Tyrosine Hydroxylase Inhibitors and Dopamine Receptor Agonists Combination Therapy for Parkinson’s Disease
Abstract
1. Parkinson’s Disease (PD)
2. The Pathological Roles of the DA-TH Pathway
2.1. Dopamine Toxicity Mechanisms
2.2. TH Inhibition-Based Strategies
3. Current Therapeutic Strategies
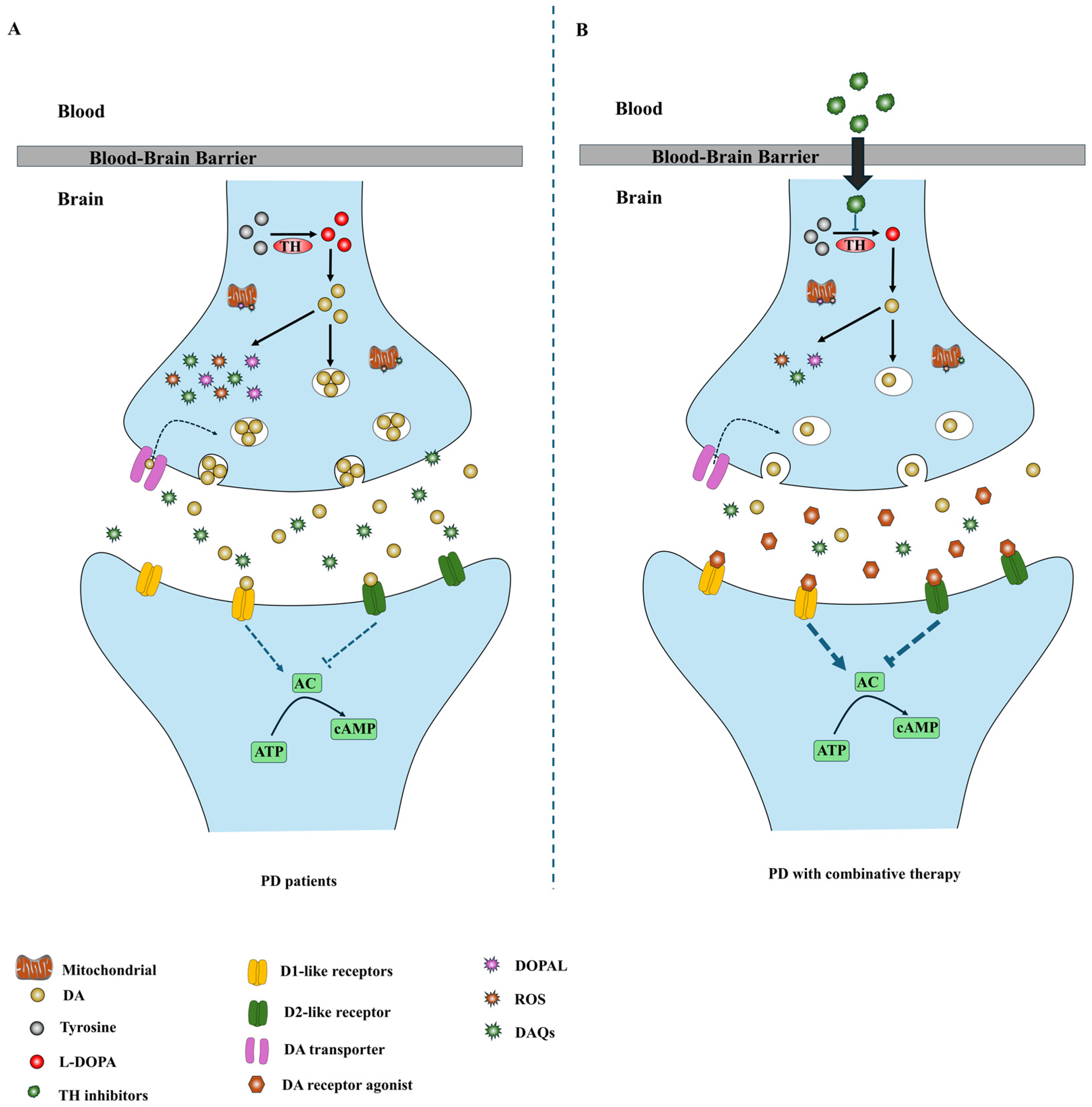
4. Combination Therapeutic Strategies Based on TH Inhibitors
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joseph, J.; Eng King, T. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nature reviews. Neuroscience 2017, 18, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, G.; Tan, K.R. Dopamine and Acetylcholine, a Circuit Point of View in Parkinson’s Disease. Front. Neural Circuits 2017, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. Jama 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Manini, P.; D’Ischia, M. Oxidation Chemistry of Catecholamines and Neuronal Degeneration: An Update. Curr. Med. Chem. 2011, 18, 1832–1845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Yi, L.X.; Wang, D.Q.; Lim, T.M.; Tan, E.K. Role of dopamine in the pathophysiology of Parkinson’s disease. Transl. Neurodegener. 2023, 12, 44. [Google Scholar] [CrossRef]
- German, C.L.; Baladi, M.G.; McFadden, L.M.; Hanson, G.R.; Fleckenstein, A.E. Regulation of the Dopamine and Vesicular Monoamine Transporters: Pharmacological Targets and Implications for Disease. Pharmacol. Rev. 2015, 67, 1005–1024. [Google Scholar] [CrossRef]
- Latif, S.; Jahangeer, M.; Maknoon Razia, D.; Ashiq, M.; Ghaffar, A.; Akram, M.; El Allam, A.; Bouyahya, A.; Garipova, L.; Ali Shariati, M.; et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta 2021, 522, 114–126. [Google Scholar] [CrossRef]
- Salazar, J.; Mena, N.; Núñez, M.T. Iron dyshomeostasis in Parkinson’s disease. J. Neural Transm. Suppl. 2006, 71, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Lan, Y.H.; Tan, E.K.; Lim, T.M. Iron species-mediated dopamine oxidation, proteasome inhibition, and dopaminergic cell demise: Implications for iron-related dopaminergic neuron degeneration. Free Radic. Biol. Med. 2010, 49, 1856–1871. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Mounsey, R.B.; Teismann, P. MPP+-induced toxicity in the presence of dopamine is mediated by COX-2 through oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 384, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhou, W.; Symmes, B.; Freed, C.R. Re-Cloning the N27 Dopamine Cell Line to Improve a Cell Culture Model of Parkinson’s Disease. PLoS ONE 2016, 11, e0160847. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E.; Gross, C.E.; Fournier, M.-C.; Dovero, S.; Bloch, B.; Jaber, M. Absence of MPTP-Induced Neuronal Death in Mice Lacking the Dopamine Transporter. Exp. Neurol. 1999, 155, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Haavik, J.; Le Bourdelles, B.; Martinez, A.; Flatmark, T.; Mallet, J. Recombinant human tyrosine hydroxylase isozymes. Eur. J. Biochem. 1991, 199, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhao, M.; Liu, Z.; Du, F.; Zhou, B. Zinc antagonizes iron-regulation of tyrosine hydroxylase activity and dopamine production in Drosophila melanogaster. BMC Biol. 2021, 19, 236. [Google Scholar] [CrossRef]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.S.; Karunakaran, R.; Rajasekaran, R.; Srikumar, P.S. Alpha-Synuclein Aggregation in Parkinson’s Disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Tamjar, J.; Waddell, A.D.; Woodroof, H.I.; Raimi, O.G.; Shaw, A.M.; Peggie, M.; Muqit, M.M.; van Aalten, D.M. Structure of PINK1 and mechanisms of Parkinson’s disease-associated mutations. eLife 2017, 6, e29985. [Google Scholar] [CrossRef]
- Beavan, M.; Schapira, A. Glucocerebrosidase mutations and the pathogenesis of Parkinson disease. Ann. Med. 2013, 45, 511–521. [Google Scholar] [CrossRef]
- Repici, M.; Giorgini, F. DJ-1 in Parkinson’s Disease: Clinical Insights and Therapeutic Perspectives. J. Clin. Med. 2019, 8, 1377. [Google Scholar] [CrossRef]
- Taymans, J.M. The GTPase function of LRRK2. Biochem. Soc. Trans. 2012, 40, 1063–1069. [Google Scholar] [CrossRef]
