Abstract
In the absence of naturally available galactofuranose-specific lectin, we report herein the bioengineering of GalfNeoLect, from the first cloned wild-type galactofuranosidase (Streptomyces sp. strain JHA19), which recognises and binds a single monosaccharide that is only related to nonmammalian species, usually pathogenic microorganisms. We kinetically characterised the GalfNeoLect to confirm attenuation of hydrolytic activity and used competitive inhibition assay, with close structural analogues of Galf, to show that it conserved interaction with its original substrate. We synthetised the bovine serum albumin-based neoglycoprotein (GalfNGP), carrying the multivalent Galf units, as a suitable ligand and high-avidity system for the recognition of GalfNeoLect which we successfully tested directly with the galactomannan spores of Aspergillus brasiliensis (ATCC 16404). Altogether, our results indicate that GalfNeoLect has the necessary versatility and plasticity to be used in both research and diagnostic lectin-based applications.
1. Introduction
Glycans, oligosaccharide moieties that originate from a small set of monosaccharides, are typically found conjugated to glycoproteins and glycolipids and are exposed to the extracellular side of membranes, where they mediate a tremendous variety of cell interactions and signalling [1,2,3]. Among the putative set of monosaccharide constituents of glycans in organisms, galactofuranose (Galf), the five-membered ring form of aldohexose galactose, is completely absent from mammalian glycan motifs, including humans. Galactofuranose is by far the most widespread in other domains of life, ranging from archaea and bacteria to protozoa, fungi, and plants [4,5,6,7,8]. Often found in pathogenic organisms such as the bacterium Mycobacterium tuberculosis, the fungus Aspergillus fumigatus, and the protozoan Leishmania major as examples, it is hypothesised to be an advantageous element for their survival, reproduction, and virulence in the host [9]. As such, Galf–deficient mutants in Aspergillus have significant modifications of the cell surface that result in aberrant morphological changes and growth reduction, whilst the virulence capacity of Leishmania species is reduced [10]. Therefore, exploiting the presence of Galf in the glycome of pathogenic microorganisms makes it a promising target for biomedical research since it circumvents the potential risk of interference with the human glycans [11]. The distinctiveness of Galf as a component of important surface glycoconjugates of human pathogens has led to increased interest in targeting it in diagnostic or imaging techniques. Recent diagnostic techniques are predominantly based on production of Galf-specific antibodies. The presence of Aspergillus exoantigens of galactomannan (GM) origin is a specific indicator of invasive pulmonary aspergillosis [12]. Monoclonal antibody detection methods for early serological diagnosis of GM antigens of invasive pulmonary aspergillosis have been experimentally developed since the 1980s. In 1995, a double-direct sandwich enzyme-linked immunosorbent assay (ELISA), that uses a rat anti-GM monoclonal antibody (EB-A2) directed against the β-Galf-(1 → 3)-β-Galf epitopes of GM, was developed. Today, two GM antigen-detection kits are commercially available, the ELISA Pastorex® Aspergillus test (Sanofi Diagnostics Pasteur, Marnes-La-Coquette, France) and the latex agglutination test (LAT) Platelia™ Aspergillus (Biorad, Marnes-la-Coquette, France) [10]. The bibliographical overview of different types of Galf-specific antibodies is summarised in Table 1.

Table 1.
Comparative summary of antibodies specific for different Galf-containing glycan motifs.
In addition to Galf-specific antibodies, there is another class of carbohydrate-binding proteins of nonimmune origin, lectins, that can be used as tools for detecting Galf-bearing motifs. Unfortunately, only a few natural lectins have been characterised so far as Galf-binding and have mostly been used in research studies, among which human intelectin-1 (hIntL-1) has been closely studied [32,33,34,35]. In addition, all of them do not specifically recognise Galf, but also multiple glycan epitopes engaged in host–pathogen recognition interactions (Table 2). We focus herein on another type of Galf-recognising lectin, neolectin, bioengineered from naturally occuring Galf-ase. First described in 1977 and purified in crude form from the culture supernatants and cell lysates of filamentous fungi, bacteria, and protozoa, it was only in 2015 that an open reading frame (ORF) encoding a Galf-specific enzyme from Gram-positive bacteria Streptomyces sp. (strain JHA19) was identified, cloned, expressed, and completely biochemically characterised [36,37,38,39,40].

Table 2.
Comparative summary of natural lectins interacting with different Galf-containing glycan motifs.
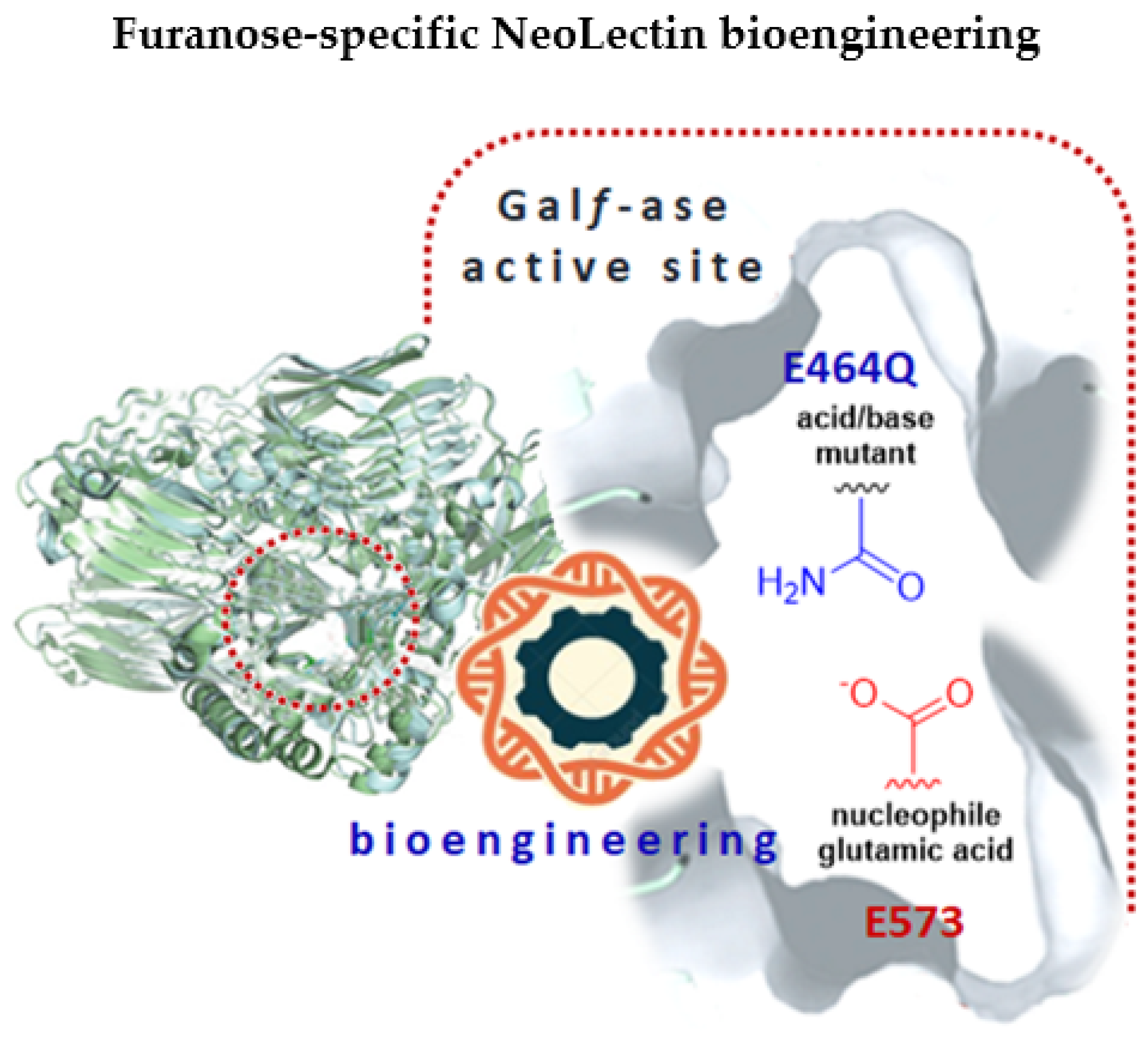
In this work, we have bioengineered, from Galf-specific carbohydrate-processing enzyme, through site-directed mutagenesis, an inactivated Galf-binding neolectin (GalfNeoLect) (Figure 1) [44]. As a probe to investigate and validate its binding specificity, we synthesised the bovine serum albumin (BSA)-based neoglycoprotein carrying the Galf, called GalfNGP. Using an integrated approach that combined enzyme kinetic assays, inhibition assays, and GLYctPROFILE® assays, we validated the GalfNGP and GalfNeoLect systems for their use to determine and study the Galf-specific recognition and binding.
