On the Re-Creation of Protoribosome Analogues in the Lab
Abstract
1. Introduction
2. Results
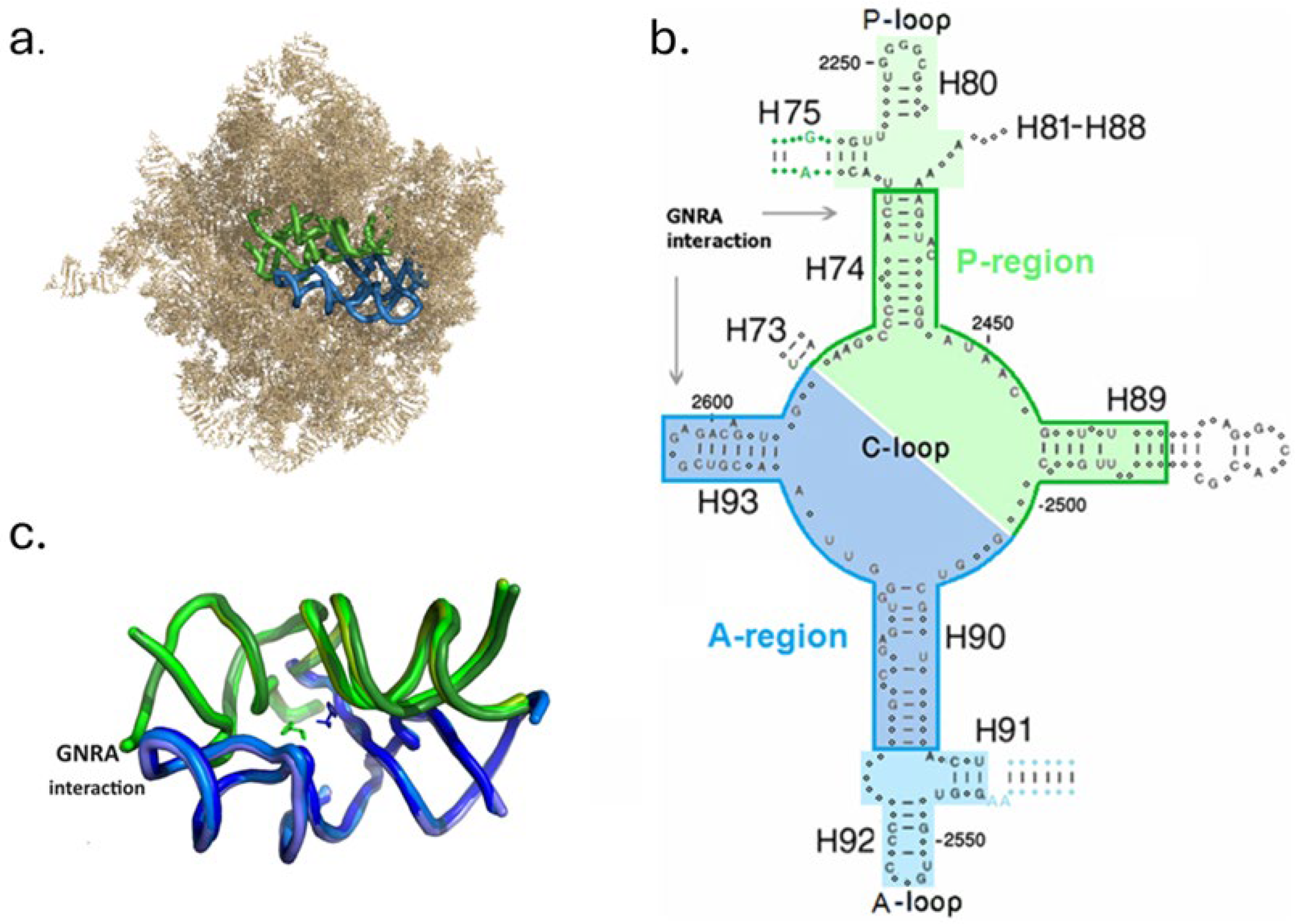
2.1. Structural Characterization of the Models
2.2. Functional Characterization of the Models
3. Discussion
3.1. Assessing Protoribosome Analogues
- The monomer sequence should be capable of folding spontaneously into a 2D scheme akin to the corresponding part of the PTC region.