- Bisaglia, M.; Greggio, E.; Maric, D.; Miller, D.W.; Cookson, M.R.; Bubacco, L. α-Synuclein overexpression increases dopamine toxicity in BE(2)-M17 cells. BMC Neurosci. 2010, 11, 41–47. [Google Scholar] [CrossRef]
- Follmer, C.; Coelho-Cerqueira, E.; Yatabe-Franco, D.Y.; Araujo, G.D.; Pinheiro, A.S.; Domont, G.B.; Eliezer, D. Oligomerization and Membrane-binding Properties of Covalent Adducts Formed by the Interaction of α-Synuclein with the Toxic Dopamine Metabolite 3,4-Dihydroxyphenylacetaldehyde (DOPAL). J. Biol. Chem. 2015, 290, 27660–27679. [Google Scholar] [CrossRef]
- Masato, A.; Plotegher, N.; Terrin, F.; Sandre, M.; Faustini, G.; Thor, A.; Adams, S.; Berti, G.; Cogo, S.; De Lazzari, F.; et al. DOPAL initiates αSynuclein-dependent impaired proteostasis and degeneration of neuronal projections in Parkinson’s disease. Npj Parkinsons Dis. 2023, 9, 42. [Google Scholar] [CrossRef]
- Conway, K.A.; Rochet, J.-C.; Bieganski, R.M.; Lansbury, P.T. Kinetic stabilization of the alpha -synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 2001, 294, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Mor, D.E.; Tsika, E.; Mazzulli, J.R.; Gould, N.S.; Kim, H.; Daniels, M.J.; Doshi, S.; Gupta, P.; Grossman, J.L.; Tan, V.X.; et al. Dopamine induces soluble α-synuclein oligomers and nigrostriatal degeneration. Nat. Neurosci. 2017, 20, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Bisaglia, M.; Tosatto, L.; Munari, F.; Tessari, I.; de Laureto, P.P.; Mammi, S.; Bubacco, L. Dopamine quinones interact with α-synuclein to form unstructured adducts. Biochem. Biophys. Res. Commun. 2010, 394, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, M.J.; Ostaszewski, B.L.; Weihofen, A.; Schlossmacher, M.G.; Selkoe, D.J. Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 2005, 11, 1214–1221. [Google Scholar] [CrossRef]
- Tokarew, J.M.; El-Kodsi, D.N.; Lengacher, N.A.; Fehr, T.K.; Nguyen, A.P.; Shutinoski, B.; O’nuallain, B.; Jin, M.; Khan, J.M.; Ng, A.C.H.; et al. Age-associated insolubility of parkin in human midbrain is linked to redox balance and sequestration of reactive dopamine metabolites. Acta Neuropathol. 2021, 141, 725–754. [Google Scholar] [CrossRef]
- Lonskaya, I.; Hebron, M.; Algarzae, N.; Desforges, N.; Moussa, C.-H. Decreased parkin solubility is associated with impairment of autophagy in the nigrostriatum of sporadic Parkinson’s disease. Neuroscience 2013, 232, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, Oxidative Stress and Protein–Quinone Modifications in Parkinson’s and Other Neurodegenerative Diseases. Angew. Chem. Int. Ed. Engl. 2019, 58, 6512–6527. [Google Scholar] [CrossRef] [PubMed]
- Girotto, S.; Sturlese, M.; Bellanda, M.; Tessari, I.; Cappellini, R.; Bisaglia, M.; Bubacco, L.; Mammi, S. Dopamine-derived Quinones Affect the Structure of the Redox Sensor DJ-1 through Modifications at Cys-106 and Cys-53. J. Biol. Chem. 2012, 287, 18738–18749. [Google Scholar] [CrossRef] [PubMed]
- Canet-Avilés, R.M.; Wilson, M.A.; Miller, D.W.; Ahmad, R.; McLendon, C.; Bandyopadhyay, S.; Baptista, M.J.; Ringe, D.; Petsko, G.A.; Cookson, M.R. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA 2004, 101, 9103–9108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Saw, W.T.; Ho, P.G.H.; Zhang, Z.W.; Zeng, L.; Chang, Y.Y.; Sun, A.X.Y.; Ma, D.R.; Wang, H.Y.; Zhou, L.; et al. The role of tyrosine hydroxylase–dopamine pathway in Parkinson’s disease pathogenesis. Cell Mol. Life Sci. 2022, 79, 599. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Carrasco, M.T.; Cuéllar, J.; Flydal, M.I.; Santiago, C.; Kråkenes, T.-A.; Kleppe, R.; López-Blanco, J.R.; Marcilla, M.; Teigen, K.; Alvira, S.; et al. Structural mechanism for tyrosine hydroxylase inhibition by dopamine and reactivation by Ser40 phosphorylation. Nat. Commun. 2022, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F.; Waymire, J.C.; Haycock, J.W. Depolarization-stimulated catecholamine biosynthesis: Involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J. Neurochem. 2001, 79, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, L.M.; Sullivan, P.; Dunn, A.R.; Bermejo, M.K.; Fu, R.; Masoud, S.T.; Gregersen, E.; Urs, N.M.; Nazari, R.; Jensen, P.H.; et al. Enhanced tyrosine hydroxylase activity induces oxidative stress, causes accumulation of autotoxic catecholamine metabolites, and augments amphetamine effects in vivo. J. Neurochem. 2021, 158, 960–979. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Refai, F.S.; Xie, S.P.; Ng, S.H.; Chan, C.H.S.; Ho, P.G.H.; Zhang, X.D.; Lim, T.M.; Tan, E.K. Mutant PINK1 upregulates tyrosine hydroxylase and dopamine levels, leading to vulnerability of dopaminergic neurons. Free Radic. Biol. Med. 2014, 68, 220–233. [Google Scholar] [CrossRef]
