drought stress by regulating the antioxidant systems and sugar contents in sorghum seedlings. Biochem. Syst. Ecol. 2023, 107, 104620. [Google Scholar] [CrossRef]
- Hwang, O.J.; Back, K. Exogenous Gibberellin Treatment Enhances Melatonin Synthesis for Melatonin-Enriched Rice Production. Biomolecules 2022, 12, 198. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Lee, K.; Park, S.; Back, K. Cellular localization and kinetics of the rice melatonin biosynthetic enzymes SNAT and ASMT. J. Pineal Res. 2014, 56, 107–114. [Google Scholar] [CrossRef]
- Liu, J.; Shabala, S.; Zhang, J.; Ma, G.; Chen, D.; Shabala, L.; Zeng, F.; Chen, Z.H.; Zhou, M.; Venkataraman, G. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ. 2020, 43, 2591–2605. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Park, S.; Lee, H.Y.; Kim, Y.-S.; Back, K. Elevated production of melatonin in transgenic rice seeds expressing rice tryptophan decarboxylase. J. Pineal Res. 2014, 56, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Back, K. Melatonin synthesis in rice seedlings in vivo is enhanced at high temperatures and under dark conditions due to increased serotonin N-acetyltransferase and N-acetylserotonin methyltransferase activities. J. Pineal Res. 2014, 56, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O.J.; Kang, K.; Back, K. Effects of Light Quality and Phytochrome Form on Melatonin Biosynthesis in Rice. Biomolecules 2020, 10, 523. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Brestic, M.; Deshmukh, R.; Vaculik, M. Priming-mediated abiotic stress management in plants: Recent avenues and future directions. Plant Stress 2022, 5, 100097. [Google Scholar] [CrossRef]
- Zou, C.; Li, L.; Miki, D.; Li, D.; Tang, Q.; Xiao, L.; Rajput, S.; Deng, P.; Peng, L.; Jia, W. The genome of broomcorn millet. Nat. Commun. 2019, 10, 436. [Google Scholar] [CrossRef]
- Rajput, S.G.; Santra, D.K.; Schnable, J. Mapping QTLs for morpho-agronomic traits in proso millet (Panicum miliaceum L.). Mol. Breed. 2016, 36, 1–18. [Google Scholar] [CrossRef]
- Zhang, R.; Yue, Z.; Chen, X.; Wang, Y.; Zhou, Y.; Xu, W.; Huang, R. Foliar applications of urea and melatonin to alleviate waterlogging stress on photosynthesis and antioxidant metabolism in sorghum seedlings. Plant Growth Regul. 2022, 97, 429–438. [Google Scholar] [CrossRef]
- Liang, J.; Wusiman, M.; Fang, Z. Effects of Exogenous Growth Substances on Seed Germination of Sweet Sorghum under Drought Stress. Acta Agrestia Sin. 2021, 29, 610–617. [Google Scholar]
- Koo, A.J.; Arimura, G.-i. Molecular biology of chemical defenses. Plant Mol. Biol. 2022, 109, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yang, X.; Ma, C.; Wang, Y.; Zhao, J. Melatonin enhances drought stress tolerance in maize through coordinated regulation of carbon and nitrogen assimilation. Plant Physiol. Biochem. 2021, 167, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Guo, H.; Wu, X.; Hao, M.; Zhang, R. Integrative transcriptome and metabolome analysis reveals the mechanism of exogenous melatonin alleviating drought stress in maize roots. Plant Physiol. Biochem. 2023, 199, 107723. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, P.; Yan, D.; Zhang, Z.; Xu, X.; Wang, T.; Wang, Y.; Peng, Z.; Yu, C.; Gao, Y.; et al. Exogenous Melatonin Improves Seed Germination of Wheat (Triticum aestivum L.) under Salt Stress. Int. J. Mol. Sci. 2022, 23, 8436. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Li, J.; Sun, F.; Song, T.; Han, B.; Liu, Z.; Su, P. Comparative transcriptome analysis reveals the key genes and pathways involved in drought stress response of two wheat (Triticum aestivum L.) varieties. Genomics 2023, 115, 110688. