Coenzyme Q10 and Autoimmune Disorders: An Overview
Abstract
1. Introduction
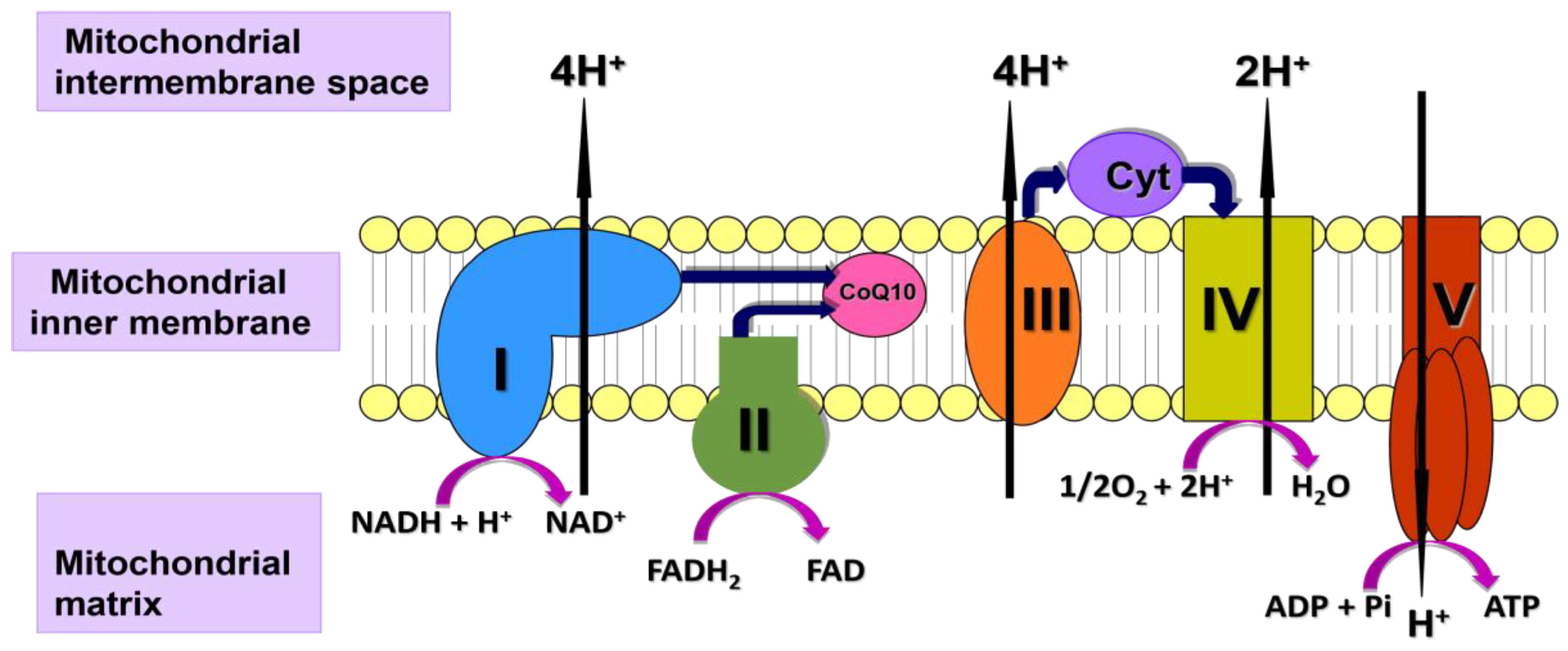
2. Mitochondrial Function in Autoimmune Disorders
3. CoQ10 and Autoimmune Disorders
3.1. Autoimmune Cardiovascular Lymphatic and Blood Clotting Disorders
3.2. Autoimmune Neuromuscular and Musculoskeletal Disorders
3.3. Autoimmune Endocrine Disorders
3.4. Gastrointestinal Autoimmune Disorders
3.5. Autoimmune Disorders of the Skin
3.6. Autoimmune Respiratory Disorders
3.7. Autoimmune Urinary Disorders
3.8. Autoimmune Visual Disorders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Samuels, H.; Malov, M.; Saha Detroja, T.; Ben Zaken, K.; Bloch, N.; Gal-Tanamy, M.; Avni, O.; Polis, B.; Samson, A.O. Autoimmune Disease Classification Based on PubMed Text Mining. J. Clin. Med. 2022, 11, 4345. [Google Scholar] [CrossRef] [PubMed]
- Bieber, K.; Hundt, J.E.; Yu, X.; Ehlers, M.; Petersen, F.; Karsten, C.M.K.; Köhl, J.; Kridin, K.; Kalies, K.; Kasprick, A.; et al. Autoimmune pre-disease. Autoimmun. Rev. 2023, 22, 103236. [Google Scholar] [CrossRef] [PubMed]
- Barrera, M.J.; Aguilera, S.; Castro, I.; Carvajal, P.; Jara, D.; Molina, C.; González, S.; González, M.J. Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: Potential role in Sjögren’s syndrome. Autoimmun. Rev. 2021, 20, 102867. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Janardhan, K.S.; Meacham, J.; Madenspacher, J.H.; Lin, W.C.; Karmaus, P.W.F.; Martinez, J.; Li, Q.Z.; Yan, M.; Zeng, J.; et al. IRGM1 links mitochondrial quality control to autoimmunity. Nat. Immunol. 2021, 22, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Chávez, M.D.; Tse, H.M. Targeting Mitochondrial-Derived Reactive Oxygen Species in T Cell-Mediated Autoimmune Diseases. Front. Immunol. 2021, 12, 703972. [Google Scholar] [CrossRef] [PubMed]
- Niedbala, W.; Cai, B.; Liew, F.Y. Role of nitric oxide in the regulation of T cell functions. Ann. Rheum. Dis. 2006, 65 (Suppl. 3), iii37–iii40. [Google Scholar] [CrossRef]
- Yamamoto, T.; Maruyama, W.; Kato, Y.; Yi, H.; Shamoto-Nagai, M.; Tanaka, M.; Sato, Y.; Naoi, M. Selective nitration of mitochondrial complex I by peroxynitrite: Involvement in mitochondria dysfunction and cell death of dopaminergic SH-SY5Y cells. J. Neural Transm. 2002, 109, 1–13. [Google Scholar] [CrossRef]
- Hargreaves, I.; Mody, N.; Land, J.; Heales, S. Blood Mononuclear Cell Mitochondrial Respiratory Chain Complex IV Activity Is Decreased in Multiple Sclerosis Patients: Effects of β-Interferon Treatment. J. Clin. Med. 2018, 7, 36. [Google Scholar] [CrossRef]
- Armon-Omer, A.; Waldman, C.; Simaan, N.; Neuman, H.; Tamir, S.; Shahien, R. New Insights on the Nutrition Status and Antioxidant Capacity in Multiple Sclerosis Patients. Nutrients 2019, 11, 427. [Google Scholar] [CrossRef]
- Yambire, K.F.; Rostosky, C.; Watanabe, T.; Pacheu-Grau, D.; Torres-Odio, S.; Sanchez-Guerrero, A.; Senderovich, O.; Meyron-Holtz, E.G.; Milosevic, I.; Frahm, J.; et al. Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. eLife 2019, 8, e51031. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.A.; Heales, S.; Rahman, K.; Sexton, D.W.; Hargreaves, I. The Effect of Cellular Coenzyme Q10 Deficiency on Lysosomal Acidification. J. Clin. Med. 2020, 9, 1923. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, E.; Costantino, A.; Leoni, M.; Pieretti, R.; Di Paolo, M.; Frati, P.; Maiese, A.; Fineschi, V. Autoimmune Heart Disease: A Comprehensive Summary for Forensic Practice. Medicina 2023, 59, 1364. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, N.; Zahra, F. Castleman Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sammaritano, L.R. Antiphospholipid syndrome. Best Pract. Res. Clin. Rheumatol. 2019, 34, 101463. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, C.; Ruiz-Limon, P.; Aguirre, M.A.; Bertolaccini, M.L.; Khamashta, M.A.; Rodriguez-Ariza, A.; Segui, P.; Collantes-Estevez, E.; Barbarroja, N.; Khraiwesh, H.; et al. Mitochondrial dysfunction in antiphospholipid syndrome: Implications in the pathogenesis of the disease and effects of coenzyme Q10 treatment. Blood 2012, 119, 5859–5870. [Google Scholar] [CrossRef] [PubMed]
- Nocella, C.; Bartimoccia, S.; Cammisotto, V.; D’Amico, A.; Pastori, D.; Frati, G.; Sciarretta, S.; Rosa, P.; Felici, C.; Riggio, O.; et al. Oxidative Stress in the Pathogenesis of Antiphospholipid Syndrome: Implications for the Atherothrombotic Process. Antioxidants 2021, 10, 1790. [Google Scholar] [CrossRef] [PubMed]