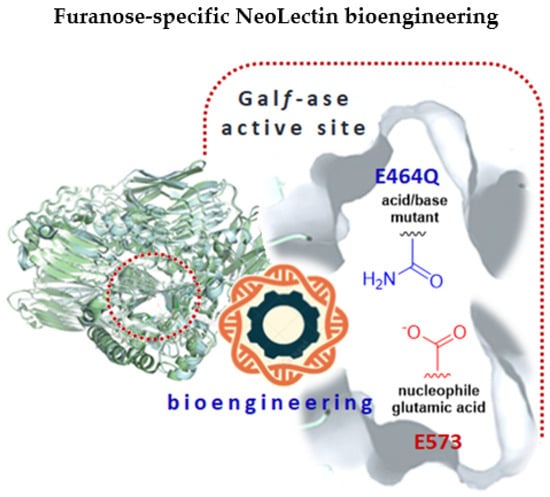
Figure 1.
A graphical abstract depicting the bioengineering of NeoLectins from galactofuranosidase by the site-directed mutagenesis method. The glutamic acid/base catalytic residue (E464) was specifically changed to glutamine (Q), resulting in galactofuranosidase E464Q mutant with attenuated hydrolytic activity that can act as galactofuranose-binding neolectin (GalfNeoLect).
2. Results
2.1. Design and Production of Novel Galf-Specific Neolectin (GalfNeoLect)
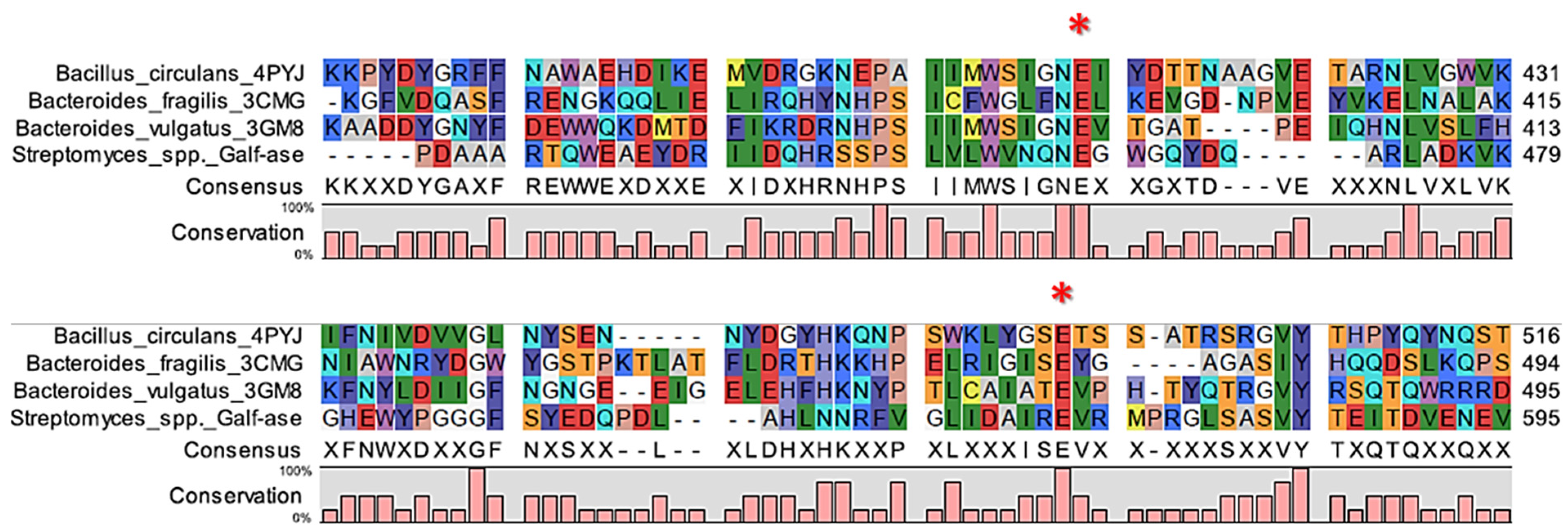
Our strategy was to generate several mutant variants of the wild-type Galf-ase characterised by attenuated hydrolytic activity towards the pNP β-D-Galf substrate. Since the deduced Galf-ase amino acid sequence analysis, as previously reported [38,39,40,45], revealed that it belongs to the GH2 family, to identify the catalytic residues of interest, we carried out in silico sequence alignment on three bacterial β-galactosidases from the GH2 family with determined crystal structures. The selected homologous amino acid sequences from Bacillus circulans (PDB accession code 4YPJ), Bacteroides fragilis (PDB accession code 3CMG), and Bacteroides vulgatus (PDB accession code 3GM8), sharing between 24% and 26% of their identities, revealed two sets of absolutely conserved glutamic acid residues (Figure 2, Supplemental Figure S3).

Figure 2.
Alignment of partial ORF of Galf-ase (Streptomyces spp.) amino acid sequence and corresponding regions of its homologs. The conserved E464 and E573 amino acid residues are indicated with asterisks.
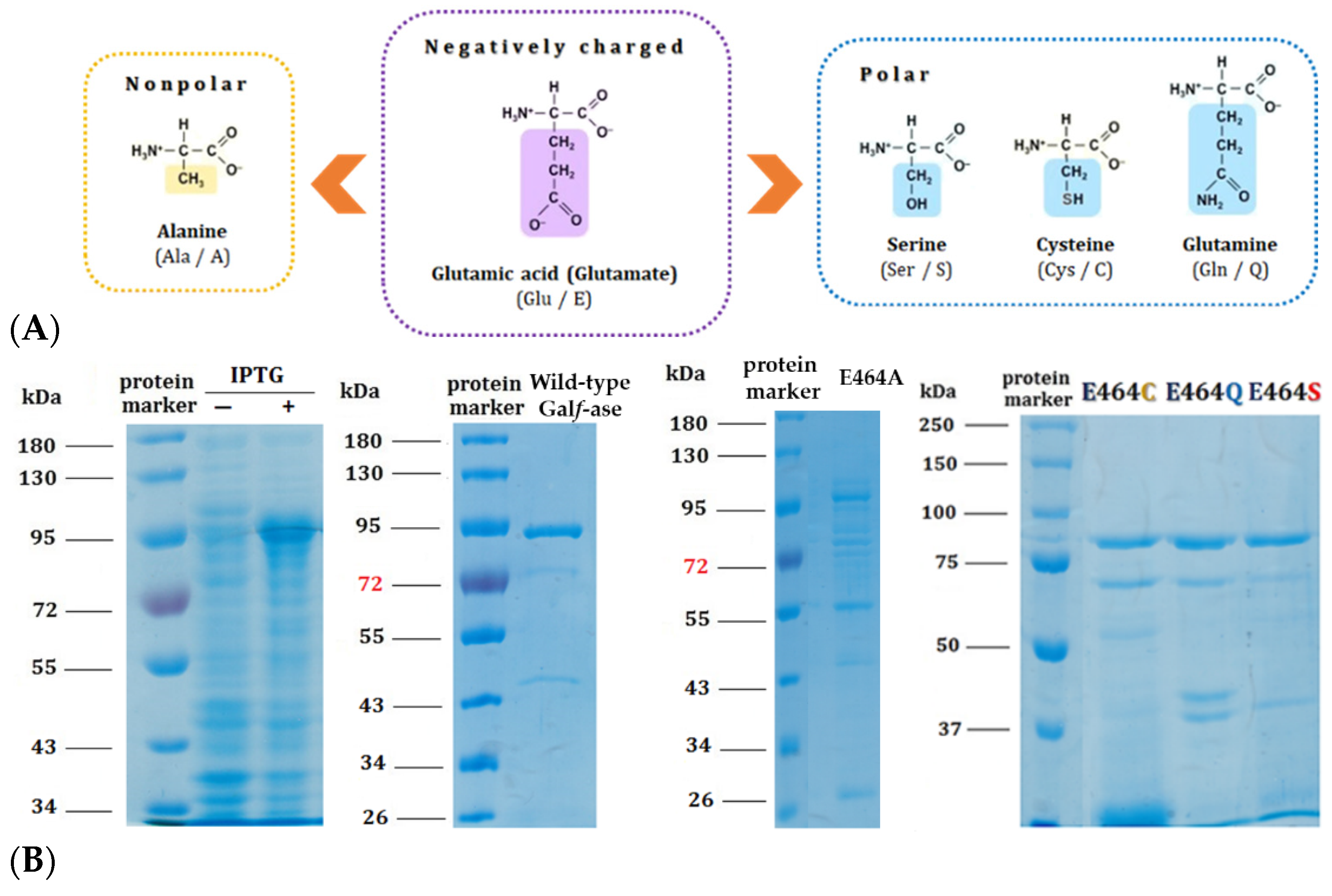
Based on mechanistic grounds of the GH2 family, the conserved residues represent the catalytic nucleophile (E573) and the catalytic acid/base (E464). Identified glutamic acid residues were expected to be the catalytic ones [40] and, therefore, we focused our mutagenesis studies on the acid/base glutamic acid residue (E464) that has a crucial step in glycohydrolitic catalysis. Therefore, the acid/base catalytic residue (E464) has been specifically substituted to alanine (E464A), serine (E464S), cysteine (E464C), and glutamine (E464Q) (Table 3). The acid/base carboxylic acid residue (E464) has been replaced by the amino acids that have the residues of specific interest—briefly, that have no negative charge—and, subsequently, the hydrolytic mechanism rate should be attenuated whilst conserving the substrate specificity, in order to generate and explore their potential role as Galf-specific neolectins (Figure 3A). All of the Galf-ase mutant protein variants, except E464A, were produced and purified under the same conditions as wild-type Galf-ase. It appears that the E464A mutation probably affected the folding and stability of protein, resulting in the formation of insoluble protein aggregates (Figure 3B).

