The Evolving Landscape of Flowcytometric Minimal Residual Disease Monitoring in B-Cell Precursor Acute Lymphoblastic Leukemia
Abstract
:1. MRD in BCP-ALL
1.1. Molecular Techniques for MRD Assessment
1.2. Flow Cytometry-Based MRD Assessment
1.3. Immunophenotype of B-Cell Precursors
| Marker | Expression on Normal Cells | Expression on BCP-ALL Blasts | Remarks | Reference |
|---|---|---|---|---|
| CD10 | Pre-B1 to immature B-cell precursors Mature neutrophils | Overexpressed in 70% of pre-B-ALL and common B-ALL patients | Negative on pro-B-cells and pro-B-ALL cells | [45,52,53] |
| CD13 | Expressed on myeloid cells Negative on lymphoid cells | Expressed in 30% of BCP-ALL cases | [47,50] | |
| CD19 | Pre-B1 to mature B-cells Negative on most other leukocytes | Expressed on >90% of BCP-ALL cases | Expression can be lost after CD19-targeted immunotherapy | [41,42,43,57,58,59] |
| CD20 | Expressed on pre-B2-Large cells Highly expressed on immature and mature B-cells Negative on all other leukocytes | Expressed in 40% of BCP-ALL cases | [45,46,47,51] | |
| CD22 | Expressed on B-cells from pro-B-cell stage onwards Negative on most other leukocytes | Expressed on >90% of BCP-ALL cases | Can be used as B-cell maker after CD19-targeted therapy | [46,47,60,61] |
| CD24 | Expressed on B-cell precursors Mature neutrophils | Expressed on >80–90% of BCP-ALL cases | Can be used as B-cell maker after CD19-targeted therapy | [60,61,62] |
| CD33 | Expressed on myeloid cells Negative on lymphoid cells | Expressed in 30% of BCP-ALL cases | [47,50] | |
| CD34 | Hematopoetic stem cells and early hematopoetic progenitors Pro-B and Pre-B1-cells | Expressed in 60% of BCP-ALL cases | Expression can be heterogenous in BCP-ALL | [44,49,54,55] |
| CD45 | Expressed on all leukocytes | Underexpressed in 30% of BCP-ALL cases | [45,50] | |
| CD58 | Expressed on antigen-presenting cells | Overexpressed on >90% of BCP-ALL cases | [63,64] | |
| CD66c | Expressed on myeloid cells Negative on lymphoid cells | Expressed on 36–81% of BCP-ALL cases | Expression correlated with BCR::ABL and hyperdiploid cases Negative in KMT2A-rearranged BCP-ALL | [65,66,67,68,69] |
| CD73 | Expressed on B-cells, T-cells and folliciular dendritic cells | Overexpressed in 42–70% of BCP-ALL cases | Expression is higher in common- and pre-B-ALL compared to pro-B-ALL patients | [62,70,71,72] |
| CD81 | Highly expressed on B-cells Negative on erytrocytes and neutrophils | Underexpressed in 82% of BCP-ALL cases | [73] | |
| CD123 | Expressed on hematopoetic progenitor cells Expressed on CD34-negative B-cell precursors Expressed on plasmacytoid dendritic cells and basophils | Aberrantly expressed in 80% of CD34-positive BCP-ALL cases | CD123 levels are higher in patients harboring a BCR::ABL translocation or with an hyperdiploid karyotype | [74,75,76,77,78,79] |
| CD304 | Highly expressed on plasmacytoid dendritic cells | Overexpressed in 40–59% of BCP-ALL cases | Overexpression is associated with ETV6::RUNX1 and BCR::ABL karyotypes | [70,80,81,82,83] |
2. First Generation Flow Cytometry MRD Panels
2.1. Introduction of Novel Markers
2.2. Second Generation Flow Cytometry MRD Panels
2.3. Next-Generation Flow Cytometry Protocols
2.4. Immunotherapy and Escape in BCP-ALL
2.5. Challenges and Novel Approaches for Flowcytometric MRD Assessment
| Panel (Reference) | Tubes/ Colors | B-Cell and Maturation Markers | Aberrancy Makers | Viability Dye | # Cells | Minimal Cluster of (MRD) Cells (LOD) | Sensitivity (LLOQ) | Suitable for Targeted Therapy Treated Patients? | Gating Strategy for Normal BCPs and BCP-ALL Cells Provided | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD10 | CD19 | CD20 | CD22 | CD24 | CD34 | CD38 | CD45 | cyCD79a | CD81 | HLA-DR | CD13 | CD33 | CD58 | CD66b | CD66c | CD73 | CD86 | CD123 | CD304 | ||||||||
