Multiple Sclerosis Onset before and after COVID-19 Vaccination: Can HLA Haplotype Be Determinant?
Abstract
1. Introduction
2. Results
2.1. Comparison between Patients with a Disease before and during the COVID-19 Pandemic
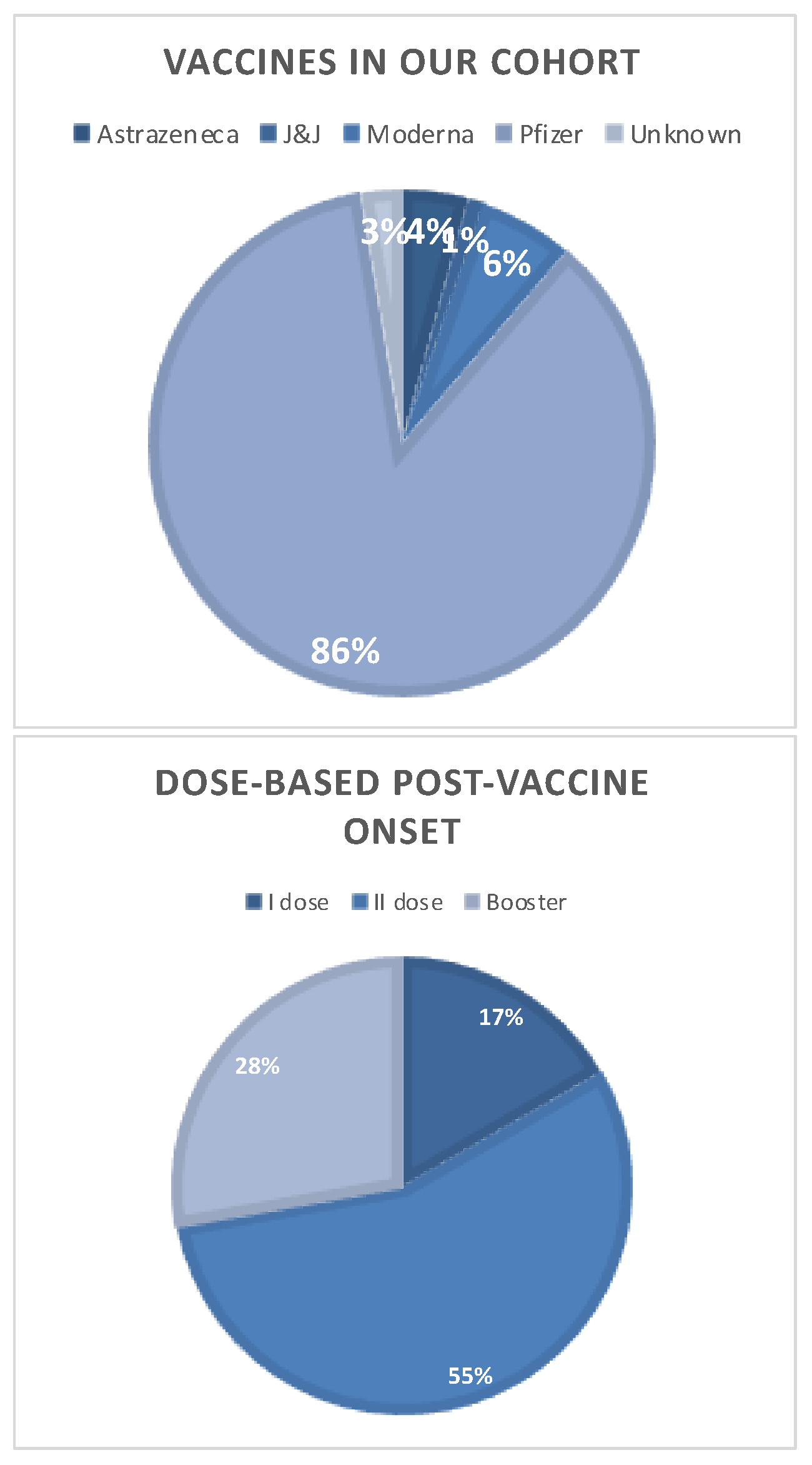
2.2. Analysis of MS Cohort with a SARS-CoV-2 Vaccine-Related Disease Onset
2.3. Comparison of HLA-DRB1 Genotyping before and during COVID-19 Pandemic
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. SARS-CoV-2 Vaccination
4.3. HLA-DR Genotyping
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ascherio, A. Environmental Factors in Multiple Sclerosis. Expert Rev. Neurother. 2013, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of Multiple Sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Bordi, I.; Ricigliano, V.A.G.; Umeton, R.; Ristori, G.; Grassi, F.; Crisanti, A.; Sutera, A.; Salvetti, M. Noise in Multiple Sclerosis: Unwanted and Necessary. Ann. Clin. Transl. Neurol. 2014, 1, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Kakalacheva, K.; Münz, C.; Lünemann, J.D. Viral Triggers of Multiple Sclerosis. Biochim. Biophys. Acta 2011, 1812, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ricigliano, V.A.G.; Handel, A.E.; Sandve, G.K.; Annibali, V.; Ristori, G.; Mechelli, R.; Cader, M.Z.; Salvetti, M. EBNA2 Binds to Genomic Intervals Associated with Multiple Sclerosis and Overlaps with Vitamin D Receptor Occupancy. PLoS ONE 2015, 10, e0119605. [Google Scholar] [CrossRef] [PubMed]
- Mechelli, R.; Romano, C.; Reniè, R.; Manfrè, G.; Buscarinu, M.C.; Romano, S.; Marrone, A.; Bigi, R.; Bellucci, G.; Ballerini, C.; et al. Viruses and Neuroinflammation in Multiple Sclerosis. Neurosciences 2021, 8, 269. [Google Scholar] [CrossRef]
- Frau, J.; Coghe, G.; Lorefice, L.; Fenu, G.; Cocco, E. Infections and Multiple Sclerosis: From the World to Sardinia, from Sardinia to the World. Front. Immunol. 2021, 12, 728677. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal Analysis Reveals High Prevalence of Epstein-Barr Virus Associated with Multiple Sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Langer-Gould, A.; Qian, L.; Tartof, S.Y.; Brara, S.M.; Jacobsen, S.J.; Beaber, B.E.; Sy, L.S.; Chao, C.; Hechter, R.; Tseng, H.F. Vaccines and the Risk of Multiple Sclerosis and Other Central Nervous System Demyelinating Diseases. JAMA Neurol. 2014, 71, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Farez, M.F.; Correale, J. Immunizations and Risk of Multiple Sclerosis: Systematic Review and Meta-Analysis. J. Neurol. 2011, 258, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Mailand, M.T.; Frederiksen, J.L. Vaccines and Multiple Sclerosis: A Systematic Review. J. Neurol. 2017, 264, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K.; Mahanty, S. Respiratory Virus Infections: Understanding COVID-19. Immunity 2020, 52, 905–909. [Google Scholar] [CrossRef] [PubMed]
- McCuddy, M.; Kelkar, P.; Zhao, Y.; Wicklund, D. Acute Demyelinating Encephalomyelitis (ADEM) in COVID-19 Infection: A Case Series. Neurol. India 2020, 68, 1192–1195. [Google Scholar] [CrossRef]