- Bayersdorfer, F.; Voigt, A.; Schneuwly, S.; Botella, J.A. Dopamine-dependent neurodegeneration in Drosophila models of familial and sporadic Parkinson’s disease. Neurobiol. Dis. 2010, 40, 113–119. [Google Scholar] [CrossRef]
- Lenartowski, R.; Goc, A. Epigenetic, transcriptional and posttranscriptional regulation of the tyrosine hydroxylase gene. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2011, 29, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.E.; Seo, J.S.; Yang, J.W.; Kim, M.W.; Kausar, R.; Joe, E.; Kim, B.Y.; Lee, M.A. Nurr1 Represses Tyrosine Hydroxylase Expression via SIRT1 in Human Neural Stem Cells. PLoS ONE 2013, 8, e71469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reddy, S.D.; Rayala, S.K.; Ohshiro, K.; Pakala, S.B.; Kobori, N.; Dash, P.; Yun, S.; Qin, J.; O’Malley, B.W.; Kumar, R. Multiple coregulatory control of tyrosine hydroxylase gene transcription. Proc. Natl. Acad. Sci. USA 2011, 108, 4200–4205. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Wang, M.; Cai, E.; Fujiwara, N.; Baker, H.; Cave, J.W. Regulation of tyrosine hydroxylase transcription by hnRNP K and DNA secondary structure. Nat. Commun. 2014, 5, 5769. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F.; Calipari, E.S.; Jones, S.R. Regulation of Tyrosine Hydroxylase Expression and Phosphorylation in Dopamine Transporter-Deficient Mice. ACS Chem. Neurosci. 2016, 7, 941–951. [Google Scholar] [CrossRef]
- Akahoshi, E.; Yoshimura, S.; Uruno, S.; Ishihara-Sugano, M. Effect of dioxins on regulation of tyrosine hydroxylase gene expression by aryl hydrocarbon receptor: A neurotoxicology study. Environ. Health A Glob. Access Sci. Source 2009, 8, 24. [Google Scholar] [CrossRef]
- Mashayekhi, F.J.; Rasti, M.; Khoshdel, Z.; Owji, A.A. Expression Levels of the Tyrosine Hydroxylase Gene and Histone Modifications Around its Promoter in the Locus Coeruleus and Ventral Tegmental Area of Rats during Forced Abstinence from Morphine. Eur. Addict. Res. 2018, 24, 304–311. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Yap, B.P.; Gung, A.Y.; Leong, S.M.; Ang, S.T.; Lim, T.M. Dopamine-related and caspase-independent apoptosis in dopaminergic neurons induced by overexpression of human wild type or mutant α-synuclein. Exp. Cell Res. 2006, 312, 156–170. [Google Scholar] [CrossRef]
- Zhou, Z.; Kerk, S.; Lim, T.M. Endogenous dopamine (DA) renders dopaminergic cells vulnerable to challenge of proteasome inhibitor MG132. Free Radic. Res. 2008, 42, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Ankenman, R.; Salvatore, M.F. Low dose alpha-methyl-para-tyrosine (AMPT) in the treatment of dystonia and dyskinesia. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 65–69. [Google Scholar] [CrossRef]
- Naruse, M.; Satoh, F.; Tanabe, A.; Okamoto, T.; Ichihara, A.; Tsuiki, M.; Katabami, T.; Nomura, M.; Tanaka, T.; Matsuda, T.; et al. Efficacy and safety of metyrosine in pheochromocytoma/paraganglioma: A multi-center trial in Japan. Endocr. J. 2018, 65, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. alpha-Methyl-p-tyrosine: A review of its pharmacology and clinical use. Drugs 1981, 21, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Fahn, S.; Mayeux, R.; Weinberg, H.; Louis, K.; Willner, J.H. Neuroleptic Malignant syndrome caused by dopamine-depleting drugs in a patient with Huntington disease. Neurology 1981, 31, 1022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Kerk, S.Y.; Xiong, G.G.; Lim, T.M. Dopamine auto-oxidation aggravates non-apoptotic cell death induced by over-expression of human A53T mutant alpha-synuclein in dopaminergic PC12 cells. J. Neurochem. 2009, 108, 601–610. [Google Scholar] [CrossRef]