| Theunissen et al. [98] | 2/8 | 4 × 106 | 10 | 10−5 | Yes, with adapted gating strategy | Yes, based on expression of CD10 and CD34 [120] | |||||||||||||||||||||
| Cherian et al. [60] | 2/7 & 8 | 5 × 105 | NA | NA | Yes | Yes, based on expression of CD22 and CD24 in absence of CD66b | |||||||||||||||||||||
| Mikhailova et al. [121] | 1/11 | a | 3 × 105 | 10 | 10−4 | Yes | Yes, based on expression of CD22, cyCD79a and CD24 | ||||||||||||||||||||
| Singh et al. [122] | 1/10 | 1 × 106 | 20 | 10−4 | Not evaluated | Yes, based on expression of CD19 and/or CD22, CD10 and CD22 | |||||||||||||||||||||
| Chatterjee et al. [123] | 1/15 | 5 × 106 | 20 | 10−5 | Yes | Yes, based on expression of CD22, CD24 and CD81. Exclusion of myeloid cells via CD33 | |||||||||||||||||||||
| Gao et al. [124] | 1/14 | b | 2 × 106 | 12 | 10−5 | Yes | Yes, based on expression of CD22 and CD24 in absence of CD66b | ||||||||||||||||||||
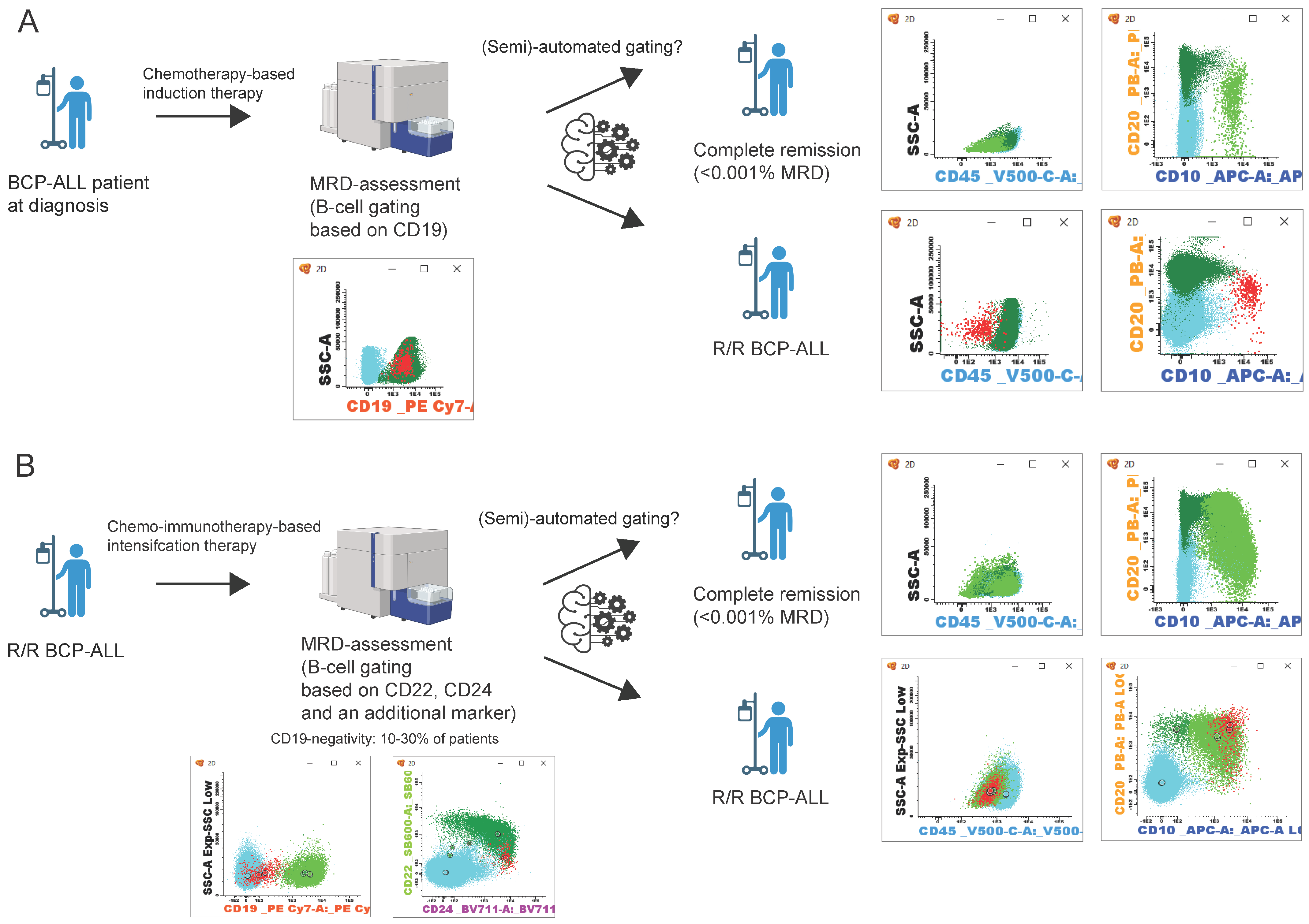
2.6. (Semi-)Automated MRD Assessment
| (Semi-)Automated Tool | Algorithm | Training Data Set | Input | Sensitivity (LLOQ) | Suitable for Targeted Therapy Treated Patients? | Challenges in Analysis |
|---|---|---|---|---|---|---|
| Verbeek et al. [125] | Database driven Automated Gating & Identification tool | Normal bone marrow samples | FCS-file of MRD sample stained with EuroFlow 8-color BCP-ALL MRD protocol (n = 174) | 10−5 | Yes | Requires manual evaluation of unassigned events (checks) |
| Fiser et al. [126] | Hierarchical clustering analysis & suport vector machine learning | Leukemic blast populations in diagnostic samples | Raw data from day 15 MRD patient FCS-files (n = 123) | 10−4 | NA | Difficulties with distinction between BCP-ALL cells and normal B-cell precursors |
| DiGuiseppe et al. [127] | t-Distributed Stochastic Neighbor Embedding-based viSNE | FCS-file containing viable CD19-positive singlets (n = 24) | 10−5 | No, since algoritm requires manual gating of CD19-positive cells | Algorithm requires gating of CD19-postive events prior to automated analysis | |
| Reiter et al. [128] | Gaussian Mixture Models | data from manual gated day 15 patient MRD samples | Data from day 15 MRD patient FCS-files (n = 337) | 10−4 | NA | Only evaluated with MRD samples at day 15 |
| Wodlinger et al. [129] | Neural network approach based on the transformer architecture | data from manual gated day 15 patient MRD samples | Data from day 15 MRD patient FCS-files (n = 519) | 10−4 | NA | Only evaluated with MRD samples at day 15 |
| Shopsowitz et al. [130] | Radar plots | Data from manual gated patient MRD samples (day 29 or later) | Raw data from MRD patient FCS-files (day 29 or later) (n = 20) | 10−4 | NA |
3. Conclusions and Future Perspectives
4. Take Home Messages
- The presence of MRD is the most important prognostic marker in the clinical management of pediatric and adult BCP-ALL.
- BCP-ALL cells can be distinguished from normal B-cells by abnormal expression of known maturation makers (e.g., CD10, CD20, CD34, and CD45) combined with aberrant expression of other markers (e.g., CD58, CD81, CD304, CD73, CD66c, and CD123).
- The use of CD19 as a B-cell B-cell-specific marker may become less reliable in the context of CD19-targeted therapies, particularly for patients with loss of CD19.
- Most next-generation flow cytometry panels include at least CD22 and CD24, along with an additional B-cell marker, for the accurate identification of BCP-ALL cells after CD19-targeted therapies.
- (Semi-)automated analysis of flow cytometry data likely will facilitate MRD assessment following targeted therapies.
