The Role of Oxygen Homeostasis and the HIF-1 Factor in the Development of Neurodegeneration
Abstract
1. Introduction
2. The Role of Hypoxia in the Development of Neurodegenerative Diseases
2.1. The Role of Hypoxia in the Pathogenesis of Alzheimer’s Disease
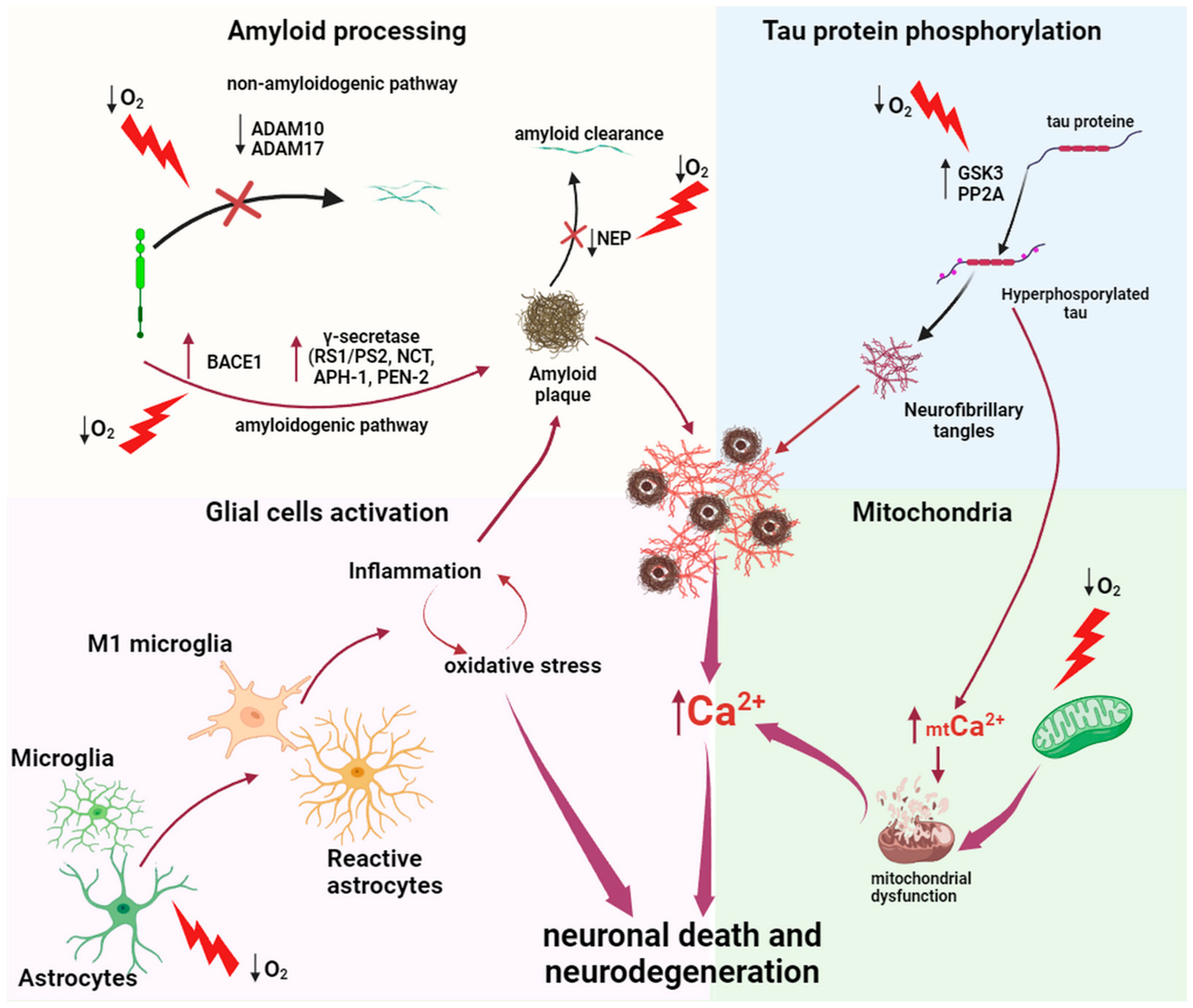
2.1.1. Amyloid Processing
2.1.2. Mitochondria
2.1.3. Tau Protein Hyperphosphorylation
2.1.4. Neuroinflammation
2.1.5. Sleep Apnea as a Risk Factor in the Development of Alzheimer’s Diseases
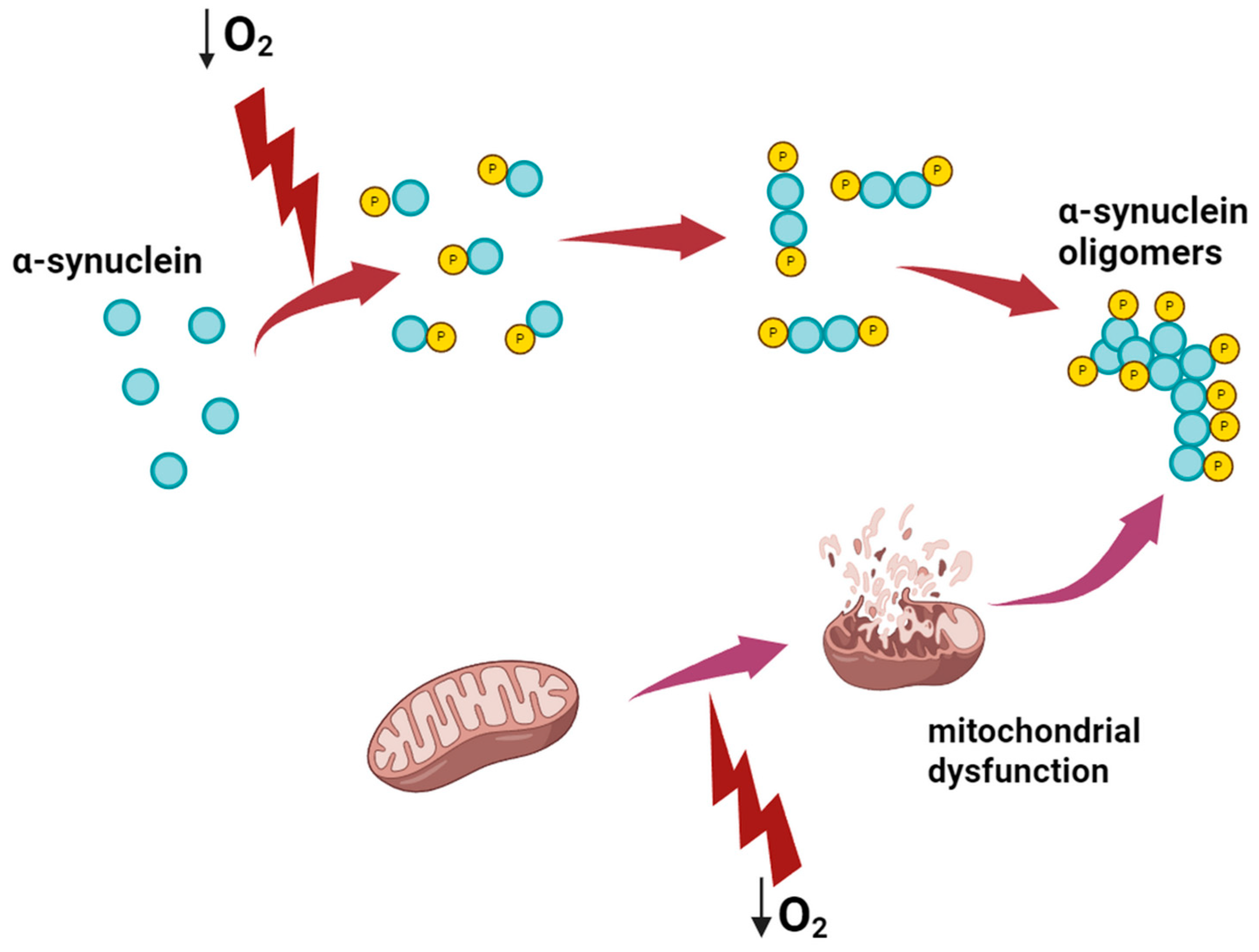
2.2. The Influence of Hypoxia on the Development of Parkinson’s Disease
3. Hypoxia-Inducible Factor (HIF) in Neurodegenerative Diseases
3.1. Structure and Functions of HIF
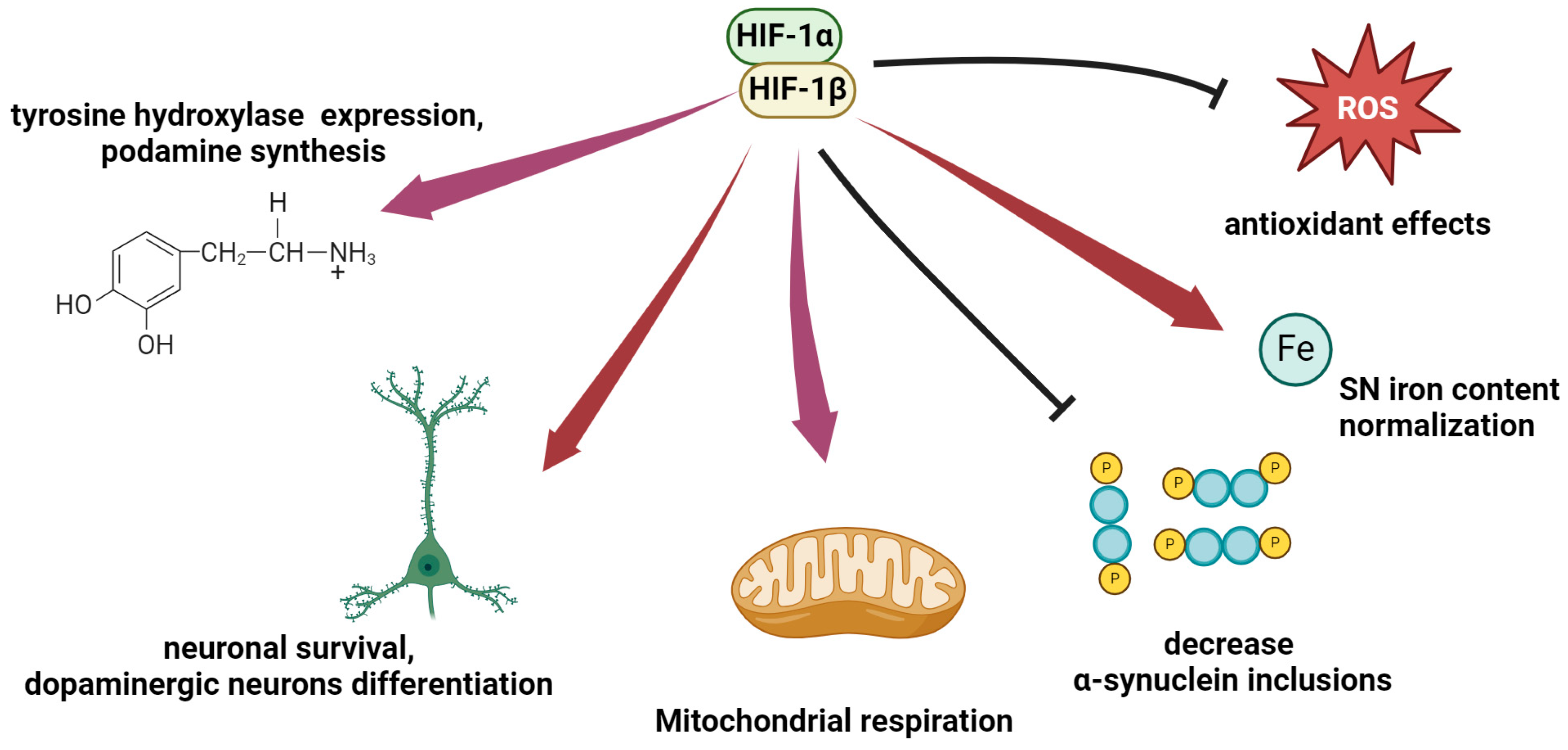
3.2. HIF for Alzheimer’s Disease
3.3. HIF-1 in Parkinson’s Disease
4. Modulation of HIF Activity as a Therapeutic Approach to Neurodegeneration
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| 3,4-DHB—3,4-dihydroxybenzonate; |
| 6-OHDA—6-hydroxydopamine; |
| α-syn—α-synuclein. |
| AAV—adeno-associated virus; |
| AD—Alzheimer’s disease; |
| AICD—APP intracellular domain; |
| ApoE—apolipoprotein E; |
| APP—amyloid precursor protein; |
| ARNT—aryl hydrocarbon receptor nuclear translocator—HIF-β subunit; |
| Aβ—amyloid-β; |
| BACE-1—beta-site APP-cleaving enzyme 1; |
| DA—dopamine; |
| DFO—deferoxamine; |
| EPO—erythropoietin; |
| FIH—FIH—asparaginyl hydroxylase; |
| GLUT 1—glucose transporter-1; |
| GLUT 3—glucose transporter-3; |
| HIF—hypoxia-inducible factor; |
| HO-1—heme oxygenase-1; |
| HRE—hypoxia-responsive elements, short DNA sequences within the gene promoter; |
| IH—intermittent hypoxia; |
| LDHA—lactate dehydrogenase A; |
| Lf—lactoferrin; |
| MPP+—1-methyl-4-phenylpyridine; |
| MPTP—1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; |
| NFT—neurofibrillary tangles; |
| ODDD—oxygen-dependent degradation domain; |
| O-GlcNAc—O-linked N-acetylglucosamine; |
| OSA—obstructive sleep apnea; |
| PD—Parkinson’s disease; |
| PDK1—pyruvate dehydrogenase 1; |
| PHD—prolyl hydroxylase; |
| pVHL—von Hippel–Lindau protein; |
| ROS—reactive oxygen species; |
| sAPPβ—soluble APPβ; |
| SNc—compact part of the substantia nigra; |
| TH—tyrosine hydroxylase; |
| VEGFA—vascular endothelial growth factor A |
References
- Reith, W. Neurodegenerative Erkrankungen. Radiologe 2018, 58, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Cong, W.; Mu, S.; Zhan, R.; Zhong, S.; Zhao, M.; Zhao, C.; Kang, K.; Zhou, Z. Effect of Physical Activity on Risk of Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Twenty-Nine Prospective Cohort Studies. Ageing Res. Rev. 2023, 92, 102127. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tam, H.L.; Quint, J.; Chen, M.; Ding, R.; Zhang, X. Epidemiology of Dementia in China in 2010–2020: A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Wimo, A.; Seeher, K.; Cataldi, R.; Cyhlarova, E.; Dielemann, J.L.; Frisell, O.; Guerchet, M.; Jönsson, L.; Malaha, A.K.; Nichols, E.; et al. The Worldwide Costs of Dementia in 2019. Alzheimer’s Dement. 2023, 19, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and Brain Aging: Neurodegeneration or Neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar] [CrossRef] [PubMed]
- Tarkowska, A. Hypoxic-Ischemic Brain Injury after Perinatal Asphyxia as a Possible Factor in the Pathology of Alzheimer’s Disease. In Cerebral Ischemia; Exon Publications: Brisbane, QLD, Australia, 2021; pp. 45–60. [Google Scholar]
- Hambali, A.; Kumar, J.; Hashim, N.F.M.; Maniam, S.; Mehat, M.Z.; Cheema, M.S.; Mustapha, M.; Adenan, M.I.; Stanslas, J.; Hamid, H.A. Hypoxia-Induced Neuroinflammation in Alzheimer’s Disease: Potential Neuroprotective Effects of Centella Asiatica. Front. Physiol. 2021, 12, 712317. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Costa, M. Hypoxia-Inducible Factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Iyalomhe, O.; Swierczek, S.; Enwerem, N.; Chen, Y.; Adedeji, M.O.; Allard, J.; Ntekim, O.; Johnson, S.; Hughes, K.; Kurian, P.; et al. The Role of Hypoxia-Inducible Factor 1 in Mild Cognitive Impairment. Cell. Mol. Neurobiol. 2017, 37, 969–977. [Google Scholar] [CrossRef]
- Lv, B.; Li, F.; Fang, J.; Xu, L.; Sun, C.; Han, J.; Hua, T.; Zhang, Z.; Feng, Z.; Jiang, X. Hypoxia Inducible Factor 1α Promotes Survival of Mesenchymal Stem Cells under Hypoxia. Am. J. Transl. Res. 2017, 9, 1521–1529. [Google Scholar]
- Vingtdeux, V.; Marambaud, P. Identification and Biology of A-secretase. J. Neurochem. 2012, 120, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Cho, Y.Y.; Kwon, O.H.; Zhao, D.; Yang, H.O.; Chung, S. Substrate-Specific Activation of α-Secretase by 7-Deoxy-Trans-Dihydronarciclasine Increases Non-Amyloidogenic Processing of β-Amyloid Protein Precursor. Molecules 2020, 25, 646. [Google Scholar] [CrossRef] [PubMed]
