The Potential of the Fibronectin Inhibitor Arg-Gly-Asp-Ser in the Development of Therapies for Glioblastoma
Abstract
1. Introduction
2. Results
2.1. Characterization of LUVs
2.2. HA and RGDS-Functionalized HA Hydrogels Characterization
2.2.1. Nuclear Magnetic Resonance and Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy Analyses
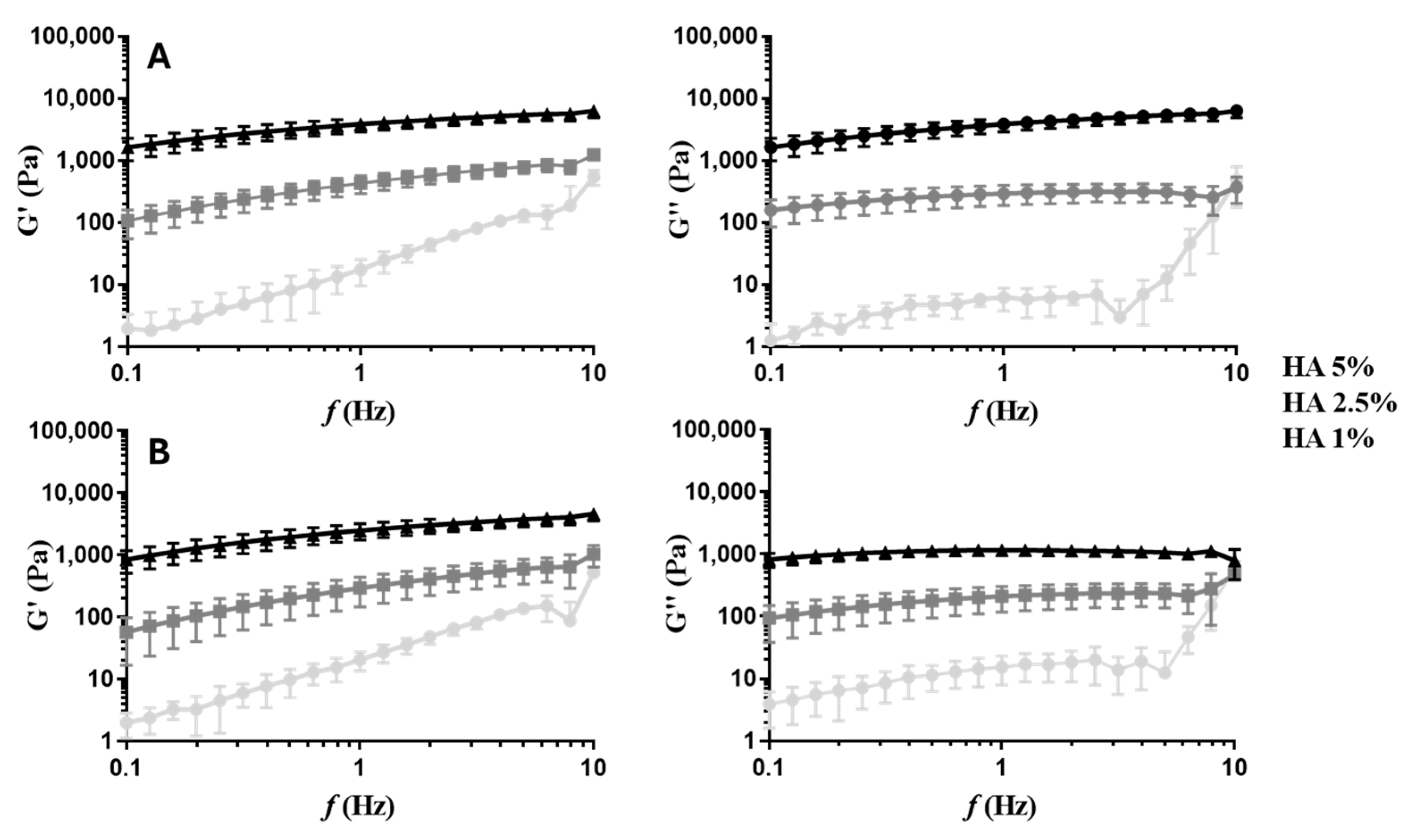
2.2.2. Rheological Properties
2.2.3. Thermal Properties
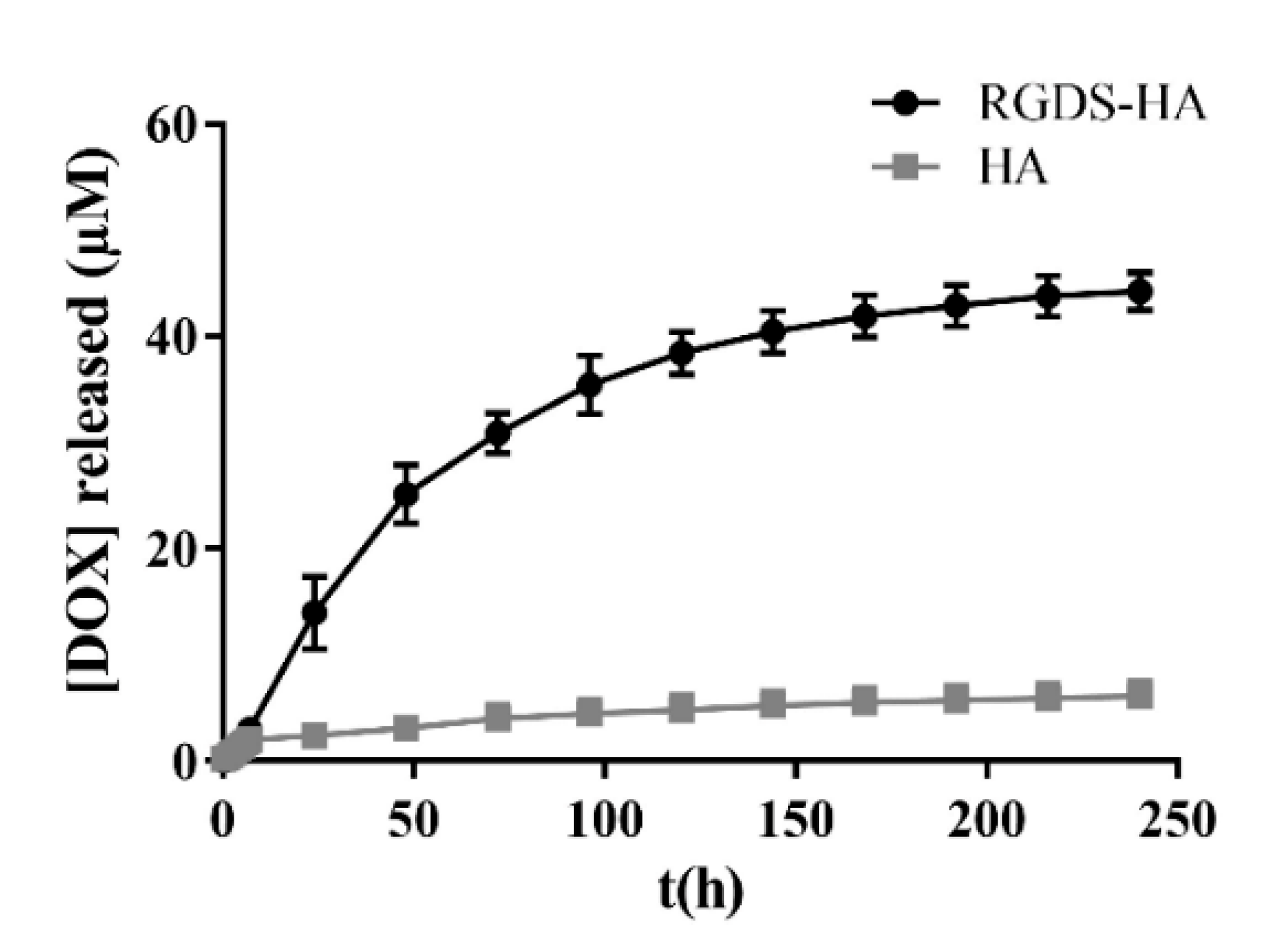
2.2.4. DOX Release by HA and RGDS-Functionalized HA Hydrogels
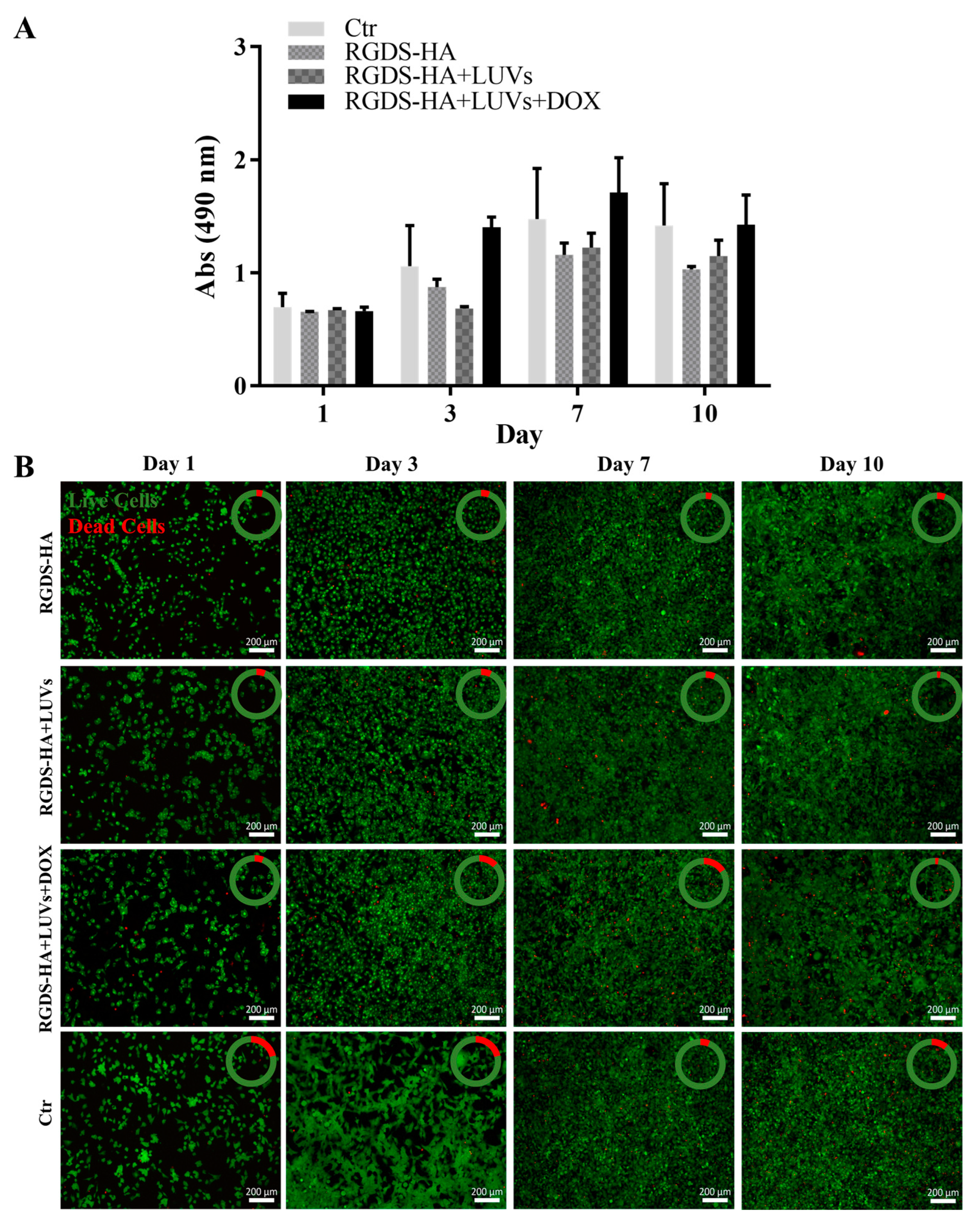
2.3. Biological Performance
2.3.1. DOX IC50 Determination
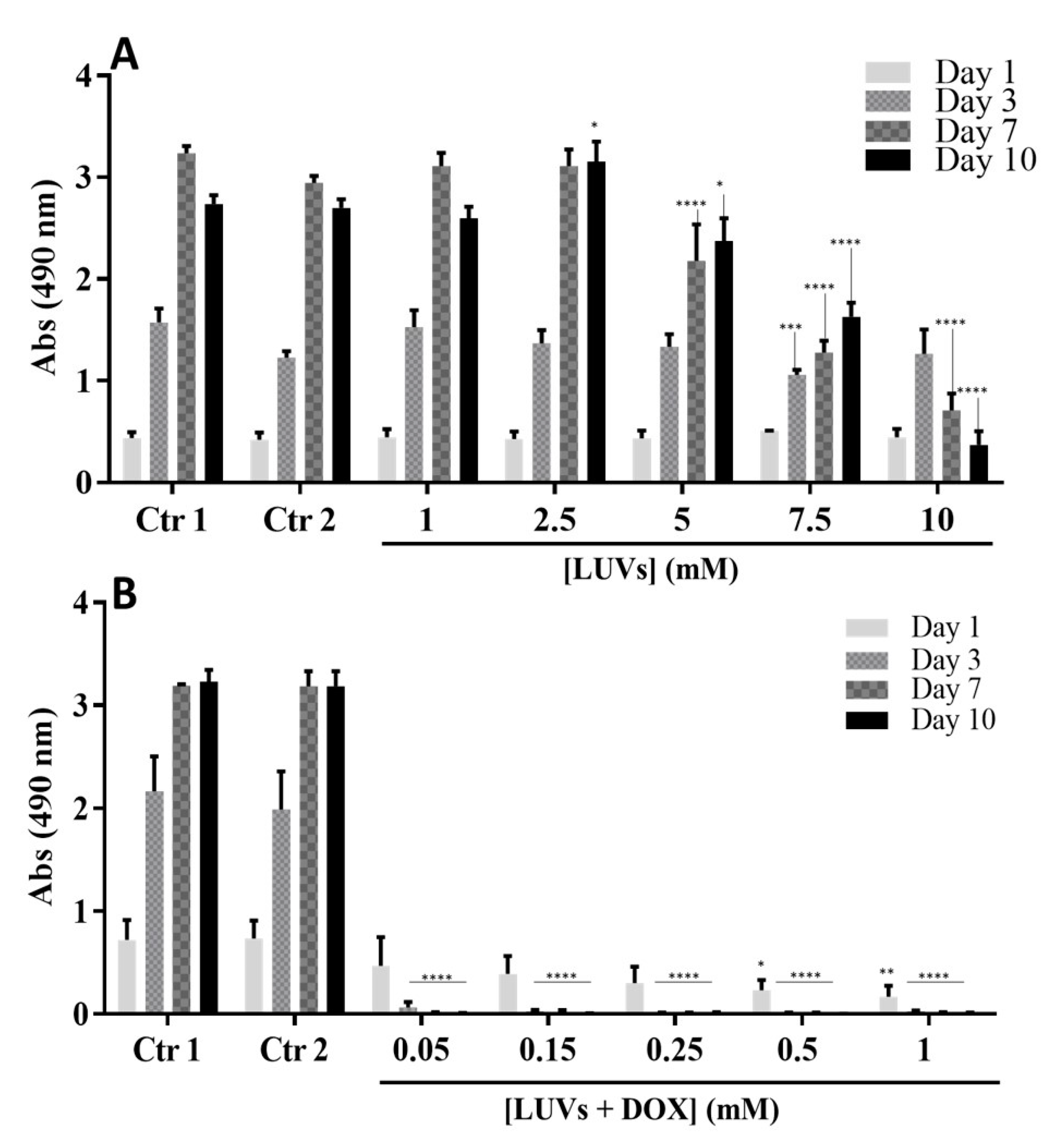
2.3.2. Metabolic Activity of GBML42 Cells in the Presence of LUVs with and without DOX
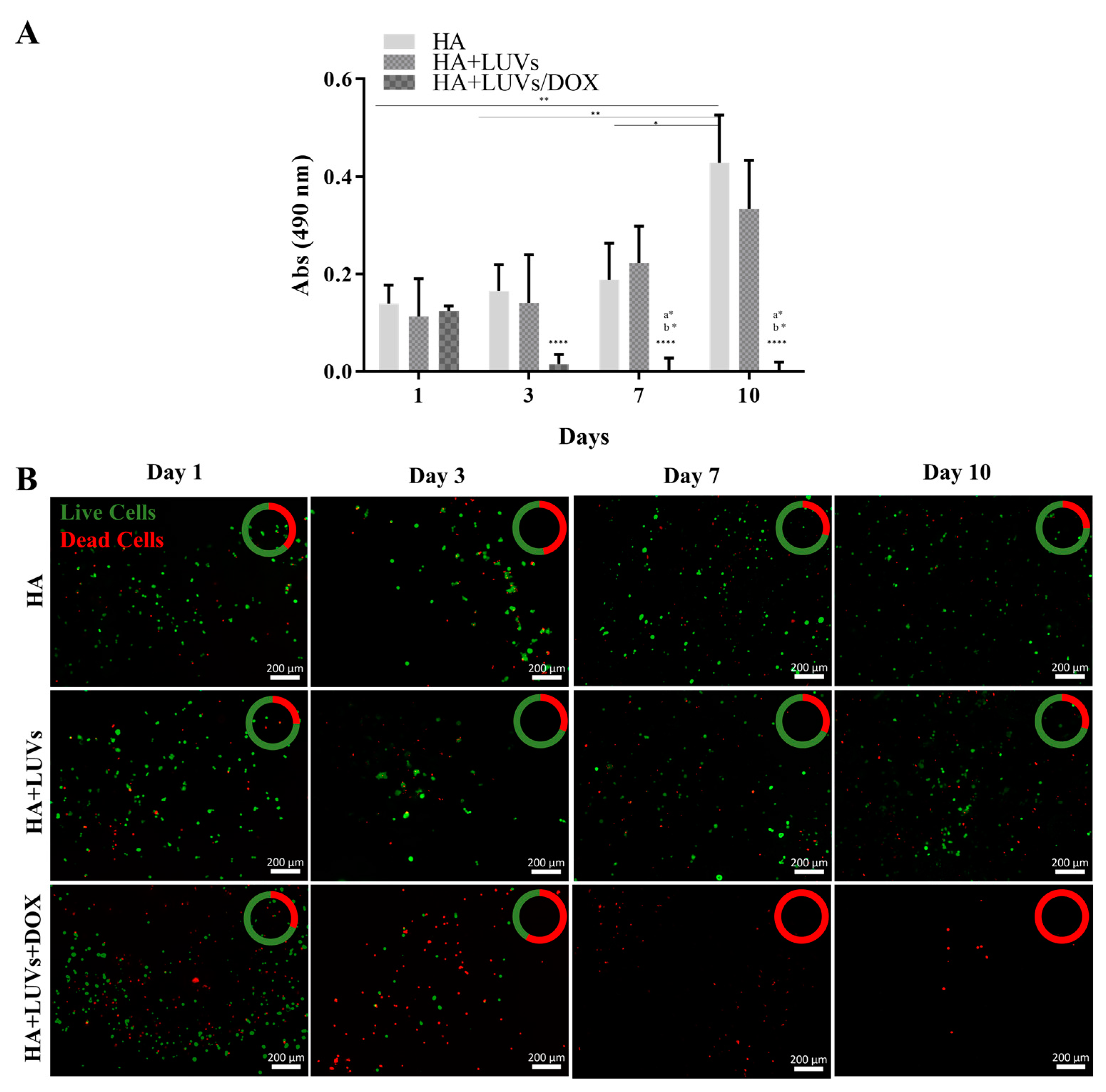
2.3.3. Efficacy of the Hydrogel in Damaging GBML42 Cells
2.3.4. Expression of MMP-2 by GBML42 Cells
2.3.5. Hydrogel Safety for Astrocytes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Production of DPPC Liposomes with and without DOX
4.3. Characterization of LUVs
4.3.1. Determination of DOX Concentration in LUVs
4.3.2. Determination of Size, PDI, Zeta Potential, and Stability
4.3.3. Thermal Characterization
4.3.4. LUV Morphology
4.4. RGDS Synthesis
4.5. Functionalization of HA with RGDS
4.6. Preparation of Hydrogels
4.7. Characterization of HA and RGDS-Functionalized HA Hydrogels
4.7.1. Mechanical Characterization
4.7.2. Thermal Characterization
4.7.3. Cleavage of the RGDS-HA Bond by MMP-2
4.7.4. Determination of DOX Release by the Hydrogel
4.8. In Vitro Assays to Assess the Safety and Efficacy of the Hydrogel
4.8.1. Cell Culture
4.8.2. Determination of DOX IC50 in GBML42 Cells
4.8.3. Metabolic Activity of GBML42 Cells in the Presence of LUVs Encapsulating or Not DOX
4.8.4. Efficacy of Hydrogels in Damaging GBML42 Cells
4.8.5. Cytotoxicity of RGDS
4.8.6. MMP-2 Production by GBML42 Cells
4.8.7. Hydrogel Efficacy and Safety in a Co-Culture of GBML42 Cells and Astrocytes