- Fahn, S. Treatment of Tardive Dyskinesia: Use of Dopamine-Depleting Agents. Clin. Neuropharmacol. 1983, 6, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Marsden, C.D. Alphamethylparatyrosine and Tetrabenazine in Movement Disorders. Clin. Neuropharmacol. 1982, 5, 375–388. [Google Scholar] [CrossRef]
- Wålinder, J.; Skott, A.; Carlsson, A.; Roos, B.-E. Potentiation by Metyrosine of Thioridazine Effects in Chronic Schizophrenics: A Long-Term Trial Using Double-Blind Crossover Technique. Arch. Gen. Psychiatry 1976, 33, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Hrachovy, R.A.; Frost, J.D.; Glaze, D.G.; Rose, D. Treatment of Infantile Spasms with Methysergide and α-Methylparatyrosine. Epilepsia 1989, 30, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, U.; Del Dotto, P.; Rascol, O. Role of dopamine receptor agonists in the treatment of early Parkinson’s disease. Park. Relat. Disord. 2009, 15, S44–S53. [Google Scholar] [CrossRef]
- Obeso, J.A.; Rodríguez-Oroz, M.C.; Benitez-Temino, B.; Blesa, F.J.; Guridi, J.; Marin, C.; Rodriguez, M. Functional organization of the basal ganglia: Therapeutic implications for Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, S548–S559. [Google Scholar] [CrossRef]
- Mao, Q.; Qin, W.-Z.; Zhang, A.; Ye, N. Recent advances in dopaminergic strategies for the treatment of Parkinson’s disease. Acta Pharmacol. Sin. 2020, 41, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Ledonne, A.; Mercuri, N.B. Current Concepts on the Physiopathological Relevance of Dopaminergic Receptors. Front. Cell. Neurosci. 2017, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Agid, Y.; Mizuno, Y.; Albanese, A.; Bonuccelli, U.; Damier, P.; De Yebenes, J.; Gershanik, O.; Guttman, M.; Grandas, F.; et al. Levodopa in the treatment of Parkinson’s disease: Current controversies. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Lester, D.B.; Rogers, T.D.; Blaha, C.D. Acetylcholine–dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 2010, 16, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 2649–2653. [Google Scholar] [CrossRef] [PubMed]
- Bargiotas, P.; Konitsiotis, S. Levodopa-induced dyskinesias in Parkinson’s disease: Emerging treatments. Neuropsychiatr. Dis. Treat. 2013, 9, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Cedarbaum, J.M.; Kutt, H.; Dhar, A.K.; Watkins, S.; McDowell, F.H. Effect of Supplemental Carbidopa on Bioavailability of l-Dopa. Clin. Neuropharmacol. 1986, 9, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Rinne, U.K.; Birket-Smith, E.; Dupont, E.; Hansen, E.; Hyyppä, M.; Marttila, R.; Mikkelsen, B.; Pakkenberg, H.; Presthus, J. Levodopa alone and in combination with a peripheral decarboxylase inhibitor benserazide (madopar®) in the treatment of Parkinson’s disease. J. Neurol. 1975, 211, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Rocha, J.-F.; Ferreira, J.J.; Rascol, O.; Soares-da-Silva, P. Redefining the strategy for the use of COMT inhibitors in Parkinson’s disease: The role of opicapone. Expert Rev. Neurother. 2021, 21, 1019–1033. [Google Scholar] [CrossRef]
- Jing, X.-Z.; Yang, H.-J.; Taximaimaiti, R.; Wang, X.-P. Advances in the Therapeutic Use of Non-Ergot Dopamine Agonists in the Treatment of Motor and Non-Motor Symptoms of Parkinson’s Disease. Curr. Neuropharmacol. 2023, 21, 1224–1240. [Google Scholar] [CrossRef]
- Maharaj, H.; Maharaj, D.S.; Scheepers, M.; Mokokong, R.; Daya, S. l-DOPA administration enhances 6-hydroxydopamine generation. Brain Res. 2005, 1063, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Melamed, E.; Offen, D.; Shirvan, A.; Djaldetti, R.; Barzilai, A.; Ziv, I. Levodopa Toxicity and Apoptosis. Ann. Neurol. 1998, 44 (Suppl. S1), S149–S154. [Google Scholar] [CrossRef]
- Stansley, B.J.; Yamamoto, B.K. l-dopa-induced dopamine synthesis and oxidative stress in serotonergic cells. Neuropharmacology 2013, 67, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Muddapu, V.R.-J.; Vijayakumar, K.; Ramakrishnan, K.; Chakravarthy, V.S. A Multi-Scale Computational Model of Levodopa-Induced Toxicity in Parkinson’s Disease. Front. Neurosci. 2022, 16, 797127. [Google Scholar] [CrossRef]
- Niall, Q. Fortnightly Review: Drug treatment of Parkinson’s disease. BMJ 1995, 310, 575. [Google Scholar] [CrossRef]