- Novi, G.; Rossi, T.; Pedemonte, E.; Saitta, L.; Rolla, C.; Roccatagliata, L.; Inglese, M.; Farinini, D. Acute Disseminated Encephalomyelitis after SARS-CoV-2 Infection. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e797. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.J.; Barr, M.J.; Lukens, J.R.; McGargill, M.A.; Chi, H.; Mak, T.W.; Kanneganti, T.-D. Signaling via the RIP2 Adaptor Protein in Central Nervous System-Infiltrating Dendritic Cells Promotes Inflammation and Autoimmunity. Immunity 2011, 34, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, D.G.; de Souza Lima, F.C.; da Cruz Bezerra, D.; Coutinho, A.C.; Hygino da Cruz, L.C. COVID-19 Associated with Encephalomyeloradiculitis and Positive Anti-Aquaporin-4 Antibodies: Cause or Coincidence? Mult. Scler. 2021, 27, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Naser Moghadasi, A. A 31-Year-Old Female Patient with Concurrent Clinical Onset of Multiple Sclerosis and COVID-19: Possible Role of SARS-CoV-2 in the Pathogenesis of Multiple Sclerosis. Autoimmun. Rev. 2021, 20, 102803. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Ghannam, M.; Manousakis, G. A First Presentation of Multiple Sclerosis with Concurrent COVID-19 Infection. eNeurologicalSci 2021, 22, 100299. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, Y.D.; Pacheco, F.A.S.; Silveira, G.L.; Oliveira, R.A.; Carvalho, V.M.; Martimbianco, A.L.C. COVID-19 in a Temporal Relation to the Onset of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2021, 50, 102863. [Google Scholar] [CrossRef] [PubMed]
- Palao, M.; Fernández-Díaz, E.; Gracia-Gil, J.; Romero-Sánchez, C.M.; Díaz-Maroto, I.; Segura, T. Multiple Sclerosis Following SARS-CoV-2 Infection. Mult. Scler. Relat. Disord. 2020, 45, 102377. [Google Scholar] [CrossRef]
- Lotan, I.; Nishiyama, S.; Manzano, G.S.; Lydston, M.; Levy, M. COVID-19 and the Risk of CNS Demyelinating Diseases: A Systematic Review. Front. Neurol. 2022, 13, 970383. [Google Scholar] [CrossRef] [PubMed]
- Kaulen, L.D.; Doubrovinskaia, S.; Mooshage, C.; Jordan, B.; Purrucker, J.; Haubner, C.; Seliger, C.; Lorenz, H.-M.; Nagel, S.; Wildemann, B.; et al. Neurological Autoimmune Diseases Following Vaccinations against SARS-CoV-2: A Case Series. Eur. J. Neurol. 2022, 29, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Raventós, B.; Roel, E.; Pistillo, A.; Martinez-Hernandez, E.; Delmestri, A.; Reyes, C.; Strauss, V.; Prieto-Alhambra, D.; Burn, E.; et al. Association between Covid-19 Vaccination, SARS-CoV-2 Infection, and Risk of Immune Mediated Neurological Events: Population Based Cohort and Self-Controlled Case Series Analysis. BMJ 2022, 376, e068373. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Handunnetthi, L.; Saatci, D.; Pan, J.; Katikireddi, S.V.; Razvi, S.; Hunt, D.; Mei, X.W.; Dixon, S.; Zaccardi, F.; et al. Neurological Complications after First Dose of COVID-19 Vaccines and SARS-CoV-2 Infection. Nat. Med. 2021, 27, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Colò, F.; Falso, S.; Russo, R.; Carlà, M.M.; Minucci, A.; Cadoni, G.; Lucchini, M.; Cicia, A.; Calabresi, P.; et al. New Onset of Susac Syndrome after mRNA COVID-19 Vaccine: A Case Report. J. Neurol. 2023, 270, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Havla, J.; Schultz, Y.; Zimmermann, H.; Hohlfeld, R.; Danek, A.; Kümpfel, T. First Manifestation of Multiple Sclerosis after Immunization with the Pfizer-BioNTech COVID-19 Vaccine. J. Neurol. 2022, 269, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Khayat-Khoei, M.; Bhattacharyya, S.; Katz, J.; Harrison, D.; Tauhid, S.; Bruso, P.; Houtchens, M.K.; Edwards, K.R.; Bakshi, R. COVID-19 mRNA Vaccination Leading to CNS Inflammation: A Case Series. J. Neurol. 2022, 269, 1093–1106. [Google Scholar] [CrossRef]
- Toljan, K.; Amin, M.; Kunchok, A.; Ontaneda, D. New Diagnosis of Multiple Sclerosis in the Setting of mRNA COVID-19 Vaccine Exposure. J. Neuroimmunol. 2022, 362, 577785. [Google Scholar] [CrossRef] [PubMed]