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 Mutations in Gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.T.C.; Sudlow, C.L.M.; Figueroa, J.D.; Brennan, P.M. Longer-Term (≥2 Years) Survival in Patients with Glioblastoma in Population-Based Studies Pre- and Post-2005: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 11622. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Hopkins, K.; Tonn, J.C.; Stupp, R.; Falini, A.; Cohen-Jonathan-Moyal, E.; Frappaz, D.; Henriksson, R.; Balana, C.; et al. EANO Guideline for the Diagnosis and Treatment of Anaplastic Gliomas and Glioblastoma. Lancet Oncol. 2014, 15, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Lemée, J.-M.; Clavreul, A.; Mennea, P. Intratumoral Heterogeneity in Glioblastoma: Don’t Forget the Peritumoral Brain Zone. Neuro Oncol. 2015, 17, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Sherriff, J.; Tamangani, J.; Senthil, L.; Cruickshank, G.; Spooner, D.; Jones, B.; Brookes, C.; Sanghera, P. Patterns of Relapse in Glioblastoma Multiforme Following Concomitant Chemoradiotherapy with Temozolomide. Br. J. Radiol. 2013, 86, 20120414. [Google Scholar] [CrossRef] [PubMed]
- Pirzkall, A.; McGue, C.; Saraswathy, S.; Cha, S.; Liu, R.; Vandenberg, S.; Lamborn, K.R.; Berger, M.S.; Chang, S.M.; Nelson, S.J. Tumor Regrowth between Surgery and Initiation of Adjuvant Therapy in Patients with Newly Diagnosed Glioblastoma. Neuro Oncol. 2009, 11, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma Stem Cells Promote Radioresistance by Preferential Activation of the DNA Damage Response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of Gene Expression and Chemoresistance of CD133+ Cancer Stem Cells in Glioblastoma. Mol. Cancer 2006, 5, 67. [Google Scholar] [CrossRef]
- Osuka, S.; Van Meir, E.G. Overcoming Therapeutic Resistance in Glioblastoma: The Way Forward. J. Clin. Investig. 2017, 127, 415–426. [Google Scholar] [CrossRef]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.-O.; Schöning, T.; Hüsing, J.; Beelte, B.; et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Strebe, J.K.; Lubin, J.A.; Kuo, J.S. “Tag Team” Glioblastoma Therapy. Neurosurgery 2016, 79, N18–N20. [Google Scholar] [CrossRef] [PubMed]