Author Contributions
Funding
Conflicts of Interest
References
- Pastorczak, A.; Domka, K.; Fidyt, K.; Poprzeczko, M.; Firczuk, M. Mechanisms of Immune Evasion in Acute Lymphoblastic Leukemia. Cancers 2021, 13, 1536. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Harrison, C.J. Advances in B-cell Precursor Acute Lymphoblastic Leukemia Genomics. Hemasphere 2018, 2, e53. [Google Scholar] [CrossRef] [PubMed]
- Kakaje, A.; Alhalabi, M.M.; Ghareeb, A.; Karam, B.; Mansour, B.; Zahra, B.; Hamdan, O. Rates and trends of childhood acute lymphoblastic leukaemia: An epidemiology study. Sci. Rep. 2020, 10, 6756. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Mullighan, C.G. Pediatric acute lymphoblastic leukemia. Haematologica 2020, 105, 2524–2539. [Google Scholar] [CrossRef] [PubMed]
- Reedijk, A.M.J.; Coebergh, J.W.W.; de Groot-Kruseman, H.A.; van der Sluis, I.M.; Kremer, L.C.; Karim-Kos, H.E.; Pieters, R. Progress against childhood and adolescent acute lymphoblastic leukaemia in the Netherlands, 1990–2015. Leukemia 2021, 35, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Short, N.J.; Jain, N.; Haddad, F.G.; Welch, M.A.; Ravandi, F.; Kantarjian, H. The evolution of acute lymphoblastic leukemia research and therapy at MD Anderson over four decades. J. Hematol. Oncol. 2023, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Pinkel, D. Five-year follow-up of “total therapy” of childhood lymphocytic leukemia. JAMA 1971, 216, 648–652. [Google Scholar] [CrossRef] [PubMed]
- ALLTogether. ALLTogether1—A Treatment Study Protocol of the ALLTogether Consortium for Children and Young Adults (1–45 Years of Age) with Newly Diagnosed Acute Lymphoblastic Leukaemia (ALL); Cancer Research UK & UCL Cancer Trials Centre: London, UK, 2021. [Google Scholar]
- Secker-Walker, L.M.; Lawler, S.D.; Hardisty, R.M. Prognostic implications of chromosomal findings in acute lymphoblastic leukaemia at diagnosis. Br. Med. J. 1978, 2, 1529–1530. [Google Scholar] [CrossRef]
- Swansbury, G.J.; Secker-Walker, L.M.; Lawler, S.D.; Hardisty, R.M.; Sallan, S.E.; Garson, O.M.; Sakurai, M. Chromosomal findings in acute lymphoblastic leukaemia of childhood: An independent prognostic factor. Lancet 1981, 2, 249–250. [Google Scholar] [CrossRef]
- Bloomfield, C.D.; Lindquist, L.L.; Arthur, D.; McKenna, R.W.; LeBien, T.W.; Nesbit, M.E.; Peterson, B.A. Chromosomal abnormalities in acute lymphoblastic leukemia. Cancer Res. 1981, 41 11 Pt 2, 4838–4843. [Google Scholar]
- Shago, M. Recurrent Cytogenetic Abnormalities in Acute Lymphoblastic Leukemia. Methods Mol. Biol. 2017, 1541, 257–278. [Google Scholar] [PubMed]
- Williams, D.L.; Look, A.T.; Melvin, S.L.; Roberson, P.K.; Dahl, G.; Flake, T.; Stass, S. New chromosomal translocations correlate with specific immunophenotypes of childhood acute lymphoblastic leukemia. Cell 1984, 36, 101–109. [Google Scholar] [CrossRef]
- Roberts, K.G.; Mullighan, C.G. The Biology of B-Progenitor Acute Lymphoblastic Leukemia. Cold Spring Harb. Perspect. Med. 2020, 10, a034835. [Google Scholar] [CrossRef] [PubMed]
- Den Boer, M.L.; van Slegtenhorst, M.; De Menezes, R.X.; Cheok, M.H.; Buijs-Gladdines, J.G.; Peters, S.T.; Van Zutven, L.J.; Beverloo, H.B.; Van der Spek, P.J.; Escherich, G.; et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: A genome-wide classification study. Lancet Oncol. 2009, 10, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Su, X.; Zhang, J.; Radtke, I.; Phillips, L.A.; Miller, C.B.; Ma, J.; Liu, W.; Cheng, C.; Schulman, B.A.; et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N. Engl. J. Med. 2009, 360, 470–480. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.J.; Szczepanski, T.; de Bruijn, M.A.; van den Beemd, M.W.; de Bruin-Versteeg, S.; Wijkhuijs, J.M.; Tibbe, G.J.; van Gastel-Mol, E.J.; Groeneveld, K.; Hooijkaas, H. Detection of minimal residual disease in acute leukemia patients. Cytokines Mol. Ther. 1996, 2, 121–133. [Google Scholar]
- Pui, C.H.; Campana, D.; Evans, W.E. Childhood acute lymphoblastic leukaemia--current status and future perspectives. Lancet Oncol. 2001, 2, 597–607. [Google Scholar] [CrossRef]
- Moppett, J.; Burke, G.A.; Steward, C.G.; Oakhill, A.; Goulden, N.J. The clinical relevance of detection of minimal residual disease in childhood acute lymphoblastic leukaemia. J. Clin. Pathol. 2003, 56, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.N.; Steward, C.G.; Oakhill, A. The significance of detection of minimal residual disease in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 1993, 83, 412–418. [Google Scholar] [CrossRef]
- Coustan-Smith, E.; Behm, F.G.; Sanchez, J.; Boyett, J.M.; Hancock, M.L.; Raimondi, S.C.; Rubnitz, J.E.; Rivera, G.K.; Sandlund, J.T.; Pui, C.H.; et al. Immunological detection of minimal residual disease in children with acute lymphoblastic leukaemia. Lancet 1998, 351, 550–554. [Google Scholar] [CrossRef]
- Cavé, H.; van der Werff ten Bosch, J.; Suciu, S.; Guidal, C.; Waterkeyn, C.; Otten, J.; Bakkus, M.; Thielemans, K.; Grandchamp, B.; Vilmer, E. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer--Childhood Leukemia Cooperative Group. N. Engl. J. Med. 1998, 339, 591–598. [Google Scholar] [CrossRef]
- van Dongen, J.J.; Seriu, T.; Panzer-Grumayer, E.R.; Biondi, A.; Pongers-Willemse, M.J.; Corral, L.; Stolz, F.; Schrappe, M.; Masera, G.; Kamps, W.A.; et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet 1998, 352, 1731–1738. [Google Scholar] [CrossRef]
- Bruggemann, M.; Schrauder, A.; Raff, T.; Pfeifer, H.; Dworzak, M.; Ottmann, O.G.; Asnafi, V.; Baruchel, A.; Bassan, R.; Benoit, Y.; et al. Standardized MRD quantification in European ALL trials: Proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18–20 September 2008. Leukemia 2010, 24, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Conter, V.; Bartram, C.R.; Valsecchi, M.G.; Schrauder, A.; Panzer-Grumayer, R.; Moricke, A.; Arico, M.; Zimmermann, M.; Mann, G.; De Rossi, G.; et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: Results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 2010, 115, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Campana, D.; Pui, C.-H. Detection of Minimal Residual Disease in Acute Leukemia: Methodologic Advances and Clinical Significance. Blood 1995, 85, 1416–1434. [Google Scholar] [CrossRef]