- The monomer should dimerize.
- The dimer should catalyze the formation of a peptide bond.
- 4.
- The monomer’s sequence should have a realistic probability of accidentally occurring among the random RNA chains of the required length, while retaining the structural and functional characteristics of the modern PTC.
- 5.
- To ensure evolutionary continuity, the process inferred from the dimer structure should be equivalent to that taking place in the modern ribosome. This entails the accommodation of the reactants akin to the positioning of the amino acids in the PTC, thereby preserving the current mechanism of the peptide bond formation.
3.2. Heterodimer or Homodimer?
3.2.1. Stereochemistry
3.2.2. Dynamics
3.2.3. Sequence
3.2.4. Origin of Life Perspective
3.3. Emergence of an Active Prebiotic Protoribosome
- Dimer formation—a dimer, probably a minimal heterodimer composed of two autonomously folded L-shaped monomers of 60–70 mer each, which harbors two substrates, could have been formed either through the dimerization of two monomers, each accommodating a substrate, or via the dimerization of the RNA monomers followed by substrates accommodation.
- Substrates—aminoacylated RNA segments comprising one to three nucleotides would be sufficiently long to act as substrates in vitro, because the remaining nucleotides, in longer RNA stretches, have nothing to pair with in the minimal, DPR-like, dimer. Nevertheless, longer RNA segments might have been present in the prebiotic substrate, possibly because they were involved in the aminoacylation of the RNA moiety [31,33].
- Peptide bond formation—reactants would be accommodated in the dimer akin to the modern PTC, in the required proximity and stereochemistry. A nucleophilic attack of one reactant on the carbonyl carbon of the second one would result in peptide bond formation.
- Processivity would possibly take place, if following the formation of a peptide bond, the deacylated substrate would depart and the next acylated substrate inhabit, allowing the formation of the next peptide bond. The reiteration of this dynamic process could have led to the production of peptides with random composition, distinguished from prebiotic mineral-catalyzed peptides by being homochiral, owing to the preference of the PTC, and its predecessor, the protoribosome, for L-amino acids [34].
4. Materials and Methods
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nissen, P.; Hansen, J.; Ban, N.; Moore, P.B.; Steitz, T.A. The Structural Basis of Ribosome Activity in Peptide Bond Synthesis. Science 2000, 289, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Noller, H.F.; Hoffarth, V.; Zimniak, L. Unusual Resistance of Peptidyl Transferase to Protein Extraction Procedures. Science 1992, 256, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.T.; Dahlberg, A.E. Peptide bond formation is all about proximity. Nat. Struct. Mol. Biol. 2004, 11, 586–587. [Google Scholar] [CrossRef] [PubMed]
- Agmon, I.; Bashan, A.; Zarivach, R.; Yonath, A. Symmetry at the active site of the ribosome: Structural and functional implications. Biol. Chem. 2005, 386, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Agmon, I.; Bashan, A.; Yonath, A. On Ribosome Conservation and Evolution. Isr. J. Ecol. Evol. 2006, 52, 359–374. [Google Scholar] [CrossRef]
- Bokov, K.; Steinberg, S.V. A hierarchical model for evolution of 23S ribosomal RNA. Nature 2009, 457, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Agmon, I.; Davidovich, C.; Bashan, A.; Yonath, A. Identification of the prebiotic translation apparatus within the contemporary ribosome. Nat. Précéd. 2009. [Google Scholar] [CrossRef]