- Young, D.; Popiolek, M.; Trapa, P.; Fonseca, K.R.; Brevard, J.; Gray, D.L.; Kozak, R. D1 Agonist Improved Movement of Parkinsonian Nonhuman Primates with Limited Dyskinesia Side Effects. ACS Chem. Neurosci. 2020, 11, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Gurrell, R.; Duvvuri, S.; Sun, P.; DeMartinis, N. A Phase I Study of the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Novel Dopamine D1 Receptor Partial Agonist, PF-06669571, in Subjects with Idiopathic Parkinson’s Disease. Clin. Drug Investig. 2018, 38, 509–517. [Google Scholar] [CrossRef]
- Papapetropoulos, S.; Liu, W.; Duvvuri, S.; Thayer, K.; Gray, D.L. Evaluation of D1/D5 Partial Agonist PF-06412562 in Parkinson’s Disease following Oral Administration. Neurodegener. Dis. 2018, 18, 262–269. [Google Scholar] [CrossRef]
- Neusch, C.; Böhme, V.; Riesland, N.; Althaus, M.; Moser, A. The dopamine D2 receptor agonist alpha-dihydroergocryptine modulates voltage-gated sodium channels in the rat caudate-putamen. J. Neural Transm. 2000, 107, 531–541. [Google Scholar] [CrossRef]
- Borovac, J.A. Side effects of a dopamine agonist therapy for Parkinson’s disease: A mini-review of clinical pharmacology. Yale J. Biol. Med. 2016, 89, 37–47. [Google Scholar]
- Montastruc, J.L.; Rascol, O.; Senard, J.M.; Rascol, A. A randomised controlled study comparing bromocriptine to which levodopa was later added, with levodopa alone in previously untreated patients with Parkinson’s disease: A five year follow up. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1034. [Google Scholar] [CrossRef]
- Laihinen, A.; Rinne, U.; Suchy, I.J.A. Comparison of lisuride and bromocriptine in the treatment of advanced Parkinson’s disease. Acta Neurol. Scand. 1992, 86, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Del Dotto, P.; Colzi, A.; Musatti, E.; Strolin Benedetti, M.; Persiani, S.; Fariello, R.; Bonuccelli, U. Clinical and pharmacokinetic evaluation of L-dopa and cabergoline cotreatment in Parkinson’s disease. Clin. Neuropharmacol. 1997, 20, 455–465. [Google Scholar] [CrossRef]
- Antonini, A.; Poewe, W. Fibrotic heart-valve reactions to dopamine-agonist treatment in Parkinson’s disease. Lancet Neurol. 2007, 6, 826–829. [Google Scholar] [CrossRef]
- Müller, T.; Fritze, J. Fibrosis associated with dopamine agonist therapy in Parkinson’s disease. Clin. Neuropharmacol. 2003, 26, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Rascol, O.; Dubois, B.; Caldas, A.C.; Senn, S.; Del Signore, S.; Lees, A. Early piribedil monotherapy of Parkinson’s disease: A planned seven-month report of the REGAIN study. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 2110–2115. [Google Scholar] [CrossRef]
- Ziegler, M.; Castro-Caldas, A.; Del Signore, S.; Rascol, O. Efficacy of piribedil as early combination to levodopa in patients with stable Parkinson’s disease: A 6-month, randomized, placebo-controlled study. Mov. Disord. Off. J. Mov. Disord. Soc. 2003, 18, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Castro-Caldas, A.; Delwaide, P.; Jost, W.; Merello, M.; Williams, A.; Lamberti, P.; Aguilar, M.; Del Signore, S.; Cesaro, P. The Parkinson–Control study: A 1-year randomized, double-blind trial comparing piribedil (150 mg/day) with bromocriptine (25 mg/day) in early combination with levodopa in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 500–509. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A.; Lyons, K.E.; Pahwa, R. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology 2007, 68, 1262–1267. [Google Scholar] [CrossRef]
- Lau, Y.H.; Leta, V.; Rukavina, K.; Parry, M.; Natividad, J.A.; Metta, V.; Chung-Faye, G.; Chaudhuri, K.R. Tolerability of overnight rotigotine transdermal patch combined with intrajejunal levodopa infusion at 1 year: A 24-h treatment option in Parkinson’s disease. J. Neural Transm. 2022, 129, 889–894. [Google Scholar] [CrossRef]
- Weiner, W.J.; Factor, S.A.; Jankovic, J.; Hauser, R.A.; Tetrud, J.W.; Waters, C.H.; Shulman, L.M.; Glassman, P.M.; Beck, B.; Paume, D.; et al. The long-term safety and efficacy of pramipexole in advanced Parkinson’s disease. Park. Relat. Disord. 2001, 7, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Stocchi, F.; Hersh, B.P.; Scott, B.L.; Nausieda, P.A.; Giorgi, L. Ropinirole 24-hour prolonged release and ropinirole immediate release in early Parkinson’s disease: A randomized, double-blind, non-inferiority crossover study. Curr. Med. Res. Opin. 2008, 24, 2883–2895. [Google Scholar] [CrossRef] [PubMed]