- Alluqmani, M. New Onset Multiple Sclerosis Post-COVID-19 Vaccination and Correlation With Possible Predictors in a Case-Control Study. Cureus 2023, 15, e36323. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 nCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, V.; Bellucci, G.; Buscarinu, M.C.; Reniè, R.; Marrone, A.; Nasello, M.; Zancan, V.; Nistri, R.; Palumbo, R.; Salerno, A.; et al. CNS Inflammatory Demyelinating Events after COVID-19 Vaccines: A Case Series and Systematic Review. Front. Neurol. 2022, 13, 1018785. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, M.R.; Montpetit, A.; Cader, M.Z.; Saarela, J.; Dyment, D.A.; Tiislar, M.; Ferretti, V.; Tienari, P.J.; Sadovnick, A.D.; Peltonen, L.; et al. A Predominant Role for the HLA Class II Region in the Association of the MHC Region with Multiple Sclerosis. Nat. Genet. 2005, 37, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Ballerini, C.; Guerini, F.R.; Rombolà, G.; Rosati, E.; Massacesi, L.; Ferrante, P.; Caputo, D.; Talamanca, L.F.; Naldi, P.; Liguori, M.; et al. HLA-Multiple Sclerosis Association in Continental Italy and Correlation with Disease Prevalence in Europe. J. Neuroimmunol. 2004, 150, 178–185. [Google Scholar] [CrossRef] [PubMed]
- The International Multiple Sclerosis Genetics Consortium & The Wellcome Trust Case Control Consortium 2. Genetic Risk and a Primary Role for Cell-Mediated Immune Mechanisms in Multiple Sclerosis. Nature 2011, 476, 214–219. [Google Scholar] [CrossRef] [PubMed]
- International Multiple Sclerosis Genetics Consortium; Patsopoulos, N.A.; Baranzini, S.E.; Santaniello, A.; Shoostari, P.; Cotsapas, C.; Wong, G.; Beecham, A.H.; James, T.; Replogle, J.; et al. Multiple Sclerosis Genomic Map Implicates Peripheral Immune Cells and Microglia in Susceptibility. Science 2019, 365, eaav7188. [Google Scholar] [CrossRef]
- Li, H.; Hou, X.; Liang, Y.; Xu, F.; Zhang, X.; Cui, P.; Xing, G.; Wang, X.; Jiang, W. Gene-Based Tests of a Genome-Wide Association Study Dataset Highlight Novel Multiple Sclerosis Risk Genes. Front. Neurosci. 2021, 15, 614528. [Google Scholar] [CrossRef] [PubMed]
- Raine, C.S. Multiple Sclerosis: Immune System Molecule Expression in the Central Nervous System. J. Neuropathol. Exp. Neurol. 1994, 53, 328–337. [Google Scholar] [CrossRef]
- Zhang, J.; Markovic-Plese, S.; Lacet, B.; Raus, J.; Weiner, H.L.; Hafler, D.A. Increased Frequency of Interleukin 2-Responsive T Cells Specific for Myelin Basic Protein and Proteolipid Protein in Peripheral Blood and Cerebrospinal Fluid of Patients with Multiple Sclerosis. J. Exp. Med. 1994, 179, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Staun-Ram, E.; Miller, A. Effector and Regulatory B Cells in Multiple Sclerosis. Clin. Immunol. 2017, 184, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, M.; Derfuss, T.; Hohlfeld, R.; Meinl, E. B Cells and Antibodies in Multiple Sclerosis Pathogenesis and Therapy. Nat. Rev. Neurol. 2012, 8, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Ransohoff, R.M. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. N. Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Bartholomäus, I.; Kawakami, N.; Odoardi, F.; Schläger, C.; Miljkovic, D.; Ellwart, J.W.; Klinkert, W.E.F.; Flügel-Koch, C.; Issekutz, T.B.; Wekerle, H.; et al. Effector T Cell Interactions with Meningeal Vascular Structures in Nascent Autoimmune CNS Lesions. Nature 2009, 462, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Greter, M.; Heppner, F.L.; Lemos, M.P.; Odermatt, B.M.; Goebels, N.; Laufer, T.; Noelle, R.J.; Becher, B. Dendritic Cells Permit Immune Invasion of the CNS in an Animal Model of Multiple Sclerosis. Nat. Med. 2005, 11, 328–334. [Google Scholar] [CrossRef]