Table 3.
Codon changes and respective amino acid changes introduced at 464 positions in the wild-type Galf-ase amino acid sequence by site-directed mutagenesis. Color indicates the mutation.
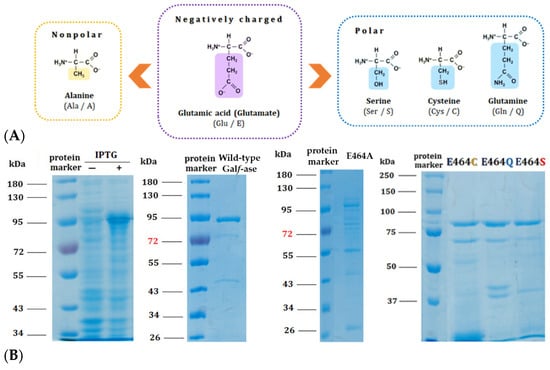
Figure 3.
(A) Structural formulas of amino acids selected for the generation of E464X Galf-ase mutant variants. (B) SDS–PAGE analysis of wild-type Galf-ase after IPTG induction and Ni-NTA chromatography purification, as well as Galf-ase E464A, E464C, E464Q, and E464S mutants. Lane 1: protein marker. Lanes 2 and 3: cells’ extract before and after IPTG’s induction of wild-type Galf-ase. Lane 4: protein marker. Lane 5: purified wild-type Galf-ase. Lane 6: protein marker. Lane 7: traces of E464A mutant (not obtained in purified form). Lane 8: protein marker. Lane 9 to 11: purified E464C, E464Q, and E464S mutants.
2.2. Kinetic Characterisation of Galf-Ase Mutant Variants—Identification of Novel Galf-Specific Neolectin (GalfNeoLect)
In analysing the hydrolitic activity and the hydrolysis rate-limiting steps of the three Galf-ase mutant variants E464S, E464C, and E464Q, the Michaelis–Menten kinetic parameters were determined under the similar conditions used for wild-type enzyme [46]. All the mutants gave typical Michaelis–Menten saturation curves (Supplemental Figure S4) and exhibited slightly decreased KM values compared to the one determined for wild-type, apart from E464S, which stands out for its sevenfold decrease in the KM value (Table 4). Altogether, the obtained values are in the same order of magnitude. Indeed, the replacement of glutamic acid residue to serine, cysteine, and glutamine has resulted in a 2000-orders-of-magnitude decrease in the hydrolytic activity compared to the wild-type Galf-ase enzyme, confirming that this residue is essential for catalysis. The significant decrease in catalytic efficiency by a factor of 3000–1400 times for E464S and E464C mutants, respectively, and 56 times lower for E464Q mutant is observed. These kinetic observations are in accordance with the other data obtained in similar assays used for the identification of the catalytic residues in retaining and inverting several GHs [46,47,48]. The Galf-ase mutant variant E464Q featured only a minor change in the KM value, 166 µM compared to 250 µM, of the wild-type Galf-ase and, given the advantage that, contrary to other mutant variants, it was purified in sufficient quantities (0.3 mg/L), it was selected as a promising candidate to be tested as a novel Galf-binding lectin (GalfNeoLect). Further in the article, the E464Q mutant variant is addressed as GalfNeoLect.

Table 4.
Comparison of Michaelis–Menten kinetics constants for hydrolysis of β-D-Galf by E464X Galf-ases mutant variants. Color indicates the mutation.
2.3. Galf-Conjugated BSA Neoglycoprotein (GalfNGP) as Novel Ligand for GalfNeoLect
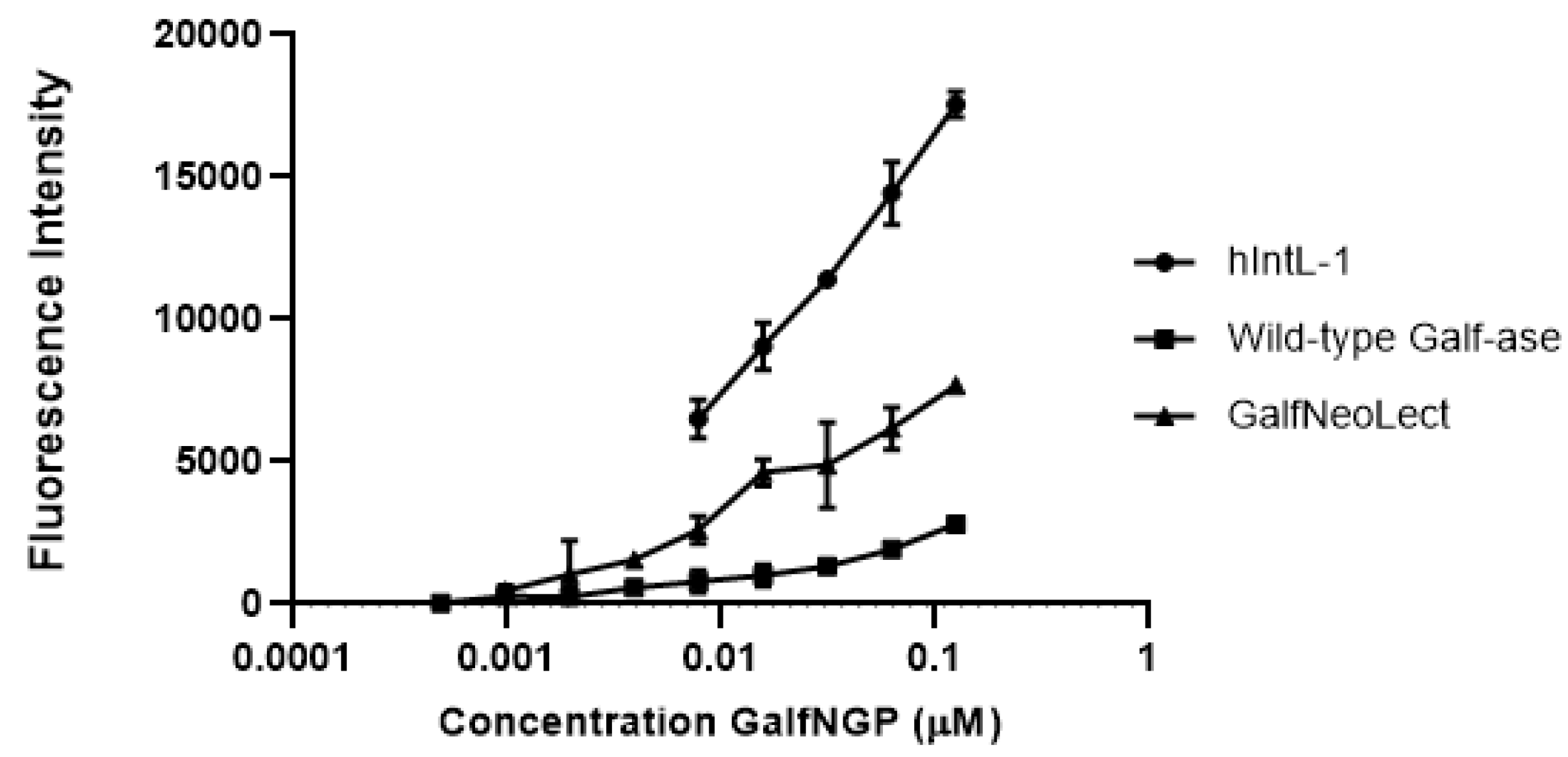
To study and evaluate the binding interactions of Galf-ase E464Q mutant variant, as novel Galf-binding lectin (GalfNeoLect), we synthetised novel neoglycoproteins (NGPs) to serve as the Galf-bearing scaffold. Bovine serum albumin (BSA), a well-known, naturally unglycosylated protein that has up to 60 primary amino functional groups available for click-conjugation chemistry [49], has been functionalised with Galf motifs. Galf-N3, involved in the corresponding click reaction with propargyl functionalised BSA, was obtained from the corresponding per-O-acetyl galactofuranose [50] using TMSN3 as a source of azide and TMSOTf as the Lewis acid (see ESI for chemical synthesis). After acetyl cleavage, the resulting/expected Galf-conjugated BSA neoglycoprotein (GalfNGP) synthesis has been first characterised for its functionality by direct binding assay with human Intelectin-1 (hIntL-1), wild-type Galf-ase, and GalfNeoLect. As hIntL-1 has been reported to bind furanose residues (five-membered-ring saccharide isomers), including a β-Galf–containing disaccharide, it served as a positive control for the evaluation of newly synthetised GalfNGP. The binding ability towards GalfNGP was tested directly by an enzyme-linked lectin assay (ELLA), where the immobilised hIntL-1, wild-type Galf-ase, and GalfNeoLect were incubated in the presence of increasing concentrations of GalfNGP. Under these conditions, the all three tested proteins gave dose-dependent responses (Figure 4) towards GalfNGP with BC50 (concentration to obtain 50% GalfNGP binding with the selected proteins) values in the same range of magnitude (Table 5). Indeed, BC50 values at 0.16 μM for GalfNeoLect and hIntL-1 were obtained. These values are comparable and in accordance with the 0.085 ± 0.014 μM functional affinity value obtained with β-D-Galf-substituted surface for hIntL-1 in a previous study [33]. These results demonstrate that GalfNGP is a novel and relevant ligand for testing the binding recognition towards Galf.

