Stem Cell and Regenerative Therapies for the Treatment of Osteoporotic Vertebral Compression Fractures
Abstract
:1. Introduction
2. Regenerative Therapy for Managing OVCFs
| Study Aim | Cell Type and Origin | Model | Delivery Method | Therapeutic Outcomes | Ref. |
|---|---|---|---|---|---|
| Clinical Study | |||||
| - 12-month, open-label, randomized controlled phase I/IIa clinical trial - To evaluate the safety and effectiveness of WJ-MSCs combined with teriparatide for treating patients with OVCFs - Enrolled 20 subjects, randomized into two groups: 10 in the experimental group and 10 in the control group | WJ-MSCs (allogenic) | Human | Intramedullary injection (4.0 × 107 cells) into fracture site, followed by intravenous injection (2.0 × 108 cells) after 1 week | - Significant improvements in the visual analog scale, Oswestry Disability Index, and 36-Item Short Form Survey. - Improved microarchitecture of spine and hip. | [34] |
| Preclinical Study | |||||
| - To develop a biocompatible treatment to address the limitations of vertebroplasty in OVCFs with PMMA-spheroid gel | BM-MSCs (allogenic) | Rat | Direct PMMA-doped MSC spheroid gel implantation into vertebral compression fracture site (1.0 × 106 cells) | - Increase in bone volume and BMD. - Decrease in pain markers in dorsal root ganglia. | [35] |
| - To evaluate the therapeutic potential of BM-MSCs for managing neural defects associated with VCFs | BM-MSCs (allogenic) | Canine | Percutaneous intraspinal injection (1.0 × 106 cells) every 15 days | - Improvement in loco-motor status and sensory functions in all cases. | [36] |
| - To investigate bone regeneration in a vertebral defect by MSCs overexpressing BMP-6 | Genetically modified BM-MSCs overexpressing BMP-6 (allogenic) | Minipig | Implanted into the vertebral defects (4.0 × 106 cells) | - Increased bone regeneration in response to the implantation of MSCs over-expressing BMP6 compared to minor bone formation in the control. - Enhanced bone regeneration in the BMP6-MSC group versus control group ex vivo. | [37] |
| - To study the capability of gene-modified adult stem cells overexpressing rhBMP-6 to regenerate vertebral bone in a rat model | Porcine ASCs (xenogeneic) | Rat | Implanted into the vertebral defects (1.0 × 106 cells) | - Considerable defect repair by 2 weeks post implantation, with bone formation rate and final bone volume. | [38] |
| - To analyze the effects of Sr-β-TCP combined with BM-MSCs or ASCs for spinal fusion - 15 OVX and 15 sham-operated rats divided into groups receiving Sr-β-TCP alone, Sr-β-TCP + BM-MSCs, and Sr-β-TCP + ASCs | BM-MSCs ASCs (syngeneic) | Rat | Direct Sr-β TCP scaffold with MSC implantation at the site of spinal fusion (1.5 × 106 cells) | - Formation of more solid fusion tissue in the Sr-β-TCP + BM-MSC group compared to Sr-β-TCP + ADSCs for both sham and OVX animals. - BMSCs’ superiority over ADSCs in promoting spinal fusion in radiographical scores and histological analysis. | [39] |
| - To assess the therapeutic potential of the systemic transplantation of MSCs in an age-related osteoporosis model | BM-MSCs (allogenic) | Rat | Intravenous injection (2.0 × 106 to 4.0 × 106 cells) | - Improved bone quality and microarchitectural competence. - Long-term engraftment and increased bone formation. | [40] |
| - To demonstrate the effectiveness of autologous BM-MSCs combined with porous β-TCP in repairing bone defects in the medial femoral condyle of osteoporotic goats | BM-MSCs (autologous) | Goat | Direct MSCs combined with porous β-TCP implantation into medial femoral condyle defects | - Improved bone formation and critical-sized bone defect repair in osteoporotic conditions. - Significant integration of MSC-β-TCP complex with the surrounding bone. | [41] |
| - To investigate the effect of an intra-bone marrow injection of BM-MSCs on femur bone mass in osteoporotic female rats | BM-MSCs (autologous) | Rat | Intra-bone marrow injection of BM-MSCs into the femurs of osteoporotic rats (7.5 × 105 cells) | - Increased femur bone mass in treated rats compared to untreated osteoporotic rats. - Similar trabecular bone percentage in treated rats to that of healthy control rats. | [42] |
| - To determine the effects of BM-MSCs on BMD and mechanical strength in the femurs of ovariectomized rats | BM-MSCs (allogenic) | Rat | Direct injection (1.0 × 107 cells) into each femur | - Significantly increased BMD in BM-MSC-injected femurs versus controls. - Increased mechanical strength with sustained improvements in rats receiving a second injection at 24 weeks. | [43] |