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, L.; Zhang, Z.; Zhou, Y.; Zhang, E.; Chen, R.; Fang, H.; Li, P.; Xu, Y.; Yao, Y.; Zhu, M. Integrated physiological, metabolomic and transcriptomic analyses provide insights into the roles of exogenous melatonin in promoting rice seed germination under salt stress. Plant Growth Regul. 2021, 95, 19–31. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, M.; Wu, X.; Wang, Y.; Zhang, R. Physiological and transcriptomic analyses of the effects of exogenous melatonin on drought tolerance in maize (Zea mays L.). Plant Physiol. Biochem. 2021, 168, 128–142. [Google Scholar] [CrossRef]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef]
- Ren, J.; Yang, X.; Zhang, N.; Feng, L.; Ma, C.; Wang, Y.; Yang, Z.; Zhao, J. Melatonin alleviates aluminum-induced growth inhibition by modulating carbon and nitrogen metabolism, and reestablishing redox homeostasis in Zea mays L. J. Hazard. Mater. 2022, 423, 127159. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ren, J.; Lin, X.; Yang, Z.; Deng, X.; Ke, Q. Melatonin Alleviates Chromium Toxicity in Maize by Modulation of Cell Wall Polysaccharides Biosynthesis, Glutathione Metabolism, and Antioxidant Capacity. Int. J. Mol. Sci. 2023, 24, 3816. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, X.; Wang, X.; Song, W.; Wang, Q.; Wang, X.; Li, S.; Fu, B. Exogenous melatonin ameliorates drought stress in Agropyron mongolicum by regulating flavonoid biosynthesis and carbohydrate metabolism. Front. Plant Sci. 2022, 13, 1051165. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, J.; Ma, J.; Wang, Z.; Zhang, L.; Wang, Z.; Meng, M.; Zhang, C.; Sun, F.; Xi, Y. Comprehensive Transcriptomic and Metabolic Profiling of Agrobacterium-tumefaciens-Infected Immature Wheat Embryos. Int. J. Mol. Sci. 2023, 24, 8449. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chung, C.-T.; Hong, W.-J.; Lee, Y.-S.; Lee, J.-H.; Koh, H.-J.; Jung, K.-H. Transcriptional changes in the developing rice seeds under salt stress suggest targets for manipulating seed quality. Front. Plant Sci. 2021, 12, 748273. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, J.; Wang, W.; Wang, Y.; Xu, J.; Li, Z.; Zhao, X.; Fu, B. Integrated analysis of the transcriptome and metabolome revealed the molecular mechanisms underlying the enhanced salt tolerance of rice due to the application of exogenous melatonin. Front. Plant Sci. 2021, 11, 618680. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.-G.; Tan, D.-X.; et al. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xu, J.; He, X.; Yang, Z.; Fang, W.; Tao, J. Role of exogenous melatonin involved in phenolic acid metabolism of germinated hulless barley under NaCl stress. Plant Physiol. Biochem. 2022, 170, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yan, Y.; Zeng, X.; Wang, Y.; Zhang, Y. Quantitative Proteomics Analysis Reveals Proteins Associated with High Melatonin Content in Barley Seeds under NaCl-Induced Salt Stress. J. Agric. Food Chem. 2022, 70, 8492–8510. [Google Scholar] [CrossRef]
- Awan, S.A.; Khan, I.; Rizwan, M.; Irshad, M.A.; Xiaosan, W.; Zhang, X.; Huang, L. Reduction in the cadmium (Cd) accumulation and toxicity in pearl millet (Pennisetum glaucum L.) by regulating physio-biochemical and antioxidant defense system via soil and foliar application of melatonin. Environ. Pollut. 2023, 328, 121658. [Google Scholar] [CrossRef]
- Wang, Y.F.; Guo, Y.Y.; Zhao, C.F.; Li, H.J.; Zhang, R.H. Exogenous Melatonin Achieves Drought Tolerance by Improving Photosynthesis in Maize Seedlings Leaves. Russ. J. Plant Physiol. 2021, 68, 718–727. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.-D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Desouhant, E.; Gomes, E.; Mondy, N.; Amat, I. Mechanistic, ecological, and evolutionary consequences of artificial light at night for insects: Review and prospective. Entomol. Exp. Appl. 2019, 167, 37–58. [Google Scholar] [CrossRef]