- Tanner, P.G.; Holtmannspötter, M.; Tonn, J.-C.; Goldbrunner, R. Effects of Drug Efflux on Convection-Enhanced Paclitaxel Delivery to Malignant Gliomas: Technical Note. Neurosurgery 2007, 61, E880–E882. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Bozzato, E.; Joudiou, N.; Ghiassinejad, S.; Danhier, F.; Gallez, B.; Préat, V. Codelivery of Paclitaxel and Temozolomide through a Photopolymerizable Hydrogel Prevents Glioblastoma Recurrence after Surgical Resection. J. Control. Release 2019, 309, 72–81. [Google Scholar] [CrossRef]
- Fourniols, T.; Randolph, L.D.; Staub, A.; Vanvarenberg, K.; Leprince, J.G.; Préat, V.; des Rieux, A.; Danhier, F. Temozolomide-Loaded Photopolymerizable PEG-DMA-Based Hydrogel for the Treatment of Glioblastoma. J. Control. Release 2015, 210, 95–104. [Google Scholar] [CrossRef]
- Bastiancich, C.; Lemaire, L.; Bianco, J.; Franconi, F.; Danhier, F.; Préat, V.; Bastiat, G.; Lagarce, F. Evaluation of Lauroyl-Gemcitabine-Loaded Hydrogel Efficacy in Glioblastoma Rat Models. Nanomedicine 2018, 13, 1999–2013. [Google Scholar] [CrossRef] [PubMed]
- Bregy, A.; Shah, A.H.; Diaz, M.V.; Pierce, H.E.; Ames, P.L.; Diaz, D.; Komotar, R.J. The Role of Gliadel Wafers in the Treatment of High-Grade Gliomas. Expert Rev. Anticancer Ther. 2013, 13, 1453–1461. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Q.; Shen, M.; Sun, Y.; Zhang, X.; Huang, C.; Chen, J.; Li, R.; Duan, Y. Thermoresponsive Nanocomposite Gel for Local Drug Delivery to Suppress the Growth of Glioma by Inducing Autophagy. Autophagy 2017, 13, 1176–1190. [Google Scholar] [CrossRef]
- Nih, L.R.; Gojgini, S.; Carmichael, S.T.; Segura, T. Dual-Function Injectable Angiogenic Biomaterial for the Repair of Brain Tissue Following Stroke. Nat. Mater. 2018, 17, 642–651. [Google Scholar] [CrossRef]
- Hou, S.; Xu, Q.; Tian, W.; Cui, F.; Cai, Q.; Ma, J.; Lee, I.-S. The Repair of Brain Lesion by Implantation of Hyaluronic Acid Hydrogels Modified with Laminin. J. Neurosci. Methods 2005, 148, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Tsui, C.; Koss, K.; Churchward, M.A.; Todd, K.G. Biomaterials and Glia: Progress on Designs to Modulate Neuroinflammation. Acta Biomater. 2019, 83, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Lingasamy, P.; Tobi, A.; Kurm, K.; Kopanchuk, S.; Sudakov, A.; Salumäe, M.; Rätsep, T.; Asser, T.; Bjerkvig, R.; Teesalu, T. Tumor-Penetrating Peptide for Systemic Targeting of Tenascin-C. Sci. Rep. 2020, 10, 5809. [Google Scholar] [CrossRef] [PubMed]