| - To evaluate whether the introduction of MSCs into sites at risk of osteoporosis in rabbits subjected to OVX can improve the architecture and mechanical properties of bone | BM-MSCs (autologous) | Rabbit | MSCs embedded in calcium alginate gels transplantation into the cancellous space of the distal femur (5.0 × 106 cells) | - Increased BMD in treated femurs. - Increased trabecular thickness and improved microstructures, including newly formed osteoid. - Stronger biomechanical stiffness. | [44] |
| - To address joint replacement complications due to osteoporosis with a three-dimensional inorganic–organic supramolecular bioactive interface combining a three-dimensional printed porous metal scaffold and a multifunctional supramolecular polysaccharide hydrogel encapsulating BM-MSCs and BMP-2 | BM-MSCs (autologous) | Rabbit | Implanted the bioactive interface containing encapsulated BMSCs and/or BMP-2 within the supramolecular hydrogel-filled pTi scaffold into distal femur defects (2.0 × 105 cells) | - Induced proliferation and osteogenic differentiation of BM-MSCs. - Promoted integration of the metal microspore–bone interface both in vitro and in vivo. | [45] |
| - To investigate the effect of BM-MSCs modified with bFGF on bone regeneration in distraction osteogenesis in rabbits. | BM-MSCs with and without bFGF gene modification (autologous) | Rabbit | Injection of BM-MSCs-(1.0 × 107 cells) with or without bFGF modification into the distraction gaps of the mandibles of rabbits | - Improved bone formation and mineralization with the highest BMD and bone mineral content observed in the bFGF-modified BM-MSC group. | [46] |
| - To evaluate the efficacy of OPG gene-modified BM-MSCs combined with an HA scaffold in treating critical-sized mandibular defects in osteoporotic rats induced by OVX | BM-MSCs modified to express OPG (autologous) | Rat | OPG gene-modified BM-MSC seeding on HA scaffold implantation (2.0 × 105 cells/cm2) into mandibular defects | - Improved bone formation and mineralization in the defect area. - Increased BMD and mineralized volume and reduced osteoclastogenesis. | [47] |
| - To investigate the feasibility of using CCB coated with BM-MSCs-sheet as a three-dimensional scaffold material in bone repair tissue engineering | BM-MSCs (allogenic) | Rat | - Implantation of CBB coated with allograft BMSC sheets (over 107 cells) in a sandwich structure for cranial defects | - Enhanced osteogenic differentiation and mineralized formation of the CBB-BMSC-sheet combination both in vitro and in vivo. - Significantly higher mRNA expressions of osteogenic markers such as BMP-2, b-FGF, Col1a1, OSX, and Runx-2. | [48] |
| - To evaluate the effectiveness of SrHA scaffolds, engineered with ASC, on osteogenesis and osteointegration in an osteoporotic sheep model | ASCs (allogenic) | Sheep | Implantation of ASCs on SrHA scaffolds in distal femur defects | - Increased osteogenic activity and mature lamellar bone formation. - Higher regeneration ratio and bone volume and improved osteointegration. | [49] |
| - To assess the ability of autologous ASCs to improve bone regeneration in a rabbit model of osteoporosis by promoting osteogenesis and reducing adipogenesis | ASCs (autologous) | Rabbit | Implantation of ASCs encapsulated in calcium alginate gel (5.0 × 106 cells) into the distal femurs | - Increased BMD and new bone formation. - Improvements in bone volume/total volume, connectivity density, and trabecular number metrics | [50] |
3. Therapeutic Mechanism of MSCs in OVCF Treatment
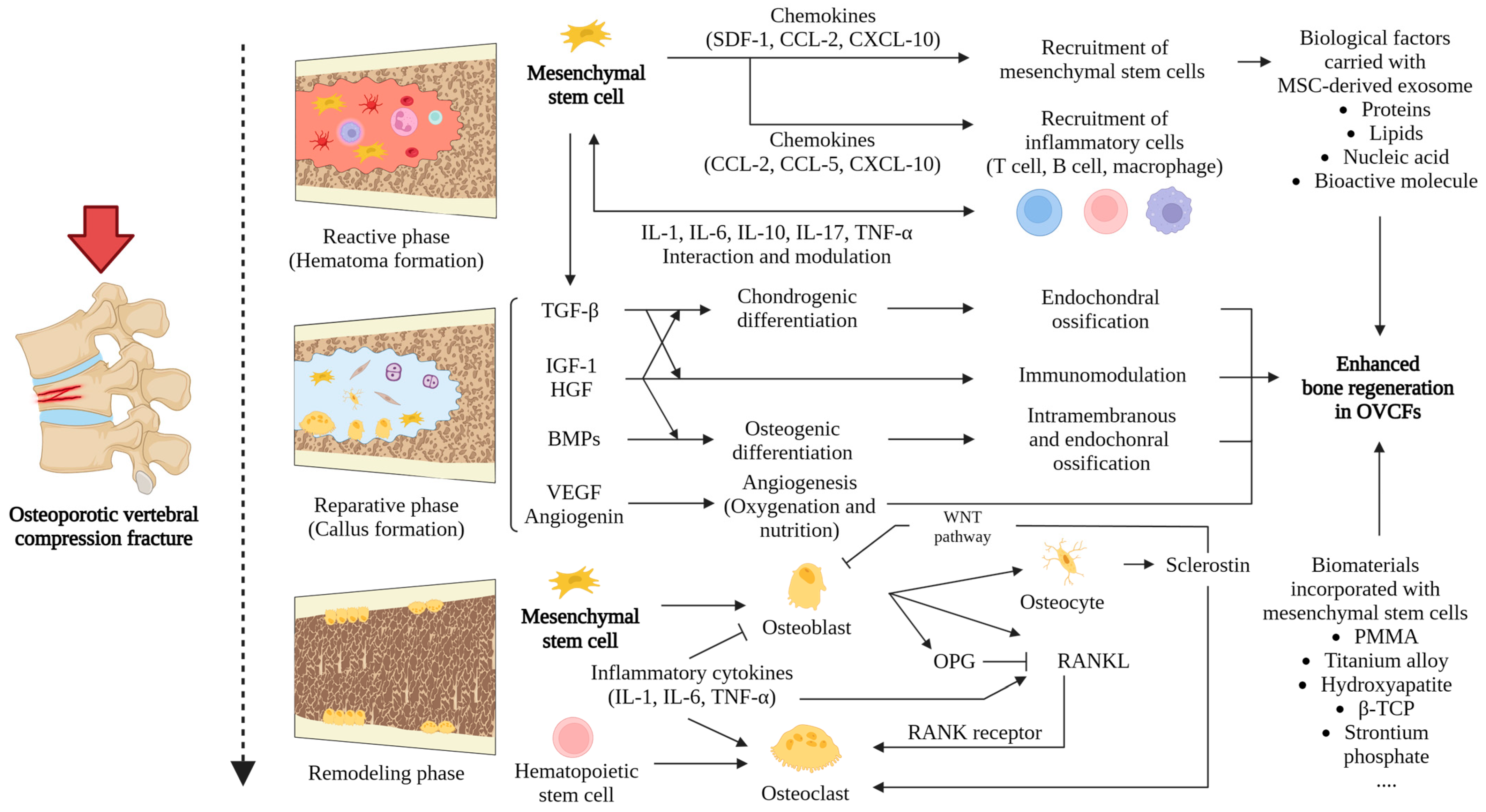
4. The Impact of MSCs on the Healing of OVCFs
5. MSC-Based Therapies for Treating OVCFs
6. MSC-Derived Exosomes in the Treatment of OVCFs
7. Biomaterials Loaded with Stem Cells in the Treatment of OVCFs
8. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OVCFs | osteoporotic vertebral compression fractures |
| MSCs | mesenchymal stem cells |
| PVP | percutaneous vertebroplasty |
| PKP | percutaneous kyphoplasty |
| SERMs | selective estrogen receptor modulators |
| BMD | bone mineral density |
| miRNAs | microRNAs |
| BMP | bone morphogenetic protein |
| TNF-α | tumor necrosis factor alpha |
| VEGF | vascular endothelial growth factor |
| IL-17 | interleukin 17 |
| ROS | reactive oxygen species |
| NRF2 | nuclear factor erythroid-2 related factor 2 |