- Agüero, P.; Sainz, M.J.; García-Ayllón, M.-S.; Sáez-Valero, J.; Téllez, R.; Guerrero-López, R.; Pérez-Pérez, J.; Jiménez-Escrig, A.; Gómez-Tortosa, E. α-Secretase Nonsense Mutation (ADAM10 Tyr167*) in Familial Alzheimer’s Disease. Alzheimers Res. Ther. 2020, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; May, P.; Gu, W.; Mayhaus, M.; Pichler, S.; Spaniol, C.; Glaab, E.; Bobbili, D.R.; Antony, P.; Koegelsberger, S.; et al. A Rare Loss-of-Function Variant of ADAM17 Is Associated with Late-Onset Familial Alzheimer Disease. Mol. Psychiatry 2020, 25, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Rybnikova, E.; Gluschenko, T.; Galeeva, A.; Tulkova, E.; Nalivaeva, N.N.; Makova, N.Z.; Turner, A.J.; Samoilov, M. Differential Expression of ADAM15 and ADAM17 Metalloproteases in the Rat Brain after Severe Hypobaric Hypoxia and Hypoxic Preconditioning. Neurosci. Res. 2012, 72, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhou, T.; Zhou, L.; Chen, Q.; Yu, Y.; Yang, H.; Zhong, K.; Zhang, X.; Xu, F.; Cai, S.; et al. Formononetin Protects Neurons Against Hypoxia-Induced Cytotoxicity Through Upregulation of ADAM10 and SAβPPα. J. Alzheimer’s Dis. 2012, 28, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, I.D.; Vinters, H.V. Effects of Anoxia and Hypoxia on Amyloid Precursor Protein Processing in Cerebral Microvascular Smooth Muscle Cells. J. Neuropathol. Exp. Neurol. 2006, 65, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.; Mohammed, R.; Ojha, U. What Are the Links between Hypoxia and Alzheimer’s Disease? Neuropsychiatr. Dis. Treat. 2019, 15, 1343–1354. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Hypoxia/Ischemia Activate Processing of Amyloid Precursor Protein: Impact of Vascular Dysfunction in the Pathogenesis of Alzheimer’s Disease. J. Neurochem. 2017, 140, 536–549. [Google Scholar] [CrossRef]
- Wong, E.; Frost, G.R.; Li, Y.-M. γ-Secretase Modulatory Proteins: The Guiding Hand Behind the Running Scissors. Front. Aging Neurosci. 2020, 12, 614690. [Google Scholar] [CrossRef]
- Villa, J.C.; Chiu, D.; Brandes, A.H.; Escorcia, F.E.; Villa, C.H.; Maguire, W.F.; Hu, C.-J.; de Stanchina, E.; Simon, M.C.; Sisodia, S.S.; et al. Nontranscriptional Role of Hif-1α in Activation of γ-Secretase and Notch Signaling in Breast Cancer. Cell Rep. 2014, 8, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Yang, D.; Luo, G.; Chen, S.; Le, W. Hypoxia Increases Aβ Generation by Altering β- and γ-Cleavage of APP. Neurobiol. Aging 2009, 30, 1091–1098. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, H.; Yang, J.; Ni, J.; Le, W. Chronic Hypoxia Facilitates Alzheimer’s Disease through Demethylation of Γ-secretase by Downregulating DNA Methyltransferase 3b. Alzheimer’s Dement. 2016, 12, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Kerridge, C.; Kozlova, D.I.; Nalivaeva, N.N.; Turner, A.J. Hypoxia Affects Neprilysin Expression Through Caspase Activation and an APP Intracellular Domain-Dependent Mechanism. Front. Neurosci. 2015, 9, 163395. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Rodríguez-Arellano, J.J.; Parpura, V.; Zorec, R. Astroglial Calcium Signalling in Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2017, 483, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A. Astroglial Calcium Signaling in Aging and Alzheimer’s Disease. Cold Spring Harb. Perspect. Biol. 2019, 11, a035188. [Google Scholar] [CrossRef] [PubMed]
- Cascella, R.; Cecchi, C. Calcium Dyshomeostasis in Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4914. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Rodriguez, M.; Kharitonova, E.K.; Bacskai, B.J. In Vivo Brain Imaging of Mitochondrial Ca2+ in Neurodegenerative Diseases with Multiphoton Microscopy. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118998. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Yamada, M.; Suematsu, N.; Matsushita, M.; Otomo, E. An Immunohistochemical Study of Centenarian Brains: A Comparison. J. Neurol. Sci. 1998, 157, 73–81. [Google Scholar] [CrossRef]
- Hauw, J.J.; Zekry, D.; Seilhean, D.; Forette, B.; Gallinari, C.; Laurent, M.; Moulias, R.; Piette, F.; Sachet, A.; Duyckaerts, C. Neuropathology of the Cerebral Vessels of Centenarians. J. Mal. Vasc. 2002, 27, S13–S18. [Google Scholar]
- Fang, B.; Zhao, Q.; Ling, W.; Zhang, Y.; Ou, M. Hypoxia Induces HT-22 Neuronal Cell Death via Orai1/CDK5 Pathway-Mediated Tau Hyperphosphorylation. Am. J. Transl. Res. 2019, 11, 7591–7603. [Google Scholar] [PubMed]
- Pena, E.; San Martin-Salamanca, R.; El Alam, S.; Flores, K.; Arriaza, K. Tau Protein Alterations Induced by Hypobaric Hypoxia Exposure. Int. J. Mol. Sci. 2024, 25, 889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Niu, L.; Li, S.; Le, W. Pathological Impacts of Chronic Hypoxia on Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Le, W. Pathological Role of Hypoxia in Alzheimer’s Disease. Exp. Neurol. 2010, 223, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, N.; Zhang, Q.; Li, C.; Sandhu, A.F.; Williams, G., III; Lin, S.; Lv, P.; Liu, Y.; Wu, Q.; et al. Inflammatory Factors and Amyloid β-Induced Microglial Polarization Promote Inflammatory Crosstalk with Astrocytes. Aging 2020, 12, 22538. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Hypoxia/Ischemia Impairs CD33 (Siglec-3)/TREM2 Signaling: Potential Role in Alzheimer’s Pathogenesis. Neurochem. Int. 2021, 150, 105186. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.S.; Gumb, T.; Pirraglia, E.; Varga, A.W.; Lu, S.; Lim, J.; Wohlleber, M.E.; Ducca, E.L.; Koushyk, V.; Glodzik, L.; et al. Sleep-Disordered Breathing Advances Cognitive Decline in the Elderly. Neurology 2015, 84, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Mullins, A.E.; Kam, K.; Parekh, A.; Bubu, O.M.; Osorio, R.S.; Varga, A.W. Obstructive Sleep Apnea and Its Treatment in Aging: Effects on Alzheimer’s Disease Biomarkers, Cognition, Brain Structure and Neurophysiology. Neurobiol. Dis. 2020, 145, 105054. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.; Ayappa, I.; Ayas, N.; Beaudin, A.E.; Hoyos, C.; Kushida, C.A.; Kaminska, M.; Mullins, A.; Naismith, S.L.; Osorio, R.S.; et al. The Link between Obstructive Sleep Apnea and Neurocognitive Impairment: An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2022, 19, 1245–1256. [Google Scholar] [CrossRef]