- Lingasamy, P.; Tobi, A.; Haugas, M.; Hunt, H.; Paiste, P.; Asser, T.; Rätsep, T.; Kotamraju, V.R.; Bjerkvig, R.; Teesalu, T. Bi-Specific Tenascin-C and Fibronectin Targeted Peptide for Solid Tumor Delivery. Biomaterials 2019, 219, 119373. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Lal, B.; Tung, B.; Wang, S.; Goodwin, C.R.; Laterra, J. Tumor Microenvironment Tenascin-C Promotes Glioblastoma Invasion and Negatively Regulates Tumor Proliferation. Neuro Oncol. 2016, 18, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Shannon, S.; Vaca, C.; Jia, D.; Entersz, I.; Schaer, A.; Carcione, J.; Weaver, M.; Avidar, Y.; Pettit, R.; Nair, M.; et al. Dexamethasone-Mediated Activation of Fibronectin Matrix Assembly Reduces Dispersal of Primary Human Glioblastoma Cells. PLoS ONE 2015, 10, e0135951. [Google Scholar] [CrossRef] [PubMed]
- Ellert-Miklaszewska, A.; Poleszak, K.; Pasierbinska, M.; Kaminska, B. Integrin Signaling in Glioma Pathogenesis: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 888. [Google Scholar] [CrossRef] [PubMed]
- Bello, L.; Francolini, M.; Marthyn, P.; Zhang, J.; Carroll, R.S.; Nikas, D.C.; Strasser, J.F.; Villani, R.; Cheresh, D.A.; Black, P.M. Avβ3 and Avβ5 Integrin Expression in Glioma Periphery. Neurosurgery 2001, 49, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; DeCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e6. [Google Scholar] [CrossRef]
- Wang, J.P.; Hielscher, A. Fibronectin: How Its Aberrant Expression in Tumors May Improve Therapeutic Targeting. J. Cancer 2017, 8, 674–682. [Google Scholar] [CrossRef]
- Giancotti, F.G. Integrin Signaling. Science 1999, 285, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Sani, S.; Messe, M.; Fuchs, Q.; Pierrevelcin, M.; Laquerriere, P.; Entz-Werle, N.; Reita, D.; Etienne-Selloum, N.; Bruban, V.; Choulier, L.; et al. Biological Relevance of RGD-Integrin Subtype-Specific Ligands in Cancer. ChemBioChem 2021, 22, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.-J.; Mas-Moruno, C.; et al. A Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-Binding Integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [CrossRef]