- Tektonidou, M.G. Cardiovascular disease risk in antiphospholipid syndrome: Thrombo-inflammation and atherothrombosis. J. Autoimmun. 2022, 128, 102813. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, C.; Aguirre, M.; Ruiz-Limón, P.; Ábalos-Aguilera, M.C.; Jiménez-Gómez, Y.; la Rosa, I.A.-D.; Rodriguez-Ariza, A.; Río, L.F.-D.; González-Reyes, J.A.; Segui, P.; et al. Ubiquinol Effects on Antiphospholipid Syndrome Prothrombotic Profile: A Randomized, Placebo-Controlled Trial. Arter. Thromb. Vasc. Biol. 2017, 37, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, J.; Hurley, J.A. Fibromyalgia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Súkeníková, L.; Mallone, A.; Schreiner, B.; Ripellino, P.; Nilsson, J.; Stoffel, M.; Ulbrich, S.E.; Sallusto, F.; Latorre, D. Autoreactive T cells target peripheral nerves in Guillain-Barré syndrome. Nature 2024, 626, 160–168. [Google Scholar] [CrossRef]
- Jayarangaiah, A.; Lui, F.; Theetha Kariyanna, P. Lambert-Eaton Myasthenic Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Tafti, D.; Ehsan, M.; Xixis, K.L. Multiple sclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Beloor Suresh, A.; Asuncion, R.M.D. Myasthenia gravis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Shumway, C.L.; Patel, B.C.; Tripathy, K.; De Jesus, O. Neuromyelitis optica. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sarwar, A.; Dydyk, A.M.; Jatwani, S. Polymyositis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mansur, A.; Castillo, P.R.; Rocha Cabrero, F.; Bokhari, S.R.A. Restless legs syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Muranova, A.; Shanina, E. Stiff person syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Beier, K.; Lui, F.; Pratt, D.P. Sydenham’s chorea. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Haschka, D.; Volani, C.; Stefani, A.; Tymoszuk, P.; Mitterling, T.; Holzknecht, E.; Heidbreder, A.; Coassin, S.; Sumbalova, Z.; Seifert, M.; et al. Association of mitochondrial iron deficiency and dysfunction with idiopathic restless legs syndrome. Mov. Disord. 2019, 34, 114–123. [Google Scholar] [CrossRef]
- Cikrikcioglu, M.A.; Hursitoglu, M.; Erkal, H.; Kınas, B.E.; Sztajzel, J.; Cakirca, M.; Arslan, A.G.; Erek, A.; Halac, G.; Tukek, T. Oxidative stress and autonomic nervous system functions in restless legs syndrome. Eur. J. Clin. Investig. 2011, 41, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C. Autoimmune inflammatory myopathies. Handb. Clin. Neurol. 2023, 195, 425–460. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Alcocer-Gómez, E.; de Miguel, M.; Culic, O.; Carrión, A.M.; Alvarez-Suarez, J.M.; Bullón, P.; Battino, M.; Fernández-Rodríguez, A.; Sánchez-Alcazar, J.A. Can coenzyme q10 improve clinical and molecular parameters in fibromyalgia? Antioxid. Redox Signal 2013, 19, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Miyamae, T.; Seki, M.; Naga, T.; Uchino, S.; Asazuma, H.; Yoshida, T.; Iizuka, Y.; Kikuchi, M.; Imagawa, T.; Natsumeda, Y.; et al. Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: Amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation. Redox Rep. 2013, 18, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; Sánchez-Alcázar, J.A.; Cordero, M.D. Coenzyme q10 regulates serotonin levels and depressive symptoms in fibromyalgia patients: Results of a small clinical trial. J. Clin. Psychopharmacol. 2014, 34, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; Culic, O.; Navarro-Pando, J.M.; Sánchez-Alcázar, J.A.; Bullón, P. Effect of Coenzyme Q10 on Psychopathological Symptoms in Fibromyalgia Patients. CNS Neurosci. Ther. 2017, 23, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Cano-García, F.J.; Alcocer-Gómez, E.; De Miguel, M.; Sánchez-Alcázar, J.A. Oxidative stress correlates with headache symptoms in fibromyalgia: Coenzyme Q10 effect on clinical improvement. PLoS ONE 2012, 7, e35677. [Google Scholar] [CrossRef] [PubMed]
- Lemus, H.N.; Warrington, A.E.; Rodriguez, M. Multiple Sclerosis: Mechanisms of Disease and Strategies for Myelin and Axonal Repair. Neurol. Clin. 2018, 36, 1–11. [Google Scholar] [CrossRef]
- Dimitriou, N.G.; Meuth, S.G.; Martinez-Lapiscina, E.H.; Albrecht, P.; Menge, T. Treatment of Patients with Multiple Sclerosis Transitioning Between Relapsing and Progressive Disease. CNS Drugs 2023, 37, 69–92. [Google Scholar] [CrossRef]