- Astara, K.; Tsimpolis, A.; Kalafatakis, K.; Vavougios, G.D.; Xiromerisiou, G.; Dardiotis, E.; Christodoulou, N.G.; Samara, M.T.; Lappas, A.S. Sleep Disorders and Alzheimer’s Disease Pathophysiology: The Role of the Glymphatic System. A Scoping Review. Mech. Ageing Dev. 2024, 217, 111899. [Google Scholar] [CrossRef]
- Beschorner, N.; Nedergaard, M. Glymphatic System Dysfunction in Neurodegenerative Diseases. Curr. Opin. Neurol. 2024, 37, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Szlufik, S.; Kopeć, K.; Szleszkowski, S.; Koziorowski, D. Glymphatic System Pathology and Neuroinflammation as Two Risk Factors of Neurodegeneration. Cells 2024, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Berdina, O.N.; Madaeva, I.M.; Bolshakova, S.E.; Sholokhov, L.F.; Rychkova, L.V. Obstructive Sleep Apnea and Amyloid-Β42 in Adolescents: The Results of a Pilot Study. Acta Biomed. Sci. 2022, 7, 12–21. [Google Scholar] [CrossRef]
- Ju, Y.S.; Finn, M.B.; Sutphen, C.L.; Herries, E.M.; Jerome, G.M.; Ladenson, J.H.; Crimmins, D.L.; Fagan, A.M.; Holtzman, D.M. Obstructive Sleep Apnea Decreases Central Nervous System–Derived Proteins in the Cerebrospinal Fluid. Ann. Neurol. 2016, 80, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Ulland, T.K.; Ewald, A.C.; Knutson, A.O.; Marino, K.M.; Smith, S.M.C.; Watters, J.J. Alzheimer’s Disease, Sleep Disordered Breathing, and Microglia: Puzzling out a Common Link. Cells 2021, 10, 2907. [Google Scholar] [CrossRef] [PubMed]
- Macheda, T.; Roberts, K.; Lyons, D.N.; Higgins, E.; Ritter, K.J.; Lin, A.; Alilain, W.J.; Bachstetter, A.D. Chronic Intermittent Hypoxia Induces Robust Astrogliosis in an Alzheimer’s Disease-Relevant Mouse Model. Neuroscience 2019, 398, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Peng, Y.-J.; Nanduri, J. Hypoxia-Inducible Factors and Obstructive Sleep Apnea. J. Clin. Investig. 2020, 130, 5042–5051. [Google Scholar] [CrossRef] [PubMed]
- Row, B.W.; Kheirandish, L.; Neville, J.J.; Gozal, D. Impaired Spatial Learning and Hyperactivity in Developing Rats Exposed to Intermittent Hypoxia. Pediatr. Res. 2002, 52, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.L.; Wang, Y.; Gozal, D. Pathological Consequences of Intermittent Hypoxia in the Central Nervous System. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2012; pp. 1767–1777. [Google Scholar]
- Payne, R.S.; Goldbart, A.; Gozal, D.; Schurr, A. Effect of Intermittent Hypoxia on Long-Term Potentiation in Rat Hippocampal Slices. Brain Res. 2004, 1029, 195–199. [Google Scholar] [CrossRef]
- Nair, D.; Dayyat, E.A.; Zhang, S.X.; Wang, Y.; Gozal, D. Intermittent Hypoxia-Induced Cognitive Deficits Are Mediated by NADPH Oxidase Activity in a Murine Model of Sleep Apnea. PLoS ONE 2011, 6, e19847. [Google Scholar] [CrossRef]
- Arias-Cavieres, A.; Khuu, M.A.; Nwakudu, C.U.; Barnard, J.E.; Dalgin, G.; Garcia, A.J. A HIF1a-Dependent Pro-Oxidant State Disrupts Synaptic Plasticity and Impairs Spatial Memory in Response to Intermittent Hypoxia. eNeuro 2020, 7, ENEURO.0024-20.2020. [Google Scholar] [CrossRef] [PubMed]
- Wall, A.M.; Corcoran, A.E.; O’Halloran, K.D.; O’Connor, J.J. Effects of Prolyl-Hydroxylase Inhibition and Chronic Intermittent Hypoxia on Synaptic Transmission and Plasticity in the Rat CA1 and Dentate Gyrus. Neurobiol. Dis. 2014, 62, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.-M.; Lau, C.-F.; Fung, M.-L. Melatonin Reduces Hippocampal β-Amyloid Generation in Rats Exposed to Chronic Intermittent Hypoxia. Brain Res. 2010, 1354, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, S.; Hirasawa, A. Intermittent Hypoxia Produces Alzheimer Disease? Oncotarget 2017, 8, 41786–41787. [Google Scholar] [CrossRef] [PubMed]
- Shiota, S.; Takekawa, H.; Matsumoto, S.; Takeda, K.; Nurwidya, F.; Yoshioka, Y.; Takahashi, F.; Hattori, N.; Tabira, T.; Mochizuki, H.; et al. Chronic Intermittent Hypoxia/Reoxygenation Facilitate Amyloid-β Generation in Mice. J. Alzheimer’s Dis. 2013, 37, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Sochal, M. Evaluation of HIF-1 Involvement in the BDNF and ProBDNF Signaling Pathways among Obstructive Sleep Apnea Patients. Int. J. Mol. Sci. 2022, 23, 14876. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Fujiwara, H.; Nonaka, T.; Wakabayashi, K.; Takahashi, H.; Lee, V.M.-Y.; Trojanowski, J.Q.; Mann, D.; Iwatsubo, T. Phosphorylated α-Synuclein Is Ubiquitinated in α-Synucleinopathy Lesions. J. Biol. Chem. 2002, 277, 49071–49076. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Feany, M.B. α-Synuclein Phosphorylation Controls Neurotoxicity and Inclusion Formation in a Drosophila Model of Parkinson Disease. Nat. Neurosci. 2005, 8, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Bengoa-Vergniory, N.; Roberts, R.F.; Wade-Martins, R.; Alegre-Abarrategui, J. Alpha-Synuclein Oligomers: A New Hope. Acta Neuropathol. 2017, 134, 819–838. [Google Scholar] [CrossRef]
- Winner, B.; Jappelli, R.; Maji, S.K.; Desplats, P.A.; Boyer, L.; Aigner, S.; Hetzer, C.; Loher, T.; Vilar, M.; Campioni, S.; et al. In Vivo Demonstration That α-Synuclein Oligomers Are Toxic. Proc. Natl. Acad. Sci. USA 2011, 108, 4194–4199. [Google Scholar] [CrossRef]
- Muddapu, V.R.; Chakravarthy, V.S. Influence of Energy Deficiency on the Subcellular Processes of Substantia Nigra Pars Compacta Cell for Understanding Parkinsonian Neurodegeneration. Sci. Rep. 2021, 11, 1754. [Google Scholar] [CrossRef]
- Guo, M.; Liu, W.; Luo, H.; Shao, Q.; Li, Y.; Gu, Y.; Guan, Y.; Ma, W.; Chen, M.; Yang, H.; et al. Hypoxic Stress Accelerates the Propagation of Pathological Alpha-synuclein and Degeneration of Dopaminergic Neurons. CNS Neurosci. Ther. 2023, 29, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ji, X.; Liu, J. Hypoxia and Alpha-Synuclein: Inextricable Link Underlying the Pathologic Progression of Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 919343. [Google Scholar] [CrossRef]