- Aloisi, F.; Ria, F.; Adorini, L. Regulation of T-Cell Responses by CNS Antigen-Presenting Cells: Different Roles for Microglia and Astrocytes. Immunol. Today 2000, 21, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Tredicine, M.; Camponeschi, C.; Pirolli, D.; Lucchini, M.; Valentini, M.; Geloso, M.C.; Mirabella, M.; Fidaleo, M.; Righino, B.; Moliterni, C.; et al. A TLR/CD44 Axis Regulates T Cell Trafficking in Experimental and Human Multiple Sclerosis. iScience 2022, 25, 103763. [Google Scholar] [CrossRef]
- Steinman, L.; Martin, R.; Bernard, C.; Conlon, P.; Oksenberg, J.R. Multiple Sclerosis: Deeper Understanding of Its Pathogenesis Reveals New Targets for Therapy. Annu. Rev. Neurosci. 2002, 25, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Cepok, S.; Zhou, D.; Srivastava, R.; Nessler, S.; Stei, S.; Büssow, K.; Sommer, N.; Hemmer, B. Identification of Epstein-Barr Virus Proteins as Putative Targets of the Immune Response in Multiple Sclerosis. J. Clin. Investig. 2005, 115, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Absinta, M.; Maric, D.; Gharagozloo, M.; Garton, T.; Smith, M.D.; Jin, J.; Fitzgerald, K.C.; Song, A.; Liu, P.; Lin, J.-P.; et al. A Lymphocyte-Microglia-Astrocyte Axis in Chronic Active Multiple Sclerosis. Nature 2021, 597, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Koeniger, T.; Tacke, S.; Kuerten, S. Characterization of Blood–Brain Barrier Integrity in a B-Cell-Dependent Mouse Model of Multiple Sclerosis. Histochem. Cell Biol. 2019, 151, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Ebers, G.C.; Kukay, K.; Bulman, D.E.; Sadovnick, A.D.; Rice, G.; Anderson, C.; Armstrong, H.; Cousin, K.; Bell, R.B.; Hader, W.; et al. A Full Genome Search in Multiple Sclerosis. Nat. Genet. 1996, 13, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.H.; Sama, P.; LaBarre, B.A.; Lokhande, H.; Balibalos, J.; Chu, C.; Du, X.; Kheradpour, P.; Kim, C.C.; Oniskey, T.; et al. Dissection of Multiple Sclerosis Genetics Identifies B and CD4+ T Cells as Driver Cell Subsets. Genome Biol. 2022, 23, 127. [Google Scholar] [CrossRef] [PubMed]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 Lymphocytes Promote Blood-Brain Barrier Disruption and Central Nervous System Inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Kebir, H.; Ifergan, I.; Alvarez, J.I.; Bernard, M.; Poirier, J.; Arbour, N.; Duquette, P.; Prat, A. Preferential Recruitment of Interferon-γ-Expressing T H 17 Cells in Multiple Sclerosis. Ann. Neurol. 2009, 66, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, E.; Pichiecchio, A.; Colombo, E.; Rigoni, E.; Asteggiano, C.; Vegezzi, E.; Masi, F.; Greco, G.; Bastianello, S.; Bergamaschi, R. The Potential Role of SARS-CoV-2 Infection and Vaccines in Multiple Sclerosis Onset and Reactivation: A Case Series and Literature Review. Viruses 2023, 15, 1569. [Google Scholar] [CrossRef] [PubMed]
- Harel, T.; Gorman, E.F.; Wallin, M.T. New Onset or Relapsing Neuromyelitis Optica Temporally Associated with SARS-CoV-2 Infection and COVID-19 Vaccination: A Systematic Review. Front. Neurol. 2023, 14, 1099758. [Google Scholar] [CrossRef] [PubMed]