- El Kechai, N.; Bochot, A.; Huang, N.; Nguyen, Y.; Ferrary, E.; Agnely, F. Effect of Liposomes on Rheological and Syringeability Properties of Hyaluronic Acid Hydrogels Intended for Local Injection of Drugs. Int. J. Pharm. 2015, 487, 187–196. [Google Scholar] [CrossRef]
- Cagel, M.; Grotz, E.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: Nanotechnological Overviews from Bench to Bedside. Drug Discov. Today 2017, 22, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Schiweck, J.; Eickholt, B.J.; Murk, K. Important Shapeshifter: Mechanisms Allowing Astrocytes to Respond to the Changing Nervous System During Development, Injury and Disease. Front. Cell. Neurosci. 2018, 12, 261. [Google Scholar] [CrossRef]
- Gilli, R.; Kacuráková, M.; Mathlouthi, M.; Navarini, L.; Paoletti, S. FTIR Studies of Sodium Hyaluronate and Its Oligomers in the Amorphous Solid Phase and in Aqueous Solution. Carbohydr. Res. 1994, 263, 315–326. [Google Scholar] [CrossRef]
- Gřundělová, L.; Gregorova, A.; Mráček, A.; Vícha, R.; Smolka, P.; Minařík, A. Viscoelastic and Mechanical Properties of Hyaluronan Films and Hydrogels Modified by Carbodiimide. Carbohydr. Polym. 2015, 119, 142–148. [Google Scholar] [CrossRef]
- Parker, F.S. Amides and Amines. In Applications of Infrared Spectroscopy in Biochemistry, Biology, and Medicine; Springer: Boston, MA, USA, 1971; pp. 165–172. [Google Scholar]
- Sewald, N.; Jakubke, H. Peptides: Chemistry and Biology; Wiley: Hoboken, NJ, USA, 2009; ISBN 9783527318674. [Google Scholar]
- Wilson, D.I. What Is Rheology? Eye 2018, 32, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.E.; Gentleman, S.M.; Dini, D. On the Characterization of the Heterogeneous Mechanical Response of Human Brain Tissue. Biomech. Model. Mechanobiol. 2017, 16, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, M.S.; Fortugno, P.; Giampietri, C.; Ragone, G.; Capogrossi, M.C.; Facchiano, A. Intracellular Targets of RGDS Peptide in Melanoma Cells. Mol. Cancer 2010, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, T.; Liu, S.; Yoshida, D.; Teramoto, A. The Expression of Matrix Metalloproteinase-2 and-9 in Human Gliomas of Different Pathological Grades. Brain Tumor Pathol. 2003, 20, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as Potential Drug Carrier Systems for Drug Delivery. In Application of Nanotechnology in Drug Delivery; InTech: London, UK, 2014. [Google Scholar]