- Stocchi, F.; Giorgi, L.; Hunter, B.; Schapira, A.H. PREPARED: Comparison of prolonged and immediate release ropinirole in advanced Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, A.; Olanow, C.W.; Sethi, K.; Swanson, P.; Waters, C.H.; Fahn, S.; Hurtig, H.; Yahr, M. A multicenter trial of ropinirole as adjunct treatment for Parkinson’s disease. Ropinirole Study Group. Neurology 1998, 51, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Rascol, O.; Lees, A.J.; Senard, J.M.; Pirtosek, Z.; Montastruc, J.L.; Fuell, D. Ropinirole in the treatment of levodopa-induced motor fluctuations in patients with Parkinson’s disease. Clin. Neuropharmacol. 1996, 19, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.R.; Moro, A.; Munhoz, R.P.; Teive, H.A.G.; Lees, A.J. Apomorphine in the treatment of Parkinson’s disease: A review. Arq. De Neuro-Psiquiatr. 2018, 76, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.; Ondo, W. Role of Apomorphine in the Treatment of Parkinson’s Disease. CNS Drugs 2015, 29, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lloret, S.; Rascol, O. Piribedil for the Treatment of Motor and Non-motor Symptoms of Parkinson Disease. CNS Drugs 2016, 30, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Shang, H.F.; Hu, X.; Chen, S.; Zhao, Z.; Du, X.; Surmann, E.; Bauer, L.; Asgharnejad, M. Rotigotine transdermal patch in Chinese patients with early Parkinson’s disease: A randomized, double-blind, placebo-controlled pivotal study. Park. Relat. Disord. 2016, 28, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-X.; Liu, C.-F.; Tao, E.-X.; Shao, M.; Liu, Y.-M.; Wang, J.; Asgharnejad, M.; Xue, H.-B.; Surmann, E.; Bauer, L. Rotigotine transdermal patch in Chinese patients with advanced Parkinson’s disease: A randomized, double-blind, placebo-controlled pivotal study. Park. Relat. Disord. 2017, 44, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, M.; Mizuno, Y.; Kondo, T.; Hasegawa, K.; Murata, M.; Takeuchi, M.; Ikeda, J.; Tomida, T.; Hattori, N. Transdermal rotigotine in advanced Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. J. Neurol. 2014, 261, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Hubble, J.P.; Koller, W.C.; Cutler, N.R.; Sramek, J.J.; Friedman, J.; Goetz, C.; Ranhosky, A.; Korts, D.; Elvin, A. Pramipexole in patients with early Parkinson’s disease. Clin. Neuropharmacol. 1995, 18, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hong, W.; Yang, Z.; Ding, J.; Ren, Y. Efficacy of pramipexole combined with levodopa for Parkinson’s disease treatment and their effects on QOL and serum TNF-α levels. J. Int. Med. Res. 2020, 48, 300060520922449. [Google Scholar] [CrossRef]
- Kujawa, K.; Leurgans, S.; Raman, R.; Blasucci, L.; Goetz, C. Acute Orthostatic Hypotension When Starting Dopamine Agonists in Parkinson’s Disease. Arch. Neurol. 2000, 57, 1461–1463. [Google Scholar] [CrossRef] [PubMed]
- Dušek, P.; Bušková, J.; Růžička, E.; Majerová, V.; Srp, A.; Jech, R.; Roth, J.; Šonka, K. Effects of ropinirole prolonged-release on sleep disturbances and daytime sleepiness in Parkinson disease. Clin. Neuropharmacol. 2010, 33, 186–190. [Google Scholar] [CrossRef]
- Dewey, J.R.B.; Hutton, J.T.; LeWitt, P.A.; Factor, S.A. A Randomized, Double-blind, Placebo-Controlled Trial of Subcutaneously Injected Apomorphine for Parkinsonian Off-State Events. Arch. Neurol. 2001, 58, 1385–1392. [Google Scholar] [CrossRef]
- Katzenschlager, R.; Poewe, W.; Rascol, O.; Trenkwalder, C.; Deuschl, G.; Chaudhuri, K.R.; Henriksen, T.; van Laar, T.; Spivey, K.; Vel, S.; et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): A multicentre, double-blind, randomised, placebo-controlled trial. The Lancet. Neurology 2018, 17, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Gudelsky, G.A.; Thornburg, J.E.; Moore, K.E. Blockade of α-methyltyrosine-induced supersensitivity to apomorphine by chronic administration of L-DOPA. Life Sci. 1975, 16, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.K.; Mark, S.S.; Richard, D.P. Dopamine-Deficient Mice Are Hypersensitive to Dopamine Receptor Agonists. The J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 4405. [Google Scholar] [CrossRef]