| M-CSF | macrophage colony-stimulating factor |
| RANKL | receptor activator of nuclear factor kappa B ligand |
| OPG | osteoprotegerin |
| TGF-β | transforming growth factor-beta |
| IL-1 | interleukin-1 |
| BM-MSCs | bone marrow-derived mesenchymal stem cells |
| OVX | ovariectomized |
| aBM-MSCs | allogenic bone marrow-derived mesenchymal stem cells |
| WJ-MSCs | Wharton’s jelly-derived MSCs |
| MSCs-Exos | exosomes sourced from MSCs |
| GPNMB | glycoprotein non-melanoma clone B |
| BMSCs | bone marrow stem cells |
| Β-TCP | β-tricalcium phosphate |
| OPG | osteoprotegerin |
| AD-MSC | adipose tissue-derived mesenchymal stem cell |
| PMMA | polymethyl methacrylate |
References
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern. Med. 2017, 167, Itc17–Itc32. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Yi, W.; Yang, D. Advances in Vertebral Augmentation Systems for Osteoporotic Vertebral Compression Fractures. Pain. Res. Manag. 2020, 2020, 3947368. [Google Scholar] [CrossRef]
- Buchbinder, R.; Johnston, R.V.; Rischin, K.J.; Homik, J.; Jones, C.A.; Golmohammadi, K.; Kallmes, D.F. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database Syst. Rev. 2018, 11, Cd006349. [Google Scholar] [CrossRef]
- Allam, A.K.; Anand, A.; Flores, A.R.; Ropper, A.E. Computer Vision in Osteoporotic Vertebral Fracture Risk Prediction: A Systematic Review. Neurospine 2023, 20, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Zileli, M.; Fornari, M.; Costa, F.; Anania, C.D.; Parthiban, J.; Sharif, S. Epidemiology, natural course, and preventive measures of osteoporotic vertebral fractures: WFNS Spine Committee Recommendations. J. Neurosurg. Sci. 2022, 66, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, D.Y.; Koo, J.W.; Lee, S.G.; Jeong, S.Y.; Kang, C.N. Incidence and Management Trends of Osteoporotic Vertebral Compression Fractures in South Korea: A Nationwide Population-Based Study. Asian Spine J. 2020, 14, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Son, H.J.; Park, S.J.; Kim, J.K.; Park, J.S. Mortality risk after the first occurrence of osteoporotic vertebral compression fractures in the general population: A nationwide cohort study. PLoS ONE 2023, 18, e0291561. [Google Scholar] [CrossRef] [PubMed]
- Park, M.W.; Park, S.J.; Chung, S.G. Relationships Between Skeletal Muscle Mass, Lumbar Lordosis, and Chronic Low Back Pain in the Elderly. Neurospine 2023, 20, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.S.; Cho, K.J.; Park, J.W. Early Adjacent Vertebral Fractures after Balloon Kyphoplasty for Osteoporotic Vertebral Compression Fractures. Asian Spine J. 2019, 13, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.L. Overview of treatment approaches to osteoporosis. Br. J. Pharmacol. 2021, 178, 1891–1906. [Google Scholar] [CrossRef]
- Xu, Z.; Hao, D.; Dong, L.; Yan, L.; He, B. Surgical options for symptomatic old osteoporotic vertebral compression fractures: A retrospective study of 238 cases. BMC Surg. 2021, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S. Molecular understanding of pharmacological treatment of osteoporosis. EFORT Open Rev. 2019, 4, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Sindel, D. Osteoporosis: Spotlight on current approaches to pharmacological treatment. Turk. J. Phys. Med. Rehabil. 2023, 69, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Engelke, K.; Keaveny, T.M.; Chines, A.; Chapurlat, R.; Foldes, A.J.; Nogues, X.; Civitelli, R.; De Villiers, T.; Massari, F.; et al. Romosozumab improves lumbar spine bone mass and bone strength parameters relative to alendronate in postmenopausal women: Results from the Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk (ARCH) trial. J. Bone Miner. Res. 2021, 36, 2139–2152. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Shin, W.C.; Kim, J.W. Effect of Osteoporosis Medication on Fracture Healing: An Evidence Based Review. J. Bone Metab. 2020, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.S.; Moon, B.G.; Kang, H.I.; Kim, J.H.; Jwa, C.; Kim, D.R. Difference in the Cobb Angle Between Standing and Supine Position as a Prognostic Factor after Vertebral Augmentation in Osteoporotic Vertebral Compression Fractures. Neurospine 2022, 19, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Inose, H.; Tamai, K.; Iwamae, M.; Terai, H.; Nakamura, H. Risk of Revision after Vertebral Augmentation for Osteoporotic Vertebral Fracture: A Narrative Review. Neurospine 2023, 20, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Jindal, V.; Binyala, S.; Kohli, S.S. Balloon kyphoplasty versus percutaneous vertebroplasty for osteoporotic vertebral body compression fractures: Clinical and radiological outcomes. Spine J. 2023, 23, 579–584. [Google Scholar] [CrossRef]