Figure 4.
Binding interaction of biotinylated GalfNGP to immobilised hIntL-1, wild-type Galf-ase, and GalfNeoLect. Galf-ase E464Q mutant variant was selected as a novel Galf-binding lectin (GalfNeoLect).

Table 5.
Comparison of BC50 of GalfNGP towards different Galf-ase-binding proteins and their respective values.
In parallel, hIntL-1, wild-type Galf-ase, and GalfNeoLect were tested in direct binding assay with standard NGPs that carry out the following carbohydrates: α-D-Galactose, α-L-Fucose, α-D-Mannose, and D-Glucose, as negative controls, which have not shown binding properties towards hIntL-1, wild-type Galf-ase, and GalfNeoLect (Supplemental Table S1).
2.4. Profiling GalfNeoLect Specificity
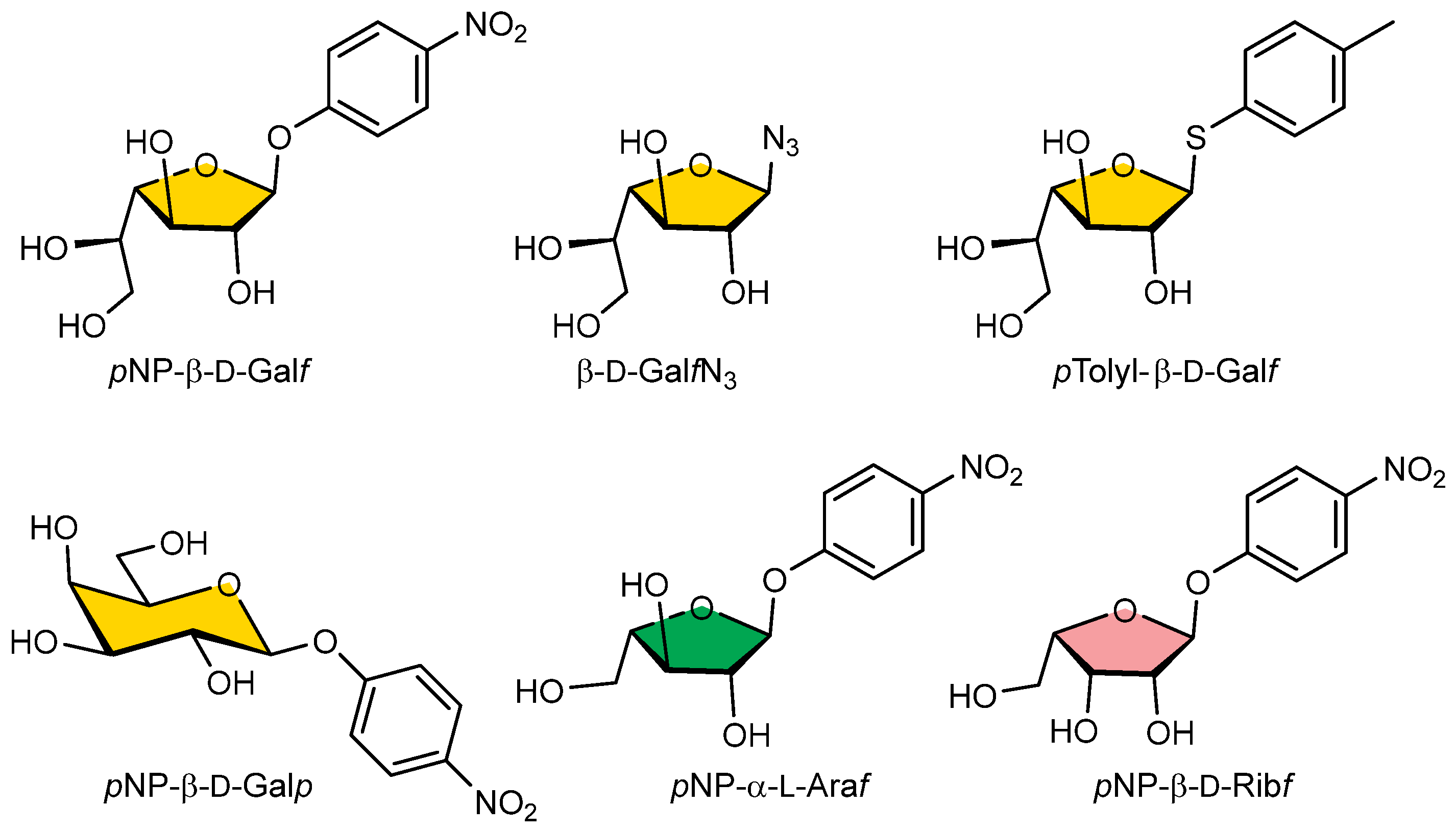
In the competitive inhibition assay, we investigated the ability of selected pNP, azido, or thyoaryl monosaccharide substrates (Galf-N3, Galf-thioaryl, pNP-β-D-Galf, pNP-β-D-Galp, pNP-α-L-Araf, pNP-β-D-Ribf) (Figure 5) for their ability to inhibit the binding of GalfNGP to immobilised GalfNeoLect.
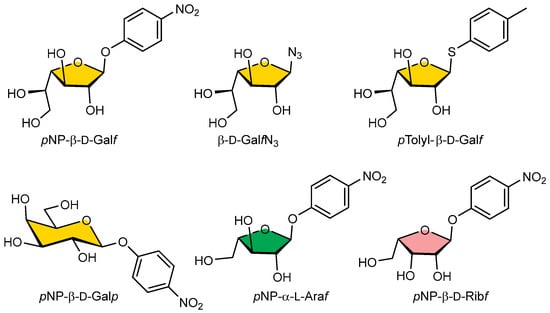
Figure 5.
Structural formulas of pNP-pyranosyl, -furanosyl, azido, or thioaryl substrates used for wild-type Galf-ase and GalfNeoLect substrate specificity screening.
The presence of increasing concentrations of Galf-N3, Galf-thioaryl, and pNP-β-D-Gal during incubation clearly resulted in a progressive decrease in the fluorescence signals for GalfNeoLect in a dose-dependent manner (Supplemental Figure S5). Indeed, Table 6 summarises the 50% inhibitory concentrations (IC50) obtained from all compounds studied. The lowest IC50 was obtained for Galf-thioaryl with 0.18 mM, followed by Galf-N3 with 0.60 mM. On the contrary, feeble responses in terms of detectability and no inhibition were obtained with pNP-β-D-Galp, pNP-α-L-Araf, and pNP-β-D-Ribf monosaccharide substrates (Supplemental Figure S5, and Table 6), which demonstrate their absence of specificity for the tested GalfNeoLectin. These results are in accordance with the IC50 values (Table 6, Supplemental Figure S6) obtained in the competitive inhibition assay with the wild-type Galf-ase, which interacts with the identical type of inhibitors (Galf-thioaryl) and in the same range of values as shown in a previous study [46]. These results demonstrate that the GalfNeoLectin binds specifically to only Galf-motifs and that the site-directed mutagenesis did not affect the substrate specificity.

Table 6.
IC50 of inhibitors towards wild-type Galf-ase and GalfNeoLect, expressed in mM.