- Kim, T.; Mehta, S.L.; Kaimal, B.; Lyons, K.; Dempsey, R.J.; Vemuganti, R. Poststroke Induction of -Synuclein Mediates Ischemic Brain Damage. J. Neurosci. 2016, 36, 7055–7065. [Google Scholar] [CrossRef]
- Unal-Cevik, I.; Gursoy-Ozdemir, Y.; Yemisci, M.; Lule, S.; Gurer, G.; Can, A.; Müller, V.; Kahle, P.J.; Dalkara, T. Alpha-Synuclein Aggregation Induced by Brief Ischemia Negatively Impacts Neuronal Survival in Vivo: A Study in [A30P]Alpha-Synuclein Transgenic Mouse. J. Cereb. Blood Flow. Metab. 2011, 31, 913–923. [Google Scholar] [CrossRef]
- Hu, X.; Rea, H.C.; Wiktorowicz, J.E.; Perez-Polo, J.R. Proteomic Analysis of Hypoxia/Ischemia-Induced Alteration of Cortical Development and Dopamine Neurotransmission in Neonatal Rat. J. Proteome Res. 2006, 5, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, Q.; Chao, D.; Xia, T.C.; Sheng, S.; Li, Z.-R.; Zhao, J.-N.; Wen, G.-Q.; Ding, G.; Xia, Y. δ-Opioid Receptor Activation Attenuates the Oligomer Formation Induced by Hypoxia and/or α-Synuclein Overexpression/Mutation Through Dual Signaling Pathways. Mol. Neurobiol. 2019, 56, 3463–3475. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sun, B.; Chen, D.; Chen, Y.; Li, W.; Xu, M.; Shen, Y.; Xu, Z.; Wang, Y.; Bu, X. Plasma α-synuclein Levels Are Increased in Patients with Obstructive Sleep Apnea Syndrome. Ann. Clin. Transl. Neurol. 2019, 6, 788–794. [Google Scholar] [CrossRef]
- Elfil, M.; Bahbah, E.I.; Attia, M.M.; Eldokmak, M.; Koo, B.B. Impact of Obstructive Sleep Apnea on Cognitive and Motor Functions in Parkinson’s Disease. Mov. Disord. 2021, 36, 570–580. [Google Scholar] [CrossRef]
- Pacelli, C.; Giguère, N.; Bourque, M.-J.; Lévesque, M.; Slack, R.S.; Trudeau, L.-É. Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Curr. Biol. 2015, 25, 2349–2360. [Google Scholar] [CrossRef]
- Burtscher, J.; Syed, M.M.K.; Keller, M.A.; Lashuel, H.A.; Millet, G.P. Fatal Attraction—The Role of Hypoxia When Alpha-Synuclein Gets Intimate with Mitochondria. Neurobiol. Aging 2021, 107, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef]
- Lestón Pinilla, L.; Ugun-Klusek, A.; Rutella, S.; De Girolamo, L.A. Hypoxia Signaling in Parkinson’s Disease: There Is Use in Asking “What HIF?”. Biology 2021, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hydroxylation of HIF-1: Oxygen Sensing at the Molecular Level. Physiology 2004, 19, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Regulation of Oxygen Homeostasis by Hypoxia-Inducible Factor 1. Physiology 2009, 24, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, M.; Pasanen, A.; Kivirikko, K.I.; Myllyharju, J. Roles of the Human Hypoxia-Inducible Factor (HIF)-3α Variants in the Hypoxia Response. Cell. Mol. Life Sci. 2011, 68, 3885–3901. [Google Scholar] [CrossRef] [PubMed]
- Leu, T.; Schützhold, V.; Fandrey, J.; Ferenz, K.B. When the Brain Yearns for Oxygen. Neurosignals 2019, 27, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Masoud, G.N.; Li, W. HIF-1α Pathway: Role, Regulation and Intervention for Cancer Therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Bruick, R.K.; McKnight, S.L. A Conserved Family of Prolyl-4-Hydroxylases That Modify HIF. Science 2001, 294, 1337–1340. [Google Scholar] [CrossRef]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G., Jr. HIFα Targeted for VHL-Mediated Destruction by Proline Hydroxylation: Implications for O2 Sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef]
- Pereira, T.; Zheng, X.; Poellinger, L. Degradation of the Hypoxia-Inducible Factor 1α, Where Does It Happen? Cell Cycle 2006, 5, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-α to the von Hippel-Lindau Ubiquitylation Complex by O2 -Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Oxygen Sensing, Hypoxia-Inducible Factors, and Disease Pathophysiology. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Dengler, V.L.; Galbraith, M.D.; Espinosa, J.M. Transcriptional Regulation by Hypoxia Inducible Factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Nakao, S.; Ueyama, T.; Harada, Y.; Kawamura, T. Metabolic Remodeling during Somatic Cell Reprogramming to Induced Pluripotent Stem Cells: Involvement of Hypoxia-Inducible Factor 1. Inflamm. Regen. 2020, 40, 8. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Jiang, B.-H.; Leung, S.W.; Passantino, R.; Concordet, J.-P.; Maire, P.; Giallongo, A. Hypoxia Response Elements in the Aldolase A, Enolase 1, and Lactate Dehydrogenase A Gene Promoters Contain Essential Binding Sites for Hypoxia-Inducible Factor 1. J. Biol. Chem. 1996, 271, 32529–32537. [Google Scholar] [CrossRef]
- Kim, J.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-Mediated Expression of Pyruvate Dehydrogenase Kinase: A Metabolic Switch Required for Cellular Adaptation to Hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Tello, D.; Balsa, E.; Acosta-Iborra, B.; Fuertes-Yebra, E.; Elorza, A.; Ordóñez, Á.; Corral-Escariz, M.; Soro, I.; López-Bernardo, E.; Perales-Clemente, E.; et al. Induction of the Mitochondrial NDUFA4L2 Protein by HIF-1α Decreases Oxygen Consumption by Inhibiting Complex I Activity. Cell Metab. 2011, 14, 768–779. [Google Scholar] [CrossRef]
- Raut, S.; Bhalerao, A.; Powers, M.; Gonzalez, M.; Mancuso, S.; Cucullo, L. Hypometabolism, Alzheimer’s Disease, and Possible Therapeutic Targets: An Overview. Cells 2023, 12, 2019. [Google Scholar] [CrossRef]
