Unraveling a Small Secreted Peptide SUBPEP3 That Positively Regulates Salt-Stress Tolerance in Pyrus betulifolia
Abstract
:1. Introduction
2. Results
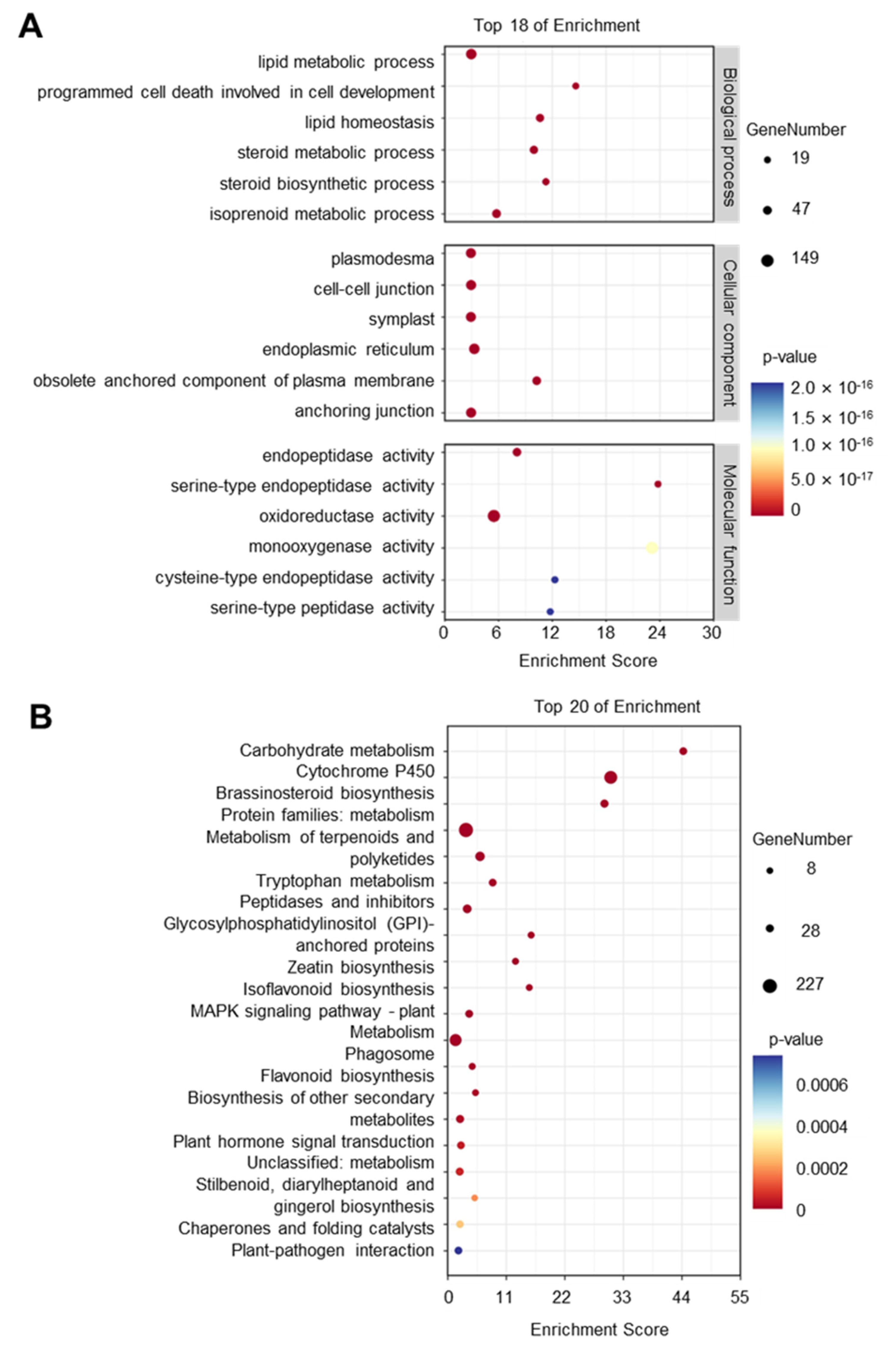
2.1. Analysis of SSP Genes in the Pyrus betulifolia Genome
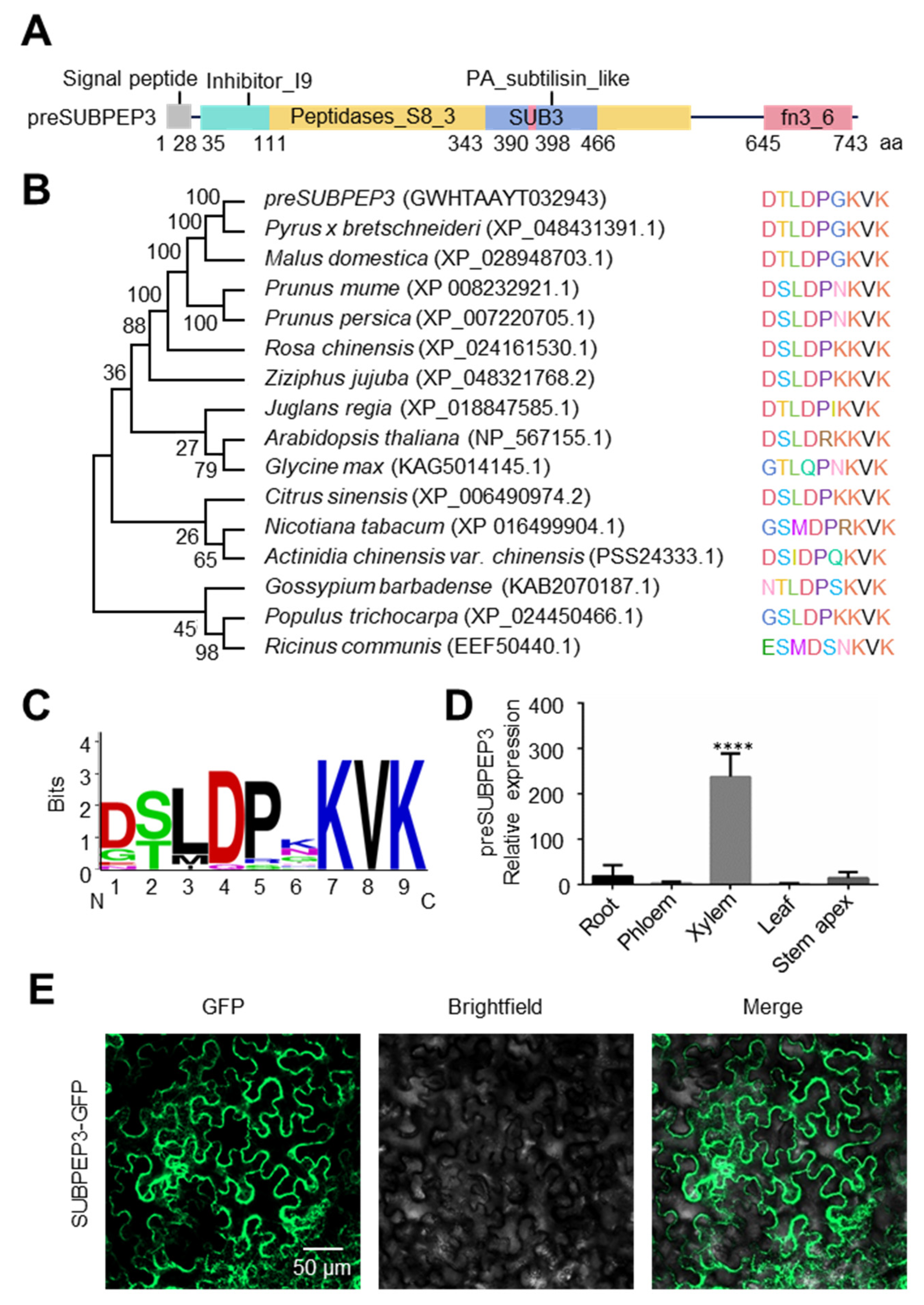
2.2. The Characteristic of SUBPEP3
2.3. preSUBPEP3 Response to Salt Stress
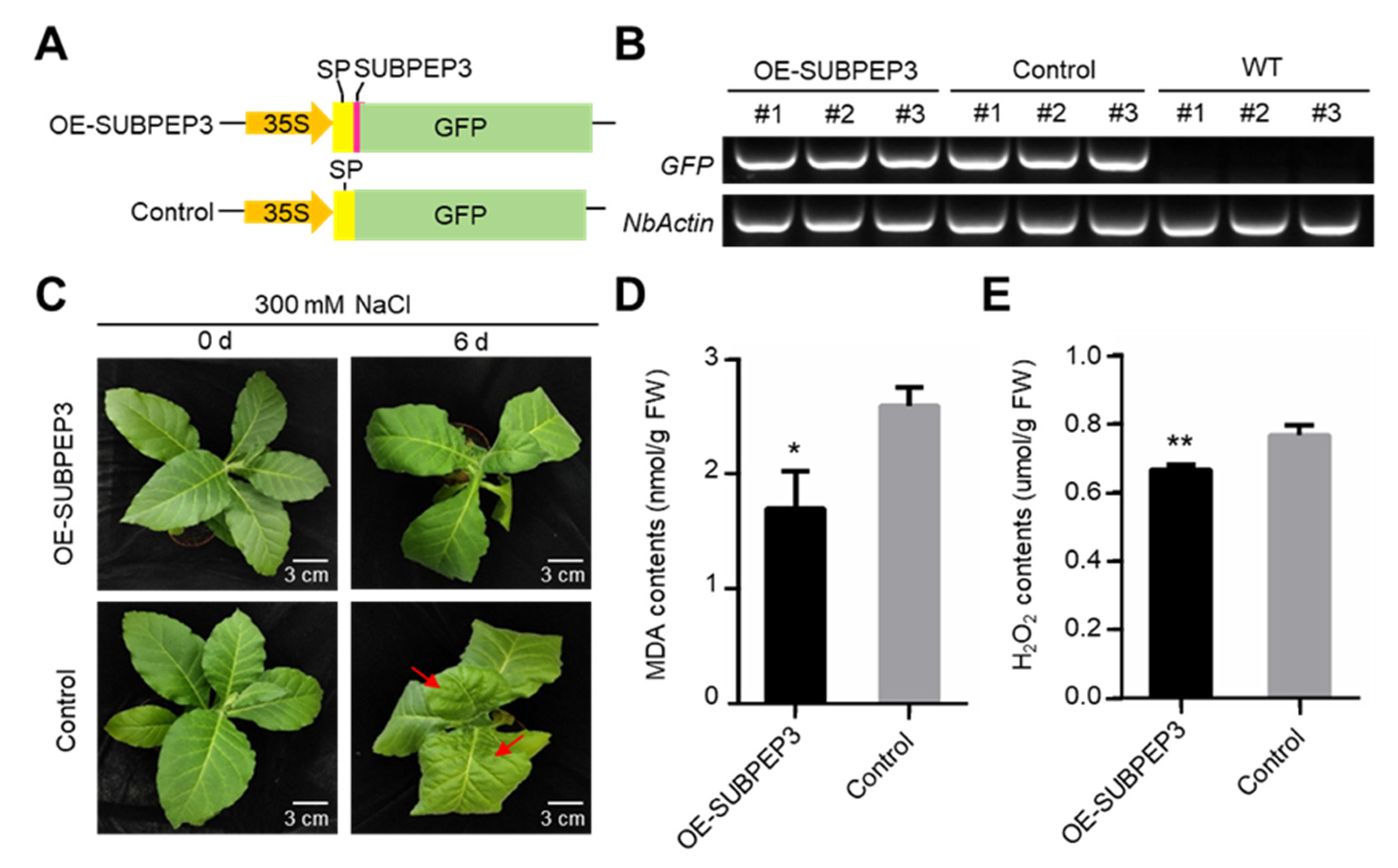
2.4. SUBPEP3 Could Improve the Salt Tolerance of Pears
2.5. Overexpression of SUBPEP3 Enhanced Salt Tolerance
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. SSP Genes Annotation
4.3. GO and KEGG Enrichment Analysis
4.4. Domain Prediction and Phylogenetic Analysis
4.5. RNA Extraction and qRT-PCR
4.6. Peptide Synthesis
4.7. Plasmid Construction
4.8. Transient Expression and Plant Transformation
4.9. Western Blot
4.10. Salt Treatment and Salt Tolerance Assay
4.11. Subcellular Localization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, M.; Augstein, F.; Kareem, A.; Melnyk, C.W. Plant grafting: Molecular mechanisms and applications. Mol. Plant 2024, 17, 75–91. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, T.; Lin, Z.; Gu, B.; Xing, C.; Zhao, L.; Dong, H.; Gao, J.; Xie, Z.; Zhang, S.; et al. A WRKY transcription factor PbrWRKY53 from Pyrus betulaefolia is involved in drought tolerance and AsA accumulation. Plant Biotechnol. J. 2019, 17, 1770–1787. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, T.; Xing, C.; Dong, H.; Qi, K.; Gao, J.; Tao, S.; Wu, J.; Wu, J.; Zhang, S.; et al. The β-amylase PbrBAM3 from pear (Pyrus betulaefolia) regulates soluble sugar accumulation and ROS homeostasis in response to cold stress. Plant Sci. 2019, 287, 110184. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, S.; Zhang, Y.; Xu, C.; Yu, Y.; Xiang, L.; Huang, W.; Tian, B.; Li, T.; Wang, S. Long-distance transport of the pear HMGR1 mRNA via the phloem is associated with enhanced salt tolerance. Plant Sci. 2023, 332, 111705. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yuan, K.; Xing, C.; Qiao, Q.; Chen, Q.; Dong, H.; Qi, K.; Xie, Z.; Chen, X.; Huang, X.; et al. Transcription factor PbbZIP4 is targeted for proteasome-mediated degradation by the ubiquitin ligase PbATL18 to influence pear’s resistance to Colletotrichum fructicola by regulating the expression of PbNPR3. Plant J. 2023, 116, 903–920. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Y.; Jin, Y.; Kan, L.; Shen, C.; Malladi, A.; Nambeesan, S.; Xu, Y.; Dong, C. Transcriptome Analysis of Pyrus betulaefolia Seedling Root Responses to Short-Term Potassium Deficiency. Int. J. Mol. Sci. 2020, 21, 8857. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.Z.; Jia, Q.; Ibrahim, A.K.; Niyitanga, S.; Zhang, L. Mechanisms and Signaling Pathways of Salt Tolerance in Crops: Understanding from the Transgenic Plants. Trop. Plant Biol. 2020, 13, 297–320. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Araya, T.; Kim, D.G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef] [PubMed]
- Melino, V.; Tester, M. Salt-Tolerant Crops: Time to Deliver. Annu. Rev. Plant Biol. 2023, 74, 671–696. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Mohd-Radzman, N.A.; Corcilius, L.; Crossett, B.; Connolly, A.; Cordwell, S.J.; Ivanovici, A.; Taylor, K.; Williams, J.; Binos, S.; et al. Diverse peptide hormones affecting root growth identified in the Medicago truncatula secreted peptidome. Mol. Cell. Proteom. 2018, 17, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Olsson, V.; Joos, L.; Zhu, S.; Gevaert, K.; Butenko, M.A.; De Smet, I. Look Closely, the Beautiful May Be Small: Precursor-Derived Peptides in Plants. Annu. Rev. Plant Biol. 2019, 70, 153–186. [Google Scholar] [CrossRef] [PubMed]
- Tabata, R.; Sumida, K.; Yoshii, T.; Ohyama, K.; Shinohara, H.; Matsubayashi, Y. Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 2014, 346, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tian, L.; Liu, H.; Li, X.; Zhang, J.; Chen, X.; Jia, X.; Zheng, X.; Wu, S.; Chen, Y.; et al. Large-Scale Discovery of Non-conventional Peptides in Maize and Arabidopsis through an Integrated Peptidogenomic Pipeline. Mol. Plant 2020, 13, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Boschiero, C.; Dai, X.; Lundquist, P.K.; Roy, S.; Christian De Bang, T.; Zhang, S.; Zhuang, Z.; Torres-Jerez, I.; Udvardi, M.K.; Scheible, W.; et al. MtSSPdb: The Medicago truncatula Small Secreted Peptide Database. Plant Physiol. 2020, 183, 399–413. [Google Scholar] [CrossRef]