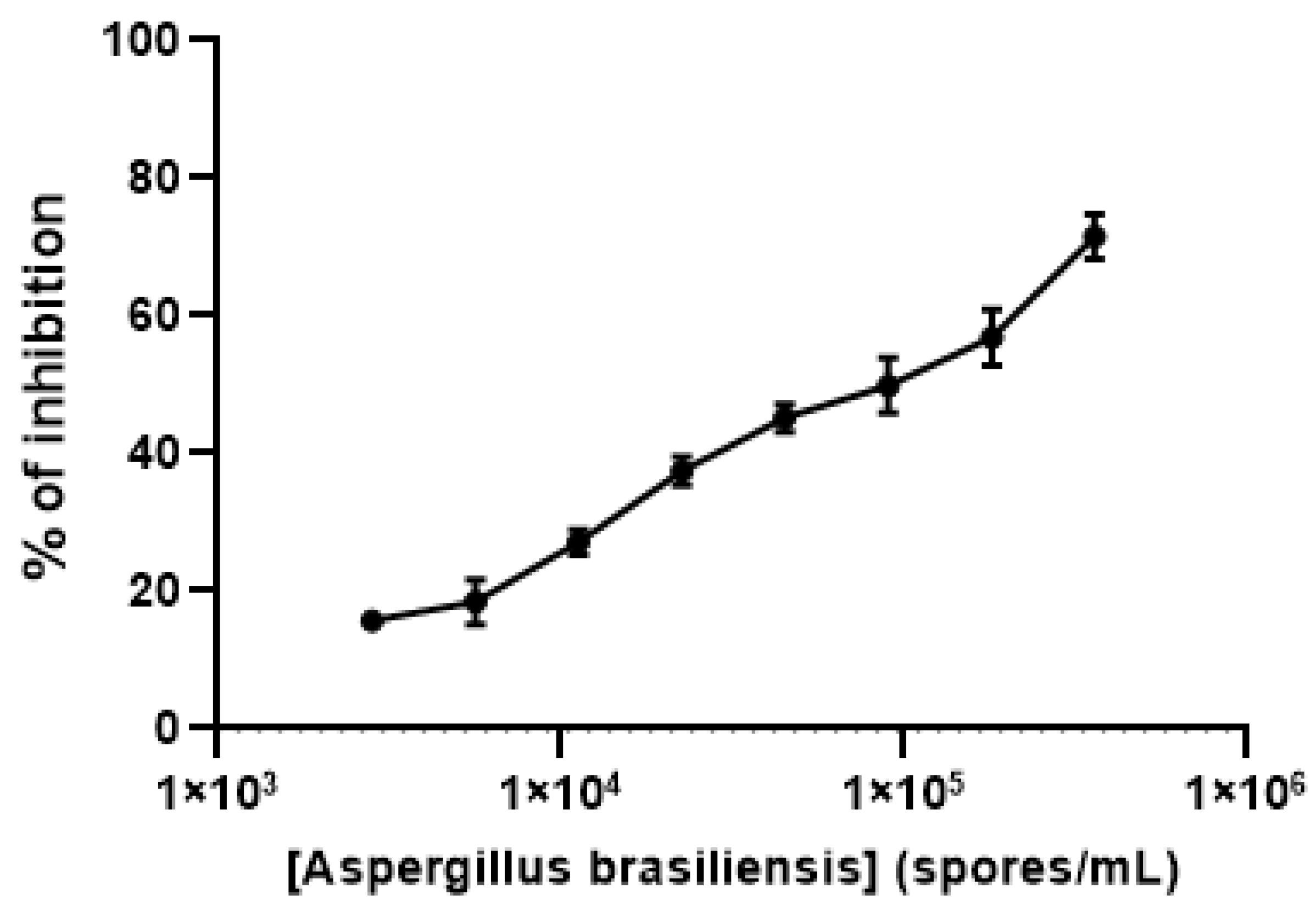
Finally, to test the biological functionality of the GalfNeoLect on crude samples, we used the viable spores from Aspergillus brasiliensis (ATCC 16404) that naturally carry Galf-containing galactomannan. By inhibition assay, we demonstrate that Aspergillus spores are able to displace the interaction between GalfNGP and GalfNeoLect in a dose-dependent manner (Figure 6).

Figure 6.
Inhibition profile of Aspergillus brasiliensis (ATCC 16404) spores with GalfNeoLect. Biotinylated GalfNGP (C = 2 μg/mL) was used as a tracer. Briefly, the A. brasiliensis (ATCC 16404) spores naturally carry on its surface Galf-containing galactomannan which is in competition for binding to GalfNeoLect with biotinylated GalfNGP.
3. Discussion
To bioengineer a galactofuranose-specific neolectin (GalfNeoLect) from a glycoside hydrolase, galactofuranosidase (Galf-ase), we undertook several steps, each followed by a qualitative procedure to determine the following parameters: (a) inactivation rate of hydrolytic activity, (b) conservation of substrate specificity, and (c) probing a robustness Galf-recognition capacity on a novel substrate. In the lack of the available resolved Galf-ase crystal structure, the prediction of the active site amino acid residues was carried out by sequence comparison with the enzymes within the same GH2 family. Based on the sequence identities between 24% and 26% with the Bacillus circulans, Bacteroides fragilis, and Bacteroides vulgatus GHs, two glutamic acid residues, E464 and E573, were highlighted as active site acid/base and nucleophile residues. In the present study, we used the site-directed mutagenesis method for direct confirmation of catalytic function of selected residues. The reduction in the catalytic activities of the mutant enzymes E464S, E464C, and E464Q was consistent with the suggested roles of the altered amino acids as catalytic residues. As indicated by kinetic studies, the catalytic activities of mutant enzymes (kcat/KM) were 3.000 to 56-fold weaker than that of the wild-type Galf-ase. According to the performance of its kinetic parameters, on-hand quantities, and the fact that, as the amide analogue is substituted to glutamic acid, it does not vary the length of aliphatic chain, the E464Q mutant variant was considered to have satisfying qualities to act as GalfNeoLect. We performed an assessment of whether the change in the catalytic acid/base residue had an impact on the substrate specificity of GalfNeoLect, the variety of pNP, azido, or thyoaryl monosaccharide substrates (Galf-N3, Galf-thioaryl, pNP-β-D-Galf, pNP-β-D-Galp, pNP-α-L-Araf, pNP-β-D-Ribf), including α-L-Araf, a stereochemical analogue of β-D-Galf, differing only by the absence of the extra C-6 hydroxymethyl group. Only three classes of substrates, all having Galf-sugar moiety, showed interaction with GalfNeoLect, bare Galf-N3, pNP-β-D-Galf and its thioaryl analogue of Galf. Data from competitive inhibition assay reveal that GalfNeoLect recognises multiple Galf-bearing molecules and can discriminate them between other monosaccharides. Also, we synthetised a novel neoglycoprotein based on Galf-decorated bovine serum albumin to investigate whether GalfNeoLect interacts with the more complex structures. BSA has been widely used as scaffold for harbouring multiple carbohydrate motifs via click chemistry [49]. The resulting polyvalent GalfNGP mimics carbohydrate presentation at the cell surface and allows one to study the GalfNeoLect–carbohydrate interactions. The Galf-binding capacity of GalfNGP was investigated with hIntL-1, a galactofuranosyl-binding lectin that served as standard, and compared with wild-type Galf-ase and GalfNeoLect in the direct binding assay. The wild-type Galf-ase and GalfNeoLect, together with hIntL-1, showed binding to GalfNGP, indicating that the Galf-moieties are able to trigger the specific interactions with GalfNGP.
4. Materials and Methods
4.1. Biological and Chemical Reagents
E. coli Rosetta™ (DE3) and plasmid vector pET-28a (+) were purchased from Novagen®. All p-nitrophenyl monosaccharides (pNP sugars) were purchased from Carbosynth (Compton, UK). All other laboratory chemicals used as the starting compounds, reagents, and solvents were analytical-grade purity and commercially available, unless otherwise specified. Neoglycoproteins (NGPs) used in direct binding assays were obtained from GLYcoDiag (Orléans, FRANCE): α-D-Galactose neoglycoprotein (NeoGa), α-L-Fucose neoglycoprotein (NeoF), α-D-Mannose neoglycoprotein (NeoM), and α-D-Glucose neoglycoprotein (NeoaG). Aspergillus brasiliensis (ATCC 16404) was obtained from an American-type culture collection (Manassas, VA, USA).
4.2. In Silico Sequence Analysis
Galf-ase nucleotide and amino acid sequences were retrieved from the National Centre for Biotechnology Information (NCBI) GenBank® (http://www.ncbi.nlm.nih.gov/genbank/, accessed on 22 July 2019) database under accession number LC073694. Amino acid sequence alignment search was conducted using a protein query BLAST® (Basic Local Alignment Search Tool) (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 22 July 2019) in the Protein Data Bank (PDB) database. The deduced protein amino acid sequences, deposited under PDB accession codes 4YPJ (B. circulans, 26% identities), 3CMG (B. fragilis, 24% identities), and 3GM8 (B. vulgatus, 25% identities), were aligned and analysed with CLC Sequence Viewer 7.7 software (Qiagen, Denmark).
4.3. Site-Directed Mutagenesis, Overexpression, and Mutant Galf-Ase Protein Purification
A pET-28a(+) plasmid construct encoding the wild-type galactofuranosidase (Galf-ase) gene from JHA 19 Streptomyces sp. [46] was used as a template to generate the mutant Galf-ase genes (E464Q, E464A, E464C, E464S) that were obtained by the site-directed mutagenesis method. The mutagenic primers (Table 7) were designed with Agilent Technologies QuikChange Primer Design tool and the reaction was performed using Agilent Technologies QuikChange Lightning Site-Directed Mutagenesis Kit (Santa Clara, CA, USA). The presence of desired mutations was confirmed by DNA sequencing performed by Eurofins Genomics. The overexpression and purification of mutant Galf-ase proteins was performed following a procedure reported previously [46]. Briefly, E. coli Rosetta™ (DE3) (Novagen®) expression strain, transformed with the plasmid construct carrying the desired Galf-ase mutant genes, was grown overnight at 37 °C in 1 L Luria–Bertani medium (LB media) supplemented with chloramphenicol (34 μg/mL) and kanamycin (30 μg/mL). Cells were grown to mid-exponential phase (OD600: 0.6), then shortly cooled on ice, and protein expression was induced by the addition of β-D-thiogalactopyranoside (IPTG) (100 μL; 1 M), after which the mixture was left on a shaker incubator (250 rpm) for 16 h at 15 °C. Afterwards, cells were harvested by centrifugation (4255× g, 30 min, 4 °C) and the cell pellets were resuspended in lysis buffer solution (1:10 v/v; 100 mM NaCl, 50 mM Tris/HCl pH 8, 1 mM phenylmethanesulfonyl fluoride (PMSF), 5% glycerol, 0.1% Triton X-100, lysozyme 1 mg/L). The suspension was incubated by stirring for 20 min at 4 °C, lysed by three freeze–thaw cycles, and subsequently sonicated on ice. Lysate was centrifuged (30,000 g, 20 min, 4 °C), supernatant was filtered (0.45 μm pore filter), and the protein was then purified from the clarified lysates using pre-equilibrated (10 mL; 50 mM Tris, 200 mM NaCl, 10 mM Imidazole) Thermo Scientific HisPur™ Ni-NTA Chromatography Cartridge (1 mL). The bound protein was eluted by an imidazole gradient (10–500 mM) and an aliquot of eluted fractions was analysed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) on 8% separating gel, according to Laemmli’s method [51], and protein bands were visualised by staining with Coomassie Brilliant Blue G250. Fractions containing pure protein were collected and concentrated by ultrafiltration (30,000 MWCO, Sartorius Vivaspin® sample concentrator), and protein quantity was determined using colorimetric Bio-Rad protein assay based on the Bradford dye-binding method, with bovine serum albumin (BSA) as a standard.