- Peng, W.; Tan, C.; Mo, L.; Jiang, J.; Zhou, W.; Du, J.; Zhou, X.; Liu, X.; Chen, L. Glucose Transporter 3 in Neuronal Glucose Metabolism: Health and Diseases. Metabolism 2021, 123, 154869. [Google Scholar] [CrossRef]
- Głuchowska, K.; Pliszka, M.; Szablewski, L. Expression of Glucose Transporters in Human Neurodegenerative Diseases. Biochem. Biophys. Res. Commun. 2021, 540, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Glucose Transporters in Brain: In Health and in Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 55, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D. Glucose Metabolism and Alzheimer’s Disease. Ageing Res. Rev. 2005, 4, 240–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, F.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.-X. Decreased Glucose Transporters Correlate to Abnormal Hyperphosphorylation of Tau in Alzheimer Disease. FEBS Lett. 2008, 582, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Soucek, T.; Cumming, R.; Dargusch, R.; Maher, P.; Schubert, D. The Regulation of Glucose Metabolism by HIF-1 Mediates a Neuroprotective Response to Amyloid Beta Peptide. Neuron 2003, 39, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, X.; Xu, S.; Pang, Z.; Li, L.; Yang, Y.; Fan, Y.; Wang, Z.; Yu, X.; Guo, C.; et al. α-Lipoic Acid Maintains Brain Glucose Metabolism via BDNF/TrkB/HIF-1α Signaling Pathway in P301S Mice. Front. Aging Neurosci. 2020, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lai, M.K.P.; Arumugam, T.V.; Jo, D.-G. O-GlcNAcylation as a Therapeutic Target for Alzheimer’s Disease. Neuromolecular Med. 2020, 22, 171–193. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jinka, S.K.A.; Khanal, S.; Bhavnani, N.; Almashhori, F.; Lallo, J.; Mathias, A.; Al-Rhayyel, Y.; Herman, D.; Holden, J.G.; et al. Cognitive Dysfunction and Increased Phosphorylated Tau Are Associated with Reduced O-GlcNAc Signaling in an Aging Mouse Model of Metabolic Syndrome. J. Neurosci. Res. 2023, 101, 1324–1344. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Huang, Z.-T.; Yuan, M.-H.; Jing, F.; Cai, R.-L.; Zou, Q.; Pu, Y.-S.; Wang, S.-Y.; Chen, F.; Yi, W.-M.; et al. Role of Hypoxia Inducible Factor-1α in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 80, 949–961. [Google Scholar] [CrossRef]
- Schubert, D.; Soucek, T.; Blouw, B. The Induction of HIF-1 Reduces Astrocyte Activation by Amyloid Beta Peptide. Eur. J. Neurosci. 2009, 29, 1323–1334. [Google Scholar] [CrossRef]
- Merelli, A.; Rodríguez, J.C.G.; Folch, J.; Regueiro, M.R.; Camins, A.; Lazarowski, A. Understanding the Role of Hypoxia Inducible Factor During Neurodegeneration for New Therapeutics Opportunities. Curr. Neuropharmacol. 2018, 16, 1484–1498. [Google Scholar] [CrossRef] [PubMed]
- Avramovich-Tirosh, Y.; Bar-Am, O.; Amit, T.; Youdim, M.B.H.; Weinreb, O. Up-Regulation of Hypoxia-Inducible Factor (HIF)-1α and HIF-Target Genes in Cortical Neurons by the Novel Multifunctional Iron Chelator Anti-Alzheimer Drug, M30. Curr. Alzheimer Res. 2010, 7, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fandrey, J.; Schümann, J.; Tiegs, G.; Brüne, B. NO and TNF-α Released from Activated Macrophages Stabilize HIF-1α in Resting Tubular LLC-PK1 Cells. Am. J. Physiol. Cell Physiol. 2003, 284, C439–C446. [Google Scholar] [CrossRef] [PubMed]
- Hellwig-Bürgel, T.; Rutkowski, K.; Metzen, E.; Fandrey, J.; Jelkmann, W. Interleukin-1beta and Tumor Necrosis Factor-Alpha Stimulate DNA Binding of Hypoxia-Inducible Factor-1. Blood 1999, 94, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Lu, J. HIF-1α Activation Attenuates IL-6 and TNF-α Pathways in Hippocampus of Rats Following Transient Global Ischemia. Cell. Physiol. Biochem. 2016, 39, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Li, B.; Subbarao Malireddi, R.K.; Lamkanfi, M.; Geiger, T.L.; Kanneganti, T.-D. Chronic TLR Stimulation Controls NLRP3 Inflammasome Activation through IL-10 Mediated Regulation of NLRP3 Expression and Caspase-8 Activation. Sci. Rep. 2015, 5, 14488. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, A.; Rao, P.; Pranay, A.; Xu, K.; LaManna, J.C.; Puchowicz, M.A. Chronic Ketosis Modulates HIF1α-Mediated Inflammatory Response in Rat Brain. In Oxygen Transport to Tissue XLII; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3–7. [Google Scholar]
- Shin, D.H.; Li, S.H.; Yang, S.; Lee, B.L.; Lee, M.K.; Park, J. Inhibitor of Nuclear Factor-kappaB Alpha Derepresses Hypoxia-inducible Factor-1 during Moderate Hypoxia by Sequestering Factor Inhibiting Hypoxia-inducible Factor from Hypoxia-inducible Factor 1α. FEBS J. 2009, 276, 3470–3480. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Kim, Y.E.; Jeon, H.S.; Yoo, M.; Kim, M.; Kim, Y.-M.; Koh, S.-H.; Choi, Y.K. Chronic Hypoxia of Endothelial Cells Boosts HIF-1α-NLRP1 Circuit in Alzheimer’s Disease. Free Radic. Biol. Med. 2023, 204, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Grammas, P.; Tripathy, D.; Sanchez, A.; Yin, X.; Luo, J. Brain Microvasculature and Hypoxia-Related Proteins in Alzheimer’s Disease. Int. J. Clin. Exp. Pathol. 2011, 4, 616–627. [Google Scholar] [PubMed]
- Alexander, C.; Li, T.; Hattori, Y.; Chiu, D.; Frost, G.R.; Jonas, L.; Liu, C.; Anderson, C.J.; Wong, E.; Park, L.; et al. Hypoxia Inducible Factor-1α Binds and Activates γ-Secretase for Aβ Production under Hypoxia and Cerebral Hypoperfusion. Mol. Psychiatry 2022, 27, 4264–4273. [Google Scholar] [CrossRef]
- Guglielmotto, M.; Aragno, M.; Autelli, R.; Giliberto, L.; Novo, E.; Colombatto, S.; Danni, O.; Parola, M.; Smith, M.A.; Perry, G.; et al. The Up-regulation of BACE1 Mediated by Hypoxia and Ischemic Injury: Role of Oxidative Stress and HIF1α. J. Neurochem. 2009, 108, 1045–1056. [Google Scholar] [CrossRef]