- Agmon, I. The Dimeric Proto-Ribosome: Structural Details and Possible Implications on the Origin of Life. Int. J. Mol. Sci. 2009, 10, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.S.; Bernier, C.R.; Hsiao, C.; Norris, A.M.; Kovacs, N.A.; Waterbury, C.C.; Stepanov, V.G.; Harvey, S.C.; Fox, G.E.; Wartell, R.M.; et al. Evolution of the ribosome at atomic resolution. Proc. Natl. Acad. Sci. USA 2014, 111, 10251–10256. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, Y. Protein-free ribosomal RNA scaffolds can assemble poly-lysine oligos from charged tRNA fragments. Biochem. Biophys. Res. Commun. 2021, 544, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Bose, T.; Fridkin, G.; Davidovich, C.; Krupkin, M.; Dinger, N.; Falkovich, A.H.; Peleg, Y.; Agmon, I.; Bashan, A.; Yonath, A. Origin of life: Protoribosome forms peptide bonds and links RNA and protein dominated worlds. Nucleic Acids Res. 2022, 50, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.; Fox, G.E. How to build a protoribosome: Structural insights from the first protoribosome constructs that have proven to be catalytically active. RNA 2023, 29, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Jerome, C.A.; Kim, H.-J.; Mojzsis, S.J.; Benner, S.A.; Biondi, E. Catalytic Synthesis of Polyribonucleic Acid on Prebiotic Rock Glasses. Astrobiology 2022, 22, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Kholod, N.S. Dimer formation by tRNAs. Biochem. Biokhimiia 1999, 64, 298–306. [Google Scholar]
- Agmon, I.C. Could a Proto-Ribosome Emerge Spontaneously in the Prebiotic World? Molecules 2016, 21, 1701. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. Origins and Early Evolution of the tRNA Molecule. Life 2015, 5, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.; Fox, G.E. Further Characterization of the Pseudo-Symmetrical Ribosomal Region. Life 2020, 10, 201. [Google Scholar] [CrossRef]
- Kawabata, M.; Kawashima, K.; Mutsuro-Aoki, H.; Ando, T.; Umehara, T.; Tamura, K. Peptide Bond Formation between Aminoacyl-Minihelices by a Scaffold Derived from the Peptidyl Transferase Center. Life 2022, 12, 573. [Google Scholar] [CrossRef]
- Ishikawa, J.; Fujita, Y.; Maeda, Y.; Furuta, H.; Ikawa, Y. GNRA/receptor interacting modules: Versatile modular units for natural and artificial RNA architectures. Methods 2011, 54, 226–238. [Google Scholar] [CrossRef]
- Müller, F.; Escobar, L.; Xu, F.; Węgrzyn, E.; Nainytė, M.; Amatov, T.; Chan, C.; Pichler, A.; Carell, T. A prebiotically plausible scenario of an RNA–peptide world. Nature 2022, 605, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Illangasekare, M.; Sanchez, G.; Nickles, T.; Yarus, M. Aminoacyl-RNA Synthesis Catalyzed by an RNA. Science 1995, 267, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Lohse, P.A.; Szostak, J.W. Ribozyme-catalysed amino-acid transfer reactions. Nature 1996, 381, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cech, T.R. Peptide bond formation by in vitro selected ribozymes. Nature 1997, 390, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Schimmel, P. Oligonucleotide-directed peptide synthesis in a ribosome- and ribozyme-free system. Proc. Natl. Acad. Sci. USA 2001, 98, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Tangpradabkul, T.; Palo, M.; Townley, J.; Hsu, K.B.; Participants, E.; Smaga, S.; Das, R.; Schepartz, A. Minimization of the E. coli ribosome, aided and optimized by community science. Nucleic Acids Res. 2024, 52, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Wekselman, I.; Davidovich, C.; Agmon, I.; Zimmerman, E.; Rozenberg, H.; Bashan, A.; Berisio, R.; Yonath, A. Ribosome’s mode of function: Myths, facts and recent results. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2009, 15, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, Y. smFRET study of rRNA dimerization at the peptidyl transfer center. Biophys. Chem. 2021, 277, 106657. [Google Scholar] [CrossRef] [PubMed]