- Ghorbani, S.; Lin, Y.; Parizot, B.; Fernandez, A.; Njo, M.F.; Van de Peer, Y.; Beeckman, T.; Hilson, P. Expanding the repertoire of secretory peptides controlling root development with comparative genome analysis and functional assays. J. Exp. Bot. 2015, 66, 5257–5269. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, Y. Posttranslationally modified small-peptide signals in plants. Annu. Rev. Plant Biol. 2014, 65, 385–413. [Google Scholar] [CrossRef]
- Okamoto, S.; Suzuki, T.; Kawaguchi, M.; Higashiyama, T.; Matsubayashi, Y. A comprehensive strategy for identifying long-distance mobile peptides in xylem sap. Plant J. 2015, 84, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, C.; Yao, J.L.; Xing, C.; Xu, K.; Zhang, Z.; Chen, Q.; Qiao, Q.; Dong, H.; Han, C.; et al. PbHsfC1a-coordinates ABA biosynthesis and H2O2 signalling pathways to improve drought tolerance in Pyrus betulaefolia. Plant Biotechnol. J. 2024, 22, 1177–1197. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, C.; Xing, C.; Yang, T.; Yan, J.; Gao, J.; Li, D.; Wang, R.; Blumwald, E.; Zhang, S.; et al. Overexpression of PbrNHX2 gene, a Na+/H+ antiporter gene isolated from Pyrus betulaefolia, confers enhanced tolerance to salt stress via modulating ROS levels. Plant Sci. 2019, 285, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yao, S.; Kosami, K.I.; Guo, T.; Li, J.; Zhang, Y.; Fukao, Y.; Kaneko Kawano, T.; Zhang, H.; She, Y.M.; et al. Identification of endogenous small peptides involved in rice immunity through transcriptomics- and proteomics-based screening. Plant Biotechnol. J. 2020, 18, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Pearce, G.; Yamaguchi, Y.; Barona, G.; Ryan, C.A. A subtilisin-like protein from soybean contains an embedded, cryptic signal that activates defense-related genes. Proc. Natl. Acad. Sci. USA 2010, 107, 14921–14925. [Google Scholar] [CrossRef] [PubMed]
