Glucosamine and Silibinin Alter Cartilage Homeostasis through Glycosylation and Cellular Stresses in Human Chondrocyte Cells
Abstract
:1. Introduction
2. Results
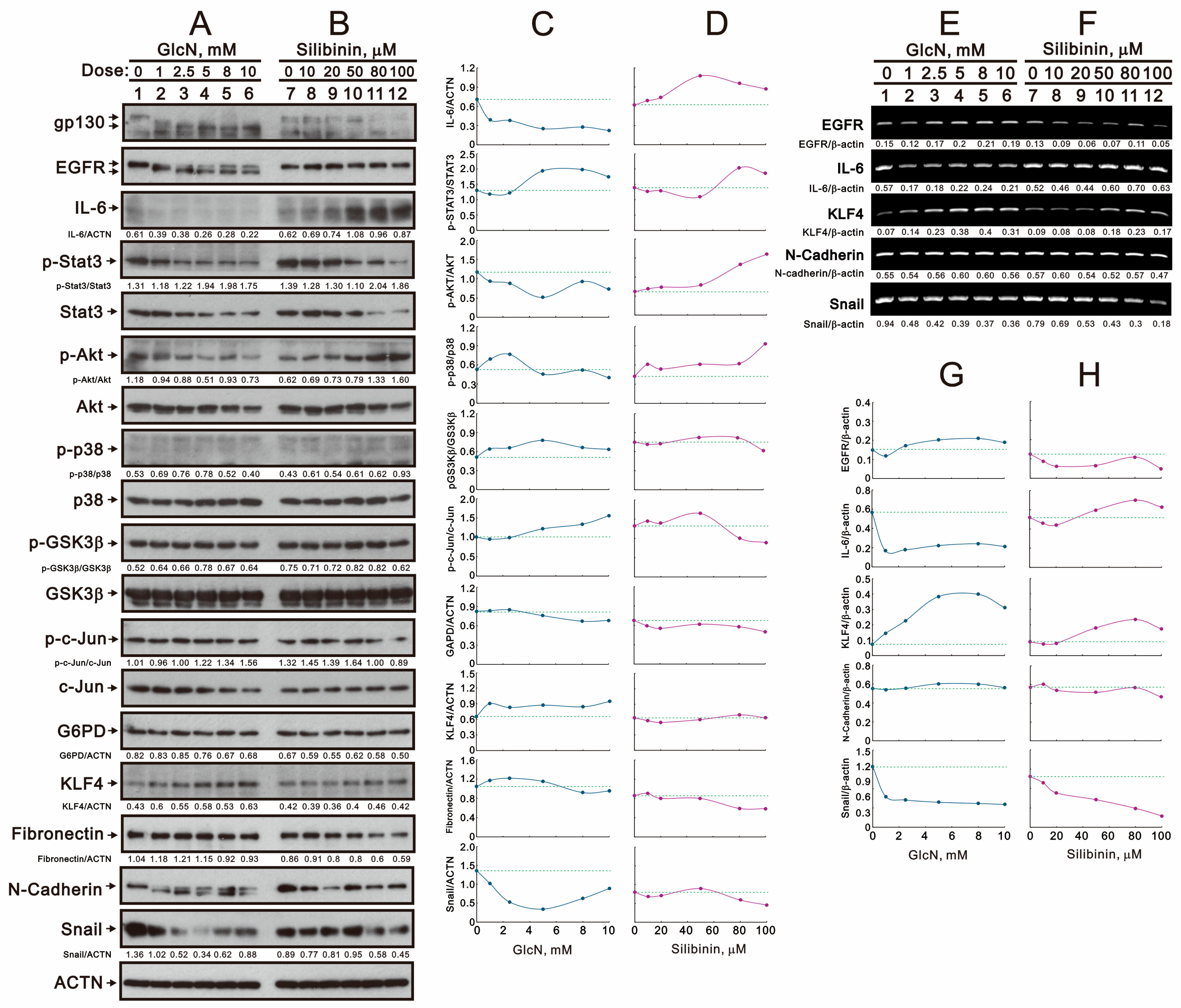
2.1. The Effects of Glucosamine and Silibinin on the Membrane Proteins and Related Signaling Pathways Were Examined in Human TC28a2 Chondrocyte Cells
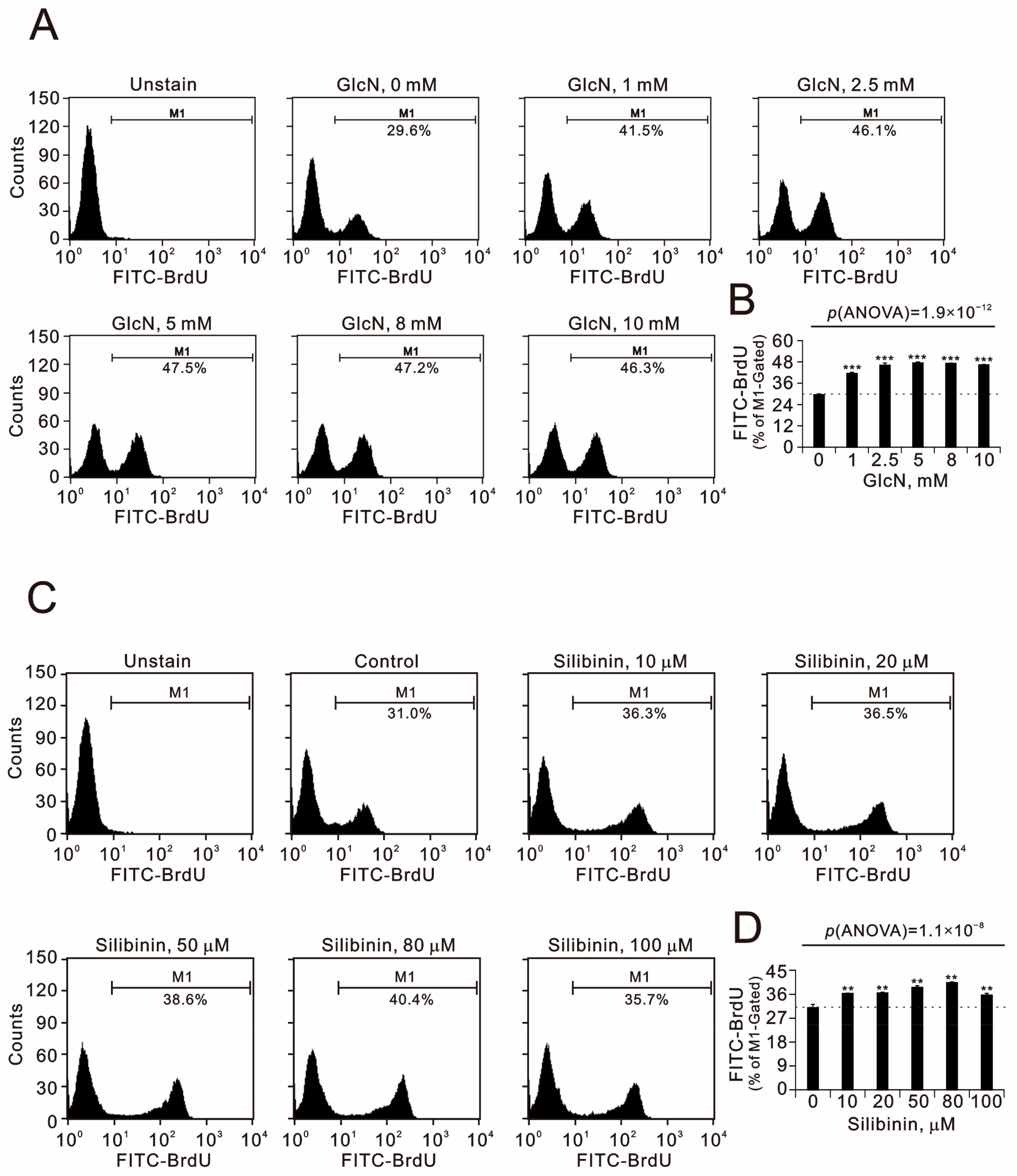
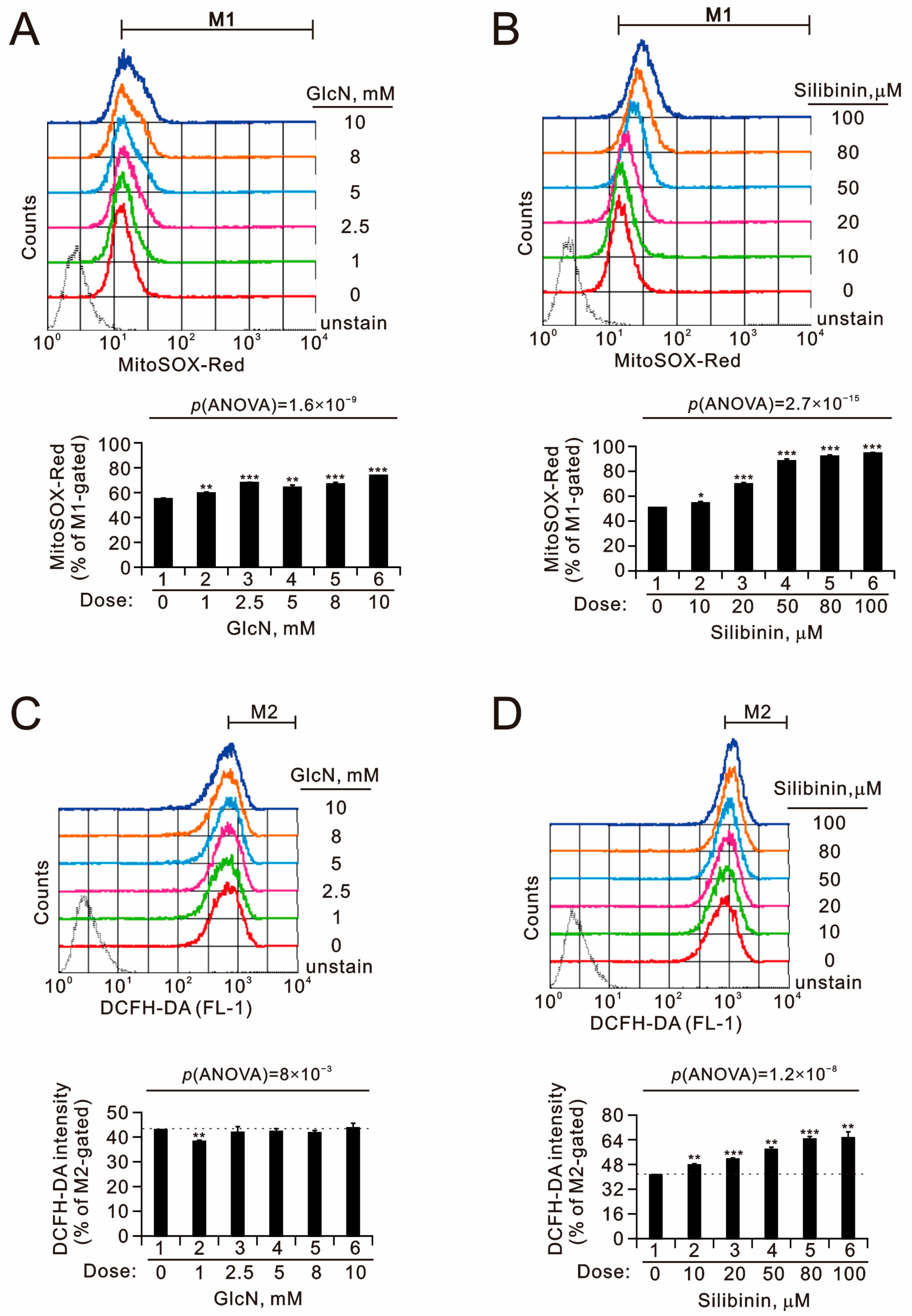
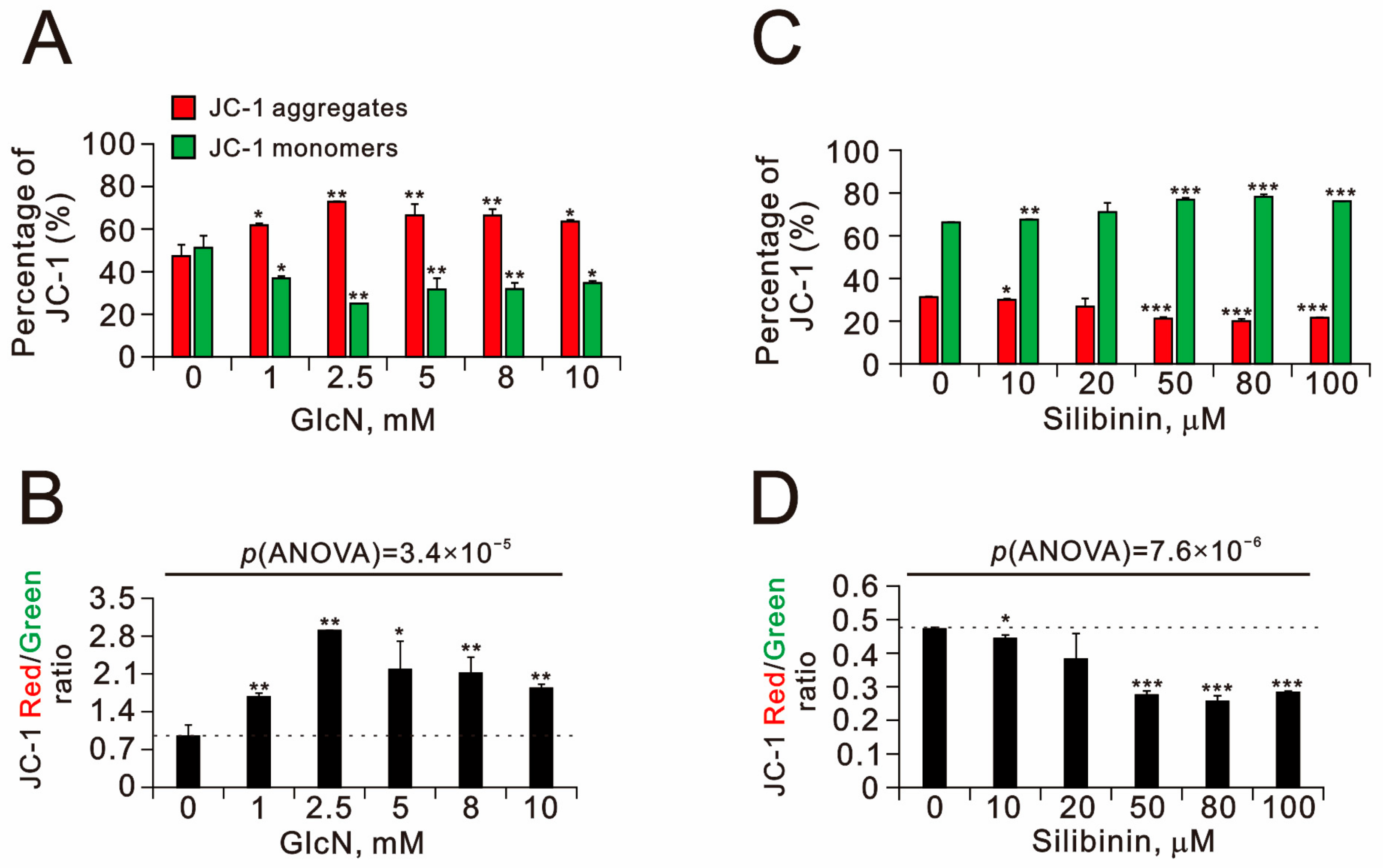
2.2. The Effects of Glucosamine and Silibinin on Cellular Proliferation, Cytosolic and Mitochondrial ROS, Mitochondrial Membrane Potential, and Autophagy Were Examined in Human TC28a2 Chondrocyte Cells
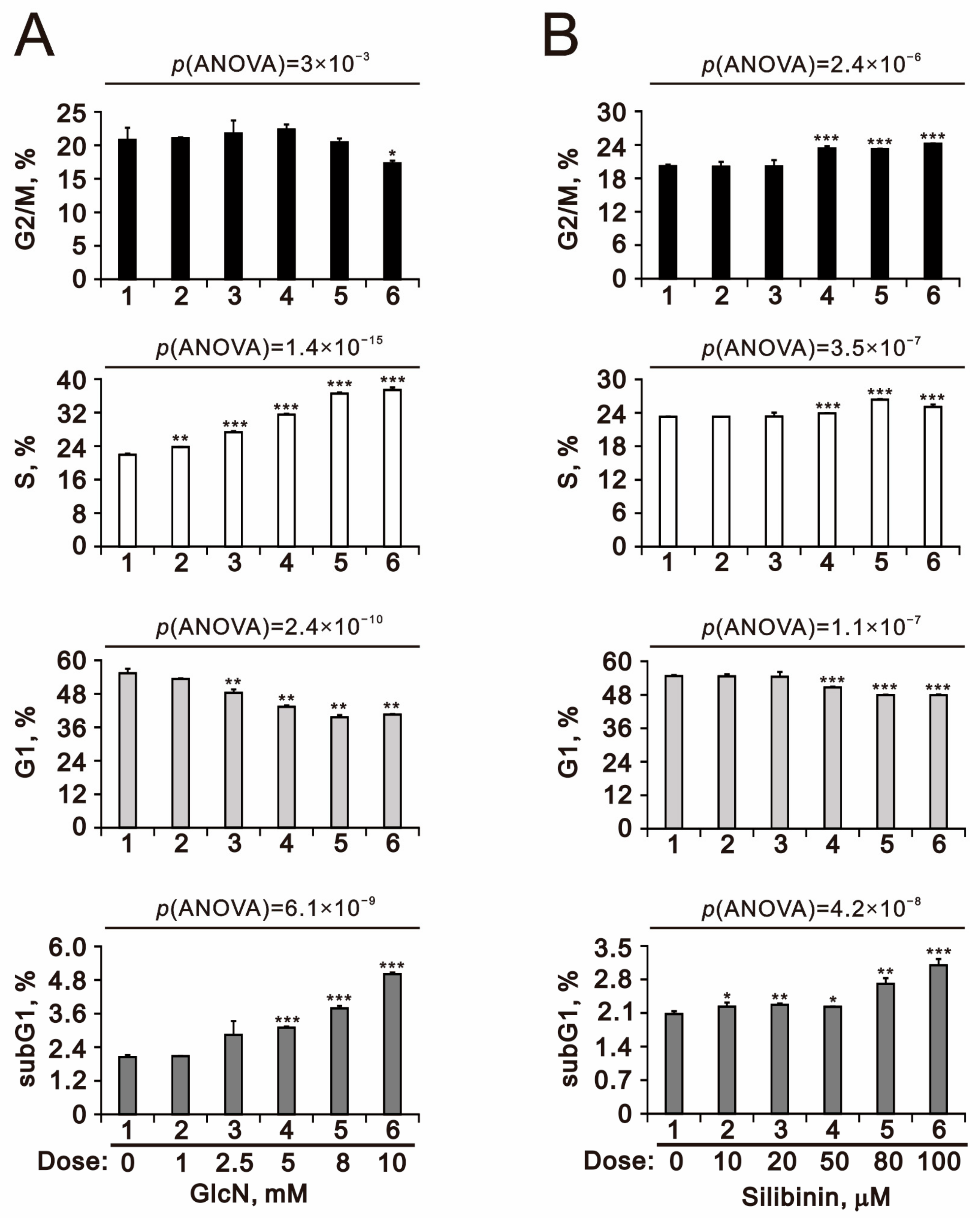
2.3. The Effects of Glucosamine and Silibinin on the Cell Cycle Profile and Cytotoxicity Were Examined in Human TC28a2 Chondrocyte Cells
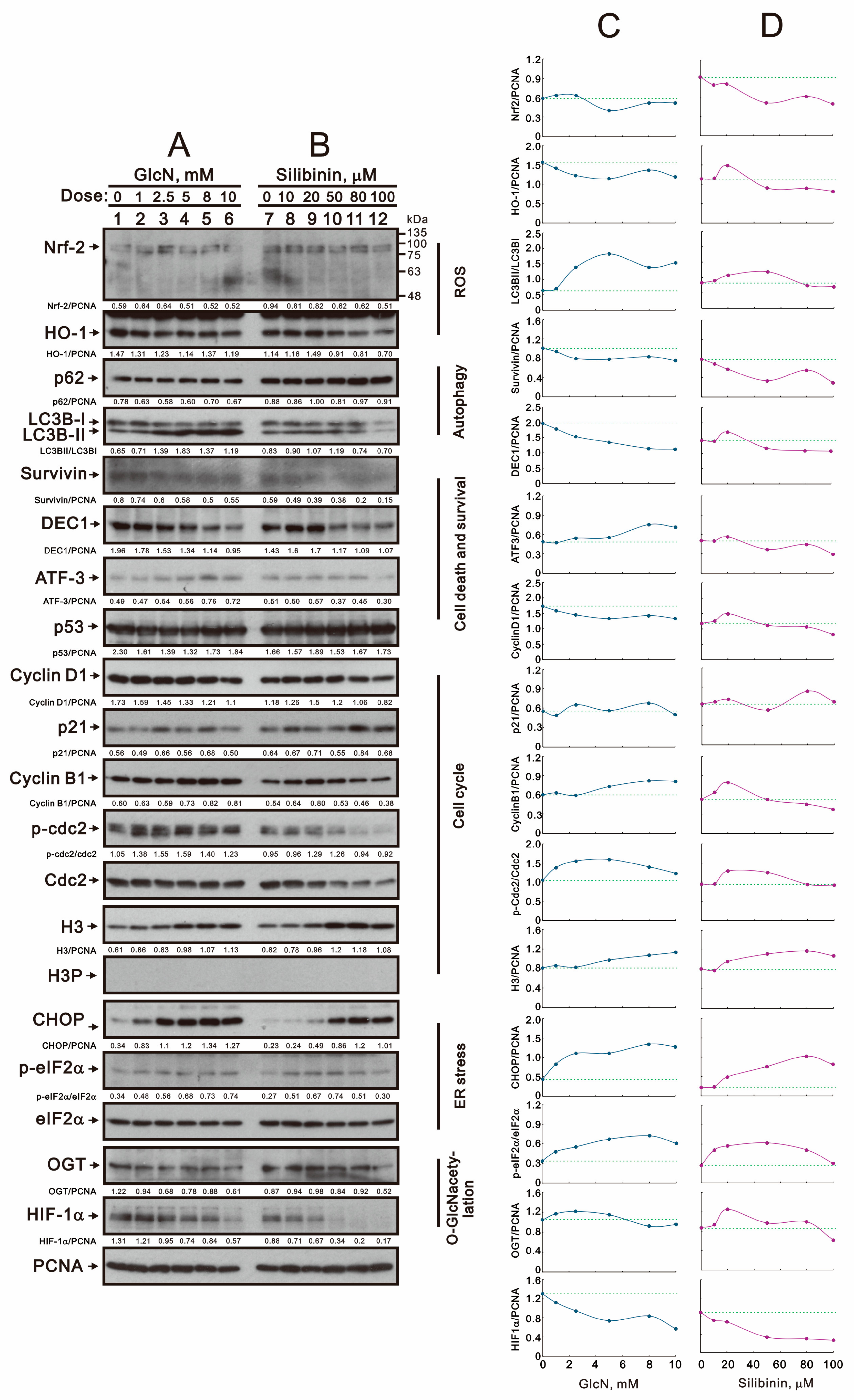
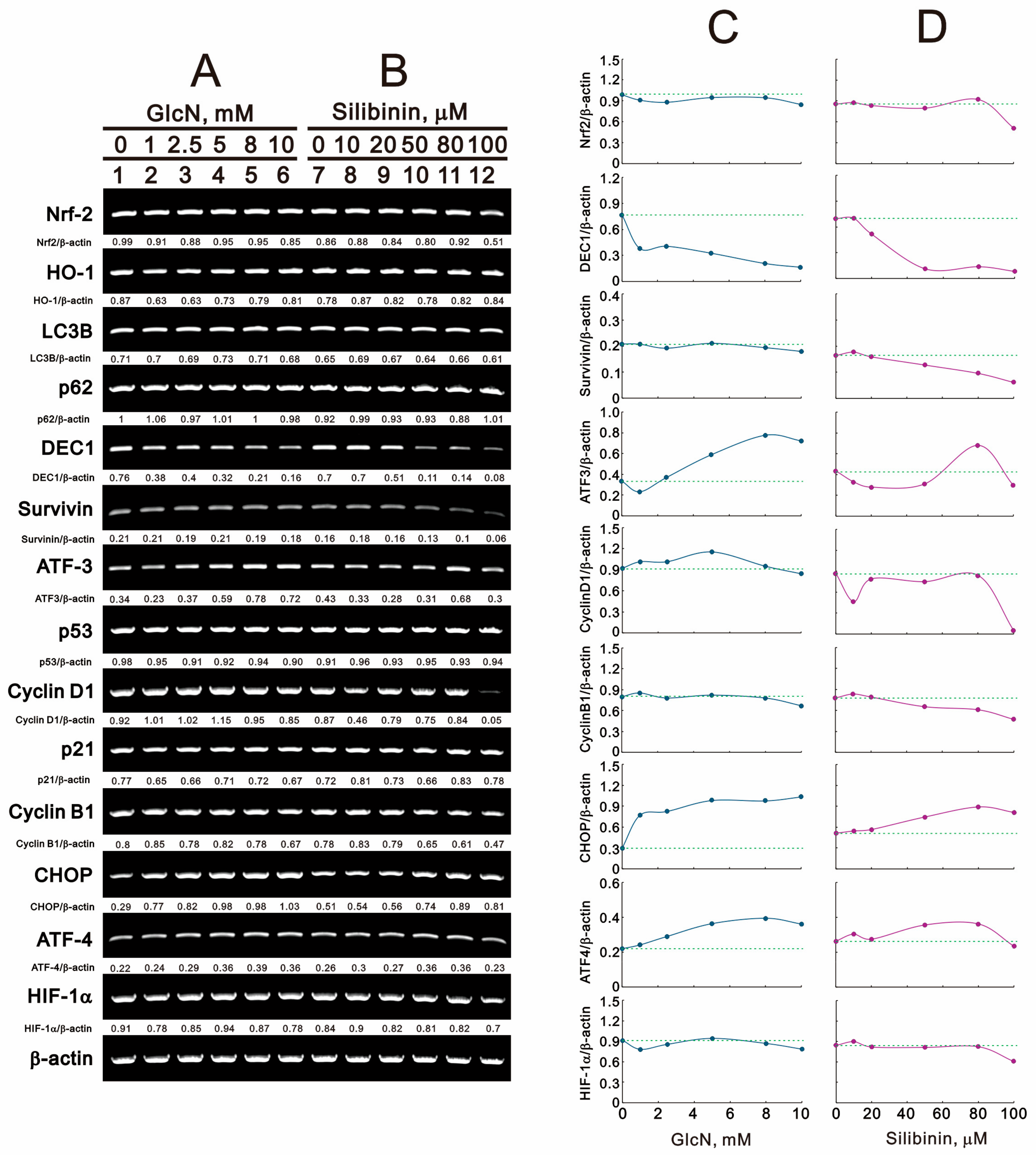
2.4. The Effects of Glucosamine and Silibinin on Specific Proteins and mRNAs Related to Cellular Stresses Were Examined in Human TC28a2 Chondrocyte Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Western Blotting Analysis
4.3. Fluorescence-Activated Cell Sorting (FACS) for Flow Cytometry Analyses of Cell Cycle Profiles, Proliferation, ROS, and Mitochondrial Membrane Potential
4.4. Flow Cytometric Quantification of Acidic Vesicular Organelles
4.5. Metabolic Activity Analysis
4.6. Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Quicke, J.G.; Conaghan, P.G.; Corp, N.; Peat, G. Osteoarthritis year in review 2021: Epidemiology & therapy. Osteoarthr. Cartil. 2022, 30, 196–206. [Google Scholar]