- Guo, D.; Bell, E.H.; Chakravarti, A. Lipid Metabolism Emerges as a Promising Target for Malignant Glioma Therapy. CNS Oncol. 2013, 2, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic Light Scattering: A Practical Guide and Applications in Biomedical Sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, T.; Shirai, S.; Okumura, T.; Morita, A. Molecular Dynamics Study of Structure and Vibrational Spectra at Zwitterionoic Lipid/Aqueous KCl, NaCl, and CaCl2 Solution Interfaces. J. Chem. Phys. 2018, 148, 222801. [Google Scholar] [CrossRef] [PubMed]
- Kraayenhof, R.; Sterk, G.J.; Wong Fong Sang, H.W. Probing Biomembrane Interfacial Potential and PH Profiles with a New Type of Float-like Fluorophores Positioned at Varying Distance from the Membrane Surface. Biochemistry 1993, 32, 10057–10066. [Google Scholar] [CrossRef]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to Improve the Scientific Value of Zeta-Potential Measurements in NanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Demetzos, C. Differential Scanning Calorimetry (DSC): A Tool to Study the Thermal Behavior of Lipid Bilayers and Liposomal Stability. J. Liposome Res. 2008, 18, 159–173. [Google Scholar] [CrossRef]
- Ohtake, S.; Schebor, C.; Palecek, S.P.; de Pablo, J.J. Phase Behavior of Freeze-Dried Phospholipid–Cholesterol Mixtures Stabilized with Trehalose. Biochim. Biophys. Acta-Biomembr. 2005, 1713, 57–64. [Google Scholar] [CrossRef]
- Tsvetkova, N.M.; Phillips, B.L.; Crowe, L.M.; Crowe, J.H.; Risbud, S.H. Effect of Sugars on Headgroup Mobility in Freeze-Dried Dipalmitoylphosphatidylcholine Bilayers: Solid-State P NMR and FTIR Studies. Biophys. J. 1998, 75, 2947–2955. [Google Scholar] [CrossRef]
- Lucio, M.; Lima, J.L.F.C.; Reis, S. Drug-Membrane Interactions: Significance for Medicinal Chemistry. Curr. Med. Chem. 2010, 17, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic Acid-Based Hydrogels: From a Natural Polysaccharide to Complex Networks. Soft Matter 2012, 8, 3280. [Google Scholar] [CrossRef] [PubMed]