| Species | Latin Name | Stress Types | Upregulated Genes/Metabolic Pathways | Downregulated Genes/Metabolic Pathways | Major Findings | References |
|---|---|---|---|---|---|---|
| Maize | Zea Mays | Drought stress | LHC, Psb, PRK, Rubisco, GAPDH, SPS, AGP, SBE, GS, NR, PetE, beta | INV, SuSy, AMY, BMY, GDH, AMT | Enhances drought tolerance in maize by protecting photosynthetic efficiency, promoting carbohydrate and N metabolism, and coordinating carbon and N assimilation, ultimately supporting plant growth and stress resilience. | [107] |
| PAL, C4H, 4CL, HCT, CHS, CHI, F3′5′H, DFR, ERFs, NACs, MYBs, bHLHs, ERF4, ERF81, ERF110 | --- | Melatonin application during drought stress leads to the upregulation of genes associated with flavonoid synthesis in roots, activation of specific transcription factors, and modulation of plant hormone signaling pathways, resulting in increased flavonoid accumulation and improved drought tolerance. | [108] | |||
| AUX1, AUX/IAA, SAUR, GID2, GID1, ABF, SIMKK, ERF1/2, BAK1, JAZ, TGA, GST, pepA, CNGCs, CDPKs, CaM/CMLs, DELLA, MYC2 | GH3, IF, EIN3, GPX, APX, PIF4, B-ARR, EIN3 | Exogenous melatonin in maize seedlings under drought stress conditions leads to increased drought tolerance by promoting growth, enhancing antioxidant defenses, modulating calcium signaling and transcription factors, and regulating the plant hormone signaling network, including jasmonic acid biosynthesis and signaling pathways. | [112] | |||
| Zmsps1, ZmPEPC, ZmrbcS, ZmrbcL, SuSy, AGPas, PEPC | GDH | Melatonin alleviates the negative impacts of drought stress on maize by enhancing photosynthesis, promoting stomatal opening, and modulating carbon and N metabolism. | [72] | |||
| IVR2, SUS2, CWI/VI, SUS, SPS | dINV, SUS1, INVINH | Melatonin can either promote or inhibit maize seedling growth, with its concentration-dependent effects on sugar metabolism and carbohydrate partitioning genes leading to alterations in photosynthesis, hexose accumulation, and sucrose phloem loading, providing novel insights into the regulation of plant growth. | [113] | |||
| ABA8ox1b, ABA8ox3a, ABA8ox3b, NCED1 | ABA8ox1a | Melatonin pre-treatment in maize seedlings mitigates the adverse effects of drought stress by maintaining leaf water content, enhancing antioxidant systems, reducing ROS accumulation, preventing chlorophyll degradation, promoting stomatal reopening, and regulating ABA levels, ultimately leading to improved drought tolerance and photosynthesis | [63] | |||
| ZmPIP1;2, ZmPIP2;2, ZmPIP1;5, ZmPIP2;5 | --- | Melatonin treatment in maize seedlings subjected to water deficiency results in increased aquaporin activity, improved root hydraulic conductance, higher leaf water potential, and enhanced tolerance to drought stress, all of which contribute to improved water uptake and transport. | [30] | |||
| Aluminum | LHC, Psb, Psa, Pet, gamma, delta, Rubisco, PGK, GAPDH, FBP, PRK, SPS, AGP, GBSS, SS, SBE, TPS, TPP, NRT, NR, GS, GOGAT, | AMY, BMY, SuSy, CWINV, GDH, AMT | Melatonin application mitigates aluminum-induced growth inhibition in maize by enhancing photosynthetic efficiency, improving carbon and N metabolism, and reducing oxidative stress, thereby highlighting its potential as an eco-friendly strategy for sustainable crop production in acidic soils. | [114] | ||
| Chromium | UGDH, GAE, GAUT, CSL, XYL, PME, GST, PCS, SOD, CAT, POD, GR, APX | RBOH, PAO | Melatonin plays a critical role by modulating osmotic balance, bolstering the plant’s antioxidant defense systems, and sustaining photosynthetic activity and mitigating cadmium toxicity in plants through its role in regulating metal transporters and antioxidant systems, and the revelation with melatonin enhances the binding capacity of cell walls in maize by influencing the biosynthesis of pectin and hemicellulose. | [115] | ||