Table 7.
Primers used for site-directed mutagenesis.
4.4. Kinetic Studies
Kinetic parameters were determined with pNP-β-D-Galf as a substrate in different concentration ranges (0.01–5 mM). The reaction (200 μL), containing buffer (20 μL; 0.1 M citric acid/0.2 M Na2HPO4 pH 4.5) and water, if required, was started by addition of the enzyme (2 μL; 0.13 mg/mL) and, after 20 min of incubation at 37 °C (water bath), the reaction was terminated (100 μL; 1 M Na2CO3) and absorbance (405 nm) of released pNP was measured. All the reactions were assayed in triplicate and absorbance values were corrected for the spontaneous hydrolysis of the substrate. The kinetic parameters (KM, Vm, kcat) were calculated using GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA).
4.5. General Procedure for the Preparation of β-d-Galactofuranosyl Azide (Galf-N3)
2,3,5,6-tetra-O-acetyl-β-d-galactofuranosyl azide: To a solution of per-O-acetylgalactofuranose [50] (500 mg, 1.28 mmol) in anhydrous dichloromethane (15 mL), we added trimethylsilyl azide (0.34 mL, 2.56 mmol) and trimethylsilyl trifluoromethanesulfonate (0.69 mL, 3.84 mmol), successively, at RT. After stirring for 3 h, Et3N (0.5 mL) was added and the solvent was evaporated under reduced pressure. The residue was purified by chromatography (cylohexane/EtOAc, 7:3) to give 2,3,5,6-tetra-O-acetylgalactofuranosyl azide (360 mg, 75%) as a mixture of anomers (β/α, 95:5 according to NMR spectra) and as a colourless oil. 1H NMR (β anomer, CDCl3): δ 5.49 (1H, s, H-1), 5.38 (1H, td, J = 6.8, 4.8 Hz, H-5), 5.06 (1H, ddd, J = 4.8, 1.7, 0.6 Hz, H-3), 4.95 (1H, dd, J = 2.0, 1.7 Hz, H-2), 4.38 (1H, t, J = 4.8 Hz, H-4), 4.35 (1H, dd, J = 12.0, 4.8 Hz, H-6), 4.21 (1H, dd, J = 12.0, 6.8 Hz, H-6′), 2.14 (3H, s, CH3CO), 2.13 (3H, s, CH3CO), 2.11 (3H, s, CH3CO), 2.07 (3H, s, CH3CO). 13C NMR (β anomer, CDCl3): δ 170.5, 170.0, 169.8, 169.5 (CO), 94.2 (C-1), 82.1 (C-4), 81.0 (C-2), 76.4 (C-3), 69.2 (C-5), 62.4 (C-6), 20.8, 20.7, 20.6 (CH3CO). HRMS (ESI): calcd for C14H19N3O9Na [M + Na]+ 396.1019, found 396.1018. Anal. Calcd for C14H19N3O9: C, 45.04; H, 5.13; N, 11.26. Found: C, 45.42; H, 5.27; N, 10.95.
β-d-galactofuranosyl azide (Galf-N3): To a solution of 2,3,5,6-tetra-O-acetylgalactofuranosyl azide (300 mg, 0.81 mmol) in anhydrous methanol (8 mL), we added a solution of sodium methoxide in methanol (0.1 M, 80 μL, 0.08 mmol). The reaction mixture was stirred at room temperature for 12 h. Then, the solution was neutralised by addition of Amberlyst IR-120 H+ resin. After filtration, the filtrate was evaporated under vacuum and the residue was dissolved in water for freeze-drying to give targeted β-d-galactofuranosyl azide (164 mg, quantitative yield) as a white powder (Supplemental Figure S1). 1H NMR (400 MHz, D2O): δ 5.31 (1H, d, J = 2.8 Hz, H-1),4.06 (1H, dd, J= 10.4, 3.6 Hz, H-3), 4.01 (1H, dd, J = 10.4, 4.4 Hz, H-4), 3.94 (1H, dd, J = 3.6, 2.8 Hz, H-2), 3.80–3.73 (1H, m, H-5), 3.64 (1H, dd, J = 11.6, 4.8Hz, H-6), 3.57 (dd, J = 11.6, 7.2 Hz, 1H, H-6′); 13C NMR (100 MHz, D2O,): δ 95.3 (C-1), 84.2 (C-4), 80.6 (C-2), 76.4 (C-3), 70.7 (C-5), 62.6 (C-6). HRMS (ESI): calcd for C6H11N3O5Na [M + Na]+ 228.0596, found 228.0602.
4.6. Synthesis of Galf-Conjugated BSA Neoglycoprotein (GalfNGP)—Conjugation of BSA-Alkynewith Galf-N3
To a solution of BSA-alkyne [49] (10 mg, 10 mg/mL, 1 equiv.) in PBS (10 mM, pH 7.2), we added, successively, GalfN3 (2 mg, 60 equiv. in solution at 10 mg/mL in PBS) L-ascorbic acid (0.5 mg, 21 equiv., in solution at 3 mg/mL in PBS), CuSO4·5H2O (0.4 mg, 10.5 equiv. in solution at 3 mg/mL in PBS), and tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA, 2.6 mg, 35 equiv. in solution at 5 mg/mL in DMSO). The solution was stirred for 24 h at room temperature, then the crude mixture was purified by size-exclusion chromatography on Sephadex G25. The purity of GalfNGP was controlled by SDS–PAGE and the number of Galf motifs presented on BSA was determined by MALDI–TOF (UltrafleXtreme, Brucker) (Supplemental Figure S2).
4.7. Direct Binding Assays
The assays were performed according to GlycoDiag’s protocol, already described in the literature [49,52,53,54,55]. Briefly, the neoglycoprotein preliminary, labelled with biotin (in a range of concentrations), was prepared in PBS supplemented with 1 mM CaCl2 and 0.5 mM MgCl2 and deposited in each well (50 μL each) of 96-well plates, previously functionalised with either Intelectin (hIntL-1, 9137-IN-050, Biotechne), wild-type Galf-ase, or GalfNeoLect (LEctPROFILE® plates from GLYcoDiag, Orléans, France) in triplicate and incubated for 2 h at room temperature. After washing with PBS buffer, the streptavidin-DTAF conjugate was added (50 μL) and incubated for 30 min. The plate was then washed again with PBS. Finally, 100 μL of PBS was added for reading the plate using a fluorescence reader (λex = 485 nm, λem = 530 nm, Fluostar OPTIMA, BMG LABTECH, France). The intensity of the signal was directly correlated with the ability of the compound to be recognised by the lectin.
4.8. Inhibition Assays
The interaction profiles of each compound were determined through an indirect method, based on the inhibition by the compound of the interaction between a specific coupled lectin–glycan (a neoglycoprotein labelled with biotin and used as a tracer [48]). Briefly, a mix of biotinylated GalfNGP (fixed concentration) and the corresponding compounds (in a range of concentrations), prepared in PBS supplemented with 1 mM CaCl2 and 0.5 mM MgCl2, are deposed in each well (50 μL each) in triplicate and incubated for 2 h at room temperature. After washing with PBS buffer, the conjugate streptavidin-DTAF was added (50 μL) and incubated for 30 min more. The plate was washed again with PBS. Finally, 100 μL of PBS was added for the readout of the fluorescent plate, performed with a fluorescence reader (λex = 485 nm, λem = 530 nm, Fluostar OPTIMA, BMG LABTECH, France). The signal intensity was inversely correlated with the capacity of the compound to be recognised by the lectin and expressed as inhibition percentage with comparison with the corresponding biotinylated GalfNGP alone.
4.9. Preparation of Aspergillus Brasiliensis (ATCC 16404) Spores
Aspergillus brasiliensis (ATCC 16404) culture was inoculated on Sabouraud agar plates and incubated at 37 °C for 4 days until the formation of a black mycelium mat, which contains spores. Fresh spores were harvested with a sterile plastic inoculation loop by rubbing the black mycelium mat and dispersed in physiological serum solution (0.8% NaCl, 10 mL). The spore solution was resuspended well by using a vortex to prevent aggregation and was calibrated using a Malassez counting chamber to 5.106 spores/mL. Spores’ solutions (5.106 spores/mL) were used directly in inhibition assays with GalfNeoLect, following the same protocol describe above (Section 4.8).