- Selyanin, M.A.; Boykov, P.Y.; Khabarov, V.N.; Polyak, F. Hyaluronic Acid; John Wiley & Sons, Ltd: Chichester, UK, 2015; ISBN 9781118695920. [Google Scholar]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical Modifications of Hyaluronic Acid for the Synthesis of Derivatives for a Broad Range of Biomedical Applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Hoefting, J.M.; Cowman, M.K.; Matsuoka, S.; Balazs, E. Temperature Effect on the Dynamic Rheological Characteristics of Hyaluronan, Hylan A and Synvisc. In Hyaluronan; Elsevier: Amsterdam, The Netherlands, 2002; pp. 103–108. [Google Scholar]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 4, 622. [Google Scholar] [CrossRef]
- Benešová, K.; Pekař, M.; Lapčík, L.; Kučerík, J. Stability Evaluation of N-Alkyl Hyaluronic Acid Derivates by DSC and TG Measurement. J. Therm. Anal. Calorim. 2006, 83, 341–348. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Comparison of the Effectiveness of Four Different Crosslinking Agents with Hyaluronic Acid Hydrogel Films for Tissue-Culture Applications. J. Appl. Polym. Sci. 2007, 104, 3183–3191. [Google Scholar] [CrossRef]
- Omidian, H.; Park, K. Introduction to Hydrogels. In Biomedical Applications of Hydrogels Handbook; Springer: New York, NY, USA, 2010; pp. 1–16. [Google Scholar]
- Novak, U.; Kaye, A.H. Extracellular Matrix and the Brain: Components and Function. J. Clin. Neurosci. 2000, 7, 280–290. [Google Scholar] [CrossRef]
- Tallant, C.; Marrero, A.; Gomis-Rüth, F.X. Matrix Metalloproteinases: Fold and Function of Their Catalytic Domains. Biochim. Biophys. Acta-Mol. Cell Res. 2010, 1803, 20–28. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, A.; Mohammed, R.; Zakieh, O. The Unique Invasiveness of Glioblastoma and Possible Drug Targets on Extracellular Matrix. Cancer Manag. Res. 2019, 11, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Pilling, D.; Henriquez, N.V.; Parsonage, G.; Threlfall, K.; Scheel-Toellner, D.; Simmons, D.L.; Akbar, A.N.; Lord, J.M.; Salmon, M. RGD Peptides Induce Apoptosis by Direct Caspase-3 Activation. Nature 1999, 397, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 17–22. [Google Scholar]
- Azari, H.; Millette, S.; Ansari, S.; Rahman, M.; Deleyrolle, L.P.; Reynolds, B.A. Isolation and Expansion of Human Glioblastoma Multiforme Tumor Cells Using the Neurosphere Assay. J. Vis. Exp. 2011, 56, e3633. [Google Scholar] [CrossRef]