- Etemadifar, M.; Sigari, A.A.; Sedaghat, N.; Salari, M.; Nouri, H. Acute Relapse and Poor Immunization Following COVID-19 Vaccination in a Rituximab-Treated Multiple Sclerosis Patient. Hum. Vaccines Immunother. 2021, 17, 3481–3483. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, G.T.; Manzo, V.; Di Battista, M.E.; Salvatore, S.; Moreggia, O.; Scavone, C.; Capuano, A. Severe Multiple Sclerosis Relapse After COVID-19 Vaccination: A Case Report. Front. Neurol. 2021, 12, 721502. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Galarza, F.F.; McCabe, A.; Santos, E.J.M.D.; Jones, J.; Takeshita, L.; Ortega-Rivera, N.D.; Cid-Pavon, G.M.D.; Ramsbottom, K.; Ghattaoraya, G.; Alfirevic, A.; et al. Allele Frequency Net Database (AFND) 2020 Update: Gold-Standard Data Classification, Open Access Genotype Data and New Query Tools. Nucleic Acids Res. 2019, 48, D783–D788. [Google Scholar] [CrossRef] [PubMed]
- Menges, D.; Zens, K.D.; Ballouz, T.; Caduff, N.; Llanas-Cornejo, D.; Aschmann, H.E.; Domenghino, A.; Pellaton, C.; Perreau, M.; Fenwick, C.; et al. Heterogenous Humoral and Cellular Immune Responses with Distinct Trajectories Post-SARS-CoV-2 Infection in a Population-Based Cohort. Nat. Commun. 2022, 13, 4855. [Google Scholar] [CrossRef] [PubMed]
- Carrat, F.; Villarroel, P.M.S.; Lapidus, N.; Fourié, T.; Blanché, H.; Dorival, C.; Nicol, J.; Deleuze, J.-F.; Robineau, O.; SAPRIS-SERO Study Group; et al. Heterogeneous SARS-CoV-2 Humoral Response after COVID-19 Vaccination and/or Infection in the General Population. Sci. Rep. 2022, 12, 8622. [Google Scholar] [CrossRef] [PubMed]
- García Borrás, S.; Moreno, J.; Abraham, N.; Tanno, H.; García Laplaca, M.; Rossi, M.C.; Racca, L.; Racca, A. HLA-DRB1 as a Risk or Resistance Factor in Autoimmune Hepatitis. Inmunología 2011, 30, 115–118. [Google Scholar] [CrossRef]
- The International Multiple Sclerosis Genetics Consortium. Class II HLA Interactions Modulate Genetic Risk for Multiple Sclerosis. Nat. Genet. 2015, 47, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Castrillo, S.; Vogrig, A.; Honnorat, J. Associations between HLA and Autoimmune Neurological Diseases with Autoantibodies. Autoimmun. Highlights 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Guimaraães Brum, D.; Barreira, A.A.; Dos Santos, A.C.; Kaimen-Maciel, D.R.; Matiello, M.; Costa, R.M.; Saloun Deghaide, N.H.; Silva Costa, L.; Louzada-Junior, P.; Beserra Diniz, P.R.; et al. HLA-DRB Association in Neuromyelitis Optica Is Different from That Observed in Multiple Sclerosis. Mult. Scler. 2010, 16, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Alonso, V.R.; De Jesus Flores Rivera, J.; Garci, Y.R.; Granados, J.; Sanchez, T.; Mena-Hernandez, L.; Corona, T. Neuromyelitis Optica (NMO IgG+) and Genetic Susceptibility, Potential Ethnic Influences. Central Nerv. Syst. Agents Med. Chem. 2018, 18, 4–7. [Google Scholar] [CrossRef]
- Deschamps, R.; Paturel, L.; Jeannin, S.; Chausson, N.; Olindo, S.; Béra, O.; Bellance, R.; Smadja, D.; Césaire, D.; Cabre, P. Different HLA Class II (DRB1 and DQB1) Alleles Determine Either Susceptibility or Resistance to NMO and Multiple Sclerosis among the French Afro-Caribbean Population. Mult. Scler. 2011, 17, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and Immunogenicity of the ChAdOx1 nCoV-19 Vaccine against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Herman, C.; Mayer, K.; Sarwal, A. Scoping Review of Prevalence of Neurologic Comorbidities in Patients Hospitalized for COVID-19. Neurology 2020, 95, 77–84. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-14-april-2021_en.pdf (accessed on 10 March 2024).