- Guo, C.; Yang, Z.-H.; Zhang, S.; Chai, R.; Xue, H.; Zhang, Y.-H.; Li, J.-Y.; Wang, Z.-Y. Intranasal Lactoferrin Enhances α-Secretase-Dependent Amyloid Precursor Protein Processing via the ERK1/2-CREB and HIF-1α Pathways in an Alzheimer’s Disease Mouse Model. Neuropsychopharmacology 2017, 42, 2504–2515. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, T.; Zou, Y.; Zhang, J.H.; Feng, H. Hypoxia Induces Autophagic Cell Death through Hypoxia-Inducible Factor 1α in Microglia. PLoS ONE 2014, 9, e96509. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, N.; El-Khatib, A.H.; Costas-Rodríguez, M.; Schwab, K.; Griffin, E.; Raab, A.; Platt, B.; Theuring, F.; Vogl, J.; Vanhaecke, F. Cu, Fe, and Zn Isotope Ratios in Murine Alzheimer’s Disease Models Suggest Specific Signatures of Amyloidogenesis and Tauopathy. J. Biol. Chem. 2021, 296, 100292. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.G.; Connor, J.R.; Meadowcroft, M.D. The Relationship between Iron Dyshomeostasis and Amyloidogenesis in Alzheimer’s Disease: Two Sides of the Same Coin. Neurobiol. Dis. 2015, 81, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Tacchini, L.; Bianchi, L.; Bernelli-Zazzera, A.; Cairo, G. Transferrin Receptor Induction by Hypoxia. J. Biol. Chem. 1999, 274, 24142–24146. [Google Scholar] [CrossRef] [PubMed]
- Tacchini, L.; Gammella, E.; De Ponti, C.; Recalcati, S.; Cairo, G. Role of HIF-1 and NF-ΚB Transcription Factors in the Modulation of Transferrin Receptor by Inflammatory and Anti-Inflammatory Signals. J. Biol. Chem. 2008, 283, 20674–20686. [Google Scholar] [CrossRef] [PubMed]
- Petralla, S.; Saveleva, L.; Kanninen, K.M.; Oster, J.S.; Panayotova, M.; Fricker, G.; Puris, E. Increased Expression of Transferrin Receptor 1 in the Brain Cortex of 5xFAD Mouse Model of Alzheimer’s Disease Is Associated with Activation of HIF-1 Signaling Pathway. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s Disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Burbulla, L.F.; Song, P.; Mazzulli, J.R.; Zampese, E.; Wong, Y.C.; Jeon, S.; Santos, D.P.; Blanz, J.; Obermaier, C.D.; Strojny, C.; et al. Dopamine Oxidation Mediates Mitochondrial and Lysosomal Dysfunction in Parkinson’s Disease. Science 2017, 357, 1255–1261. [Google Scholar] [CrossRef]
- Hauser, D.N.; Hastings, T.G. Mitochondrial Dysfunction and Oxidative Stress in Parkinson’s Disease and Monogenic Parkinsonism. Neurobiol. Dis. 2013, 51, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, R.; Barrett, P.J.; Hoffman, E.K.; Barrett, C.W.; Zharikov, A.; Borah, A.; Hu, X.; McCoy, J.; Chu, C.T.; Burton, E.A.; et al. α-Synuclein Binds to TOM20 and Inhibits Mitochondrial Protein Import in Parkinson’s Disease. Sci. Transl. Med. 2016, 8, 342ra78. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Esteves, A.R.; Silva, D.F.; Januário, C.; Cardoso, S.M. The Impact of Mitochondrial Fusion and Fission Modulation in Sporadic Parkinson’s Disease. Mol. Neurobiol. 2015, 52, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. α-Synuclein in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, L.; Liu, J.; Jin, Y.; Lin, Z.; Du, S.; Fu, Z.; Chen, T.; Qin, Y.; Sui, F.; et al. HIF-1α/MicroRNA-128-3p Axis Protects Hippocampal Neurons from Apoptosis via the Axin1 -Mediated Wnt/β-Catenin Signaling Pathway in Parkinson’s Disease Models. Aging 2020, 12, 4067–4081. [Google Scholar] [CrossRef] [PubMed]
- Agani, F.H.; Pichiule, P.; Chavez, J.C.; LaManna, J.C. The Role of Mitochondria in the Regulation of Hypoxia-Inducible Factor 1 Expression during Hypoxia. J. Biol. Chem. 2000, 275, 35863–35867. [Google Scholar] [CrossRef] [PubMed]
- Czyzyk-Krzeska, M.F.; Furnari, B.A.; Lawson, E.E.; Millhorn, D.E. Hypoxia Increases Rate of Transcription and Stability of Tyrosine Hydroxylase MRNA in Pheochromocytoma (PC12) Cells. J. Biol. Chem. 1994, 269, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, J.; Chang, Y.; ShiDu Yan, S.; Shi, H. Hypoxia Inducible Factor-1 as a Target for Neurodegenerative Diseases. Curr. Med. Chem. 2011, 18, 4335–4343. [Google Scholar] [CrossRef]
- Milosevic, J.; Maisel, M.; Wegner, F.; Leuchtenberger, J.; Wenger, R.H.; Gerlach, M.; Storch, A.; Schwarz, J. Lack of Hypoxia-Inducible Factor-1α Impairs Midbrain Neural Precursor Cells Involving Vascular Endothelial Growth Factor Signaling. J. Neurosci. 2007, 27, 412–421. [Google Scholar] [CrossRef]
- Weng, M.; Xie, X.; Liu, C.; Lim, K.-L.; Zhang, C.; Li, L. The Sources of Reactive Oxygen Species and Its Possible Role in the Pathogenesis of Parkinson’s Disease. Park. Dis. 2018, 2018, 9163040. [Google Scholar] [CrossRef]
- Parandavar, E.; Yazdanparast, R. Differential Impact of Various Reactive Oxygen Species (ROS) on HIF-1α/P53 Direct Interaction in SK-N-MC Neuroblastoma Cells. Cell Biosci. 2017, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Sanjuán-Pla, A.; Cervera, A.M.; Apostolova, N.; Garcia-Bou, R.; Víctor, V.M.; Murphy, M.P.; McCreath, K.J. A Targeted Antioxidant Reveals the Importance of Mitochondrial Reactive Oxygen Species in the Hypoxic Signaling of HIF-1α. FEBS Lett. 2005, 579, 2669–2674. [Google Scholar] [CrossRef] [PubMed]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.J.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 Gene Associated with Autosomal Recessive Early-Onset Parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Parsanejad, M.; Zhang, Y.; Qu, D.; Irrcher, I.; Rousseaux, M.W.C.; Aleyasin, H.; Kamkar, F.; Callaghan, S.; Slack, R.S.; Mak, T.W.; et al. Regulation of the VHL/HIF-1 Pathway by DJ-1. J. Neurosci. 