- Dalirfardouei, R.; Karimi, G.; Jamialahmadi, K. Molecular mechanisms and biomedical applications of glucosamine as a potential multifunctional therapeutic agent. Life Sci. 2016, 152, 21–29. [Google Scholar] [CrossRef]
- Kouri, J.B.; Jimenez, S.A.; Quintero, M.; Chico, A. Ultrastructural study of chondrocytes from fibrillated and non-fibrillated human osteoarthritic cartilage. Osteoarthr. Cartil. 1996, 4, 111–125. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, D.R.; Wylie, J.D.; Longpre, J.M.; Leduc, R.; Apte, S.S. 10 mM glucosamine prevents activation of proADAMTS5 (aggrecanase-2) in transfected cells by interference with post-translational modification of furin. Osteoarthr. Cartil. 2010, 18, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Sandy, J.D.; Gamett, D.; Thompson, V.; Verscharen, C. Chondrocyte-mediated catabolism of aggrecan: Aggrecanase-dependent cleavage induced by interleukin-1 or retinoic acid can be inhibited by glucosamine. Biochem. J. 1998, 335 Pt 1, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, E.J.; Jahr, H.; Koevoet, J.L.; Jenniskens, Y.M.; Bierma-Zeinstra, S.M.; Degroot, J.; Verhaar, J.A.; Weinans, H.; van Osch, G.J. Glucosamine decreases expression of anabolic and catabolic genes in human osteoarthritic cartilage explants. Osteoarthr. Cartil. 2006, 14, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Chesnokov, V.; Gong, B.; Sun, C.; Itakura, K. Anti-cancer activity of glucosamine through inhibition of N-linked glycosylation. Cancer Cell Int. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Rainone, F. Milk thistle. Am. Fam. Physician 2005, 72, 1285–1288. [Google Scholar]