| Wheat | Triticum aestivum L. | Salt stress | Oxalate oxidase activity, glutathione transferase activity, oxidoreductase activity, and establishment | Sodium ion import across plasma membrane, potassium ion transmembrane transporter activity, cellular chemical homeostasis, cellular lipid catabolic process, and hormone catabolic process | Melatonin enhances wheat seed germination by increasing antioxidant enzyme activities, modifying phytohormone responses, regulating ion transport pathways, and influencing the synthesis of protective substances such as flavonoids, ultimately improving salt tolerance during germination. | [109] |
| Drought stress | NCED, PP2C, SnRK2, ARF, ARG, ODC1, ROCD, ARD, PRDX6, HK | crtZ, PYR/PYL, AROK, SAUR, CD13, ASO, NADH, scrK, PFP, ALDO | Drought stress significantly impacts wheat production and quality; several genes related to wheat drought tolerance have been identified through transcriptome analysis that revealed key tolerance mechanisms involved in flavonoid biosynthesis, plant hormone signaling, phenolamide production, and antioxidant responses. | [110] | ||
| GAS, C4H, CHS, | CHI, RAFS, STS, FRSs, SUS | Significant improvement in drought tolerance in wheat seedlings through exogenous melatonin, as evidenced by enhanced physiological parameters, transcriptomic and metabolomic analyses revealing the key pathways involved in drought response, and the identification of potential molecular mechanisms related to flavonoid biosynthesis and carbohydrate metabolism. | [116] | |||
| Agrobacterium-tumefaciens | PFK, gapN, gpmI, pyk, RAFS, tktA, IDH1, GLT1, POP2 | PDHA, PDHB, DLAT, DLD, LSC1, LSC2, ACO, fumC, MDH2, GSS, SDHA | The identification of the key pathways and genes involved in the response of immature wheat embryos to Agrobacterium infection, highlighting the activation of energy and stress-related pathways, changes in redox substances, and the complex regulatory network. | [117] | ||
| Rice | Oryza sativa | Salt stress | PAL, GA2, RCI3, PRX4, PRX6, PRX10, bHLH TFs | NECD | Exogenous melatonin treatment enhances rice seed germination under salt stress by promoting antioxidant activity, modulating metabolic pathways, and influencing phytohormone concentrations, suggesting its potential use in improving salt tolerance in rice. | [111] |
| TDC, T5H, ASMT, T6PP, TRE, GoIS, HSF, | GRAS, WRKY, PLATZ | Salt stress negatively impacts the growth and seed quality of rice plants, leading to changes in gene expression, mineral accumulation, and the upregulation of various metabolic pathways and transcription factors in developing rice seeds. | [118] | |||
| OsEXPB2, Hsp40, TFs, auxin, ABA | OsBBX20, OsLTP2.12, | Exogenous melatonin in rice seedlings leads to the upregulation of specific transcription factors, activation of phytohormone signaling pathways, and modulation of metabolite profiles, collectively contributing to enhanced salt tolerance and improved stress responses. | [119] | |||
| Tify, TRAF, SRS, RWP-PK, mTTERF, HMG, GRAS, C2C2-YABBY, C2C2-CO, | bZIP, NAC, TFs, GRAS, mTERF, Tify, HSF, MYB, WRKY | Melatonin delays leaf senescence and cell death in rice by enhancing oxidative stress tolerance, reducing hydrogen peroxide (H2O2) accumulation, and modulating gene expression and the antioxidant defense system, thus extending the longevity of leaves and improving stress resistance. | [120] | |||