2014, 34, 8043–8050. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, D.; Eshel, G.; Finberg, J.P.M.; Youdim, M.B.H. The Iron Chelator Desferrioxamine (Desferal) Retards 6-Hydroxydopamine-Induced Degeneration of Nigrostriatal Dopamine Neurons. J. Neurochem. 1991, 56, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Wohlrab, C.; Kuiper, C.; Vissers, M.C.; Phillips, E.; Robinson, B.A.; Dachs, G.U. Ascorbate Modulates the Hypoxic Pathway by Increasing Intracellular Activity of the HIF Hydroxylases in Renal Cell Carcinoma Cells. Hypoxia 2019, 7, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Flashman, E.; Davies, S.L.; Yeoh, K.K.; Schofield, C.J. Investigating the Dependence of the Hypoxia-Inducible Factor Hydroxylases (Factor Inhibiting HIF and Prolyl Hydroxylase Domain 2) on Ascorbate and Other Reducing Agents. Biochem. J. 2010, 427, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yang, E. Recent Advances in Developing Inhibitors for Hypoxia-Inducible Factor Prolyl Hydroxylases and Their Therapeutic Implications. Molecules 2015, 20, 20551–20568. [Google Scholar] [CrossRef]
- Mitroshina, E.V.; Savyuk, M.O.; Ponimaskin, E.; Vedunova, M.V. Hypoxia-Inducible Factor (HIF) in Ischemic Stroke and Neurodegenerative Disease. Front. Cell Dev. Biol. 2021, 9, 703084. [Google Scholar] [CrossRef]
- Kupershmidt, L.; Amit, T.; Bar-Am, O.; Youdim, M.B.H.; Weinreb, O. The Novel Multi-Target Iron Chelating-Radical Scavenging Compound M30 Possesses Beneficial Effects on Major Hallmarks of Alzheimer’s Disease. Antioxid. Redox Signal 2012, 17, 860–877. [Google Scholar] [CrossRef]
- Mechlovich, D.; Amit, T.; Bar-Am, O.; Mandel, S.; Youdim, M.; Weinreb, O. The Novel Multi-Target Iron Chelator, M30 Modulates HIF-1α-Related Glycolytic Genes and Insulin Signaling Pathway in the Frontal Cortex of APP/PS1 Alzheimer’s Disease Mice. Curr. Alzheimer Res. 2014, 11, 119–127. [Google Scholar] [CrossRef]
- Amit, T.; Bar-Am, O.; Mechlovich, D.; Kupershmidt, L.; Youdim, M.B.H.; Weinreb, O. The Novel Multitarget Iron Chelating and Propargylamine Drug M30 Affects APP Regulation and Processing Activities in Alzheimer’s Disease Models. Neuropharmacology 2017, 123, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gal, S.; Zheng, H.; Fridkin, M.; Youdim, M.B.H. Restoration of Nigrostriatal Dopamine Neurons in Post-MPTP Treatment by the Novel Multifunctional Brain-Permeable Iron Chelator-Monoamine Oxidase Inhibitor Drug, M30. Neurotox. Res. 2010, 17, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.H.; Kupershmidt, L.; Amit, T.; Weinreb, O. Promises of Novel Multi-Target Neuroprotective and Neurorestorative Drugs for Parkinson’s Disease. Park. Relat. Disord. 2014, 20, S132–S136. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.H. Multi Target Neuroprotective and Neurorestorative Anti-Parkinson and Anti-Alzheimer Drugs Ladostigil and M30 Derived from Rasagiline. Exp. Neurobiol. 2013, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.E.; Fernández-Pérez, E.J.; Olivos, N.; Burgos, C.F.; Boopathi, S.; Armijo-Weingart, L.; Pacheco, C.R.; González, W.; Aguayo, L.G. The Stimulatory Effects of Intracellular α-Synuclein on Synaptic Transmission Are Attenuated by 2-Octahydroisoquinolin-2(1H)-Ylethanamine. Int. J. Mol. Sci. 2021, 22, 13253. [Google Scholar] [CrossRef] [PubMed]
- Farr, A.C.; Xiong, M.P. Challenges and Opportunities of Deferoxamine Delivery for Treatment of Alzheimer’s Disease, Parkinson’s Disease, and Intracerebral Hemorrhage. Mol. Pharm. 2021, 18, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Abdelmoneem, M.A.; Hassanin, I.A.; Abd Elwakil, M.M.; Elnaggar, M.A.; Mokhtar, S.; Fang, J.-Y.; Elkhodairy, K.A. Lactoferrin, a Multi-Functional Glycoprotein: Active Therapeutic, Drug Nanocarrier & Targeting Ligand. Biomaterials 2020, 263, 120355. [Google Scholar] [CrossRef]
- Zakharova, E.T.; Kostevich, V.A.; Sokolov, A.V.; Vasilyev, V.B. Human Apo-Lactoferrin as a Physiological Mimetic of Hypoxia Stabilizes Hypoxia-Inducible Factor-1 Alpha. BioMetals 2012, 25, 1247–1259. [Google Scholar] [CrossRef]
- Xu, S.-F.; Zhang, Y.-H.; Wang, S.; Pang, Z.-Q.; Fan, Y.-G.; Li, J.-Y.; Wang, Z.-Y.; Guo, C. Lactoferrin Ameliorates Dopaminergic Neurodegeneration and Motor Deficits in MPTP-Treated Mice. Redox Biol. 2019, 21, 101090. [Google Scholar] [CrossRef]
- Nordquist, L.; Friederich-Persson, M.; Fasching, A.; Liss, P.; Shoji, K.; Nangaku, M.; Hansell, P.; Palm, F. Activation of Hypoxia-Inducible Factors Prevents Diabetic Nephropathy. J. Am. Soc. Nephrol. 2015, 26, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Taher, M.; Yin, C.; Salloum, F.; Kukreja, R.C. Cobalt Chloride Induces Delayed Cardiac Preconditioning in Mice through Selective Activation of HIF-1α and AP-1 and INOS Signaling. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2369–H2375. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Y.; Xiong, Q.-W.; Yao, X.; Liu, F.; Tang, X.; Fu, H.; Tong, T.; Mao, J.; Peng, W.-X. Roxadustat: Do We Know All the Answers? Biomol. Biomed. 2023, 23, 354. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, X.; Cao, J.; Geng, Z.; Wang, Z. Effect of Proline Analogues on Activity of Human Prolyl Hydroxylase and the Regulation of HIF Signal Transduction Pathway. PLoS ONE 2014, 9, e95692. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, V.; Parinandi, N.L.; Adluri, R.S.; Goldman, J.W.; Hussain, N.; Sanchez, J.A.; Maulik, N. Molecular Mechanisms of Action and Therapeutic Uses of Pharmacological Inhibitors of HIF–Prolyl 4-Hydroxylases for Treatment of Ischemic Diseases. Antioxid. Redox Signal 2014, 20, 2631–2665. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Rajagopalan, S.; Siddiq, A.; Gwiazda, R.; Yang, L.; Beal, M.F.; Ratan, R.R.; Andersen, J.K. Inhibition of Prolyl Hydroxylase Protects against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Neurotoxicity. J. Biol. Chem. 2009, 284, 29065–29076. [Google Scholar] [CrossRef] [PubMed]