- Kidd, P.; Head, K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: A silybin-phosphatidylcholine complex (Siliphos). Altern. Med. Rev. 2005, 10, 193–203. [Google Scholar]
- Kadoglou, N.P.E.; Panayiotou, C.; Vardas, M.; Balaskas, N.; Kostomitsopoulos, N.G.; Tsaroucha, A.K.; Valsami, G. A Comprehensive Review of the Cardiovascular Protective Properties of Silibinin/Silymarin: A New Kid on the Block. Pharmaceuticals 2022, 15, 538. [Google Scholar] [CrossRef] [PubMed]
- Radovani, B.; Gudelj, I. N-Glycosylation and Inflammation; the Not-So-Sweet Relation. Front. Immunol. 2022, 13, 893365. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Sukarieh, R.; Pelletier, J. Silibinin inhibits translation initiation: Implications for anticancer therapy. Mol. Cancer Ther. 2009, 8, 1606–1612. [Google Scholar] [CrossRef]
- Ozcan, S.; Andrali, S.S.; Cantrell, J.E. Modulation of transcription factor function by O-GlcNAc modification. Biochim. Biophys. Acta 2010, 1799, 353–364. [Google Scholar] [CrossRef]
- Hanover, J.A.; Krause, M.W.; Love, D.C. Bittersweet memories: Linking metabolism to epigenetics through O-GlcNAcylation. Nat. Rev. Mol. Cell Biol. 2012, 13, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.; Jung, E.H.; Kim, S.M.; Cho, H.; Han, I.O. Glucosamine regulates hepatic lipid accumulation by sensing glucose levels or feeding states of normal and excess. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158764. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef] [PubMed]