- Agmon, I. Sequence complementarity at the ribosomal Peptidyl Transferase Centre implies self-replicating origin. FEBS Lett. 2017, 591, 3252–3258. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.L.; Unrau, P.J.; Müller, U.F. RNA Synthesis by in Vitro Selected Ribozymes for Recreating an RNA World. Life 2015, 5, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Agmon, I. Three Biopolymers and Origin of Life Scenarios. Life 2024, 14, 277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; O’Flaherty, D.K.; Szostak, J.W. Template-directed copying of RNA by non-enzymatic ligation. Angew. Chem. Int. Ed. Engl. 2020, 59, 15682–15687. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Reichel, A.; Buguin, A.; Libchaber, A. Efficiency of a self-aminoacylating ribozyme: Effect of the length and base-composition of its 3′ extension. RNA 2007, 13, 1191–1197. [Google Scholar] [CrossRef][Green Version]
- Zarivach, R.; Bashan, A.; Berisio, R.; Harms, J.; Auerbach, T.; Schluenzen, F.; Bartels, H.; Baram, D.; Pyetan, E.; Sittner, A.; et al. Functional aspects of ribosomal architecture: Symmetry, chirality and regulation. J. Phys. Org. Chem. 2004, 17, 901–912. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. A solution for the best rotation to relate two sets of vectors. Acta Crystallogr. Sect. A 1976, 32, 922–923. [Google Scholar] [CrossRef]


| Monomer | Model I | Model II | Model III | |
|---|---|---|---|---|
| Term in Figure 1b | P | P-DPR | P-DPR + P-loop + H75 | P-DPR |
| Original names | P | tt-P1 *; tt-P1c; sa-P1c; ef-P1c | ptc1a | P1c2; P1c2UGGU |
| Nucleotides included | P | 2058–2074; 2435–2461 *; 2489–2501; | 2060–2085; 2234–2258; 2432–2463; 2487–2501; | 2055–2075; 2434–2461; 2489–2501; +UGGU |
| Term in Figure 1b | A | A-DPR + A-loop + H91 | ||
| Original names | A | ptc1b | ||
| Nucleotides included | A | 2503–2528; 2535–2610 | ||
| Loops added to truncated helix | P | H74: 5′-CUUCGG-3′ H89: 5′-CUUCGG-3′ or: 5′-GUGA-3′ | H75: 5′-GAAGAA-3′ H89: 5′-GUGAG-3′ | H74: 5′-UUCG-3′ H89: 5′-GUGA-3′ |
| Loops added to truncated helix | A | H91: 5′-GAAGAA-3′ | ||
| Length ** (nucleotides) | P | 71 or: 67 | 108 | 70; 74 |
| Length ** (nucleotides) | A | 111 | ||
| Dimerization type | Homodimer PP’ (PIPI’) | Homodimer AA’ (AIIAII’) + heterodimer AP (AIIPII) | Homodimer PP’ (PIIIPIII’) | |
| Substrate | P | CCA-pcb # | ACCCACCA-K | Ala-minihelixAla |
| Substrate | A | C-Pmn ## | ACCCACCA-K | Ala-minihelixAla |
| Product | C-Pmn-pcb | CCA-K9 to CCCACCA-K9 | alanylalanine | |
| Species | Thermus thermophilus, Staphylococcus aureus, Enterococcus faecium | Thermus thermophilus | Deinococcus radiodurans |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agmon, I. On the Re-Creation of Protoribosome Analogues in the Lab. Int. J. Mol. Sci. 2024, 25, 4960. https://doi.org/10.3390/ijms25094960
Agmon I. On the Re-Creation of Protoribosome Analogues in the Lab. International Journal of Molecular Sciences. 2024; 25(9):4960. https://doi.org/10.3390/ijms25094960
Chicago/Turabian StyleAgmon, Ilana. 2024. "On the Re-Creation of Protoribosome Analogues in the Lab" International Journal of Molecular Sciences 25, no. 9: 4960. https://doi.org/10.3390/ijms25094960
APA StyleAgmon, I. (2024). On the Re-Creation of Protoribosome Analogues in the Lab. International Journal of Molecular Sciences, 25(9), 4960. https://doi.org/10.3390/ijms25094960