- Spagni, G.; Todi, L.; Monte, G.; Valentini, M.; Di Sante, G.; Damato, V.; Marino, M.; Evoli, A.; Lantieri, F.; Provenzano, C. Human Leukocyte Antigen Class II Associations in Late-Onset Myasthenia Gravis. Ann. Clin. Transl. Neurol. 2021, 8, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Di Sante, G.; Tolusso, B.; Fedele, A.L.; Gremese, E.; Alivernini, S.; Nicolò, C.; Ria, F.; Ferraccioli, G. Collagen Specific T-Cell Repertoire and HLA-DR Alleles: Biomarkers of Active Refractory Rheumatoid Arthritis. eBioMedicine 2015, 2, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Maiuri, M.T.; Di Sante, G.; Scuderi, F.; La Carpia, F.; Trakas, N.; Provenzano, C.; Zisimopoulou, P.; Ria, F.; Tzartos, S.J.; et al. T Cell Repertoire in DQ5-Positive MuSK-Positive Myasthenia Gravis Patients. J. Autoimmun. 2014, 52, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Di Sante, G.; Gremese, E.; Tolusso, B.; Cattani, P.; Di Mario, C.; Marchetti, S.; Alivernini, S.; Tredicine, M.; Petricca, L.; Palucci, I.; et al. Haemophilus Parasuis (Glaesserella parasuis) as a Potential Driver of Molecular Mimicry and Inflammation in Rheumatoid Arthritis. Front. Med. 2021, 8, 671018. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Suissa, S.; Saddier, P.; Bourdès, V.; Vukusic, S.; Vaccines in Multiple Sclerosis Study Group Vaccinations and the Risk of Relapse in Multiple Sclerosis. Vaccines in Multiple Sclerosis Study Group. N. Engl. J. Med. 2001, 344, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between Genetic, Lifestyle and Environmental Risk Factors for Multiple Sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Alfredsson, L.; Olsson, T. Lifestyle and Environmental Factors in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a028944. [Google Scholar] [CrossRef] [PubMed]
- Goodin, D.S.; Khankhanian, P.; Gourraud, P.-A.; Vince, N. The Nature of Genetic and Environmental Susceptibility to Multiple Sclerosis. PLoS ONE 2021, 16, e0246157. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, F.; Cianci, R.; Casciano, F.; Pagliari, D.; De Pasquale, T.; Landolfi, R.; Di Sante, G.; Kurnick, J.T.; Ria, F. Skewed T-Cell Receptor Repertoire: More than a Marker of Malignancy, a Tool to Dissect the Immunopathology of Inflammatory Diseases. J. Biol. Regul. Homeost. Agents 2011, 25, 153–161. [Google Scholar] [PubMed]
- Nicolò, C.; Di Sante, G.; Procoli, A.; Migliara, G.; Piermattei, A.; Valentini, M.; Delogu, G.; Cittadini, A.; Constantin, G.; Ria, F. M tuberculosis in the Adjuvant Modulates Time of Appearance of CNS-Specific Effector T Cells in the Spleen through a Polymorphic Site of TLR2. PLoS ONE 2013, 8, e55819. [Google Scholar] [CrossRef] [PubMed]



| Before COVID-19 Era | During COVID-19 Era | p-Value | |
|---|---|---|---|
| Patients with a new diagnosis of MS | 143 | 123 | |
| Women (%) | 105/143 (73.4%) | 80/123 (65.0%) | 0.178 |
| Age in years—median (IQR) | 33.0 (25.82–39.89) | 33.0 (25.82–39.89) | 0.052 |
| Family history of MS (%) | 8/143 (5.6%) | 10/123 (8.1%) | 0.565 |