- Grienenberger, E.; Fletcher, J.C. Polypeptide signaling molecules in plant development. Curr. Opin. Plant Biol. 2015, 23, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.C. Recent Advances in Arabidopsis CLE Peptide Signaling. Trends Plant Sci. 2020, 25, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Taleski, M.; Imin, N.; Djordjevic, M.A. CEP peptide hormones: Key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. J. Exp. Bot. 2018, 69, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Amano, Y.; Tsubouchi, H.; Shinohara, H.; Ogawa, M.; Matsubayashi, Y. Tyrosine-sulfated glycopeptide involved in cellular proliferation and expansion in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 18333–18338. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, S.; Piepho, H.P.; Stintzi, A.; Schaller, A. Peptide signaling for drought-induced tomato flower drop. Science 2020, 367, 1482–1485. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Di, Q.; Luo, L.; Yu, L. Phytosulfokine peptides, their receptors, and functions. Front. Plant Sci. 2024, 14, 1326964. [Google Scholar] [CrossRef] [PubMed]
- Doll, N.M.; Royek, S.; Fujita, S.; Okuda, S.; Chamot, S.; Stintzi, A.; Widiez, T.; Hothorn, M.; Schaller, A.; Geldner, N.; et al. A two-way molecular dialogue between embryo and endosperm is required for seed development. Science 2020, 367, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Shinohara, H.; Tanaka, M.; Baba, K.; Ogawa-Ohnishi, M.; Matsubayashi, Y. A peptide hormone required for Casparian strip diffusion barrier formation in Arabidopsis roots. Science 2017, 355, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Fujita, S.; Moretti, A.; Hohmann, U.; Doblas, V.G.; Ma, Y.; Pfister, A.; Brandt, B.; Geldner, N.; Hothorn, M. Molecular mechanism for the recognition of sequence- divergent CIF peptides by the plant receptor kinases GSO1/SGN3 and GSO2. Proc. Natl. Acad. Sci. USA 2020, 5, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Schardon, K.; Hohl, M.; Graff, L.; Pfannstiel, J.; Schulze, W.; Stintzi, A.; Schaller, A. Precursor processing for plant peptide hormone maturation by subtilisin-like serine proteinases. Science 2016, 354, 1594–1597. [Google Scholar] [CrossRef] [PubMed]