- Gabert, J.; Beillard, E.; van der Velden, V.H.; Bi, W.; Grimwade, D.; Pallisgaard, N.; Barbany, G.; Cazzaniga, G.; Cayuela, J.M.; Cave, H.; et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—A Europe Against Cancer program. Leukemia 2003, 17, 2318–2357. [Google Scholar] [CrossRef]
- Szczepanski, T.; Orfao, A.; van der Velden, V.H.; San Miguel, J.F.; van Dongen, J.J. Minimal residual disease in leukaemia patients. Lancet Oncol. 2001, 2, 409–417. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.J.; van der Velden, V.H.; Bruggemann, M.; Orfao, A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: Need for sensitive, fast, and standardized technologies. Blood 2015, 125, 3996–4009. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, R.P.; Hoogeveen, P.G.; Bladergroen, R.; van Dijk, F.; Sonneveld, E.; van Leeuwen, F.N.; Boer, J.; Sergeeva, I.; Feitsma, H.; den Boer, M.L.; et al. Minimal residual disease (MRD) detection in acute lymphoblastic leukaemia based on fusion genes and genomic deletions: Towards MRD for all. Br. J. Haematol. 2021, 194, 888–892. [Google Scholar] [CrossRef]
- Height, S.E.; Swansbury, G.J.; Matutes, E.; Treleaven, J.G.; Catovsky, D.; Dyer, M.J. Analysis of clonal rearrangements of the Ig heavy chain locus in acute leukemia. Blood 1996, 87, 5242–5250. [Google Scholar] [CrossRef]
- van Dongen, J.J.; Wolvers-Tettero, I.L. Analysis of immunoglobulin and T cell receptor genes. Part II: Possibilities and limitations in the diagnosis and management of lymphoproliferative diseases and related disorders. Clin. Chim. Acta 1991, 198, 93–174. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, V.H.; Panzer-Grumayer, E.R.; Cazzaniga, G.; Flohr, T.; Sutton, R.; Schrauder, A.; Basso, G.; Schrappe, M.; Wijkhuijs, J.M.; Konrad, M.; et al. Optimization of PCR-based minimal residual disease diagnostics for childhood acute lymphoblastic leukemia in a multi-center setting. Leukemia 2007, 21, 706–713. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, V.H.; van Dongen, J.J. MRD detection in acute lymphoblastic leukemia patients using Ig/TCR gene rearrangements as targets for real-time quantitative PCR. Methods Mol. Biol. 2009, 538, 115–150. [Google Scholar] [PubMed]
- Svaton, M.; Skotnicova, A.; Reznickova, L.; Rennerova, A.; Valova, T.; Kotrova, M.; van der Velden, V.H.J.; Bruggemann, M.; Darzentas, N.; Langerak, A.W.; et al. NGS better discriminates true MRD positivity for the risk stratification of childhood ALL treated on an MRD-based protocol. Blood 2023, 141, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Kotrova, M.; van der Velden, V.H.J.; van Dongen, J.J.M.; Formankova, R.; Sedlacek, P.; Bruggemann, M.; Zuna, J.; Stary, J.; Trka, J.; Fronkova, E. Next-generation sequencing indicates false-positive MRD results and better predicts prognosis after SCT in patients with childhood ALL. Bone Marrow Transplant. 2017, 52, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Pulsipher, M.A.; Carlson, C.; Langholz, B.; Wall, D.A.; Schultz, K.R.; Bunin, N.; Kirsch, I.; Gastier-Foster, J.M.; Borowitz, M.; Desmarais, C.; et al. IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood 2015, 125, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, N.; Lim, E.H.; Chin, W.H.N.; Lu, Y.; Chiew, K.H.; Kham, S.K.Y.; Yang, W.; Quah, T.C.; Lin, H.P.; et al. Identifying IGH disease clones for MRD monitoring in childhood B-cell acute lymphoblastic leukemia using RNA-Seq. Leukemia 2020, 34, 2418–2429. [Google Scholar] [CrossRef]
- Faham, M.; Zheng, J.; Moorhead, M.; Carlton, V.E.; Stow, P.; Coustan-Smith, E.; Pui, C.H.; Campana, D. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood 2012, 120, 5173–5180. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, M.C.; van der Burg, M.; de Ridder, D.; Barendregt, B.H.; de Haas, E.F.; Reinders, M.J.; Lankester, A.C.; Revesz, T.; Staal, F.J.; van Dongen, J.J. Ig gene rearrangement steps are initiated in early human precursor B cell subsets and correlate with specific transcription factor expression. J. Immunol. 2005, 175, 5912–5922. [Google Scholar] [CrossRef]
- Kehrl, J.H.; Riva, A.; Wilson, G.L.; Thevenin, C. Molecular mechanisms regulating CD19, CD20 and CD22 gene expression. Immunol. Today 1994, 15, 432–436. [Google Scholar] [CrossRef]
- Zhou, L.J.; Ord, D.C.; Omori, S.A.; Tedder, T.F. Structure of the genes encoding the CD19 antigen of human and mouse B lymphocytes. Immunogenetics 1992, 35, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Tedder, T.F.; Klejman, G.; Schlossman, S.F.; Saito, H. Structure of the gene encoding the human B lymphocyte differentiation antigen CD20 (B1). J. Immunol. 1989, 142, 2560–2568. [Google Scholar] [CrossRef]
- Hurwitz, C.A.; Loken, M.R.; Graham, M.L.; Karp, J.E.; Borowitz, M.J.; Pullen, D.J.; Civin, C.I. Asynchronous antigen expression in B lineage acute lymphoblastic leukemia. Blood 1988, 72, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Dworzak, M.N.; Froschl, G.; Printz, D.; Mann, G.; Potschger, U.; Muhlegger, N.; Fritsch, G.; Gadner, H.; Austrian Berlin-Frankfurt-Munster Study Group. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood 2002, 99, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Lucio, P.; Parreira, A.; van den Beemd, M.W.; van Lochem, E.G.; van Wering, E.R.; Baars, E.; Porwit-MacDonald, A.; Bjorklund, E.; Gaipa, G.; Biondi, A.; et al. Flow cytometric analysis of normal B cell differentiation: A frame of reference for the detection of minimal residual disease in precursor-B-ALL. Leukemia 1999, 13, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Ciudad, J.; San Miguel, J.F.; Lopez-Berges, M.C.; Vidriales, B.; Valverde, B.; Ocqueteau, M.; Mateos, G.; Caballero, M.D.; Hernandez, J.; Moro, M.J.; et al. Prognostic value of immunophenotypic detection of minimal residual disease in acute lymphoblastic leukemia. J. Clin. Oncol. 1998, 16, 3774–3781. [Google Scholar] [CrossRef] [PubMed]
- Coustan-Smith, E.; Sancho, J.; Hancock, M.L.; Boyett, J.M.; Behm, F.G.; Raimondi, S.C.; Sandlund, J.T.; Rivera, G.K.; Rubnitz, J.E.; Ribeiro, R.C.; et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood 2000, 96, 2691–2696. [Google Scholar] [CrossRef]