- Smets, I.; Van Deun, L.; Bohyn, C.; van Pesch, V.; Vanopdenbosch, L.; Dive, D.; Bissay, V.; Dubois, B.; Belgian Study Group for Multiple Sclerosis. Corticosteroids in the management of acute multiple sclerosis exacerbations. Acta Neurol. Belg. 2017, 117, 623–633. [Google Scholar] [CrossRef]
- Gironi, M.; Borgiani, B.; Mariani, E.; Cursano, C.; Mendozzi, L.; Cavarretta, R.; Saresella, M.; Clerici, M.; Comi, G.; Rovaris, M.; et al. Oxidative stress is differentially present in multiple sclerosis courses, early evident, and unrelated to treatment. J. Immunol. Res. 2014, 2014, 961863. [Google Scholar] [CrossRef] [PubMed]
- Steen, G.; Axelsson, H.; Bowallius, M.; Holthuis, N.; Molander, B.M. Isoprenoid biosynthesis in multiple sclerosis. Acta Neurol. Scand. 1985, 72, 328–335. [Google Scholar] [CrossRef] [PubMed]
- de Bustos, F.; Jiménez-Jiménez, F.J.; Molina, J.A.; Gómez-Escalonilla, C.; de Andrés, C.; del Hoyo, P.; Zurdo, M.; Tallón-Barranco, A.; Berbel, A.; Porta-Etessam, J.; et al. Serum levels of coenzyme Q10 in patients with multiple sclerosis. Acta Neurol. Scand. 2000, 101, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Khalilian, B.; Madadi, S.; Fattahi, N.; Abouhamzeh, B. Coenzyme Q10 enhances remyelination and regulate inflammation effects of cuprizone in corpus callosum of chronic model of multiple sclerosis. J. Mol. Histol. 2021, 52, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Jameie, S.B.; Barati, M.; Mehdizadeh, M.; Kerdari, M. Effects of coenzyme Q10 on the ratio of TH1/TH2 in experimental autoimmune encephalomyelitis model of multiple sclerosis in C57BL/6. Iran. Biomed. J. 2014, 18, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Sanoobar, M.; Eghtesadi, S.; Azimi, A.; Khalili, M.; Khodadadi, B.; Jazayeri, S.; Gohari, M.R.; Aryaeian, N. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: A double blind, placebo, controlled randomized clinical trial. Nutr. Neurosci. 2015, 18, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Sanoobar, M.; Dehghan, P.; Khalili, M.; Azimi, A.; Seifar, F. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: A double blind randomized clinical trial. Nutr. Neurosci. 2016, 19, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Moccia, M.; Capacchione, A.; Lanzillo, R.; Carbone, F.; Micillo, T.; Perna, F.; De Rosa, A.; Carotenuto, A.; Albero, R.; Matarese, G.; et al. Coenzyme Q10 supplementation reduces peripheral oxidative stress and inflammation in interferon-β1a-treated multiple sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1756286418819074. [Google Scholar] [CrossRef] [PubMed]
- van Delft, M.A.M.; Huizinga, T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef]
- Abdollahzad, H.; Aghdashi, M.A.; Asghari Jafarabadi, M.; Alipour, B. Effects of Coenzyme Q10 Supplementation on Inflammatory Cytokines (TNF-α, IL-6) and Oxidative Stress in Rheumatoid Arthritis Patients: A Randomized Controlled Trial. Arch. Med. Res. 2015, 46, 527–533. [Google Scholar] [CrossRef]
- Nachvak, S.M.; Alipour, B.; Mahdavi, A.M.; Aghdashi, M.A.; Abdollahzad, H.; Pasdar, Y.; Samadi, M.; Mostafai, R. Effects of coenzyme Q10 supplementation on matrix metalloproteinases and DAS-28 in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Clin. Rheumatol. 2019, 38, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Lee, S.H.; Byun, J.K.; Jeong, J.H.; Kim, E.K.; Lee, J.; Jung, Y.O.; Shin, D.; Park, S.H.; Cho, M.L. Coenzyme Q10 suppresses Th17 cells and osteoclast differentiation and ameliorates experimental autoimmune arthritis mice. Immunol. Lett. 2015, 166, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Lee, S.; Kim, S.Y.; Na, H.S.; Kim, E.K.; Kim, J.K.; Jeong, J.H.; Park, S.H.; Cho, M.L. Combination therapy with metformin and coenzyme Q10 in murine experimental autoimmune arthritis. Immunopharmacol. Immunotoxicol. 2016, 38, 103–112. [Google Scholar] [CrossRef]
- Kucharská, J.; Poništ, S.; Vančová, O.; Gvozdjáková, A.; Uličná, O.; Slovák, L.; Taghdisiesfejir, M.; Bauerová, K. Treatment with coenzyme Q10, omega-3-polyunsaturated fatty acids and their combination improved bioenergetics and levels of coenzyme Q9 and Q10 in skeletal muscle mitochondria in experimental model of arthritis. Physiol. Res. 2021, 70, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Giuffrida, G.; Campennì, A. Autoimmune endocrine diseases. Minerva Endocrinol. 2018, 43, 305–322. [Google Scholar] [CrossRef]