- Lyapina, I.; Filippova, A.; Kovalchuk, S.; Ziganshin, R.; Mamaeva, A.; Lazarev, V.; Latsis, I.; Mikhalchik, E.; Panasenko, O.; Ivanov, O.; et al. Possible role of small secreted peptides (SSPs) in immune signaling in bryophytes. Plant Mol. Biol. 2021, 106, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, Z.; Song, W.; Chai, J. Structural Insight into Recognition of Plant Peptide Hormones by Receptors. Mol. Plant 2016, 9, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, H.; Matsubayashi, Y. Reevaluation of the CLV3-receptor interaction in the shoot apical meristem: Dissection of the CLV3 signaling pathway from a direct ligand-binding point of view. Plant J. 2015, 82, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Liu, L.; Wang, J.; Wu, Z.; Zhang, H.; Tang, J.; Lin, G.; Wang, Y.; Wen, X.; Li, W.; et al. Signature motif-guided identification of receptors for peptide hormones essential for root meristem growth. Cell Res. 2016, 26, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M.; Sabat, G.; Stecker, K.; Minkoff, B.B.; Sussman, M.R. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 2014, 343, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Asami, T. Chemical regulators of plant hormones and their applications in basic research and agriculture. Biosci. Biotechnol. Biochem. 2018, 82, 1265–1300. [Google Scholar] [CrossRef] [PubMed]
- Almagro, A.J.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Muller, J.; Szklarczyk, D.; Julien, P.; Letunic, I.; Roth, A.; Kuhn, M.; Powell, S.; von Mering, C.; Doerks, T.; Jensen, L.J.; et al. eggNOG v2.0: Extending the evolutionary genealogy of genes with enhanced non-supervised orthologous groups, species and functional annotations. Nucleic Acids Res. 2010, 38, D190–D195. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Perrone, I.; Gribaudo, I. A Rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem. Anal. 2008, 19, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Duan, X.; Wang, S.; Hao, L.; Zhang, Y.; Xu, C.; Yu, Y.; Xiang, L.; Jiang, F.; Heinlein, M.; et al. A chaperonin containing T-complex polypeptide-1 facilitates the formation of the PbWoxT1-PbPTB3 ribonucleoprotein complex for long-distance RNA trafficking in Pyrus betulaefolia. New Phytol. 