- Ciudad, J.; San Miguel, J.F.; Lopez-Berges, M.C.; Garcia Marcos, M.A.; Gonzalez, M.; Vazquez, L.; del Canizo, M.C.; Lopez, A.; Van Dongen, J.J.; Orfao, A. Detection of abnormalities in B-cell differentiation pattern is a useful tool to predict relapse in precursor-B-ALL. Br. J. Haematol. 1999, 104, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, F.; Piro-Noack, M.; Kaib, N.; Falk, M.; Renziehausen, A.; Troff, C.; Grove, D.; Schnittger, S.; Buchner, T.; Ritter, J.; et al. Leukaemia-associated immunophenotypes (LAIP) are observed in 90% of adult and childhood acute lymphoblastic leukaemia: Detection in remission marrow predicts outcome. Br. J. Haematol. 1999, 105, 241–255. [Google Scholar]
- Gokbuget, N.; Hoelzer, D. Treatment with monoclonal antibodies in acute lymphoblastic leukemia: Current knowledge and future prospects. Ann. Hematol. 2004, 83, 201–205. [Google Scholar] [CrossRef]
- Szczepanski, T.; van der Velden, V.H.; van Dongen, J.J. Classification systems for acute and chronic leukaemias. Best. Pract. Res. Clin. Haematol. 2003, 16, 561–582. [Google Scholar] [CrossRef]
- Chiaretti, S.; Zini, G.; Bassan, R. Diagnosis and subclassification of acute lymphoblastic leukemia. Mediterr. J. Hematol. Infect. Dis. 2014, 6, e2014073. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Eaves, C.J.; Lansdorp, P.M. Expression of CD34 on human B cell precursors. Clin. Exp. Immunol. 1991, 85, 168–173. [Google Scholar] [CrossRef]
- De Zen, L.; Orfao, A.; Cazzaniga, G.; Masiero, L.; Cocito, M.G.; Spinelli, M.; Rivolta, A.; Biondi, A.; Zanesco, L.; Basso, G. Quantitative multiparametric immunophenotyping in acute lymphoblastic leukemia: Correlation with specific genotype. I. ETV6/AML1 ALLs identification. Leukemia 2000, 14, 1225–1231. [Google Scholar] [CrossRef]
- Caldwell, C.W.; Patterson, W.P. Relationship between CD45 antigen expression and putative stages of differentiation in B-cell malignancies. Am. J. Hematol. 1991, 36, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Perez, C.; Carmona, M.; Benabdellah, K.; Herrera, C. Failure of ALL recognition by CAR T cells: A review of CD 19-negative relapses after anti-CD 19 CAR-T treatment in B-ALL. Front. Immunol. 2023, 14, 1165870. [Google Scholar] [CrossRef] [PubMed]
- Sotillo, E.; Barrett, D.M.; Black, K.L.; Bagashev, A.; Oldridge, D.; Wu, G.; Sussman, R.; Lanauze, C.; Ruella, M.; Gazzara, M.R.; et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015, 5, 1282–1295. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Q.; Liang, X.; Chen, Z.; Zhang, X.; Zhou, X.; Li, M.; Tu, H.; Liu, Y.; Tu, S.; et al. Mechanisms of Relapse After CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies. Front. Immunol. 2019, 10, 2664. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.; Miller, V.; McCullouch, V.; Dougherty, K.; Fromm, J.R.; Wood, B.L. A novel flow cytometric assay for detection of residual disease in patients with B-lymphoblastic leukemia/lymphoma post anti-CD19 therapy. Cytom. B Clin. Cytom. 2018, 94, 112–120. [Google Scholar] [CrossRef]
- Mikhailova, E.; Itov, A.; Zerkalenkova, E.; Roumiantseva, J.; Olshanskaya, Y.; Karachunskiy, A.; Novichkova, G.; Maschan, M.; Popov, A. B-lineage antigens that are useful to substitute CD19 for minimal residual disease monitoring in B cell precursor acute lymphoblastic leukemia after CD19 targeting. Cytom. B Clin. Cytom. 2022, 102, 353–359. [Google Scholar] [CrossRef]
- Coustan-Smith, E.; Song, G.; Clark, C.; Key, L.; Liu, P.; Mehrpooya, M.; Stow, P.; Su, X.; Shurtleff, S.; Pui, C.H.; et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood 2011, 117, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Veltroni, M.; De Zen, L.; Sanzari, M.C.; Maglia, O.; Dworzak, M.N.; Ratei, R.; Biondi, A.; Basso, G.; Gaipa, G.; Group, I.B.-A.-F.-M.-S. Expression of CD58 in normal, regenerating and leukemic bone marrow B cells: Implications for the detection of minimal residual disease in acute lymphocytic leukemia. Haematologica 2003, 88, 1245–1252. [Google Scholar] [PubMed]
- Lee, R.V.; Braylan, R.C.; Rimsza, L.M. CD58 expression decreases as nonmalignant B cells mature in bone marrow and is frequently overexpressed in adult and pediatric precursor B-cell acute lymphoblastic leukemia. Am. J. Clin. Pathol. 2005, 123, 119–124. [Google Scholar] [CrossRef]
- Boccuni, P.; Di Noto, R.; Lo Pardo, C.; Villa, M.R.; Ferrara, F.; Rotoli, B.; Del Vecchio, L. CD66c antigen expression is myeloid restricted in normal bone marrow but is a common feature of CD10+ early-B-cell malignancies. Tissue Antigens 1998, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ratei, R.; Karawajew, L.; Schabath, R.; Ehrfeldt, A.; Grunert, F.; Ludwig, W.D. Differential expression of the carcinoembryonic antigen-related cell adhesion molecules panCD66, CD66a, CD66c and of sialyl-Lewis x (CD15s) on blast cells of acute leukemias. Int. J. Hematol. 2008, 87, 137–143. [Google Scholar] [CrossRef]
- Guillaume, N.; Penther, D.; Vaida, I.; Gruson, B.; Harrivel, V.; Claisse, J.F.; Capiod, J.C.; Lefrere, J.J.; Damaj, G. CD66c expression in B-cell acute lymphoblastic leukemia: Strength and weakness. Int. J. Lab. Hematol. 2011, 33, 92–96. [Google Scholar] [CrossRef]
- Kiyokawa, N.; Iijima, K.; Tomita, O.; Miharu, M.; Hasegawa, D.; Kobayashi, K.; Okita, H.; Kajiwara, M.; Shimada, H.; Inukai, T.; et al. Significance of CD66c expression in childhood acute lymphoblastic leukemia. Leuk. Res. 2014, 38, 42–48. [Google Scholar] [CrossRef]
- Tang, G.S.; Wu, J.; Liu, M.; Chen, H.; Gong, S.G.; Yang, J.M.; Hu, X.X.; Wang, J.M. BCR-ABL1 and CD66c exhibit high concordance in minimal residual disease detection of adult B-acute lymphoblastic leukemia. Am. J. Transl. Res. 2015, 7, 632–639. [Google Scholar]
- Sedek, L.; Theunissen, P.; da Costa, E.S.; van der Sluijs-Gelling, A.; Mejstrikova, E.; Gaipa, G.; Sonsala, A.; Twardoch, M.; Oliveira, E.; Novakova, M.; et al. Differential expression of CD73, CD86 and CD304 in normal vs. leukemic B-cell precursors and their utility as stable minimal residual disease markers in childhood B-cell precursor acute lymphoblastic leukemia. J. Immunol. Methods 2019, 475, 112429. [Google Scholar] [CrossRef]