- Engelman, K.; Horwitz, D.; Jéquier, E.; Sjoerdsma, A. Biochemical and pharmacologic effects of α-methyltyrosine in man. J. Clin. Investig. 1968, 47, 577–594. [Google Scholar] [CrossRef]
- Schoenberger, J.A. Drug-Induced Orthostatic Hypotension. Drug Saf. 1991, 6, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Kujacic, M.; Svensson, K.; Löfberg, L.; Carlsson, A. Acute changes in dopamine levels in rat adrenal glands after administration of dopamine receptor agonists and antagonists. Eur. J. Pharmacol. 1990, 177, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Gianutsos, G.; Moore, K.E. Differential behavioral and biochemical effects of four dopaminergic agonists. Psychopharmacology 1980, 68, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mierau, J.; Schingnitz, G. Biochemical and pharmacological studies on pramipexole, a potent and selective dopamine D2 receptor agonist. Eur. J. Pharmacol. 1992, 215, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hindle, J.V. Ageing, neurodegeneration and Parkinson’s disease. Age Ageing 2010, 39, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Sechi, G.; Deledda, M.G.; Bua, G.; Satta, W.M.; Deiana, G.A.; Pes, G.M.; Rosati, G. Reduced intravenous glutathione in the treatment of early Parkinson’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1996, 20, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Banaclocha, M.A. N-acetyl-cysteine in the treatment of Parkinson’s disease. What are we waiting for? Med. Hypotheses 2012, 79, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Sepehrmanesh, Z.; Heidary, M.; Akasheh, N.; Akbari, H.; Heidary, M. Therapeutic effect of adjunctive N-acetyl cysteine (NAC) on symptoms of chronic schizophrenia: A double-blind, randomized clinical trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Farokhnia, M.; Azarkolah, A.; Adinehfar, F.; Khodaie-Ardakani, M.R.; Hosseini, S.M.; Yekehtaz, H.; Tabrizi, M.; Rezaei, F.; Salehi, B.; Sadeghi, S.M.; et al. N-acetylcysteine as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia: A randomized, double-blind, placebo-controlled study. Clin. Neuropharmacol. 2013, 36, 185–192. [Google Scholar] [CrossRef]
- Berman, A.E.; Chan, W.Y.; Brennan, A.M.; Reyes, R.C.; Adler, B.L.; Suh, S.W.; Kauppinen, T.M.; Edling, Y.; Swanson, R.A. N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1−/− mouse. Ann. Neurol. 2011, 69, 509–520. [Google Scholar] [CrossRef]
- Clark, J.; Clore, E.L.; Zheng, K.; Adame, A.; Masliah, E.; Simon, D.K. Oral N-acetyl-cysteine attenuates loss of dopaminergic terminals in α-synuclein overexpressing mice. PLoS ONE 2010, 5, e12333. [Google Scholar] [CrossRef] [PubMed]
- Holmay, M.J.; Terpstra, M.; Coles, L.D.; Mishra, U.; Ahlskog, M.; Öz, G.; Cloyd, J.C.; Tuite, P.J. N-Acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin. Neuropharmacol. 2013, 36, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.W.; Wintering, N.A.; Cai, J.; Wei, X.; Bazzan, A.J.; Zhong, L.; Bowen, B.; et al. N-Acetyl Cysteine May Support Dopamine Neurons in Parkinson’s Disease: Preliminary Clinical and Cell Line Data. PLoS ONE 2016, 11, e0157602. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.-W.; Wintering, N.A.; Bazzan, A.J.; Zhong, L.; Bowens, B.K.; Chervoneva, I.; Intenzo, C.; et al. N-Acetyl Cysteine Is Associated with Dopaminergic Improvement in Parkinson’s Disease. Clin. Pharmacol. Ther. 2019, 106, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Albin, R.L.; Young, A.B.; Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989, 12, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Carlos Giugni, J.; Rodriguez-Cruz, R.L. Anticholinergic agents in the management of Parkinson’s disease. In Parkinson’s Disease: Current and Future Therapeutics and Clinical Trials; Espay, A.J., Fernandez, H.H., Fox, S.H., Gálvez-Jiménez, N., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 5–12. [Google Scholar]