2023, 238, 1115–1128. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Li, W.; Wang, S.; Hao, L.; Xu, C.; Yu, Y.; Xiang, L.; Li, T.; Jiang, F. A long noncoding RNA HILinc1 enhances pear thermotolerance by stabilizing PbHILT1 transcripts through complementary base pairing. Commun. Biol. 2022, 5, 1114–1134. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 1, 271–278. [Google Scholar]
- Golkar, P.; Akbari, R.; Bazarganipour, M.; Javed, R. Biochemical and phytochemical responses of Ammi visnaga L. (Apiaceae) callus culture elicited by SiO2 and graphene Oxide-SiO2 nanoparticles. Plant Physiol. Biochem. 2023, 200, 107741. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Xiang, L.; Huang, W.; Zhang, X.; Mao, C.; Wu, S.; Li, T.; Wang, S.; Wang, S. Unraveling a Small Secreted Peptide SUBPEP3 That Positively Regulates Salt-Stress Tolerance in Pyrus betulifolia. Int. J. Mol. Sci. 2024, 25, 4612. https://doi.org/10.3390/ijms25094612
Xu C, Xiang L, Huang W, Zhang X, Mao C, Wu S, Li T, Wang S, Wang S. Unraveling a Small Secreted Peptide SUBPEP3 That Positively Regulates Salt-Stress Tolerance in Pyrus betulifolia. International Journal of Molecular Sciences. 2024; 25(9):4612. https://doi.org/10.3390/ijms25094612
Chicago/Turabian StyleXu, Chaoran, Ling Xiang, Wenting Huang, Xiao Zhang, Chong Mao, Shuang Wu, Tianzhong Li, Shengyuan Wang, and Shengnan Wang. 2024. "Unraveling a Small Secreted Peptide SUBPEP3 That Positively Regulates Salt-Stress Tolerance in Pyrus betulifolia" International Journal of Molecular Sciences 25, no. 9: 4612. https://doi.org/10.3390/ijms25094612
APA StyleXu, C., Xiang, L., Huang, W., Zhang, X., Mao, C., Wu, S., Li, T., Wang, S., & Wang, S. (2024). Unraveling a Small Secreted Peptide SUBPEP3 That Positively Regulates Salt-Stress Tolerance in Pyrus betulifolia. International Journal of Molecular Sciences, 25(9), 4612. https://doi.org/10.3390/ijms25094612