- Wang, W.; Gao, L.; Li, Y.; Li, Z.L.; Gong, M.; Huang, F.Z.; Chen, Y.R.; Zhang, C.X.; Gao, Y.Y.; Ma, Y.G. The application of CD73 in minimal residual disease monitoring using flow cytometry in B-cell acute lymphoblastic leukemia. Leuk. Lymphoma 2016, 57, 1174–1181. [Google Scholar] [CrossRef]
- Arumugam, J.R.; Bommannan, K.; Radhakrishnan, V.; Sagar, T.G.; Sundersingh, S. Immunophenotypic expression and immunomodulation in minimal residual disease analysis of pediatric B acute lymphoblastic leukemia by high sensitive flow cytometry. Leuk. Lymphoma 2022, 63, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Muzzafar, T.; Medeiros, L.J.; Wang, S.A.; Brahmandam, A.; Thomas, D.A.; Jorgensen, J.L. Aberrant underexpression of CD81 in precursor B-cell acute lymphoblastic leukemia: Utility in detection of minimal residual disease by flow cytometry. Am. J. Clin. Pathol. 2009, 132, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Gupta, R.; Gupta, S.K.; Bakhshi, S.; Malhotra, A.; Rai, S.; Singh, S.; Prajapati, V.K.; Sahoo, R.K.; Gogia, A.; et al. A Real-world Perspective of CD123 Expression in Acute Leukemia as Promising Biomarker to Predict Treatment Outcome in B-ALL and AML. Clin. Lymphoma Myeloma Leuk. 2020, 20, e673–e684. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, N.M.; Alcancia, F.; Perkinson, K.R.; Buckley, P.J.; Lagoo, A.S. Distinct expression patterns of CD123 and CD34 on normal bone marrow B-cell precursors (“hematogones”) and B lymphoblastic leukemia blasts. Am. J. Clin. Pathol. 2009, 132, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, M.A.; Kamal, H.M.; El Shabrawy, D.A.; Esh, A.M.; Sattar, R.H. Significance of CD34/CD123 expression in detection of minimal residual disease in B-ACUTE lymphoblastic leukemia in children. Blood Cells Mol. Dis. 2016, 59, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bras, A.E.; de Haas, V.; van Stigt, A.; Jongen-Lavrencic, M.; Beverloo, H.B.; Te Marvelde, J.G.; Zwaan, C.M.; van Dongen, J.J.M.; Leusen, J.H.W.; van der Velden, V.H.J. CD123 expression levels in 846 acute leukemia patients based on standardized immunophenotyping. Cytom. B Clin. Cytom. 2019, 96, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Djokic, M.; Bjorklund, E.; Blennow, E.; Mazur, J.; Soderhall, S.; Porwit, A. Overexpression of CD123 correlates with the hyperdiploid genotype in acute lymphoblastic leukemia. Haematologica 2009, 94, 1016–1019. [Google Scholar] [PubMed]
- Li, Z.; Chu, X.; Gao, L.; Ling, J.; Xiao, P.; Lu, J.; Wang, Y.; He, H.; Li, J.; Hu, Y.; et al. High Expression of Interleukin-3 Receptor Alpha Chain (CD123) Predicts Favorable Outcome in Pediatric B-Cell Acute Lymphoblastic Leukemia Lacking Prognosis-Defining Genomic Aberrations. Front. Oncol. 2021, 11, 614420. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, G.; Dudakia, V.; Ghogale, S.; Deshpande, N.; Girase, K.; Chaturvedi, A.; Shetty, D.; Senger, M.; Jain, H.; Bagal, B.; et al. Expression of CD304/neuropilin-1 in adult b-cell lymphoblastic leukemia/lymphoma and its utility for the measurable residual disease assessment. Int. J. Lab. Hematol. 2021, 43, 990–999. [Google Scholar] [CrossRef]
- Abaza, H.M.; Alfeky, M.A.A.; Eissa, D.S.; Abdel Fattah, M.F.; Annaka, L.M.; Ebeid, F.S. Neuropilin-1/CD304 Expression by Flow Cytometry in Pediatric Precursor B-Acute Lymphoblastic Leukemia: A Minimal Residual Disease and Potential Prognostic Marker. J. Pediatr. Hematol. Oncol. 2018, 40, 200–207. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, X.H.; Song, Y.L.; Zhou, Y.C.; Cai, R.Z.; Chi, P.D. Evaluation of diagnostic efficacy of NRP-1/CD304 in hematological diseases. Cancer Med. 2023, 12, 11284–11292. [Google Scholar] [CrossRef]
- Solly, F.; Angelot, F.; Garand, R.; Ferrand, C.; Seilles, E.; Schillinger, F.; Decobecq, A.; Billot, M.; Larosa, F.; Plouvier, E.; et al. CD304 is preferentially expressed on a subset of B-lineage acute lymphoblastic leukemia and represents a novel marker for minimal residual disease detection by flow cytometry. Cytom. A 2012, 81, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ciudad, J.; Orfao, A.; Vidriales, B.; Macedo, A.; Martinez, A.; Gonzalez, M.; Lopez-Berges, M.C.; Valverde, B.; San Miguel, J.F. Immunophenotypic analysis of CD19+ precursors in normal human adult bone marrow: Implications for minimal residual disease detection. Haematologica 1998, 83, 1069–1075. [Google Scholar] [PubMed]
- Lucio, P.; Gaipa, G.; van Lochem, E.G.; van Wering, E.R.; Porwit-MacDonald, A.; Faria, T.; Bjorklund, E.; Biondi, A.; van den Beemd, M.W.; Baars, E.; et al. BIOMED-I concerted action report: Flow cytometric immunophenotyping of precursor B-ALL with standardized triple-stainings. BIOMED-1 Concerted Action Investigation of Minimal Residual Disease in Acute Leukemia: International Standardization and Clinical Evaluation. Leukemia 2001, 15, 1185–1192. [Google Scholar] [PubMed]
- Weir, E.G.; Cowan, K.; LeBeau, P.; Borowitz, M.J. A limited antibody panel can distinguish B-precursor acute lymphoblastic leukemia from normal B precursors with four color flow cytometry: Implications for residual disease detection. Leukemia 1999, 13, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Mlcáková, A.; Babusíková, O. Multiparametric flow cytometry in detection of minimal residual disease in acute lymphoblastic leukemia of early B-cell phenotype. Neoplasma 2003, 50, 416–421. [Google Scholar]
- Irving, J.; Jesson, J.; Virgo, P.; Case, M.; Minto, L.; Eyre, L.; Noel, N.; Johansson, U.; Macey, M.; Knotts, L.; et al. Establishment and validation of a standard protocol for the detection of minimal residual disease in B lineage childhood acute lymphoblastic leukemia by flow cytometry in a multi-center setting. Haematologica 2009, 94, 870–874. [Google Scholar] [CrossRef] [PubMed]
- van Wering, E.R.; van der Linden-Schrever, B.E.; Szczepanski, T.; Willemse, M.J.; Baars, E.A.; van Wijngaarde-Schmitz, H.M.; Kamps, W.A.; van Dongen, J.J. Regenerating normal B-cell precursors during and after treatment of acute lymphoblastic leukaemia: Implications for monitoring of minimal residual disease. Br. J. Haematol. 2000, 110, 139–146. [Google Scholar] [CrossRef]