- Finger, F.; Schorle, C.; Zien, A.; Gebhard, P.; Goldring, M.B.; Aigner, T. Molecular phenotyping of human chondrocyte cell lines T/C-28a2, T/C-28a4, and C-28/I2. Arthritis Rheum. 2003, 48, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Rodriguez-Enriquez, S.; Lemasters, J.J. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 2007, 462, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Mahalingaiah, P.K.; Singh, K.P. Chronic oxidative stress increases growth and tumorigenic potential of MCF-7 breast cancer cells. PLoS ONE 2014, 9, e87371. [Google Scholar] [CrossRef]
- Millot, C.; Millot, J.M.; Morjani, H.; Desplaces, A.; Manfait, M. Characterization of acidic vesicles in multidrug-resistant and sensitive cancer cells by acridine orange staining and confocal microspectrofluorometry. J. Histochem. Cytochem. 1997, 45, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Yu, Y.; Gao, B.; Wang, D.; Sun, H.; Feng, Y.; Ma, Z.; Xie, X. Advances in protein glycosylation and its role in tissue repair and regeneration. Glycoconj. J. 2023, 40, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Q.; Meng, X.; Li, C.; Gao, Y.Y.; Li, N.; Niu, X.F.; Guan, Y.; Wang, H.Q. Glucosamine induces cell death via proteasome inhibition in human ALVA41 prostate cancer cell. Exp. Mol. Med. 2011, 43, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Dalirfardouei, R.; Karimi, G.; Jamialahmadi, K. Molecular mechanisms of anticancer effects of Glucosamine. Biomed. Pharmacother. 2017, 95, 1051–1058. [Google Scholar] [CrossRef]
- Ferrer, C.M.; Lynch, T.P.; Sodi, V.L.; Falcone, J.N.; Schwab, L.P.; Peacock, D.L.; Vocadlo, D.J.; Seagroves, T.N.; Reginato, M.J. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol. Cell 2014, 54, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, Y.; Wang, L.; Chen, B.; Chen, J.; Wang, H.; Chen, L. Hyperglycemia-induced accumulation of advanced glycosylation end products in fibroblast-like synoviocytes promotes knee osteoarthritis. Exp. Mol. Med. 2021, 53, 1735–1747. [Google Scholar] [CrossRef]