- Alkholy, U.M.; Abdalmonem, N.; Zaki, A.; Elkoumi, M.A.; Hashim, M.I.A.; Basset, M.A.A.; Salah, H.E. The antioxidant status of coenzyme Q10 and vitamin E in children with type 1 diabetes. J. Pediatr. 2019, 95, 224–230. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.G.; Archbold, G.P. Plasma ubiquinol/cholesterol ratios in patients with hyperlipidaemia, those with diabetes mellitus and in patients requiring dialysis. Clin. Chim. Acta 1996, 253, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wittenstein, B.; Klein, M.; Finckh, B.; Ullrich, K.; Kohlschütter, A. Plasma antioxidants in pediatric patients with glycogen storage disease, diabetes mellitus, and hypercholesterolemia. Free Radic. Biol. Med. 2002, 33, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.E.; Andersen, C.B.; Hother-Nielsen, O.; Vaag, A.; Mortensen, S.A.; Beck-Nielsen, H. Impact of ubiquinone (coenzyme Q10) treatment on glycaemic control, insulin requirement and well-being in patients with Type 1 diabetes mellitus. Diabet. Med. 1999, 16, 312–318. [Google Scholar] [CrossRef]
- Serag, H.; El Wakeel, L.; Adly, A. Coenzyme Q10 administration has no effect on sICAM-1 and metabolic parameters of pediatrics with type 1 diabetes mellitus. Int. J. Vitam. Nutr. Res. 2021, 91, 315–324. [Google Scholar] [CrossRef]
- Brauner, H.; Lüthje, P.; Grünler, J.; Ekberg, N.R.; Dallner, G.; Brismar, K.; Brauner, A. Markers of innate immune activity in patients with type 1 and type 2 diabetes mellitus and the effect of the anti-oxidant coenzyme Q10 on inflammatory activity. Clin. Exp. Immunol. 2014, 177, 478–482. [Google Scholar] [CrossRef] [PubMed]
- McIver, B.; Morris, J.C. The pathogenesis of Graves’ disease. Endocrinol. Metab. Clin. N. Am. 1998, 27, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.E.; Seifert, E.L. Thyroid hormone effects on mitochondrial energetics. Thyroid 2008, 18, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Sterling, K.; Campbell, G.A.; Taliadouros, G.S.; Nunez, E.A. Mitochondrial binding of triiodothyronine (T3). Demonstration by electron-microscopic radioautography of dispersed liver cells. Cell Tissue Res. 1984, 236, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Resch, U.; Helsel, G.; Tatzber, F.; Sinzinger, H. Antioxidant status in thyroid dysfunction. Clin. Chem. Lab. Med. 2002, 40, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid hormones, oxidative Stress, and inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Raimondo, S.; Di Segni, C.; Persano, M.; Gadotti, G.; Silvestrini, A.; Festa, R.; Tiano, L.; Pontecorvi, A.; Meucci, E. Thyroid hormones and antioxidant systems: Focus on oxidative stress in cardiovascular and pulmonary diseases. Int. J. Mol. Sci. 2013, 14, 23893–23909. [Google Scholar] [CrossRef]
- Ogura, F.; Morii, H.; Ohno, M.; Ueno, T.; Kitabatake, S.; Hamada, N.; Ito, K. Serum coenzyme Q10 levels in thyroid disorders. Horm. Metab. Res. 1980, 12, 537–540. [Google Scholar] [CrossRef]
- Suzuki, H.; Naitoh, T.; Kuniyoshi, S.; Banba, N.; Kuroda, H.; Suzuki, Y.; Hiraiwa, M.; Yamazaki, N.; Ishikawa, M.; Hashigami, Y.; et al. Cardiac performance and coenzyme Q10 in thyroid disorders. Endocrinol. Jpn. 1984, 31, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; De Marinis, L.; Calabrò, F.; Sciuto, R.; Oradei, A.; Lippa, S.; Sandric, S.; Littarru, G.P.; Barbarino, A. Evaluation of metabolic status in amiodarone-induced thyroid disorders: Plasma coenzyme Q10 determination. J. Endocrinol. Investig. 1989, 12, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Grossi, G.; Bargossi, A.M.; Fiorella, P.L.; Piazzi, S.; Battino, M.; Bianchi, G.P. Improved high-performance liquid chromatographic method for the determination of coenzyme Q10 in plasma. J. Chromatogr. 1992, 593, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, C.; Ferrari, D.; Stanic, I.; Pellegrini, L. Circulating levels of CoQ10 in hypo- and hyperthyroidism. Minerva Endocrinol. 1994, 19, 139–142. [Google Scholar] [PubMed]
- Bianchi, G.; Solaroli, E.; Zaccheroni, V.; Grossi, G.; Bargossi, A.M.; Melchionda, N.; Marchesini, G. Oxidative stress and anti-oxidant metabolites in patients with hyperthyroidism: Effect of treatment. Horm. Metab. Res. 1999, 31, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wu, M.; Zheng, Y.; Wang, C.; Li, Y.; Xin, J.; Xu, G. Analysis of coenzyme Q(10) in human plasma by column-switching liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 805, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Menke, T.; Niklowitz, P.; Reinehr, T.; de Sousa, G.J.; Andler, W. Plasma levels of coenzyme Q10 in children with hyperthyroidism. Horm. Res. 2004, 61, 153–158. [Google Scholar] [CrossRef]