- Griffoni, C.; Lukassen, J.N.M.; Babbi, L.; Girolami, M.; Lamartina, C.; Cecchinato, R.; Gasbarrini, A.; Barbanti Brodano, G. Percutaneous vertebroplasty and balloon kyphoplasty in the treatment of osteoporotic vertebral fractures: A prospective randomized comparison. Eur. Spine J. 2020, 29, 1614–1620. [Google Scholar] [CrossRef]
- Nieuwenhuijse, M.J.; Van Erkel, A.R.; Dijkstra, P.D. Cement leakage in percutaneous vertebroplasty for osteoporotic vertebral compression fractures: Identification of risk factors. Spine J. 2011, 11, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Ikeda, N.; Tanaka, H.; Ito, Y.; Sugie, A.; Yamada, M.; Wanibuchi, M.; Kawanishi, M. The Effectiveness of Vertebral Height Restoration Based on the Vertebroplasty Procedure Used to Treat Osteoporotic Vertebral Fractures. Neurospine 2023, 20, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cheng, J.; Yin, J.; Zhang, Z.; Liu, C.; Hao, D. Therapeutic effect of kyphoplasty and balloon vertebroplasty on osteoporotic vertebral compression fracture: A systematic review and meta-analysis of randomized controlled trials. Medicine 2019, 98, e17810. [Google Scholar] [CrossRef]
- Daher, M.; Kreichati, G.; Kharrat, K.; Sebaaly, A. Vertebroplasty versus Kyphoplasty in the Treatment of Osteoporotic Vertebral Compression Fractures: A Meta-Analysis. World Neurosurg. 2023, 171, 65–71. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.F.; Miao, D.C.; Dong, Z.; Shen, Y. Which one is more effective for the treatment of very severe osteoporotic vertebral compression fractures: PVP or PKP? J. Pain Res. 2018, 11, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, M.; Tanaka, H.; Ito, Y.; Yamada, M.; Yokoyama, K.; Sugie, A.; Ikeda, N. Treatment for Osteoporotic Vertebral Fracture—A Short Review of Orthosis and Percutaneous Vertebroplasty and Balloon Kyphoplasty. Neurospine 2023, 20, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.; Liang, Q.Y.; Ding, Y.; Han, I.; Zeng, X. Multimodal Repair of Spinal Cord Injury with Mesenchymal Stem Cells. Neurospine 2022, 19, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Wu, S.Y.; Leung, P.S. Human Fetal Bone Marrow-Derived Mesenchymal Stem Cells Promote the Proliferation and Differentiation of Pancreatic Progenitor Cells and the Engraftment Function of Islet-like Cell Clusters. Int. J. Mol. Sci. 2019, 20, 4083. [Google Scholar] [CrossRef]
- Roolfs, L.; Hubertus, V.; Spinnen, J.; Shopperly, L.K.; Fehlings, M.G.; Vajkoczy, P. Therapeutic Approaches Targeting Vascular Repair after Experimental Spinal Cord Injury: A Systematic Review of the Literature. Neurospine 2022, 19, 961–975. [Google Scholar] [CrossRef]
- Arjmand, B.; Sarvari, M.; Alavi-Moghadam, S.; Payab, M.; Goodarzi, P.; Gilany, K.; Mehrdad, N.; Larijani, B. Prospect of Stem Cell Therapy and Regenerative Medicine in Osteoporosis. Front. Endocrinol. 2020, 11, 430. [Google Scholar] [CrossRef]
- Takami, T.; Shimokawa, N.; Parthiban, J.; Zileli, M.; Ali, S. Pharmacologic and Regenerative Cell Therapy for Spinal Cord Injury: WFNS Spine Committee Recommendations. Neurospine 2020, 17, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Stamnitz, S.; Klimczak, A. Mesenchymal Stem Cells, Bioactive Factors, and Scaffolds in Bone Repair: From Research Perspectives to Clinical Practice. Cells 2021, 10, 1925. [Google Scholar] [CrossRef] [PubMed]
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front. Cell Dev. Biol. 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.; Ahsan, M.; Saleem, Z.; Iqtedar, M.; Islam, M.; Danish, Z.; Khan, A.M. Mesenchymal stem cells (MSCs) as skeletal therapeutics—An update. J. Biomed. Sci. 2016, 23, 41. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Gou, W.; Lu, Q.; Peng, J.; Lu, S. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. Int. Orthop. 2013, 37, 2491–2498. [Google Scholar] [CrossRef]