- Neubig, R.R.; Spedding, M.; Kenakin, T.; Christopoulos, A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on Terms and Symbols in Quantitative Pharmacology. Pharmacol. Rev. 2003, 55, 597–606. [Google Scholar] [CrossRef]

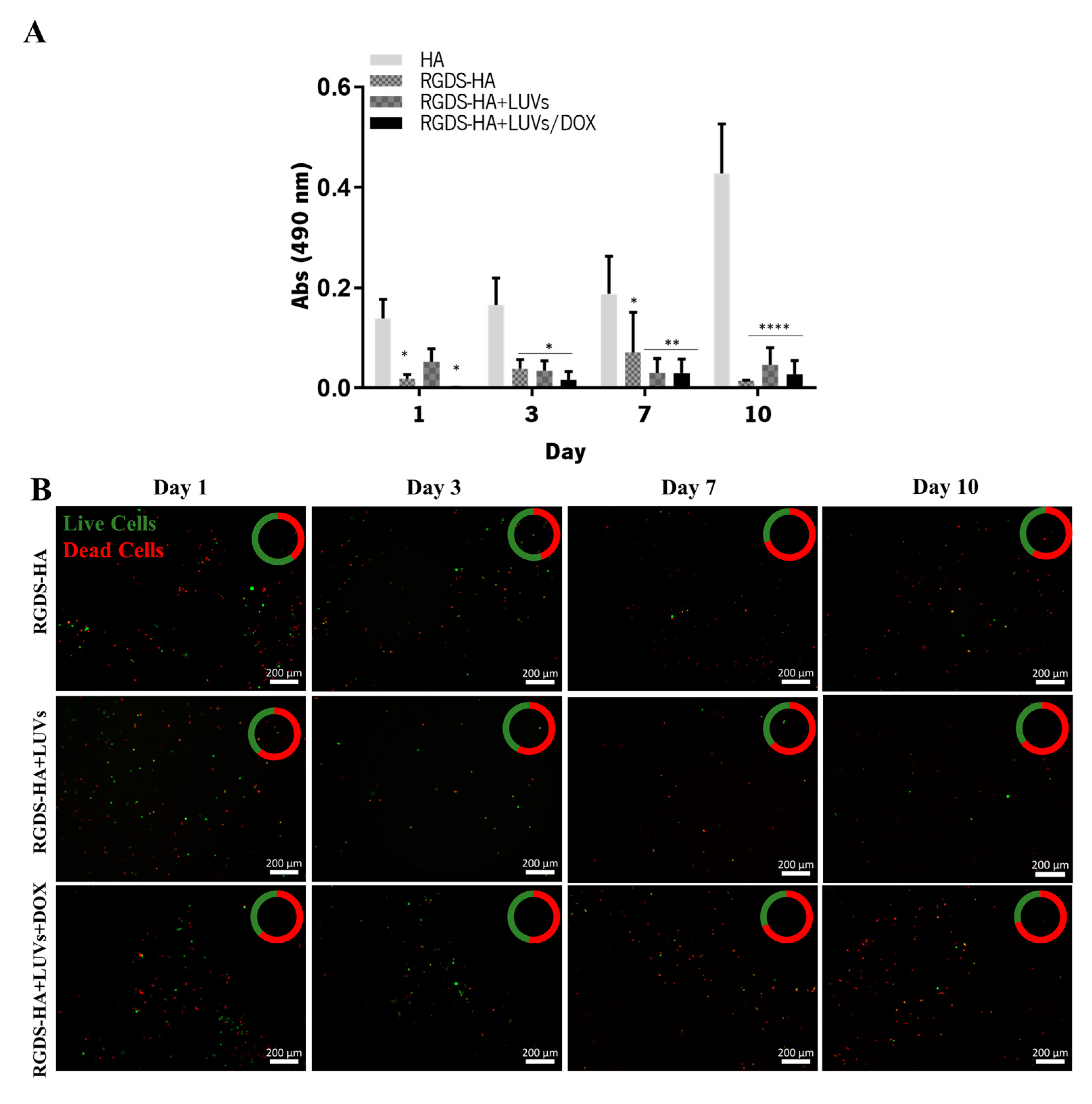
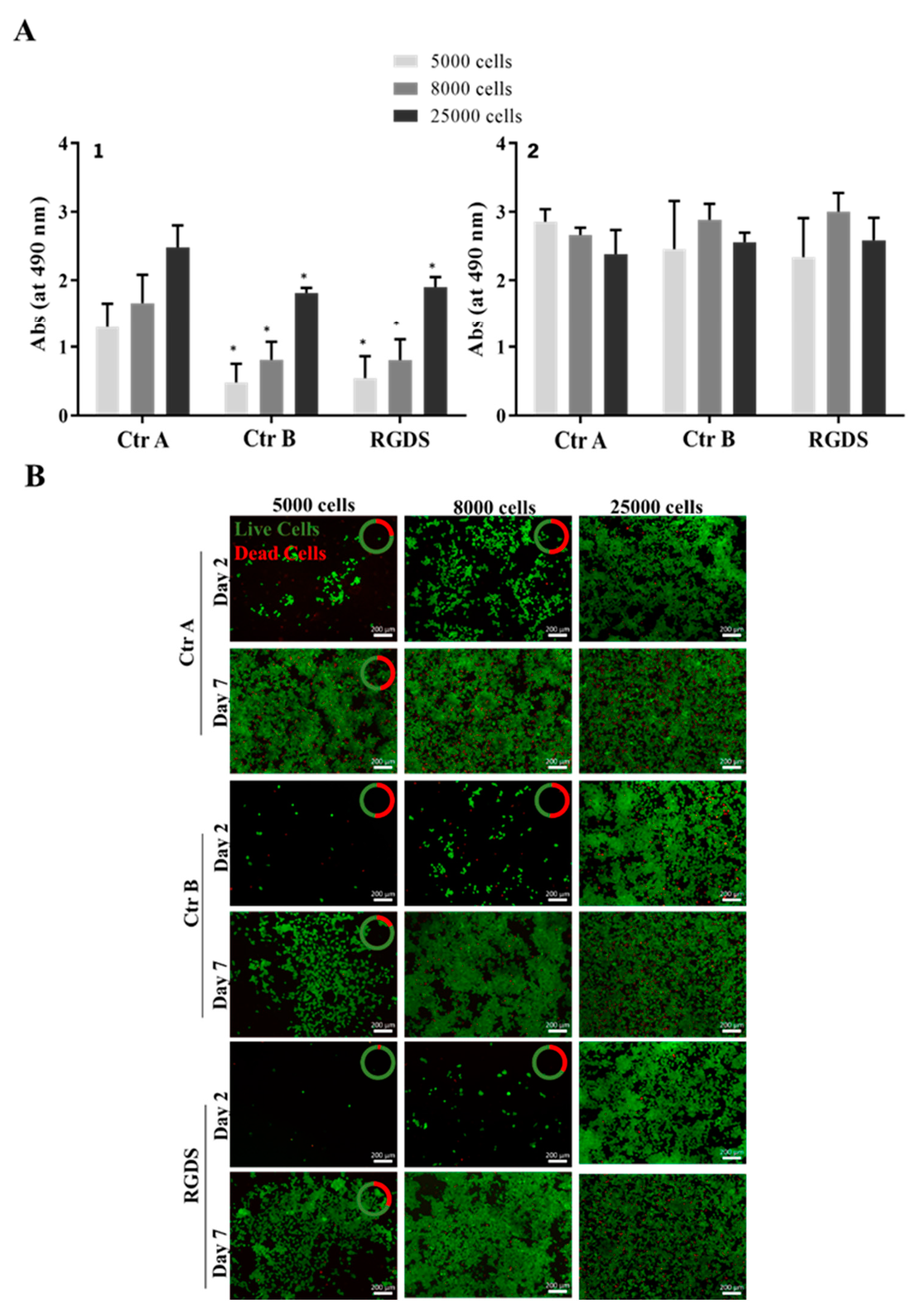
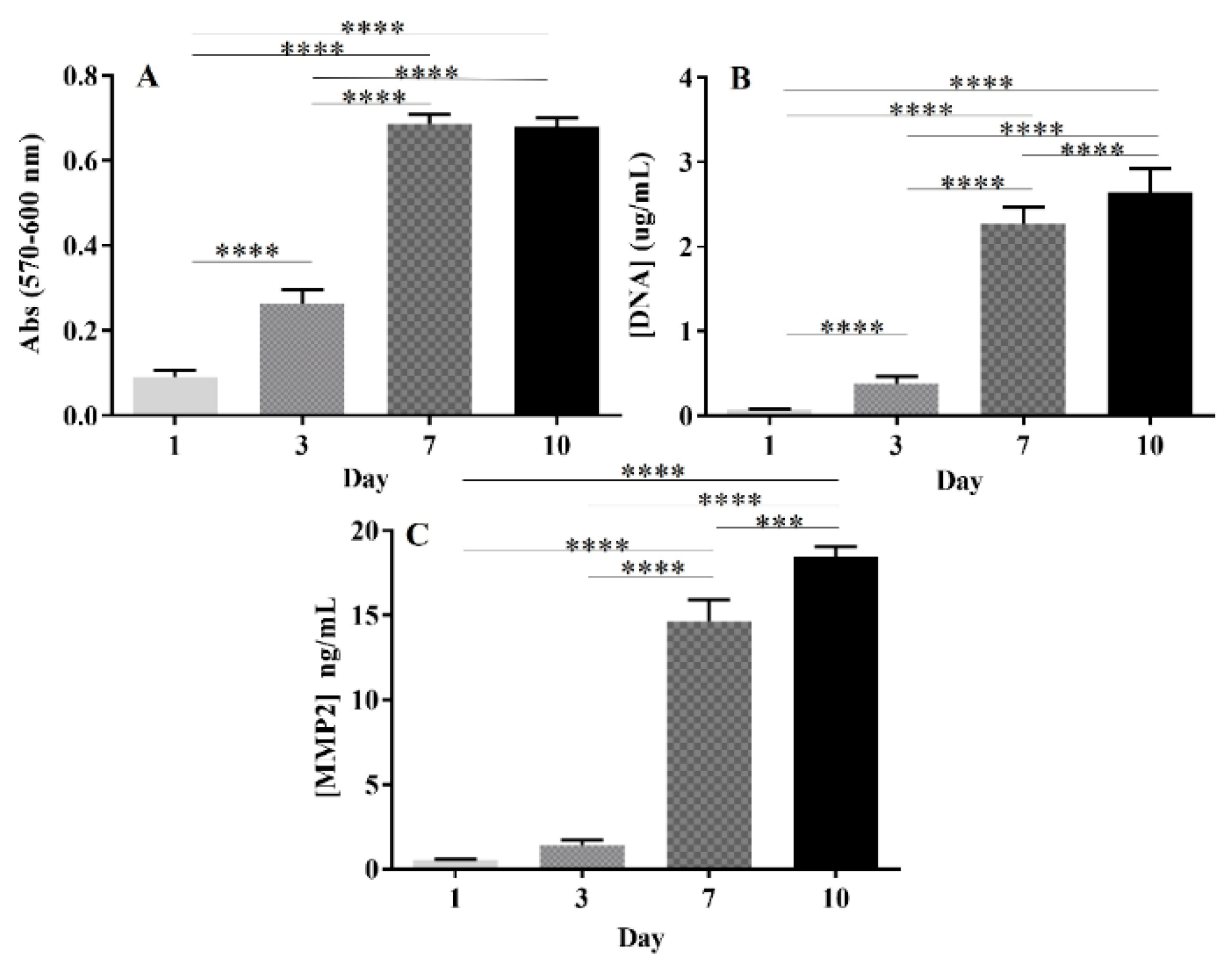
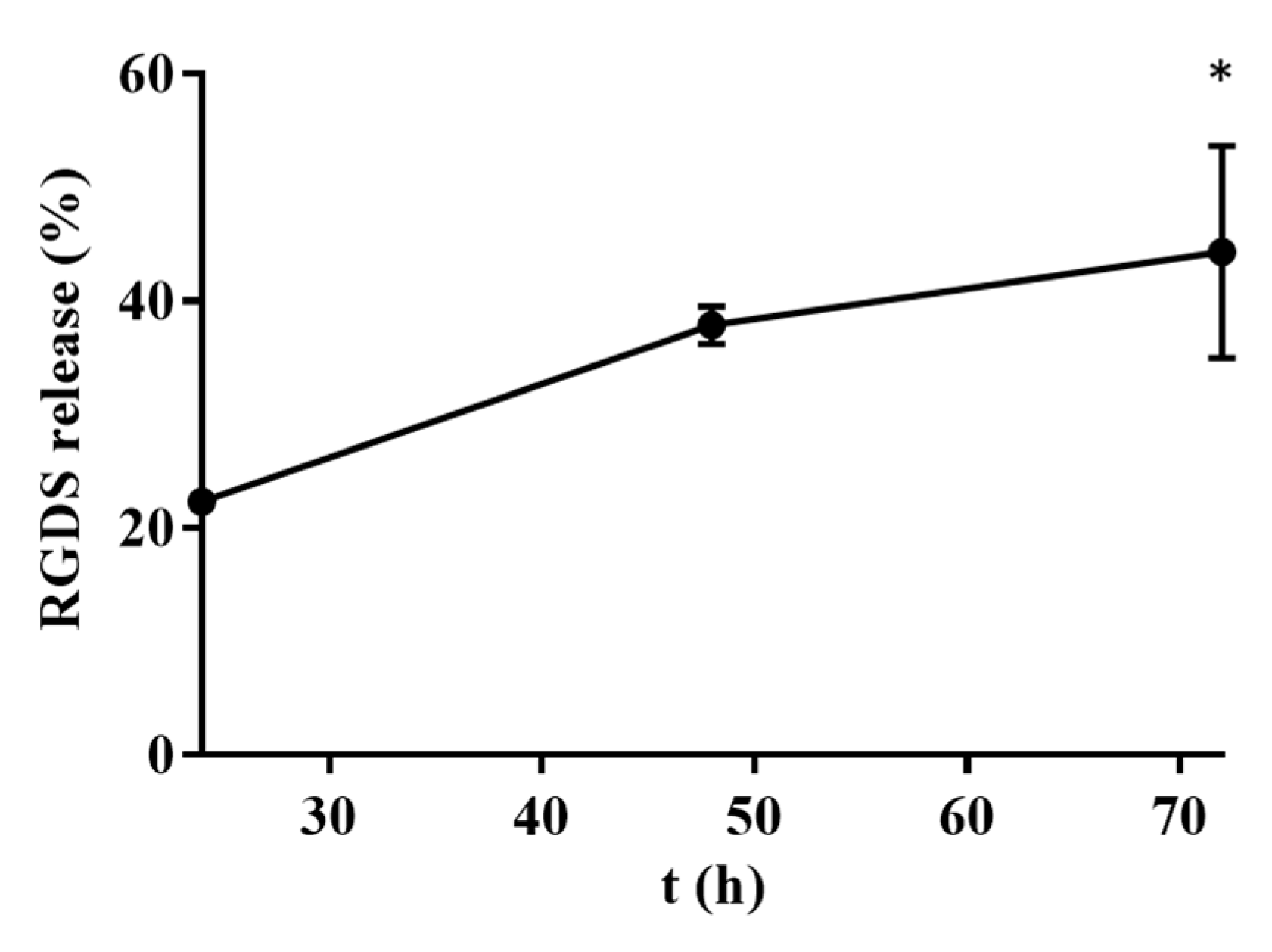
| Sample | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| LUVs | 114.7 ± 2.3 | 0.076 ± 0.023 | −1.38 ± 0.99 |
| LUVs+DOX | 121.7 ± 4.7 | 0.164 ± 0.041 | −2.43 ± 1.33 |
| Day | IC50 (µM) 1 |
|---|---|
| 1 | 3.822 (3.089–4.730) |
| 2 | 0.160 (0.136–0.189) |
| 3 | 0.128 (0.114–0.144) |
| 7 | 0.133 (0.116–0.152) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Ribeiro, M.L.; Castro, V.I.B.; Vieira de Castro, J.; Pires, R.A.; Reis, R.L.; Costa, B.M.; Ferreira, H.; Neves, N.M. The Potential of the Fibronectin Inhibitor Arg-Gly-Asp-Ser in the Development of Therapies for Glioblastoma. Int. J. Mol. Sci. 2024, 25, 4910. https://doi.org/10.3390/ijms25094910
Castro-Ribeiro ML, Castro VIB, Vieira de Castro J, Pires RA, Reis RL, Costa BM, Ferreira H, Neves NM. The Potential of the Fibronectin Inhibitor Arg-Gly-Asp-Ser in the Development of Therapies for Glioblastoma. International Journal of Molecular Sciences. 2024; 25(9):4910. https://doi.org/10.3390/ijms25094910
Chicago/Turabian StyleCastro-Ribeiro, Maria L., Vânia I. B. Castro, Joana Vieira de Castro, Ricardo A. Pires, Rui L. Reis, Bruno M. Costa, Helena Ferreira, and Nuno M. Neves. 2024. "The Potential of the Fibronectin Inhibitor Arg-Gly-Asp-Ser in the Development of Therapies for Glioblastoma" International Journal of Molecular Sciences 25, no. 9: 4910. https://doi.org/10.3390/ijms25094910
APA StyleCastro-Ribeiro, M. L., Castro, V. I. B., Vieira de Castro, J., Pires, R. A., Reis, R. L., Costa, B. M., Ferreira, H., & Neves, N. M. (2024). The Potential of the Fibronectin Inhibitor Arg-Gly-Asp-Ser in the Development of Therapies for Glioblastoma. International Journal of Molecular Sciences, 25(9), 4910. https://doi.org/10.3390/ijms25094910