- Tan, K.C.; Shiu, S.W.; Kung, A.W. Effect of thyroid dysfunction on high-density lipoprotein subfraction metabolism: Roles of hepatic lipase and cholesteryl ester transfer protein. J. Clin. Endocrinol. Metab. 1998, 83, 2921–2924. [Google Scholar] [CrossRef] [PubMed]
- Mano, T.; Iwase, K.; Hayashi, R.; Hayakawa, N.; Uchimura, K.; Makino, M.; Nagata, M.; Sawai, Y.; Oda, N.; Hamada, M.; et al. Vitamin E and coenzyme Q concentrations in the thyroid tissues of patients with various thyroid disorders. Am. J. Med. Sci. 1998, 315, 230–232. [Google Scholar] [CrossRef]
- Naito, T. Abnormal cardiac index measured by means of systolic time intervals and the effect of co-enzyme Q10 in thyroid disorders. Nihon Naibunpi Gakkai Zasshi. 1986, 62, 619–630. [Google Scholar] [CrossRef][Green Version]
- Moncayo, R.; Moncayo, H. Practical Guidelines for Diagnosing and Treating Thyroid Disease Based on the WOMED Metabolic Model of Disease Focusing on Glycolysis and Coenzyme Q10 Deficiency-A Clinical Alternative to the 2021 Retired Clinical Practice Guidelines of the Endocrine Society. Diagnostics 2022, 12, 107. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, B. The digestive system and autoimmunity. BMC Immunol. 2023, 24, 36. [Google Scholar] [CrossRef]
- Farsi, F.; Ebrahimi-Daryani, N.; Barati, M.; Janani, L.; Karimi, M.Y.; Akbari, A.; Irandoost, P.; Mesri Alamdari, N.; Agah, S.; Vafa, M. Effects of coenzyme Q10 on health-related quality of life, clinical disease activity and blood pressure in patients with mild to moderate ulcerative colitis: A randomized clinical trial. Med. J. Islam. Repub. Iran 2021, 35, 3. [Google Scholar] [CrossRef]
- El Morsy, E.M.; Kamel, R.; Ahmed, M.A. Attenuating effects of coenzyme Q10 and amlodipine in ulcerative colitis model in rats. Immunopharmacol. Immunotoxicol. 2015, 37, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Shastri, S.; Shinde, T.; Sohal, S.S.; Gueven, N.; Eri, R. Idebenone Protects against Acute Murine Colitis via Antioxidant and Anti-Inflammatory Mechanisms. Int. J. Mol. Sci. 2020, 21, 484. [Google Scholar] [CrossRef]
- Korkina, L.; Suprun, M.; Petrova, A.; Mikhal’chik, E.; Luci, A.; De Luca, C. The protective and healing effects of a natural antioxidant formulation based on ubiquinol and Aloe vera against dextran sulfate-induced ulcerative colitis in rats. Biofactors 2003, 18, 255–264. [Google Scholar] [CrossRef]
- Ewees, M.G.; Messiha, B.A.; Abo-Saif, A.A.; Abd El-Latif, H.A. Is Coenzyme Q10 Effective in Protection against Ulcerative Colitis? An Experimental Study in Rats. Biol. Pharm. Bull. 2016, 39, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Lee, S.E.; Kim, J.H. Immunopathology and Immunotherapy of Inflammatory Skin Diseases. Immune Netw. 2022, 22, e7. [Google Scholar] [CrossRef]
- Abdelsamie, M.; Zahran, F.; Hussine, A.A.; Shaker, O.; Al-Mahallawi, A.M. Clinical and biochemical assessment of the effect of topical use of coenzyme Q10 versus topical corticosteroid in management of symptomatic oral lichen planus: Randomized controlled clinical trial. BMC Oral Health 2023, 23, 506. [Google Scholar] [CrossRef] [PubMed]
- Al-Oudah, G.A.; Sahib, A.S.; Al-Hattab, M.K.; Al-Ameedee, A.A. Effect of CoQ10 Administration to Psoriatic Iraqi Patients on Biological Therapy Upon Severity Index (PASI) and Quality of Life Index (DLQI) Before and After Therapy. J. Popul. Ther. Clin. Pharmacol. 2022, 29, e52–e60. [Google Scholar] [CrossRef]
- Kharaeva, Z.; Gostova, E.; De Luca, C.; Raskovic, D.; Korkina, L. Clinical and biochemical effects of coenzyme Q(10), vitamin E, and selenium supplementation to psoriasis patients. Nutrition 2009, 25, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Passi, S.; Grandinetti, M.; Maggio, F.; Stancato, A.; De Luca, C. Epidermal oxidative stress in vitiligo. Pigment. Cell Res. 1998, 11, 81–85. [Google Scholar] [CrossRef]
- Kiriakidou, M.; Ching, C.L. Systemic Lupus Erythematosus. Ann. Intern. Med. 2020, 172, ITC81–ITC96. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Mahajan, N.; Sah, S.; Nath, S.K.; Paudyal, B. Oxidative stress and its biomarkers in systemic lupus erythematosus. J. Biomed. Sci. 2014, 21, 23. [Google Scholar] [CrossRef]
- Aringer, M. Inflammatory markers in systemic lupus erythematosus. J. Autoimmun. 2020, 110, 102374. [Google Scholar] [CrossRef]