- Sanghani-Kerai, A.; McCreary, D.; Lancashire, H.; Osagie, L.; Coathup, M.; Blunn, G. Stem Cell Interventions for Bone Healing: Fractures and Osteoporosis. Curr. Stem Cell Res. Ther. 2018, 13, 369–377. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Wang, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Osteoporosis via MicroRNA-27a-Induced Inhibition of DKK2-Mediated Wnt/β-Catenin Pathway. Inflammation 2022, 45, 780–799. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Liu, B.; Shao, C.; Shi, Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell 2022, 29, 1515–1530. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, L.; Cao, H.; Guo, J.; Zhou, X.; Zeng, Z. Application and Molecular Mechanisms of Extracellular Vesicles Derived from Mesenchymal Stem Cells in Osteoporosis. Curr. Issues Mol. Biol. 2022, 44, 6346–6367. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Hong, C.G.; Hu, W.B.; Chen, M.L.; Duan, R.; Li, H.M.; Yue, T.; Cao, J.; Wang, Z.X.; Chen, C.Y.; et al. Autophagy receptor OPTN (optineurin) regulates mesenchymal stem cell fate and bone-fat balance during aging by clearing FABP3. Autophagy 2021, 17, 2766–2782. [Google Scholar] [CrossRef]
- Deng, P.; Yuan, Q.; Cheng, Y.; Li, J.; Liu, Z.; Liu, Y.; Li, Y.; Su, T.; Wang, J.; Salvo, M.E.; et al. Loss of KDM4B exacerbates bone-fat imbalance and mesenchymal stromal cell exhaustion in skeletal aging. Cell Stem Cell 2021, 28, 1057–1073.e1057. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, J.; Yu, Y.; Ni, Y.; Wei, Y.; Cheng, Y.; Han, L.; Xiao, L.; Ma, X.; Wei, H.; et al. Mitochondrial Transfer Regulates Cell Fate through Metabolic Remodeling in Osteoporosis. Adv. Sci. 2023, 10, e2204871. [Google Scholar] [CrossRef]
- Roberts, J.L.; Paglia, D.N.; Drissi, H. Transcriptional Mechanisms of Secondary Fracture Healing. Curr. Osteoporos. Rep. 2018, 16, 146–154. [Google Scholar] [CrossRef]
- Augello, A.; De Bari, C. The regulation of differentiation in mesenchymal stem cells. Hum. Gene Ther. 2010, 211, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Nishio, Y.; Dong, Y.; Paris, M.; O’Keefe, R.J.; Schwarz, E.M.; Drissi, H. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene 2006, 372, 62–70. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhang, X.; Zhu, C.; Tang, X.; Yu, F.; Shang, G.W.; Cai, X. Molecular Mechanisms of PPAR-γ Governing MSC Osteogenic and Adipogenic Differentiation. Curr. Stem Cell Res. Ther. 2016, 11, 255–264. [Google Scholar] [CrossRef]
- Lin, F.T.; Lane, M.D. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc. Natl. Acad. Sci. USA 1994, 91, 8757–8761. [Google Scholar] [CrossRef]
- Okitsu, Y.; Takahashi, S.; Minegishi, N.; Kameoka, J.; Kaku, M.; Yamamoto, M.; Sasaki, T.; Harigae, H. Regulation of adipocyte differentiation of bone marrow stromal cells by transcription factor GATA-2. Biochem. Biophys. Res. Commun. 2007, 364, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Song, W.X.; Luo, Q.; Tang, N.; Luo, J.; Luo, X.; Chen, J.; Bi, Y.; He, B.C.; Park, J.K.; et al. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009, 18, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Glowacki, J.; Zhou, S. Inhibition of adipocytogenesis by canonical WNT signaling in human mesenchymal stem cells. Exp. Cell Res. 2011, 317, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Q.; Luo, S.; Liu, Z.; Luo, D.; Zhang, B.; Zhang, D.; Rao, P.; Xiao, J. PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2016, 11, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Eliasson, B.; Smith, U. Thiazolidinediones increase the wingless-type MMTV integration site family (WNT) inhibitor Dickkopf-1 in adipocytes: A link with osteogenesis. Diabetologia 2010, 53, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Wang, M.; Yin, Z.; Jing, Y.; Bai, L.; Su, J. Microenvironment-targeted strategy steers advanced bone regeneration. Mater. Today Biol. 2023, 22, 100741. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Yao, B.; Zhang, B.D.; Bai, Y.; Sui, W.; Wang, W.; Yu, Q. Stromal-derived Factor-1α signaling is involved in bone morphogenetic protein-2-induced odontogenic differentiation of stem cells from apical papilla via the Smad and Erk signaling pathways. Exp. Cell Res. 2019, 381, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Cho, M.L.; Park, M.K.; Yoon, C.H.; Park, S.H.; Lee, S.H.; Kim, H.Y. Increased interleukin-17 production via a phosphoinositide 3-kinase/Akt and nuclear factor kappaB-dependent pathway in patients with rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R139–R148. [Google Scholar] [CrossRef]
- Sohni, A.; Verfaillie, C.M. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013, 2013, 130763. [Google Scholar] [CrossRef]