5. Conclusions
A natural lectin that specifically binds only a Galf epitope and does not engage with other glycan epitopes has not yet been discovered. Therefore, to the best of our knowledge, we generated the first Galf-specific neolectin from the corresponding wild-type galactofuranosidase that exclusively recognises and interacts with Galf-glycan motifs. Furthermore, we synthetised the novel neoglycoprotein-carrying Galf monosaccharide units and demonstrated that it is recognised (in this study) by all available Galf-binding, hIntL-1, and Galf-specific proteins, as well as wild-type Galf-ase and GalfNeoLect. Furthermore, it may be feasible to exploit the Galf multivalency of Galf-NGP to identify enzymes involved in Galf metabolism and, together with GalfNeoLect, it is an interesting complementary Galf-oriented system to study host–pathogen interactions or for the qualitative GLYcoPROFILE® serodiagnosis of infectious diseases such as aspergillosis, leishmaniasis, borreliosis (Lyme disease), or tuberculosis.
6. Patents
Daniellou, R.; Lafite, P.; Seničar, M.; Roubinet, B.; Landemarre, L.; Néolectine spécifique et son utilisation pour détecter des microorganismes.
| Patent No. | Kind | Date | Application N° | Date |
| FR3116062 | A1 | 13 May 2022 | FR2011444 | 6 November 2020 |
| WO2022096829 | A1 | 12 May 2022 | WO2021-FR51944 | 4 November 2021 |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25094826/s1.
Author Contributions
Conceptualisation, project administration, and funding acquisition, R.D.; performed experiments, data analysis, and curation, M.S., L.L. (Laurent Legentil), B.R. and P.L.; provided access to crucial research components, L.L. (Ludovic Landemarre) and V.F.; writing—original draft preparation, M.S.; writing—review and editing, M.S., P.L. and R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the APR IR NeoLect project from the Region Centre-Val de Loire, France.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
R.D. acknowledges Orléans Métropole for supporting grants.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lindhorst, T.K. Essentials of Carbohydrate Chemistry and Biochemistry, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 5–49. ISBN 978-3-527-31528-4. [Google Scholar]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.H.; Stanley, P.; Bertozzi, C.R.; Hart, G.W.; Etzler, M.E. Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009; ISBN 9780879697709. [Google Scholar]
- Campo, V.L.; Aragão-Leoneti, V.; Teixeira, M.B.M.; Carvalho, I. Carbohydrates and Glycoproteins: Cellular Recognition and Drug Design. In New Developments in Medicinal Chemistry; Taft, C.A., Ed.; Bentham Science: Sharjah, United Arab Emirates, 2010; Volume 1, pp. 133–151. [Google Scholar] [CrossRef]
- Taylor, M.E.; Drickamer, K. Introduction to Glycobiology, 3rd ed.; Oxford University Press: Oxford, UK, 2011; pp. 1–304. ISBN 978-0199569113. [Google Scholar]
- Lichtenthaler, F.W. Carbohydrates: Occurrence, Structures and Chemistry. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 15–49. [Google Scholar] [CrossRef]
- Imamura, A.; Lowary, T. Chemical Synthesis of Furanose Glycosides. Trends Glycosci. Glycotechnol. 2011, 23, 134–152. [Google Scholar] [CrossRef]
- Lafite, P.; Daniellou, R. Rare and unusual glycosylation of peptides and proteins. Nat. Prod. Rep. 2012, 29, 729. [Google Scholar] [CrossRef] [PubMed]
- Marino, C.; Lederkremer, R. Galactose configurations in nature with emphasis on the biosynthesis of galactofuranose in glycans. In Galactose: Structure and Function in Biology and Medicine; Pomin, V.H., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 107–133. [Google Scholar]
- Tefsen, B.; Ram, A.F.; van Die, I.; Routier, F.H. Galactofuranose in eukaryotes: Aspects of biosynthesis and functional impact. Glycobiology 2012, 22, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Seničar, M.; Lafite, P.; Eliseeva, S.V.; Petoud, S.; Landemarre, L.; Daniellou, R. Galactofuranose-Related Enzymes: Challenges and Hopes. Int. J. Mol. Sci. 2020, 21, 3465. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.R.; Gagneux, P. Evolution of carbohydrate antigens—Microbial forces shaping host glycomes? Glycobiology 2007, 17, 23R–34R. [Google Scholar] [CrossRef] [PubMed]
- Stynen, D.; Sarfati, J.; Goris, A.; Prévost, M.C.; Lesourd, M.; Kamphuis, H.; Darras, V.; Latgé, J.P. Rat monoclonal antibodies against Aspergillus galactomannan. Infect. Immun. 1992, 60, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Notermans, S.; Dufrenne, J.; Wijnands, L.M.; Engel, H.W.B. Human serum antibodies to extracellular polysaccharides (EPS) of moulds. J. Med. Vet. Mycol. 1988, 26, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Notermans, S.; Veeneman, G.H.; van Zuylen, C.W.E.M.; Hoogerhout, P.; van Boom, J.H. (1→5)-linked β-D-galactofuranosides are immunodominant in extracellular polysaccharides of Penicillium and Aspergillus species. Mol. Immunol. 1988, 25, 975–979. [Google Scholar] [CrossRef] [PubMed]
- De Arruda, M.V.; Colli, W.; Zingales, B. Terminal β-D-galactofuranosyl epitopes recognized by antibodies that inhibit Trypanosoma cruzi internalization into mammalian cells. Eur. J. Biochem. 1989, 182, 413–421. [Google Scholar] [CrossRef]
- McConville, M.J.; Homans, S.W.; Thomas-Oates, J.E.; Dell, A.; Bacic, A. Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. J. Biol. Chem. 1990, 265, 7385–7394. [Google Scholar] [CrossRef]
- Golgher, D.B.; Colli, W.; Souto-Padrón, T.; Zingales, B. Galactofuranose-containing glycoconjugates of epimastigote and trypomastigote forms of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1993, 60, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.P.; Kobayashi, H.; Debeaupuis, J.P.; Diaquin, M.; Sarfati, J.; Wieruszeski, J.M.; Parra, E.; Bouchara, J.P.; Fournet, B. Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infect. Immun. 1994, 62, 5424–5433. [Google Scholar] [CrossRef] [PubMed]
- Leitão, E.A.; Bittencourt, V.C.B.; Haido, R.M.T.; Valente, A.P.; Peter-Katalinic, J.; Letzel, M.; de Souza, L.M.; Barreto-Bergter, E. β-Galactofuranose-containing O-linked oligosaccharides present in the cell wall peptidogalactomannan of Aspergillus fumigatus contain immunodominant epitopes. Glycobiology 2003, 13, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Morelle, W.; Bernard, M.; Debeaupuis, J.-P.; Buitrago, M.; Tabouret, M.; Latgé, J.-P. Galactomannoproteins of Aspergillus fumigatus. Eukaryot. Cell. 2005, 4, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Mennink-Kersten, M.A.S.H.; Ruegebrink, D.; Klont, R.R.; Warris, A.; Blijlevens, N.M.A.; Donnelly, J.P.; Verweij, P.E. Improved detection of circulating Aspergillus antigen by use of a modified pretreatment procedure. J. Clin. Microbiol. 2008, 46, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Krylov, V.B.; Solovev, A.S.; Argunov, D.A.; Latgé, J.-P.; Nifantiev, N.E. Reinvestigation of carbohydrate specificity of EB-A2 monoclonal antibody used in the immune detection of Aspergillus fumigatus galactomannan. Heliyon 2019, 31, e01173. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.S.; Suzuki, E.; Straus, A.H.; Takahashi, H.K. Glycolipids from Paracoccidioides brasiliensis. Isolation of a galactofuranose-containing glycolipid reactive with sera of patients with paracoccidioidomycosis. J. Med. Vet. Mycol. 1995, 33, 247–251. [Google Scholar] [CrossRef]
- Suzuki, E.; Toledo, M.S.; Takahashi, H.K.; Straus, A.H. A monoclonal antibody directed to terminal residue of β-galactofuranose of a glycolipid antigen isolated from Paracoccidioides brasiliensis: Cross-reactivity with Leishmania major and Trypanosoma cruzi. Glycobiology 1997, 7, 463–468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toledo, M.S.; Tagliari, L.; Suzuki, E.; Silva, C.M.; Straus, A.H.; Takahashi, H.K. Effect of anti-glycosphingolipid monoclonal antibodies in pathogenic fungal growth and differentiation. Characterization of monoclonal antibody MEST-3 directed to Manp α1→3Manp α1→2IPC. BMC Microbiol. 2010, 10, 1–12. [Google Scholar] [CrossRef]