- Nishtala, P.S.; Salahudeen, M.S.; Hilmer, S.N. Anticholinergics: Theoretical and clinical overview. Expert Opin. Drug Saf. 2016, 15, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Sansone, M.; Hano, J. Enhancement by chlordiazepoxide of the anticholinergic-induced locomotor stimulation in mice. Psychopharmacology 1979, 64, 181–184. [Google Scholar] [CrossRef]
- Thornburg, J.; Moore, K. Inhibition of anticholinergic drug-induced locomotor stimulation in mice by α-methyltyrosine. Neuropharmacology 1973, 12, 1179–1185. [Google Scholar] [CrossRef]

| Disease | Models | Clinical Outcomes | Reference |
|---|---|---|---|
| PD | Dopaminergic cell models | Ameliorates overexpression of α-syn induced neurotoxicity | [48] |
| PD | Dopaminergic cell models | Ameliorates SNCA mutant-induced neurotoxicity | [54] |
| PD | Dopaminergic cell models | Ameliorates PINK1 mutant-induced neurotoxicity | [39] |
| PD | Dopaminergic cell models | Ameliorates proteasome inhibitor-induced neurotoxicity | [49] |
| PD | Transgenic Drosophila model | Ameliorate LRRK2 mutant-induced neurodegeneration and extent of lifespan | [35] |
| Dystonia | Human patients | Well-tolerated and attenuates hallucinations and painful dystonia | [50] |
| Dystonia | Human patients | Well-tolerated and improves physical signs of tardive dystonia | [50] |
| Dyskinesia | Human patients | Well-tolerated and improves physical signs of tardive dyskinesia | [50,55] |
| Pheochromocytoma | Human patients | Well-tolerated and relieves symptoms of Pheochromocytoma | [51] |
| Huntington’s disease | Human patients | Improves movement symptoms | [56] |
| Schizophrenics | Human patients | Well-tolerated and potentiates the therapeutic effects of antipsychotic medications | [57] |
| Infantile Spasms | Human patients | Relieves physical symptoms | [58] |
| Name | Formula | Structure | Specificity | Clinical Use | Side Effects | References |
|---|---|---|---|---|---|---|
| Piribedil | C16H18N4O2 |  | D2-like receptor | Monotherapy or adjunct drug to L-DOPA therapy in early PD patients without motor fluctuations | Nausea, vomiting, confusion, agitation, dizziness, hypotension, orthostatic | [86,87,88,98] |
| Rotigotine | C19H25NOS |  | D1-like and D2-like receptors | Monotherapy or adjunct drugs to L-DOPA therapy in both early and advanced PD patients | Nausea, application site reactions, dizziness, insomnia, somnolence, vomiting, fatigue and orthostatic hypotension | [89,90,99,100,101] |
| Pramipexole | C10H17N3S |  | D2-like receptors | Monotherapy or adjunct drugs to L-DOPA therapy in both early and advanced PD patients | Sleep attack, nausea, somnolence, fatigue and orthostatic hypotension | [91,102,103,104] |
| Ropinirole | C16H24N2O |  | D2-like receptors | Monotherapy or adjunct drugs to L-DOPA therapy in both early and advanced PD patients | Orthostatic hypotension, dizziness, nausea, somnolence, sleep attacks | [92,93,94,95,105] |
| Apomorphine | C17H17NO2 |  | D1-like and D2-like receptors | Advanced PD patients who suffer from drug-resistant OFF time | Yawning, headache, drowsiness, nausea, dizziness, postural instability, injection site reactions | [105,106,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, L.X.; Tan, E.K.; Zhou, Z.D. Tyrosine Hydroxylase Inhibitors and Dopamine Receptor Agonists Combination Therapy for Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 4643. https://doi.org/10.3390/ijms25094643
Yi LX, Tan EK, Zhou ZD. Tyrosine Hydroxylase Inhibitors and Dopamine Receptor Agonists Combination Therapy for Parkinson’s Disease. International Journal of Molecular Sciences. 2024; 25(9):4643. https://doi.org/10.3390/ijms25094643
Chicago/Turabian StyleYi, Ling Xiao, Eng King Tan, and Zhi Dong Zhou. 2024. "Tyrosine Hydroxylase Inhibitors and Dopamine Receptor Agonists Combination Therapy for Parkinson’s Disease" International Journal of Molecular Sciences 25, no. 9: 4643. https://doi.org/10.3390/ijms25094643
APA StyleYi, L. X., Tan, E. K., & Zhou, Z. D. (2024). Tyrosine Hydroxylase Inhibitors and Dopamine Receptor Agonists Combination Therapy for Parkinson’s Disease. International Journal of Molecular Sciences, 25(9), 4643. https://doi.org/10.3390/ijms25094643