- Pelled, G.; Sheyn, D.; Tawackoli, W.; Jun, D.S.; Koh, Y.; Su, S.; Cohn Yakubovich, D.; Kallai, I.; Antebi, B.; Da, X.; et al. BMP6-Engineered MSCs Induce Vertebral Bone Repair in a Pig Model: A Pilot Study. Stem Cells Int. 2016, 2016, 6530624. [Google Scholar] [CrossRef] [PubMed]
- Macías, I.; Alcorta-Sevillano, N.; Rodríguez, C.I.; Infante, A. Osteoporosis and the Potential of Cell-Based Therapeutic Strategies. Int. J. Mol. Sci. 2020, 21, 1653. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Wallis, G. Endochondral ossification: A delicate balance between growth and mineralisation. Curr. Biol. 2001, 11, R589–R591. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nam, H.; Joo, K.M.; Lee, S.H. Advances in Neural Stem Cell Therapy for Spinal Cord Injury: Safety, Efficacy, and Future Perspectives. Neurospine 2022, 19, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Cortet, B.; Bousson, V.; Thomas, T. Vertebroplasty for osteoporotic vertebral fracture. RMD Open 2021, 7, e001655. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Rovella, V.; Candi, E.; Jia, W.; Bernassola, F.; Bove, P.; Piacentini, M.; Scimeca, M.; Sica, G.; et al. Aged mesenchymal stem cells and inflammation: From pathology to potential therapeutic strategies. Biol. Direct 2023, 18, 40. [Google Scholar] [CrossRef]
- Beane, O.S.; Fonseca, V.C.; Cooper, L.L.; Koren, G.; Darling, E.M. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE 2014, 9, e115963. [Google Scholar] [CrossRef] [PubMed]
- Fafián-Labora, J.A.; Morente-López, M.; Arufe, M.C. Effect of aging on behaviour of mesenchymal stem cells. World J. Stem Cells 2019, 11, 337–346. [Google Scholar] [CrossRef]
- Schell, H.; Duda, G.N.; Peters, A.; Tsitsilonis, S.; Johnson, K.A.; Schmidt-Bleek, K. The haematoma and its role in bone healing. J. Exp. Orthop. 2017, 4, 5. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Nyúl-Tóth, Á.; Kiss, T.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Benyo, Z.; Csiszar, A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: From increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience 2019, 41, 727–738. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, J.; Huang, Y.; Zhang, Y.; Liu, W.; Wang, G.; Zhang, Q.; Wang, G.; Yang, Y.; Li, H.; et al. NRF2 overexpression in mesenchymal stem cells induces stem-cell marker expression and enhances osteoblastic differentiation. Biochem. Biophys. Res. Commun. 2017, 491, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L. Changes in inflammatory factors in patients with osteoporotic vertebral compression fracture and influences of rehabilitation training on postoperative functional recovery and inflammation. J. Musculoskelet. Neuronal Interact. 2018, 18, 272–279. [Google Scholar]
- Adamopoulos, I.E. Inflammation in bone physiology and pathology. Curr. Opin. Rheumatol. 2018, 30, 59–64. [Google Scholar] [CrossRef]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Paspaliaris, V.; Kolios, G. Stem cells in Osteoporosis: From Biology to New Therapeutic Approaches. Stem Cells Int. 2019, 2019, 1730978. [Google Scholar] [CrossRef]
- Fernandes, M.B.; Guimarães, J.A.; Casado, P.L.; Cavalcanti Ados, S.; Gonçalves, N.N.; Ambrósio, C.E.; Rodrigues, F.; Pinto, A.C.; Miglino, M.A.; Duarte, M.E. The effect of bone allografts combined with bone marrow stromal cells on the healing of segmental bone defects in a sheep model. BMC Vet. Res. 2014, 10, 36. [Google Scholar] [CrossRef]
- Ocarino Nde, M.; Boeloni, J.N.; Jorgetti, V.; Gomes, D.A.; Goes, A.M.; Serakides, R. Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats. Connect. Tissue Res. 2010, 51, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shao, B.; Zhou, Z.; Shang, F.; Shuai, Y.; Wang, X.; Liao, L.; Jin, Y.; Yang, D. Role of bone marrow-derived mesenchymal stem cells in treating estrogen deficiency induced osteoporosis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2013, 29, 1267–1271. [Google Scholar]
- Wang, Z.; Goh, J.; Das De, S.; Ge, Z.; Ouyang, H.; Chong, J.S.; Low, S.L.; Lee, E.H. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006, 12, 1753–1761. [Google Scholar] [CrossRef]
- Cao, L.; Liu, G.; Gan, Y.; Fan, Q.; Yang, F.; Zhang, X.; Tang, T.; Dai, K. The use of autologous enriched bone marrow MSCs to enhance osteoporotic bone defect repair in long-term estrogen deficient goats. Biomaterials 2012, 33, 5076–5084. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, P.; Zhang, X.; Lv, L.; Zhou, Y. Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell Prolif. 2021, 54, e12956. [Google Scholar] [CrossRef]