| Autoimmune comorbidity (%) | 18/143 (12.6%) | 7/123 (5.7%) | 0.087 |
| Time from onset to diagnosis—median (IQR) | 7.0 (1.97–7.70) | 4.0 (1.97–7.70) | <0.001 |
| Type of onset | 0.081 | ||
| Optic neuritis (%) | 29/143 (20.3%) | 21/123 (17.1%) | |
| Brain stem (%) | 31/143 (21.6%) | 22/123 (17.9%) | |
| Cerebellum (%) | 3/143 (2.1%) | 1/123 (0.8%) | |
| Spinal cord (%) | 38/143 (26.6%) | 34/123 (27.6%) | |
| Supratentorial (%) | 20/143 (14.0%) | 10/123 (8.1%) | |
| Multisystemic (%) | 22/143 (15.4%) | 35/123 (28.5%) | |
| Oligoclonal bands (%) | 122/139 (87.8%) | 100/110 (90.9%) | 0.528 |
| EDSS at onset—median (IQR) | 2.0 (1.50–2.5) | 2.0 (1.50–2.5) | 0.097 |
| EDSS after relapse—median (IQR) | 1.0 (1.50–2.5) | 1.0 (1.50–2.5) | 0.905 |
| Clinical recovery after pulsed steroid therapy | 0.372 | ||
| None (%) | 7/143 (4.9%) | 10/123 (8.1%) | |
| Partial (%) | 89/143 (62.2%) | 74/123 (60.2%) | |
| Full (%) | 48/143 (33.6%) | 38/123 (30.9%) | |
| MRI brain stem (%) | 60/143 (42.0%) | 49/123 (39.8%) | 0.822 |
| MRI cerebellum | 41/143 (28.7%) | 35/123 (28.5%) | 1.000 |
| MRI spinal cord (%) | 99/143 (69.2%) | 93/123 (75.6%) | 0.308 |
| MRI infratentorial (%) | 79/143 (55.2%) | 61/123 (49.6%) | 0.506 |
| MRI number of supratentorial lesions—median (IQR) | 5 (3.0–9.0) | 5 (3.0–9.0) | 0.215 |
| MRI contrast enhancement (%) | 76/143 (53.1%) | 68/123 (55.3%) | 0.637 |
| Highly active efficacy therapy (%) | 62/143 (43.4%) | 60/114 (52.6%) | 0.218 |
| Smoke (%) | 46/143 (32.2%) | 41/123 (33.3%) | 0.943 |
| Vitamin D—median (IQR) | 23.9 (17.55–30.76) | 23.9 (17.55–30.76) | 0.564 |
| BMI (kg/m2)—median (IQR) | 23.6 (21.30–26.23) | 23.6 (21.30–26.23) | 0.383 |
| EBV positive serology (%) | 124/143 (86.7%) | 110/123 (89.4%) | 0.065 |
| Post-Vaccine New Diagnosis of MS | Non-Vaccine-Related New Diagnosis of MS | p-Value | |
|---|---|---|---|
| Number of patients with a new diagnosis of MS | 14 | 252 | |
| Women (%) | 11/14 (78.6%) | 174/252 (69.0%) | 0.623 |
| Age in years—median (IQR) | 33.0 (26.00–40.00) | 33.0 (26.00–40.00) | 0.321 |
| Family history of MS (%) | 1/14 (7.1%) | 16/252 (6.3%) | 1.000 |
| Autoimmune comorbidity (%) | 0/14 (0.0%) | 24/252 (9.5%) | 0.456 |
| Time from onset to diagnosis—median (IQR) | 4.0 (1.97–7.70) | 4.0 (2.00–8.00) | 0.869 |
| Type of onset | 0.379 | ||
| Optic neuritis (%) | 0/14 (0.0%) | 50/252 (19.8%) | |
| Brain stem (%) | 5/14 (35.7%) | 48/252 (19.0%) | |
| Cerebellum (%) | 0/14 (0.0%) | 4/252 (1.6%) | |
| Spinal cord (%) | 4/14 (28.6%) | 68/252 (27.0%) | |
| Supratentorial (%) | 1/14 (7.1%) | 29/252 (11.5%) | |
| Multisystemic (%) | 4/14 (28.6%) | 53/252 (21.0%) | |
| Oligoclonal bands (%) | 10/12 (83.3%) | 212/237 (89.5%) | 0.863 |
| EDSS at onset—median (IQR) | 2.0 (1.50–2.50) | 2.0 (1.50–2.50) | 0.328 |
| EDSS after relapse—median (IQR) | 1.0 (0.00–1.50) | 1.0 (0.00–1.50) | 0.264 |
| Clinical recovery after pulsed steroid therapy | 0.180 | ||
| None (%) | 1/14 (7.1%) | 14/252 (5.6%) | |
| Partial (%) | 3/14 (21.4%) | 151/252 (59.9%) | |