- Yang, S.K.; Zhang, H.R.; Shi, S.P.; Zhu, Y.Q.; Song, N.; Dai, Q.; Zhang, W.; Gui, M.; Zhang, H. The Role of Mitochondria in Systemic Lupus Erythematosus: A Glimpse of Various Pathogenetic Mechanisms. Curr. Med. Chem. 2020, 27, 3346–3361. [Google Scholar] [CrossRef]
- Blanco, L.P.; Pedersen, H.L.; Wang, X.; Lightfoot, Y.L.; Seto, N.; Carmona-Rivera, C.; Yu, Z.X.; Hoffmann, V.; Yuen, P.S.T.; Kaplan, M.J. Improved Mitochondrial Metabolism and Reduced Inflammation Following Attenuation of Murine Lupus with Coenzyme Q10 Analog Idebenone. Arthritis Rheumatol. 2020, 72, 454–464. [Google Scholar] [CrossRef]
- Fortner, K.A.; Blanco, L.P.; Buskiewicz, I.; Huang, N.; Gibson, P.C.; Cook, D.L.; Pedersen, H.L.; Yuen, P.S.T.; Murphy, M.P.; Perl, A.; et al. Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci. Med. 2020, 7, e000387. [Google Scholar] [CrossRef]
- DeVrieze, B.W.; Hurley, J.A. Goodpasture’s syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sève, P.; Pacheco, Y.; Durupt, F.; Jamilloux, Y.; Gerfaud-Valentin, M.; Isaac, S.; Boussel, L.; Calender, A.; Androdias, G.; Valeyre, D.; et al. Sarcoidosis: A Clinical Overview from Symptoms to Diagnosis. Cells 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Hassler, J.R. IgA nephropathy: A brief review. Semin. Diagn. Pathol. 2020, 37, 143–147. [Google Scholar] [CrossRef]
- Dong, L.; Tan, J.; Zhong, Z.; Tang, Y.; Qin, W. Altered serum metabolic profile in patients with IgA nephropathy. Clin. Chim. Acta 2023, 549, 117561. [Google Scholar] [CrossRef]
- Yang, S.M.; Ka, S.M.; Hua, K.F.; Wu, T.H.; Chuang, Y.P.; Lin, Y.W.; Yang, F.L.; Wu, S.H.; Yang, S.S.; Lin, S.H.; et al. Antroquinonol mitigates an accelerated and progressive IgA nephropathy model in mice by activating the Nrf2 pathway and inhibiting T cells and NLRP3 inflammasome. Free Radic. Biol. Med. 2013, 61, 285–297. [Google Scholar] [CrossRef]
- Dutta Majumder, P.; Marchese, A.; Pichi, F.; Garg, I.; Agarwal, A. An update on autoimmune retinopathy. Indian. J. Ophthalmol. 2020, 68, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, X. T cells in ocular autoimmune uveitis: Pathways and therapeutic approaches. Int. Immunopharmacol. 2023, 114, 109565. [Google Scholar] [CrossRef]
- Espinoza, G.M.; Wheeler, J.; Temprano, K.K.; Keller, A.P. Cogan’s Syndrome: Clinical Presentations and Update on Treatment. Curr. Allergy Asthma Rep. 2020, 20, 46. [Google Scholar] [CrossRef]
- Pereira, S.; Vieira, B.; Maio, T.; Moreira, J.; Sampaio, F. Susac’s Syndrome: An Updated Review. Neuroophthalmology 2020, 44, 355–360. [Google Scholar] [CrossRef]
- Kmeid, M.; Medrea, I. Review of Tolosa-Hunt Syndrome, Recent Updates. Curr. Pain Headache Rep. 2023, 27, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Cabellos, J.S.; Pallardó, F.V.; García-Giménez, J.L.; Seco-Cervera, M. Oxidative Stress and Epigenetics: miRNA Involvement in Rare Autoimmune Diseases. Antioxidants 2023, 12, 800. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10 and Immune Function: An Overview. Antioxidants 2021, 10, 759. [Google Scholar] [CrossRef]
- Hidaka, T.; Fujii, K.; Funahashi, I.; Fukutomi, N.; Hosoe, K. Safety assessment of coenzyme Q10 (CoQ10). Biofactors 2008, 32, 199–208. [Google Scholar] [CrossRef]
- Gvozdjáková, A.; Kucharská, J.; Bartkovjaková, M.; Gazdíková, K.; Gazdík, F.E. Coenzyme Q10 supplementation reduces corticosteroids dosage in patients with bronchial asthma. Biofactors 2005, 25, 235–240. [Google Scholar] [CrossRef] [PubMed]
| System/Disorder | Target Tissue | Autoantibody Targets | Prevalence |
|---|---|---|---|
| Cardiovascular/lymphatic Autoimmune arteritis | Arteries | Endothelial and cytoskeletal proteins | 10–100/100,000 |
| Autoimmune vasculitis | Blood vessels | ANCA (neutrophil cytoplasm) | 20–200/100,000 |
| Autoimmune rheumatic heart disease | Heart valves | Rheumatoid factor and citrullinated proteins | 2/1000 |
| Antiphospholipid syndrome | Blood | Cardiolipin and beta-2 glycoprotein | 50/100,000 |
| Thrombocytopenia | Blood platelets | Platelet membrane glycoproteins | 9/100,000 |
| Castleman disease | Lymph nodes | Nucleus, double-stranded DNA, and voltage-gated potassium channels | 1/100,000 |
| Neuromuscular: Fibromyalgia | Muscles | Satellite glia cells | 2–4/100 |
| Guillain–Barre syndrome | Nerves | Gangliosides | 5–10/100,000 |
| Lambert–Eaton syndrome | CNS (neuromuscular junction) | Voltage-gated calcium channels | 1–2/1,000,000 |
| Multiple sclerosis | CNS | Myelin | 30–90/100,000 |
| Myasthenia gravis | CNS (neuromuscular junction) | Nicotinic acetylcholine post-synaptic receptors | 15–20/100,000 |