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Uejima, S.; Okada, K.; Kagami, H.; Taguchi, A.; Ueda, M. Bone marrow stromal cell therapy improves femoral bone mineral density and mechanical strength in ovariectomized rats. Cytotherapy 2008, 10, 479–489. [Google Scholar] [CrossRef]
- Kiernan, J.; Hu, S.; Grynpas, M.D.; Davies, J.E.; Stanford, W.L. Systemic Mesenchymal Stromal Cell Transplantation Prevents Functional Bone Loss in a Mouse Model of Age-Related Osteoporosis. Stem Cells Transl. Med. 2016, 5, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Ichioka, N.; Inaba, M.; Kushida, T.; Esumi, T.; Takahara, K.; Inaba, K.; Ogawa, R.; Iida, H.; Ikehara, S. Prevention of senile osteoporosis in SAMP6 mice by intrabone marrow injection of allogeneic bone marrow cells. Stem Cells 2002, 20, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Dong, F.; Huang, G.; He, P.; Chen, H.; Qin, S.; Li, A. Risk factors for secondary fractures to percutaneous vertebroplasty for osteoporotic vertebral compression fractures: A systematic review. J. Orthop. Surg. Res. 2021, 16, 644. [Google Scholar] [CrossRef]
- Sharun, K.; Rawat, T.; Kumar, R.; Chandra, V.; Saxena, A.C.; Pawde, A.M.; Kinjavdekar, P.; Amarpal; Sharma, G.T. Clinical evaluation following the percutaneous transplantation of allogenic bone marrow-derived mesenchymal stem cells (aBM-MSC) in dogs affected by vertebral compression fracture. Vet. Anim. Sci. 2020, 10, 100152. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Shim, J.; Kim, K.T.; Kim, K.G.; Choi, U.Y.; Kyung, J.W.; Sohn, S.; Lim, S.H.; Choi, H.; Ahn, T.K.; Choi, H.J.; et al. Safety and efficacy of Wharton’s jelly-derived mesenchymal stem cells with teriparatide for osteoporotic vertebral fractures: A phase I/IIa study. Stem Cells Transl. Med. 2021, 10, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Bhujel, B.; Shin, H.E.; Choi, D.J.; Han, I. Mesenchymal Stem Cell-Derived Exosomes and Intervertebral Disc Regeneration: Review. Int. J. Mol. Sci. 2022, 23, 7306. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- Szydlak, R. Biological, chemical and mechanical factors regulating migration and homing of mesenchymal stem cells. World J. Stem Cells 2021, 13, 619–631. [Google Scholar] [CrossRef]
- Baranovskii, D.S.; Klabukov, I.D.; Arguchinskaya, N.V.; Yakimova, A.O.; Kisel, A.A.; Yatsenko, E.M.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell Investig. 2022, 9, 7. [Google Scholar] [CrossRef]
- Sinder, B.P.; Novak, S.; Wee, N.K.Y.; Basile, M.; Maye, P.; Matthews, B.G.; Kalajzic, I. Engraftment of skeletal progenitor cells by bone-directed transplantation improves osteogenesis imperfecta murine bone phenotype. Stem Cells 2020, 38, 530–541. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Khanh, V.C.; Yamashita, T.; Ohneda, K.; Tokunaga, C.; Kato, H.; Osaka, M.; Hiramatsu, Y.; Ohneda, O. Rejuvenation of mesenchymal stem cells by extracellular vesicles inhibits the elevation of reactive oxygen species. Sci. Rep. 2020, 10, 17315. [Google Scholar] [CrossRef] [PubMed]
- Sekelova, T.; Danisovic, L.; Cehakova, M. Rejuvenation of Senescent Mesenchymal Stem Cells to Prevent Age-Related Changes in Synovial Joints. Cell Transplant. 2023, 32, 9636897231200065. [Google Scholar] [CrossRef]
- Woods, K.; Guezguez, B. Dynamic Changes of the Bone Marrow Niche: Mesenchymal Stromal Cells and Their Progeny during Aging and Leukemia. Front. Cell Dev. Biol. 2021, 9, 714716. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Shi, H.; Qian, H.; Xu, W. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy 2018, 20, 291–301. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, Y.; Liu, Z.; Weng, Y.; Chen, S.; Pan, Q.; Li, Y.; Wang, H.; Lin, S.; Yu, H. Osteoporosis treatment using stem cell-derived exosomes: A systematic review and meta-analysis of preclinical studies. Stem Cell Res. Ther. 2023, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Wei, S.; Wang, Y.; Wang, Y.; Man, Y.; Qu, Y. Extracellular vesicle and mesenchymal stem cells in bone regeneration: Recent progress and perspectives. J. Biomed. Mater. Res. A 2019, 107, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Ai, K.; He, K.; Wu, B.; Peng, J.; Xiang, J.; Zhang, G.; Jiao, Z.; Zhou, R.; Zhang, H. Global Research Trends of Exosomes in the Central Nervous System: A Bibliometric and Visualized Analysis. Neurospine 2023, 20, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Gao, W.; Papadimitriou, J.M.; Zhang, C.; Gao, J.; Zheng, M. Exosomes-the enigmatic regulators of bone homeostasis. Bone Res. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Shen, E.; Shu, X.; Liu, D.; Wu, C. ERK-estrogen receptor α signaling plays a role in the process of bone marrow mesenchymal stem cell-derived exosomes protecting against ovariectomy-induced bone loss. J. Orthop. Surg. Res. 2023, 18, 250. [Google Scholar] [CrossRef]