- Heesemann, L.; Kotz, A.; Echtenacher, B.; Broniszewska, M.; Routier, F.; Hoffmann, P.; Ebel, F. Studies on galactofuranose-containing glycostructures of the pathogenic mold Aspergillus fumigatus. Int. J. Med. Microbiol. 2011, 301, 523–530. [Google Scholar] [CrossRef]
- Dufresne, S.F.; Datta, K.; Li, X.; Dadachova, E.; Staab, J.F.; Patterson, T.F.; Feldmesser, M.; Marr, K.A. Detection of urinary excreted fungal galactomannan-like antigens for diagnosis of invasive aspergillosis. PLoS One 2012, 7, e42736. [Google Scholar] [CrossRef] [PubMed]
- Rolle, A.-M.; Hasenberg, M.; Thornton, C.R.; Solouk-Saran, D.; Männ, L.; Weski, J.; Maurer, A.; Fischer, E.; Spycher, P.R.; Schibli, R.; et al. ImmunoPET/MR imaging allows specific detection of Aspergillus fumigatus lung infection in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Rolle, A.-M.; Maurer, A.; Spycher, P.R.; Schillinger, C.; Solouk-Saran, D.; Hasenberg, M.; Weski, J.; Fonslet, J.; Dubois, A.; et al. Towards translational immunoPET/MR imaging of invasive pulmonary aspergillosis: The humanised monoclonal antibody JF5 detects Aspergillus lung infections in vivo. Theranostics 2017, 7, 3398–3414. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Xue, S.; Ebel, F.; Vaggelas, A.; Krylov, V.B.; Nifantiev, N.E.; Chudobová, I.; Schillberg, S.; Nölke, G. Monoclonal antibody AP3 binds galactomannan antigens displayed by the pathogens Aspergillus flavus, A. fumigatus, and A. parasiticus. Front. Cell. Infect. Microbiol. 2019, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Matveev, A.L.; Krylov, V.B.; Emelyanova, L.A.; Solovev, A.S.; Khlusevich, Y.A.; Baykov, I.K.; Fontaine, T.; Latgé, J.-P.; Tikunova, N.V.; Nifantiev, N.E. Novel mouse monoclonal antibodies specifically recognize Aspergillus fumigatus galactomannan. PLoS One 2018, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Uehori, J.; Matsumoto, M.; Suzuki, Y.; Matsuhisa, A.; Toyoshima, K.; Seya, T. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J. Biol. Chem. 2001, 276, 23456–23463. [Google Scholar] [CrossRef] [PubMed]
- Wesener, D.A.; Wangkanont, K.; McBride, R.; Song, X.; Kraft, M.B.; Hodges, H.L.; Zarling, L.C.; Splain, R.A.; Smith, D.F.; Cummings, R.D.; et al. Recognition of microbial glycans by human intelectin-1. Nat. Struct. Mol. Biol. 2015, 22, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ramya TN, C. Saccharide binding by intelectins. Int. J. Biol. Macromol. 2018, 108, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Yamashita, M.; Nishiyama, A.; Shinohara, T.; Li, Z.; Myrvik, Q.N.; Hoffman, D.R.; Henriksen, R.A.; Shibata, Y. Differential structure and activity between human and mouse intelectin-1: Human intelectin-1 is a disulfide-linked trimer, whereas mouse homologue is a monomer. Glycobiology 2007, 17, 1045–1051. [Google Scholar] [CrossRef]
- Matsunaga, E.; Higuchi, Y.; Mori, K.; Tashiro, K.; Takegawa, K. Draft Genome Sequence of Streptomyces sp. JHA26, a Strain That Harbors a PA14 Domain Containing β-D-Galactofuranosidase. Genome Announc. 2017, 5, e00190-17. [Google Scholar] [CrossRef]
- Matsunaga, E.; Higuchi, Y.; Mori, K.; Yairo, N.; Toyota, S.; Oka, T.; Tashiro, K.; Takegawa, K. Characterization of a PA14 Domain-Containing Galactofuranose-Specific β-D-Galactofuranosidase from Streptomyces sp. Biosci. Biotechnol. Biochem. 2017, 81, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, E.; Higuchi, Y.; Mori, K.; Tashiro, K.; Kuhara, S.; Takegawa, K. Draft Genome Sequence of Streptomyces sp. JHA19, a Strain That Possesses β-d-Galactofuranosidase Activity. Genome Announc. 2015, 3, e01171-15. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, E.; Higuchi, Y.; Mori, K.; Yairo, N.; Oka, T.; Shinozuka, S.; Tashiro, K.; Izumi, M.; Kuhara, S.; Takegawa, K. Identification and Characterization of a Novel Galactofuranose-Specific β-D-Galactofuranosidase from Streptomyces Species. PLoS ONE 2015, 10, e0137230. [Google Scholar] [CrossRef]
- Seničar, M.; Legentil, L.; Ferrières, V.; Eliseeva, S.V.; Petoud, S.; Takegawa, K.; Lafite, P.; Daniellou, R. Galactofuranosidase from JHA 19 Streptomyces sp.: Subcloning and biochemical characterization. Carbohydr. Res. 2019, 480, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Abeygunawardana, C.; Bush, C.A.; Cisar, J.O. Complete structure of the cell surface polysaccharide of Streptococcus oralis ATCC 10557: A receptor for lectin-mediated interbacterial adherence. Biochemistry 1991, 30, 6528–6540. [Google Scholar] [CrossRef] [PubMed]
- Cassels, F.J.; Hughes, C.V.; Nauss, J.L. Adhesin receptors of human oral bacteria and modeling of putative adhesin-binding domains. J. Ind. Microbiol. 1995, 15, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Chiodo, F.; Marrad, M.; Park, J.; Ram, A.F.J.; Penadé, S.; van Die, I.; Tefsen, B. Galactofuranose-coated gold nanoparticles elicit a pro-inflammatory response in human monocyte-derived dendritic cells and are recognized by DC-SIGN. ACS Chem. Biol. 2014, 9, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Dureau, R.; Legentil, L.; Daniellou, R.; Ferrières, V. Two-Step Synthesis of Per-O-acetylfuranoses: Optimization and Rationalization. J. Org. Chem. 2012, 77, 1301–1307. [Google Scholar] [CrossRef]
- Arnaud, J.; Audfray, A.; Imberty, A. Binding sugars: From natural lectins to synthetic receptors and engineered neolectins. Chem. Soc. Rev. 2013, 42, 4798–4813. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- McGrath, C.E.; Vuong, T.V.; Wilson, D.B. Site-directed mutagenesis to probe catalysis by a Thermobifida fusca beta-1,3-glucanase (Lam81A). Protein Eng. Des. Sel. 2009, 22, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Shallom, D.; Leon, M.; Bravman, T.; Ben-David, A.; Zaide, G.; Belakhov, V.; Shoham, G.; Schomburg, D.; Baasov, T.; Shoham, Y. Biochemical characterization and identification of the catalytic residues of a family 43 beta-D-xylosidase from Geobacillus stearothermophilus T-6. Biochemistry 2005, 44, 387–397. [Google Scholar] [CrossRef]
- Assailly, C.; Bridot, C.; Saumonneau, A.; Lottin, P.; Roubinet, B.; Krammer, E.-M.; François FVena, F.; Landemarre, L.; Alvarez Dorta, D.; Deniaud, D.; et al. Polyvalent transition-state analogues of sialyl substrates strongly inhibit bacterial sialidases. Chem. Eur. J. 2021, 27, 3142–3150. [Google Scholar] [CrossRef] [PubMed]
- Landemarre, L.; Duverger, E. Lectin Glycoprofiling of Recombinant Therapeutic Interleukin-7. In Glycosylation Engineering of Biopharmaceuticals: Methods and Protocols, Methods in Molecular Biology; Beck, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 988, pp. 221–226. [Google Scholar] [CrossRef]
- Goyard, D.; Roubinet, B.; Vena, F.; Landemarre, L.; Renaudet, O. Homo- and Heterovalent Neoglycoproteins as Ligands for Bacterial Lectins. ChemPlusChem 2001, 87, e202100481. [Google Scholar] [CrossRef] [PubMed]
- Cauwel, M.; Sivignon, A.; Bridot, C.; Nongbe, M.C.; Deniaud, D.; Roubinet, B.; Landemarre, L.; Felpin, F.-X.; Bouckaert, J.; Barnich, N.; et al. Heptylmannose-functionalized cellulose for the binding and specific detection of pathogenic E. coli. Chem. Commun. 2019, 55, 10158–10161. [Google Scholar] [CrossRef] [PubMed]
- Brissonnet, Y.; Assailly, C.; Saumonneau, A.; Bouckaert, J.; Maillasson, M.; Petitot, C.; Roubinet, B.; Didak, B.; Landemarre, L.; Bridot, C.; et al. Multivalent Thiosialosides and Their Synergistic Interaction with Pathogenic Sialidases. Chem. Eur. J. 2019, 25, 2358. [Google Scholar] [CrossRef] [PubMed]
- Krammer, E.M.; Bridot, C.; Serna, S.; Echeverria, B.; Semwal, S.; Roubinet, B.; van Noort, K.; Wilbers, R.H.P.; Bourenkov, G.; de Ruyck, J.; et al. Structural insights into a cooperative switch between one and two FimH bacterial adhesins binding pauci- and high-mannose type N-glycan receptors. J. Biol. Chem. 2023, 299, 104627. [Google Scholar] [CrossRef] [PubMed]
- Shallom, D.; Belakhov, V.; Solomon, D.; Gilead-Gropper, S.; Baasov, T.; Shoham, G.; Shoham, Y. The identification of the acid-base catalyst of alpha-arabinofuranosidase from Geobacillus stearothermophilus T-6, a family 51 glycoside hydrolase. FEBS Lett. 2002, 514, 163–167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).