- Guan, Z.; Jin, X.; Guan, Z.; Liu, S.; Tao, K.; Luo, L. The gut microbiota metabolite capsiate regulate SLC2A1 expression by targeting HIF-1alpha to inhibit knee osteoarthritis-induced ferroptosis. Aging Cell 2023, 22, e13807. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, R.; Ma, H.; Zhao, X.; Wang, G. miRNA-411 Regulates Chondrocyte Autophagy in Osteoarthritis by Targeting Hypoxia-Inducible Factor 1 alpha (HIF-1alpha). Med. Sci. Monit. 2020, 26, e921155. [Google Scholar] [PubMed]
- Zhan, T.; Digel, M.; Kuch, E.M.; Stremmel, W.; Fullekrug, J. Silybin and dehydrosilybin decrease glucose uptake by inhibiting GLUT proteins. J. Cell Biochem. 2011, 112, 849–859. [Google Scholar] [CrossRef]
- De Roover, A.; Nunez, A.E.; Cornelis, F.M.; Cherifi, C.; Casas-Fraile, L.; Sermon, A.; Cailotto, F.; Lories, R.J.; Monteagudo, S. Hypoxia induces DOT1L in articular cartilage to protect against osteoarthritis. JCI Insight 2021, 6, e150451. [Google Scholar] [CrossRef]
- Sage, A.T.; Walter, L.A.; Shi, Y.; Khan, M.I.; Kaneto, H.; Capretta, A.; Werstuck, G.H. Hexosamine biosynthesis pathway flux promotes endoplasmic reticulum stress, lipid accumulation, and inflammatory gene expression in hepatic cells. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E499–E511. [Google Scholar] [CrossRef] [PubMed]
- Beriault, D.R.; Dang, V.T.; Zhong, L.H.; Petlura, C.I.; McAlpine, C.S.; Shi, Y.; Werstuck, G.H. Glucosamine induces ER stress by disrupting lipid-linked oligosaccharide biosynthesis and N-linked protein glycosylation. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E48–E57. [Google Scholar] [CrossRef] [PubMed]