- Chinta, S.J.; Rajagopalan, S.; Ganesan, A.; Andersen, J.K. A Possible Novel Anti-Inflammatory Mechanism for the Pharmacological Prolyl Hydroxylase Inhibitor 3,4-Dihydroxybenzoate: Implications for Use as a Therapeutic for Parkinson’s Disease. Park. Dis. 2012, 2012, 364684. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, X.-X.; Chen, Y.-J.; Wu, T.-T.; Xu, H.; Yin, H.; Wu, Y.-C. Therapeutic Potential of a Prolyl Hydroxylase Inhibitor FG-4592 for Parkinson’s Diseases in Vitro and in Vivo: Regulation of Redox Biology and Mitochondrial Function. Front. Aging Neurosci. 2018, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; You, Y.; Wang, Z.; Zhou, Y.; Chai, G.; Yan, G.; Jin, Z.; Wang, Q.; Sun, H. Orexin A Peptidergic System: Comparative Sleep Behavior, Morphology and Population in Brains between Wild Type and Alzheimer’s Disease Mice. Brain Struct. Funct. 2022, 227, 1051–1065. [Google Scholar] [CrossRef]
- Liu, M.-F.; Xue, Y.; Liu, C.; Liu, Y.-H.; Diao, H.-L.; Wang, Y.; Pan, Y.-P.; Chen, L. Orexin-A Exerts Neuroprotective Effects via OX1R in Parkinson’s Disease. Front. Neurosci. 2018, 12, 415023. [Google Scholar] [CrossRef]
- Shimizu, S.; Takenoshita, N.; Inagawa, Y.; Tsugawa, A.; Hirose, D.; Kaneko, Y.; Ogawa, Y.; Serisawa, S.; Sakurai, S.; Hirao, K.; et al. Positive Association Between Cognitive Function and Cerebrospinal Fluid Orexin A Levels in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 73, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Fronczek, R.; van Geest, S.; Frölich, M.; Overeem, S.; Roelandse, F.W.C.; Lammers, G.J.; Swaab, D.F. Hypocretin (Orexin) Loss in Alzheimer’s Disease. Neurobiol. Aging 2012, 33, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Villano, I.; La Marra, M.; Di Maio, G.; Monda, V.; Chieffi, S.; Guatteo, E.; Messina, G.; Moscatelli, F.; Monda, M.; Messina, A. Physiological Role of Orexinergic System for Health. Int. J. Environ. Res. Public Health 2022, 19, 8353. [Google Scholar] [CrossRef] [PubMed]
- Sikder, D.; Kodadek, T. The Neurohormone Orexin Stimulates Hypoxia-Inducible Factor-1 Activity. Genes Dev. 2007, 21, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ru, Y.; Wang, W.; Cai, G.; Gu, L.; Ye, J.; Zhang, W.-B.; Wang, L. Orexin-A Reverse Bone Mass Loss Induced by Chronic Intermittent Hypoxia Through OX1R-Nrf2/HIF-1α Pathway. Drug Des. Dev. Ther. 2022, 16, 2145–2160. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Liu, Y.; Zhao, Y.; Sun, X.; Fan, D.; Guo, L. Orexin A Affects HepG2 Human Hepatocellular Carcinoma Cells Glucose Metabolism via HIF-1α-Dependent and -Independent Mechanism. PLoS ONE 2017, 12, e0184213. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, T.; Li, X.-Q.; Liu, Y.; Zhu, X.-Y.; Jankovic, J.; Pan, T.-H.; Wu, Y.-C. Neuroprotection by Orexin-A via HIF-1α Induction in a Cellular Model of Parkinson’s Disease. Neurosci. Lett. 2014, 579, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Kong, W.; Liu, L.; Yu, W.; Zhang, Z.; Sun, Y. A Viral Vector Expressing Hypoxia-Inducible Factor 1 Alpha Inhibits Hippocampal Neuronal Apoptosis. Neural Regen. Res. 2014, 9, 1145. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Du, F.; Cui, Y.; Qi, M.; Zhuang, J.; Wang, J.; Zhang, M.; Zhang, X.; Liu, Z.; Zou, L.; et al. Synthesis and Biological Evaluations of 8-Biaryl-2,2-Dimethylbenzopyranamide Derivatives against Alzheimer’s Disease and Ischemic Stroke. Bioorganic Chem. 2024, 143, 107064. [Google Scholar] [CrossRef]
- Hai, Y.; Ren, K.; Zhang, Y.; Yang, L.; Cao, H.; Yuan, X.; Su, L.; Li, H.; Feng, X.; Liu, D. HIF-1α Serves as a Co-Linker between AD and T2DM. Biomed. Pharmacother. 2024, 171, 116158. [Google Scholar] [CrossRef]
- Lei, L.; Feng, J.; Wu, G.; Wei, Z.; Wang, J.-Z.; Zhang, B.; Liu, R.; Liu, F.; Wang, X.; Li, H.-L. HIF-1α Causes LCMT1/PP2A Deficiency and Mediates Tau Hyperphosphorylation and Cognitive Dysfunction during Chronic Hypoxia. Int. J. Mol. Sci. 2022, 23, 16140. [Google Scholar] [CrossRef] [PubMed]
- Kapogiannis, D.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Biragyn, A.; Masharani, U.; Frassetto, L.; Petersen, R.C.; Miller, B.L.; Goetzl, E.J. Dysfunctionally Phosphorylated Type 1 Insulin Receptor Substrate in Neural-derived Blood Exosomes of Preclinical Alzheimer’s Disease. FASEB J. 2015, 29, 589–596. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitroshina, E.V.; Vedunova, M.V. The Role of Oxygen Homeostasis and the HIF-1 Factor in the Development of Neurodegeneration. Int. J. Mol. Sci. 2024, 25, 4581. https://doi.org/10.3390/ijms25094581
Mitroshina EV, Vedunova MV. The Role of Oxygen Homeostasis and the HIF-1 Factor in the Development of Neurodegeneration. International Journal of Molecular Sciences. 2024; 25(9):4581. https://doi.org/10.3390/ijms25094581
Chicago/Turabian StyleMitroshina, Elena V., and Maria V. Vedunova. 2024. "The Role of Oxygen Homeostasis and the HIF-1 Factor in the Development of Neurodegeneration" International Journal of Molecular Sciences 25, no. 9: 4581. https://doi.org/10.3390/ijms25094581
APA StyleMitroshina, E. V., & Vedunova, M. V. (2024). The Role of Oxygen Homeostasis and the HIF-1 Factor in the Development of Neurodegeneration. International Journal of Molecular Sciences, 25(9), 4581. https://doi.org/10.3390/ijms25094581