- De Waele, M.; Renmans, W.; Jochmans, K.; Schots, R.; Lacor, P.; Trullemans, F.; Otten, J.; Balduck, N.; Vander Gucht, K.; Van Camp, B.; et al. Different expression of adhesion molecules on CD34+ cells in AML and B-lineage ALL and their normal bone marrow counterparts. Eur. J. Haematol. 1999, 63, 192–201. [Google Scholar] [CrossRef]
- Mirkowska, P.; Hofmann, A.; Sedek, L.; Slamova, L.; Mejstrikova, E.; Szczepanski, T.; Schmitz, M.; Cario, G.; Stanulla, M.; Schrappe, M.; et al. Leukemia surfaceome analysis reveals new disease-associated features. Blood 2013, 121, e149–e159. [Google Scholar] [CrossRef]
- Denys, B.; van der Sluijs-Gelling, A.J.; Homburg, C.; van der Schoot, C.E.; de Haas, V.; Philippe, J.; Pieters, R.; van Dongen, J.J.; van der Velden, V.H. Improved flow cytometric detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia 2013, 27, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Shaver, A.C.; Greig, B.W.; Mosse, C.A.; Seegmiller, A.C. B-ALL minimal residual disease flow cytometry: An application of a novel method for optimization of a single-tube model. Am. J. Clin. Pathol. 2015, 143, 716–724. [Google Scholar] [CrossRef]
- Borowitz, M.J.; Wood, B.L.; Devidas, M.; Loh, M.L.; Raetz, E.A.; Salzer, W.L.; Nachman, J.B.; Carroll, A.J.; Heerema, N.A.; Gastier-Foster, J.M.; et al. Prognostic significance of minimal residual disease in high risk B-ALL: A report from Children’s Oncology Group study AALL0232. Blood 2015, 126, 964–971. [Google Scholar] [CrossRef]
- Weng, X.Q.; Shen, Y.; Sheng, Y.; Chen, B.; Wang, J.H.; Li, J.M.; Mi, J.Q.; Chen, Q.S.; Zhu, Y.M.; Jiang, C.L.; et al. Prognostic significance of monitoring leukemia-associated immunophenotypes by eight-color flow cytometry in adult B-acute lymphoblastic leukemia. Blood Cancer J. 2013, 3, e133. [Google Scholar] [CrossRef]
- Bouriche, L.; Bernot, D.; Nivaggioni, V.; Arnoux, I.; Loosveld, M. Detection of Minimal Residual Disease in B Cell Acute Lymphoblastic Leukemia Using an Eight-Color Tube with Dried Antibody Reagents. Cytom. B Clin. Cytom. 2019, 96, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Stow, P.; Key, L.; Chen, X.; Pan, Q.; Neale, G.A.; Coustan-Smith, E.; Mullighan, C.G.; Zhou, Y.; Pui, C.H.; Campana, D. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood 2010, 115, 4657–4663. [Google Scholar] [CrossRef]
- Theunissen, P.; Mejstrikova, E.; Sedek, L.; van der Sluijs-Gelling, A.J.; Gaipa, G.; Bartels, M.; da Costa, E.S.; Kotrova, M.; Novakova, M.; Sonneveld, E.; et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood 2017, 129, 347–357. [Google Scholar] [CrossRef]
- FDA. FDA Grants Regular Approval to Blinatumomab and Expands Indication to Include Philadelphia Chromosome-Positive B Cell; FDA: Delaware, PA, USA, 2017. [Google Scholar]
- Liu, H.; Xi, R.; Mao, D.; Zhao, X.; Wu, T. Efficacy and Safety of Blinatumomab for the Treatment of Relapsed/Refractory Acute Lymphoblastic Leukemia: A Systemic Review and Meta-Analysis. Clin. Lymphoma Myeloma Leuk. 2023, 23, e139–e149. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef]
- Gardner, R.A.; Finney, O.; Annesley, C.; Brakke, H.; Summers, C.; Leger, K.; Bleakley, M.; Brown, C.; Mgebroff, S.; Kelly-Spratt, K.S.; et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017, 129, 3322–3331. [Google Scholar]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Hu, Y.; Pu, C.; Yu, J.; Luo, Y.; Shi, J.; Cui, Q.; Wu, W.; Wang, J.; Xiao, L.; et al. CD19 targeted CAR-T therapy versus chemotherapy in re-induction treatment of refractory/relapsed acute lymphoblastic leukemia: Results of a case-controlled study. Ann. Hematol. 2018, 97, 781–789. [Google Scholar] [CrossRef]
- Zinzi, A.; Gaio, M.; Liguori, V.; Cagnotta, C.; Paolino, D.; Paolisso, G.; Castaldo, G.; Nicoletti, G.F.; Rossi, F.; Capuano, A.; et al. Late relapse after CAR-T cell therapy for adult patients with hematologic malignancies: A definite evidence from systematic review and meta-analysis on individual data. Pharmacol. Res. 2023, 190, 106742. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Zhu, M.; Huang, H.; Hu, Y. Relapse after CAR-T cell therapy in B-cell malignancies: Challenges and future approaches. J. Zhejiang Univ. Sci. B 2022, 23, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Ruella, M.; Barrett, D.M.; Kenderian, S.S.; Shestova, O.; Hofmann, T.J.; Perazzelli, J.; Klichinsky, M.; Aikawa, V.; Nazimuddin, F.; Kozlowski, M.; et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J. Clin. Investig. 2016, 126, 3814–3826. [Google Scholar] [CrossRef] [PubMed]
- Braig, F.; Brandt, A.; Goebeler, M.; Tony, H.P.; Kurze, A.K.; Nollau, P.; Bumm, T.; Bottcher, S.; Bargou, R.C.; Binder, M. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood 2017, 129, 100–104. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, M.C.; Smet, J.; Adams, B.; Mascart, F.; Schandene, L.; Janssen, F.; Ferster, A.; Kuo, C.C.; Levy, S.; van Dongen, J.J.; et al. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J. Clin. Investig. 2010, 120, 1265–1274. [Google Scholar] [CrossRef]
- Hamieh, M.; Dobrin, A.; Cabriolu, A.; van der Stegen, S.J.C.; Giavridis, T.; Mansilla-Soto, J.; Eyquem, J.; Zhao, Z.; Whitlock, B.M.; Miele, M.M.; et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 2019, 568, 112–116. [Google Scholar] [CrossRef]
- Rayes, A.; McMasters, R.L.; O’Brien, M.M. Lineage Switch in MLL-Rearranged Infant Leukemia Following CD19-Directed Therapy. Pediatr. Blood Cancer 2016, 63, 1113–1115. [Google Scholar] [CrossRef]
- Zoghbi, A.; Zur Stadt, U.; Winkler, B.; Muller, I.; Escherich, G. Lineage switch under blinatumomab treatment of relapsed common acute lymphoblastic leukemia without MLL rearrangement. Pediatr. Blood Cancer 2017, 64, e26594. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, E.; Nguyen, S.M.; Fountaine, T.J.; Welp, K.; Gryder, B.; Qin, H.; Yang, Y.; Chien, C.D.; Seif, A.E.; Lei, H.; et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat. Commun. 2016, 7, 12320. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Lin, Y.; Liu, D.; Tong, C.; Liu, S. CAR-T therapy as a consolidation in remission B-ALL patients with poor prognosis. Cancer Rep. 2022, 5, e1706. [Google Scholar] [CrossRef] [PubMed]