- Beriault, D.R.; Sharma, S.; Shi, Y.; Khan, M.I.; Werstuck, G.H. Glucosamine-supplementation promotes endoplasmic reticulum stress, hepatic steatosis and accelerated atherogenesis in apoE−/− mice. Atherosclerosis 2011, 219, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Delbrel, E.; Soumare, A.; Naguez, A.; Label, R.; Bernard, O.; Bruhat, A.; Fafournoux, P.; Tremblais, G.; Marchant, D.; Gille, T.; et al. HIF-1alpha triggers ER stress and CHOP-mediated apoptosis in alveolar epithelial cells, a key event in pulmonary fibrosis. Sci. Rep. 2018, 8, 17939. [Google Scholar] [CrossRef] [PubMed]
- Ngoh, G.A.; Hamid, T.; Prabhu, S.D.; Jones, S.P. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1711–H1719. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, H.S.; Kim, N.H.; Ji, S.; Cha, S.Y.; Kang, J.G.; Ota, I.; Shimada, K.; Konishi, N.; Nam, H.W.; et al. Snail1 is stabilized by O-GlcNAc modification in hyperglycaemic condition. EMBO J. 2010, 29, 3787–3796. [Google Scholar] [CrossRef] [PubMed]
- Koditz, J.; Nesper, J.; Wottawa, M.; Stiehl, D.P.; Camenisch, G.; Franke, C.; Myllyharju, J.; Wenger, R.H.; Katschinski, D.M. Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood 2007, 110, 3610–3617. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- McCulloch, K.; Litherland, G.J.; Rai, T.S. Cellular senescence in osteoarthritis pathology. Aging Cell 2017, 16, 210–218. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology 2002, 3, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.K.; Liu, S.T.; Wu, Z.S.; Wang, Y.C.; Huang, S.M. Mechanisms of Cisplatin in Combination with Repurposed Drugs against Human Endometrial Carcinoma Cells. Life 2021, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Chang, Y.L.; Liu, S.T.; Chen, G.S.; Lee, S.P.; Huang, S.M. Differential Cytotoxicity Mechanisms of Copper Complexed with Disulfiram in Oral Cancer Cells. Int. J. Mol. Sci. 2021, 22, 3711. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Sequence (5′ → 3′) |
|---|---|
| β-actin | Forward: 5′-GTGGGGCGCCCCAGGCACCA-3′ Reverse: 5′-CTCCTTAATGTCACGCACGATTTC-3′ |
| ATF3 | Forward: 5′-GAGGATTTTGCTAACCTGAC-3′ Reverse: 5′-TAGCTCTGCAATGTTCCTTC-3′ |
| N-Cadherin | Forward: 5′-CCATTAGCCAAGGGAATTCAGC-3′ Reverse: 5′-GGTCTGGAGTTTCGCAAGTCTC-3′ |
| CHOP | Forward: 5′-CATTGCCTTTCTCCTTCGGG-3′ Reverse: 5′-GCCGTTCATTCTCTTCAGCT-3′ |
| Cyclin B1 | Forward: 5′-GTTGATACTGCCTCTCCAAG-3′ Reverse: 5′-CTTAGTATAAGTGTTGTCAGTCAC-3′ |
| cyclin D1 | Forward: 5′-ATGGAACACCAGCTCCTGTGCTGC-3′ Reverse: 5′-TCAGATGTCCACGTCCCGCACGTCGG-3′ |
| DEC1 | Forward: 5′-GTACCCTGCCCACATGTACC-3′ Reverse: 5′-GCTTGGCCAGATACTGAAGC-3′ |
| EGFR | Forward: 5′-GCTTTGGTGCCACCTGCGTG-3′ Reverse: 5′-CTCCATCACTTATCTCCTTGAG-3′ |
| HIF-1a | Forward: 5′-GAACCTGATGCTTTAACT-3′ Reverse: 5′-CAACTGATCGAAGGAACG-3′ |
| HO-1 | Forward: 5′-ATGCCCCAGGATTTGTCAGAG-3′ Reverse: 5′-AGGGCTTTCTGGGCAATCTTT-3′ |
| IL-6 | Forward: 5′-ATGAACTCCTTCTCCACAAGCGC-3′ Reverse: 5′-CTACATTTGCCGAAGAGCCCTCA-3′ |
| KLF4 | Forward: 5′-CTTGAGGAAGTGCTGAGCAG-3′ Reverse: 5′-CGGTAGTGCCTGGTCAGTTC-3′ |
| LC3B | Forward: 5′-AGCAGCATCCAACCAAAATC-3′ Reverse: 5′-TGACAATTTCATCCCGAACG-3′ |