| Neuromyelitis optica | Optic nerves | Aquaporin-4 | 1–5/100,000 |
| Restless legs syndrome | CNS | Interferon α | 5–10/100 |
| Stiff-person syndrome | CNS | Glutamic acid decarboxylase | 1–2/1,000,000 |
| Sydenham’s chorea | Brain | Basal ganglia | Rare |
| Musculoskeletal: Polymyositis | Muscles | Jo-1 | 10/100,000 |
| Rheumatoid arthritis | Joints | Rheumatoid factor, cyclic citrullinated peptide-2, and carbamylated protein | 0.5–1/100 |
| Endocrine: Addison’s disease | Adrenals | 21-hydroxylase | 1/10,000 |
| Autoimmune oophoritis | Ovaries | Multiple ovarian antigens | 4% of females with primary ovarian insufficiency |
| Autoimmune orchitis | Testes | Anti-sperm antibodies | Not accurately known, but very rare |
| Diabetes type I | Pancreas | Islet cells, insulin, glutamic acid decarboxylase, and tyrosine phosphatase | 1–4/1000 |
| Graves’ disease | Thyroid | TSH receptor, thyroglobulin, and thyroid peroxidase | 1/100 |
| Hashimoto’s disease | Thyroid | Thyroglobulin and thyroid peroxidase | 1/100 |
| Sjogren’s disease | Salivary/lacrimal glands | SS-A and SS-B | 0.1–4/100 |
| Gastrointestinal: Autoimmune enteropathy | Small intestine | Enterocytes | <1/100,000 |
| Autoimmune hepatitis | Liver | Various, including nuclear, smooth muscle | 1/10,000 |
| Celiac disease | Small intestine | Transglutaminase and endomysium | 1/100 |
| Crohn’s disease | Digestive tract | Neutrophils and saccharomyces cerevisiae | 2/1000 |
| Pernicious anaemia | Stomach | Parietal cells and intrinsic factor | 1/1000 |
| Ulcerative colitis | Colon/rectum | Integrin alpha-v/beta-6 | 2–300/100,000 |
| Skin: Alopecia areata | Hair follicles | Hair follicle antigens | 1/1000 |
| Autoimmune angioedema | Face | C1-1NH | 0.2–1/100,000 |
| Parry–Romberg syndrome | Face | Nucleus and rheumatoid factor | 0.3–1/250,000 |
| Autoimmune urticaria | Skin | IgE and high-affinity IgE receptor | 1/1000 |
| Autoimmune pemphigoid | Skin | Structural proteins of the epidermal–dermal junction | 1.5/10,000 |
| Dermatomyositis | Skin and muscles | Mi-2 nuclear antigen and Jo-1 | 0.2–1/10,000 |
| Lichen planus | Skin | Desmoglein | 1/100 |
| Lupus | Skin | Nucleus and double-stranded DNA | 20–100/100,000 |
| Psoriasis | Skin | LL-37, ADAMTS-L5 | 1–2/100 |
| Scleroderma (localised) | Skin | Centromeres, topoisomerase | 10–30/100,000 |
| Vitiligo | Skin | Melanocytes | 0.5–2/100 |
| Respiratory: Goodpasture’s syndrome | Lungs and kidneys | Alveolar or glomerular basement membranes | 1/100,000 |
| Sarcoidosis (pulmonary) | Lungs | Elevated IgG and IgM autoantibodies | 10–40/100,000 |
| Urinary: IgA nephropathy | Kidneys | IgA1 (glomerulus) | 3/100,000 |
| Visual: Autoimmune retinopathy | Retina | Retinal proteins | Extremely rare |
| Autoimmune uveitis | Uvea | Neurofilament-M | 20–60/100,000 |
| Cogan’s syndrome | Eye and inner ear | Sensory epithelia and endothelia | Not accurately known, rare |
| Susac’s syndrome | Retina and inner ear | Endothelia | Not accurately known, rare |
| Tolosa–Hunt syndrome | Orbit | Aquaporin-4 | Prevalence unknown; incidence estimated at 1–2/1,000,000 |
| System | Disorder | CoQ10 Deficiency | CoQ10 Supplementation |
|---|---|---|---|
| Cardiovascular | Autoimmune arteritis | Not investigated | Not investigated |
| Autoimmune vasculitis | Not investigated | Not investigated | |
| Autoimmune rheumatic heart disease | Not investigated | Not investigated | |
| Antiphospholipid syndrome | Not investigated | See Section 3.1 | |
| Thrombocytopenia | Not investigated | Not investigated | |
| Castleman disease | Not investigated | Not investigated | |
| Neuromuscular | Fibromyalgia | See Section 3.2 | See Section 3.2 |
| Guillain–Barre syndrome | Not investigated | Not investigated | |
| Lambert–Eaton syndrome | Not investigated | Not investigated | |
| Multiple sclerosis | See Section 3.2 | See Section 3.2 | |
| Myasthenia gravis | Not investigated | Not investigated | |
| Neuromyelitis optica | Not investigated | Not investigated | |
| Restless legs syndrome | Not investigated | Not investigated | |
| Stiff-person syndrome | Not investigated | Not investigated | |
| Sydenham’s chorea | Not investigated | Not investigated | |
| Musculoskeletal | Polymyositis | Not investigated | Not investigated |
| Rheumatoid arthritis | See Section 3.2 | See Section 3.2 | |
| Endocrine | Addison’s disease | Not investigated | Not investigated |
| Autoimmune oophoritis | Not investigated | Not investigated | |