| Full (%) | 4/14 (28.6%) | 82/252 (32.5%) | |
| MRI brain stem (%) | 6/14 (42.9%) | 103/252 (40.9%) | 1.000 |
| MRI cerebellum (%) | 5/14 (35.7%) | 71/252 (28.2%) | 0.688 |
| MRI spinal cord (%) | 10/14 (71.4%) | 182/252 (72.2%) | 1.000 |
| MRI infratentorial (%) | 9/14 (64.3%) | 132/252 (52.4%) | 0.517 |
| MRI number of supratentorial lesions—median (IQR) | 5.0 (3.00–9.00) | 5.0 (3.00–9.00) | 0.428 |
| MRI contrast enhancement (%) | 11/14 (78.6%) | 131/252 (52.0%) | 0.102 |
| Highly active efficacy therapy (%) | 8/10 (80.0%) | 114/246 (46.3%) | 0.073 |
| Smoke (%) | 4/14 (28.6%) | 83/252 (32.9%) | 0.931 |
| Vitamin D—median (IQR) | 23.9 (17.55–30.76) | 23.9 (17.55–30.76) | 0.431 |
| BMI (kg/m2)—median (IQR) | 23.6 (21.30–26.23) | 23.6 (21.30–26.23) | 0.334 |
| EBV positive serology (%) | 13/14 (92.9%) | 205/252 (81.3%) | 0.544 |
| HLA-DRB1 Alleles of Patients with a New Diagnosis of MS. | |||
|---|---|---|---|
| HLA-DRB1 Alleles | Pre-COVID-19 Era | COVID-19 Era | |
| Vaccine-Unrelated Onset | Vaccine-Related Onset | ||
| HLA-DRB1*01, n (%) | 2.0 (11.8) | 4.0 (22.2) | 2.0 (14.3) |
| HLA-DRB1*03, n (%) | 5.0 (29.4) | 6.0 (33.3) | 3.0 (21.4) |
| HLA-DRB1*04, n (%) | 3.0 (17.6) | 2.0 (11.1) | 2.0 (14.3) |
| HLA-DRB1*07, n (%) | 5.0 (29.4) | 4.0 (22.2) | 2.0 (14.3) |
| HLA-DRB1*11, n (%) | 5.0 (29.4) | 3.0 (16.7) | 2.0 (14.3) |
| HLA-DRB1*13, n (%) | 4.0 (23.5) | 3.0 (16.7) | 5 (35.7) |
| HLA-DRB1*15, n (%) | 3.0 (17.6) | 4.0 (22.2) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, A.; Di Sante, G.; Colò, F.; De Arcangelis, V.; Cicia, A.; Del Giacomo, P.; De Bonis, M.; Morganti, T.G.; Carlomagno, V.; Lucchini, M.; et al. Multiple Sclerosis Onset before and after COVID-19 Vaccination: Can HLA Haplotype Be Determinant? Int. J. Mol. Sci. 2024, 25, 4556. https://doi.org/10.3390/ijms25084556
Bianco A, Di Sante G, Colò F, De Arcangelis V, Cicia A, Del Giacomo P, De Bonis M, Morganti TG, Carlomagno V, Lucchini M, et al. Multiple Sclerosis Onset before and after COVID-19 Vaccination: Can HLA Haplotype Be Determinant? International Journal of Molecular Sciences. 2024; 25(8):4556. https://doi.org/10.3390/ijms25084556
Chicago/Turabian StyleBianco, Assunta, Gabriele Di Sante, Francesca Colò, Valeria De Arcangelis, Alessandra Cicia, Paola Del Giacomo, Maria De Bonis, Tommaso Giuseppe Morganti, Vincenzo Carlomagno, Matteo Lucchini, and et al. 2024. "Multiple Sclerosis Onset before and after COVID-19 Vaccination: Can HLA Haplotype Be Determinant?" International Journal of Molecular Sciences 25, no. 8: 4556. https://doi.org/10.3390/ijms25084556
APA StyleBianco, A., Di Sante, G., Colò, F., De Arcangelis, V., Cicia, A., Del Giacomo, P., De Bonis, M., Morganti, T. G., Carlomagno, V., Lucchini, M., Minucci, A., Calabresi, P., & Mirabella, M. (2024). Multiple Sclerosis Onset before and after COVID-19 Vaccination: Can HLA Haplotype Be Determinant? International Journal of Molecular Sciences, 25(8), 4556. https://doi.org/10.3390/ijms25084556