- Hodder, A.; Mishra, A.K.; Enshaei, A.; Baird, S.; Elbeshlawi, I.; Bonney, D.; Clesham, K.; Cummins, M.; Vedi, A.; Gibson, B.; et al. Blinatumomab for First-Line Treatment of Children and Young Persons with B-ALL. J. Clin. Oncol. 2023, 42, 907–914. [Google Scholar]
- Mikhailova, E.; Roumiantseva, J.; Illarionova, O.; Lagoyko, S.; Miakova, N.; Zerkalenkova, E.; Zharikova, L.; Olshanskaya, Y.; Novichkova, G.; Maschan, M.; et al. Strong expansion of normal CD19-negative B-cell precursors after the use of blinatumomab in the first-line therapy of acute lymphoblastic leukaemia in children. Br. J. Haematol. 2022, 196, e6–e9. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Highfill, S.L.; Shalabi, H.; Yates, B.; Jin, J.; Wolters, P.L.; Ombrello, A.; Steinberg, S.M.; Martin, S.; Delbrook, C.; et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J. Clin. Oncol. 2020, 38, 1938–1950. [Google Scholar] [PubMed]
- Pennesi, E.; Michels, N.; Brivio, E.; van der Velden, V.H.J.; Jiang, Y.; Thano, A.; Ammerlaan, A.J.C.; Boer, J.M.; Beverloo, H.B.; Sleight, B.; et al. Inotuzumab ozogamicin as single agent in pediatric patients with relapsed and refractory acute lymphoblastic leukemia: Results from a phase II trial. Leukemia 2022, 36, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, M.W.C.; Buracchi, C.; Laqua, A.; Nierkens, S.; Sedek, L.; Flores-Montero, J.; Hofmans, M.; Sobral de Costa, E.; Novakova, M.; Mejstrikova, E.; et al. Flow cytometric minimal residual disease assessment in B-cell precursor acute lymphoblastic leukaemia patients treated with CD19-targeted therapies—A EuroFlow study. Br. J. Haematol. 2022, 197, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.; Illarionova, O.; Komkov, A.; Zerkalenkova, E.; Mamedov, I.; Shelikhova, L.; Olshanskaya, Y.; Miakova, N.; Novichkova, G.; Karachunskiy, A.; et al. Reliable Flow-Cytometric Approach for Minimal Residual Disease Monitoring in Patients with B-Cell Precursor Acute Lymphoblastic Leukemia after CD19-Targeted Therapy. Cancers 2022, 14, 5445. [Google Scholar] [CrossRef]
- Singh, J.; Gorniak, M.; Grigoriadis, G.; Westerman, D.; McBean, M.; Venn, N.; Law, T.; Sutton, R.; Morgan, S.; Fleming, S. Correlation between a 10-color flow cytometric measurable residual disease (MRD) analysis and molecular MRD in adult B-acute lymphoblastic leukemia. Cytom. B Clin. Cytom. 2022, 102, 115–122. [Google Scholar] [CrossRef]
- Chatterjee, G.; Dhende, P.; Raj, S.; Shetty, V.; Ghogale, S.; Deshpande, N.; Girase, K.; Patil, J.; Kalra, A.; Narula, G.; et al. 15-color highly sensitive flow cytometry assay for post anti-CD19 targeted therapy (anti-CD19-CAR-T and blinatumomab) measurable residual disease assessment in B-lymphoblastic leukemia/lymphoma: Real-world applicability and challenges. Eur. J. Haematol. 2023, 112, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, Y.; Aypar, U.; Baik, J.; Londono, D.; Sun, X.; Zhang, J.; Zhang, Y.; Roshal, M. Highly sensitive single tube B-lymphoblastic leukemia/lymphoma minimal/measurable residual disease test robust to surface antigen directed therapy. Cytom. B Clin. Cytom. 2023, 104, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, M.W.C.; Rodriguez, B.S.; Sedek, L.; Laqua, A.; Buracchi, C.; Buysse, M.; Reiterova, M.; Oliveira, E.; Morf, D.; Oude Alink, S.R.; et al. Minimal residual disease assessment in B-cell precursor acute lymphoblastic leukemia by semi-automated identification of normal hematopoietic cells: A EuroFlow study. Cytom. B Clin. Cytom. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Sieger, T.; Schumich, A.; Wood, B.; Irving, J.; Mejstrikova, E.; Dworzak, M.N. Detection and monitoring of normal and leukemic cell populations with hierarchical clustering of flow cytometry data. Cytom. A 2012, 81, 25–34. [Google Scholar] [CrossRef] [PubMed]
- DiGiuseppe, J.A.; Tadmor, M.D.; Pe’er, D. Detection of minimal residual disease in B lymphoblastic leukemia using viSNE. Cytom. B Clin. Cytom. 2015, 88, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Reiter, M.; Diem, M.; Schumich, A.; Maurer-Granofszky, M.; Karawajew, L.; Rossi, J.G.; Ratei, R.; Groeneveld-Krentz, S.; Sajaroff, E.O.; Suhendra, S.; et al. Automated Flow Cytometric MRD Assessment in Childhood Acute B- Lymphoblastic Leukemia Using Supervised Machine Learning. Cytom. A 2019, 95, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Wodlinger, M.; Reiter, M.; Weijler, L.; Maurer-Granofszky, M.; Schumich, A.; Sajaroff, E.O.; Groeneveld-Krentz, S.; Rossi, J.G.; Karawajew, L.; Ratei, R.; et al. Automated identification of cell populations in flow cytometry data with transformers. Comput. Biol. Med. 2022, 144, 105314. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Liu, L.; Setiadi, A.; Al-Bakri, M.; Vercauteren, S. Machine learning optimized multiparameter radar plots for B-cell acute lymphoblastic leukemia minimal residual disease analysis. Cytom. B Clin. Cytom. 2022, 102, 342–352. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbeek, M.W.C.; van der Velden, V.H.J. The Evolving Landscape of Flowcytometric Minimal Residual Disease Monitoring in B-Cell Precursor Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2024, 25, 4881. https://doi.org/10.3390/ijms25094881
Verbeek MWC, van der Velden VHJ. The Evolving Landscape of Flowcytometric Minimal Residual Disease Monitoring in B-Cell Precursor Acute Lymphoblastic Leukemia. International Journal of Molecular Sciences. 2024; 25(9):4881. https://doi.org/10.3390/ijms25094881
Chicago/Turabian StyleVerbeek, Martijn W. C., and Vincent H. J. van der Velden. 2024. "The Evolving Landscape of Flowcytometric Minimal Residual Disease Monitoring in B-Cell Precursor Acute Lymphoblastic Leukemia" International Journal of Molecular Sciences 25, no. 9: 4881. https://doi.org/10.3390/ijms25094881
APA StyleVerbeek, M. W. C., & van der Velden, V. H. J. (2024). The Evolving Landscape of Flowcytometric Minimal Residual Disease Monitoring in B-Cell Precursor Acute Lymphoblastic Leukemia. International Journal of Molecular Sciences, 25(9), 4881. https://doi.org/10.3390/ijms25094881