| Autoimmune orchitis | Not investigated | Not investigated | |
| Diabetes type I | See Section 3.3 | See Section 3.3 | |
| Graves’ disease | See Section 3.3 | See Section 3.3 | |
| Hashimoto’s disease | Not investigated | Not investigated | |
| Sjogren’s disease | Not investigated | Not investigated | |
| Gastrointestinal | Autoimmune enteropathy | Not investigated | Not investigated |
| Autoimmune hepatitis | Not investigated | Not investigated | |
| Celiac disease | Not investigated | Not investigated | |
| Crohn’s disease | |||
| Pernicious anaemia | Not investigated | Not investigated | |
| Ulcerative colitis | See Section 3.4 | See Section 3.4 | |
| Skin | Alopecia areata | Not investigated | Not investigated |
| Autoimmune angioedema | Not investigated | Not investigated | |
| Parry–Romberg syndrome | Not investigated | Not investigated | |
| Autoimmune urticaria | Not investigated | Not investigated | |
| Autoimmune pemphigoid | Not investigated | Not investigated | |
| Dermatomyositis | Not investigated | Not investigated | |
| Lichen planus | Not investigated | See Section 3.5 | |
| Lupus | Not investigated | See Section 3.5 | |
| Psoriasis | Not investigated | See Section 3.5 | |
| Scleroderma (localised) | Not investigated | Not investigated | |
| Vitiligo | See Section 3.5 | Not investigated | |
| Respiratory | Goodpasture’s syndrome | Not investigated | Not investigated |
| Sarcoidosis | Not investigated | Not investigated | |
| Urinary | IgA nephropathy | ||
| Visual | Autoimmune retinopathy | Not investigated | Not investigated |
| Autoimmune uveitis | Not investigated | Not investigated | |
| Cogan’s syndrome | Not investigated | Not investigated | |
| Susac’s syndrome | Not investigated | Not investigated | |
| Tolosa–Hunt syndrome | Not investigated | Not investigated |
| Reference | Disorder | Mitochondrial Disorder |
|---|---|---|
| Rai (2021) [4] | Autoimmune diseases in general | Leakage of damaged mitochondrial components (particularly mtDNA) into the cells activates the production of type 1 interferon and results in inflammation and autoimmunity |
| Chavez and Tse (2021) [5] | Autoimmune disorders in general | Increased production of reactive oxygen species results in T cell activation |
| Hargreaves et al. (2018) [9] | Multiple sclerosis | Deficiency of Complex IV and increased oxidative stress |
| Barrera et al. (2021) [4] | Sjögren’s syndrome | Ultrastructural alterations observed in mitochondria from salivary gland cells |
| Shah et al. (2014), Aringer et al. (2019), and Yang et al. (2020) [94,95,96] | Lupus | Pathogenesis associated with mitochondrial dysfunction, oxidative stress, and systemic inflammation |
| Perez-Sanchez et al. (2017) [19] | Antiphospholipid syndrome | CoQ10 reduced levels of inflammatory markers, and endothelial function improved |
| Farsi et al. (2021) [83] | Ulcerative colitis | CoQ10 reduced levels of inflammatory markers and disease severity |
| Cordero et al. (2013) [33] | Fibromyalgia | CoQ10 increased mitochondrial ATP energy generation and reduced oxidative stress and inflammation |
| Sanoobar et al. (2015) [46] | Multiple sclerosis | CoQ10 reduced blood levels of the inflammatory biomarkers IL-6 and TNF-alpha |
| Abdollahzad et al. (2015) [50] | Rheumatoid arthritis | CoQ10 reduced blood levels of oxidative stress and inflammatory markers |
| Naito et al. (1986) [86] | Graves’ disease | CoQ10 improved cardiac performance |
| Kharaeva et al. (2009) [91] | Psoriasis | CoQ10, together with vitamin E and selenium, reduces levels of oxidative stress |
| Abdelsamie et al. (2023) [89] | Lichen planus | Topical application of CoQ10 reduced pain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantle, D.; Hargreaves, I.P. Coenzyme Q10 and Autoimmune Disorders: An Overview. Int. J. Mol. Sci. 2024, 25, 4576. https://doi.org/10.3390/ijms25084576
Mantle D, Hargreaves IP. Coenzyme Q10 and Autoimmune Disorders: An Overview. International Journal of Molecular Sciences. 2024; 25(8):4576. https://doi.org/10.3390/ijms25084576
Chicago/Turabian StyleMantle, David, and Iain P. Hargreaves. 2024. "Coenzyme Q10 and Autoimmune Disorders: An Overview" International Journal of Molecular Sciences 25, no. 8: 4576. https://doi.org/10.3390/ijms25084576
APA StyleMantle, D., & Hargreaves, I. P. (2024). Coenzyme Q10 and Autoimmune Disorders: An Overview. International Journal of Molecular Sciences, 25(8), 4576. https://doi.org/10.3390/ijms25084576