| Nrf-2 | Forward: 5′-CAGTCAGCGACGGAAAGAGT-3′ Reverse: 5′-GGCTACCTGAGCAACAGAAG-3′ |
| p21 | Forward: 5′-CTGAGCCGCGACTGTGATGCG-3′ Reverse: 5′-GGTCTGCCGCCGTTTTCGACC-3′ |
| p53 | Forward: 5′-CTCTGACTGTACCACCATCCACTA-3′ Reverse: 5′-GAGTTCCAAGGCCTCATTCAGCTC-3′ |
| p62 | Forward: 5′-CCGTGAAGGCCTACCTTCTG-3′ Reverse: 5′-GCACTTGTAGCGGGTTCCTA-3′ |
| Snail | Forward: 5′-ATGCCGCGCTCTTTCCTCGTCAGG-3′ Reverse: 5′-TCAGCGGGGACATCCTGAGCAGCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-P.; Huang, T.-H.; Liu, S.-T.; Huang, S.-M.; Chen, Y.-C.; Wu, C.-C. Glucosamine and Silibinin Alter Cartilage Homeostasis through Glycosylation and Cellular Stresses in Human Chondrocyte Cells. Int. J. Mol. Sci. 2024, 25, 4905. https://doi.org/10.3390/ijms25094905
Hsu Y-P, Huang T-H, Liu S-T, Huang S-M, Chen Y-C, Wu C-C. Glucosamine and Silibinin Alter Cartilage Homeostasis through Glycosylation and Cellular Stresses in Human Chondrocyte Cells. International Journal of Molecular Sciences. 2024; 25(9):4905. https://doi.org/10.3390/ijms25094905
Chicago/Turabian StyleHsu, Yu-Pao, Tsung-Hsi Huang, Shu-Ting Liu, Shih-Ming Huang, Yi-Chou Chen, and Chia-Chun Wu. 2024. "Glucosamine and Silibinin Alter Cartilage Homeostasis through Glycosylation and Cellular Stresses in Human Chondrocyte Cells" International Journal of Molecular Sciences 25, no. 9: 4905. https://doi.org/10.3390/ijms25094905
APA StyleHsu, Y. -P., Huang, T. -H., Liu, S. -T., Huang, S. -M., Chen, Y. -C., & Wu, C. -C. (2024). Glucosamine and Silibinin Alter Cartilage Homeostasis through Glycosylation and Cellular Stresses in Human Chondrocyte Cells. International Journal of Molecular Sciences, 25(9), 4905. https://doi.org/10.3390/ijms25094905