| Barly | Hordeum vulgare | Cold stress | HvCCA1, HvPRR73, HvELF3 | HvTOC1, HvPRR59, HvPRR95, HvLUX, HvGI | A total of 1 µM of exogenous melatonin restores the rhythmicity of circadian clock genes, enhances the accumulation of photosynthetic pigments, and reduces stress-related indicators, ultimately promoting plant growth under cold stress conditions in hulless barley. | [83] |
| HvSOD1, HvCAT2, | --- | Melatonin can re-establish circadian rhythms in H2O2 levels, antioxidant enzyme activities (SOD and CAT), and the PRX-SO2/3 rhythmic marker under cold stress conditions in hulless barley seedlings. Additionally, melatonin influences the circadian rhythmicity of MDA and soluble sugars. | [82] | |||
| Salt stress | 4CL, APL, C4H, | F5H | Melatonin significantly increases the levels of phenolic acids, including ferulic acid, p-coumaric acid, and p-hydroxybenzoic acid, while reducing oxidative damage, enhancing biomass, and promoting sprout growth of barley under salt stress. | [121] | ||
| TDC, T5H, F2CTV7 Osmotin/thaumatin-like_sf, A0A287WVK2 Tryptophan synthase, F2E7G3 methyltransferase activity, AOA287M228 delta-1-pyrroline-5-carboxylate synthase | F2D9A0 α/β hydrolase | Melatonin significantly improves salt stress tolerance in germinating hulless barley seeds, as indicated by the increased germination rate and root length and the reduced oxidative stress levels, with an underlying influence on multiple molecular and metabolic pathways related to microtubule-associated proteins, motor proteins, histone H2B, energy metabolism, amino acid metabolism, ion transport, antioxidant defenses, and vacuolar ion exchange. | [122] | |||
| Millet | Panicum miliaceum L. | Drought stress | SNAT, TDC, AUX/IAA, ABF, AUX1, | PP2Cs, MPK6, ChlH, ChlI, and ChlD | Drought-tolerant and drought-sensitive broomcorn millet varieties (DT 43 and DS 190) show the differential responses of these varieties to drought stress, the role of plant hormone signaling and MAPK pathways in conferring drought resistance to DT 43, the impact of carbon and N metabolism on senescence under drought stress, and the beneficial effects of melatonin treatment in enhancing drought resistance by improving photosynthetic and antioxidant capacities while mitigating transcriptional responses in both varieties. | [4] |
| Cadmium | superoxide dismutase SOD-[Fe] 2, Fe superoxide dismutase, Peroxiredoxin 2C | --- | Melatonin application through soil and foliar spray significantly reduces Cd accumulation, mitigates oxidative stress, improves growth parameters, enhances the expression of antioxidant-related genes, and increases Cd stress tolerance in pearl millet. | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, I.; Ahmad, S.; Shen, W. Melatonin-Mediated Molecular Responses in Plants: Enhancing Stress Tolerance and Mitigating Environmental Challenges in Cereal Crop Production. Int. J. Mol. Sci. 2024, 25, 4551. https://doi.org/10.3390/ijms25084551
Muhammad I, Ahmad S, Shen W. Melatonin-Mediated Molecular Responses in Plants: Enhancing Stress Tolerance and Mitigating Environmental Challenges in Cereal Crop Production. International Journal of Molecular Sciences. 2024; 25(8):4551. https://doi.org/10.3390/ijms25084551
Chicago/Turabian StyleMuhammad, Ihsan, Shakeel Ahmad, and Weijun Shen. 2024. "Melatonin-Mediated Molecular Responses in Plants: Enhancing Stress Tolerance and Mitigating Environmental Challenges in Cereal Crop Production" International Journal of Molecular Sciences 25, no. 8: 4551. https://doi.org/10.3390/ijms25084551
APA StyleMuhammad, I., Ahmad, S., & Shen, W. (2024). Melatonin-Mediated Molecular Responses in Plants: Enhancing Stress Tolerance and Mitigating Environmental Challenges in Cereal Crop Production. International Journal of Molecular Sciences, 25(8), 4551. https://doi.org/10.3390/ijms25084551