- Hadjiargyrou, M.; Komatsu, D.E. The Therapeutic Potential of MicroRNAs as Orthobiologics for Skeletal Fractures. J. Bone Miner. Res. 2019, 34, 797–809. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, C.; He, H.B.; Hu, R.; Zhou, H.D.; Xie, H.; Zhu, W.; Dai, R.C.; Wu, X.P.; Liao, E.Y.; et al. miR-148a regulates osteoclastogenesis by targeting V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. J. Bone Miner. Res. 2013, 28, 1180–1190. [Google Scholar] [CrossRef]
- Hassan, M.Q.; Maeda, Y.; Taipaleenmaki, H.; Zhang, W.; Jafferji, M.; Gordon, J.A.; Li, Z.; Croce, C.M.; van Wijnen, A.J.; Stein, J.L.; et al. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J. Biol. Chem. 2012, 287, 42084–42092. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Tyagi, N. Exosomes: Mediators of bone diseases, protection, and therapeutics potential. Oncoscience 2018, 5, 181–195. [Google Scholar] [CrossRef]
- Li, Y.; Jin, D.; Xie, W.; Wen, L.; Chen, W.; Xu, J.; Ding, J.; Ren, D.; Xiao, Z. Mesenchymal Stem Cells-Derived Exosomes: A Possible Therapeutic Strategy for Osteoporosis. Curr. Stem Cell Res. Ther. 2018, 13, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Guo, S.; Ren, X.; Wu, Z.; Liu, S.; Yao, X. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells 2023, 12, 1416. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef]
- Di Bella, M.A. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology 2022, 11, 804. [Google Scholar] [CrossRef]
- Gong, Y.; Bu, Y.; Li, Y.; Hao, D.; He, B.; Kong, L.; Huang, W.; Gao, X.; Zhang, B.; Qu, Z.; et al. Hydrogel-based delivery system applied in the local anti-osteoporotic bone defects. Front. Bioeng. Biotechnol. 2022, 10, 1058300. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. A 2020, 108, 1617–1633. [Google Scholar] [CrossRef]
- Wheelton, A.; Mace, J.; Khan, W.S.; Anand, S. Biomaterials and Fabrication to Optimise Scaffold Properties for Musculoskeletal Tissue Engineering. Curr. Stem Cell Res. Ther. 2016, 11, 578–584. [Google Scholar] [CrossRef]
- Meesuk, L.; Suwanprateeb, J.; Thammarakcharoen, F.; Tantrawatpan, C.; Kheolamai, P.; Palang, I.; Tantikanlayaporn, D.; Manochantr, S. Osteogenic differentiation and proliferation potentials of human bone marrow and umbilical cord-derived mesenchymal stem cells on the 3D-printed hydroxyapatite scaffolds. Sci. Rep. 2022, 12, 19509. [Google Scholar] [CrossRef]
- Girón, J.; Kerstner, E.; Medeiros, T.; Oliveira, L.; Machado, G.M.; Malfatti, C.F.; Pranke, P. Biomaterials for bone regeneration: An orthopedic and dentistry overview. Braz. J. Med. Biol. Res. 2021, 54, e11055. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Ngo, M.T.; Harley, B.A.C. Angiogenic biomaterials to promote therapeutic regeneration and investigate disease progression. Biomaterials 2020, 255, 120207. [Google Scholar] [CrossRef] [PubMed]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in Tissue Engineering: As Nature Intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Bai, H.; Zhao, Y.; Wang, C.; Wang, Z.; Wang, J.; Liu, H.; Feng, Y.; Lin, Q.; Li, Z.; Liu, H. Enhanced osseointegration of three-dimensional supramolecular bioactive interface through osteoporotic microenvironment regulation. Theranostics 2020, 10, 4779–4794. [Google Scholar] [CrossRef]
- Jiang, X.; Zou, S.; Ye, B.; Zhu, S.; Liu, Y.; Hu, J. bFGF-Modified BMMSCs enhance bone regeneration following distraction osteogenesis in rabbits. Bone 2010, 46, 1156–1161. [Google Scholar] [CrossRef]
- Liu, X.; Bao, C.; Xu, H.H.K.; Pan, J.; Hu, J.; Wang, P.; Luo, E. Osteoprotegerin gene-modified BMSCs with hydroxyapatite scaffold for treating critical-sized mandibular defects in ovariectomized osteoporotic rats. Acta Biomater. 2016, 42, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhu, H.; Li, J.; Feng, C.; Yao, Q.; Wang, L.; Chang, J.; Wu, C. Bioactive Scaffolds for Regeneration of Cartilage and Subchondral Bone Interface. Theranostics 2018, 8, 1940–1955. [Google Scholar] [CrossRef]
- Salamanna, F.; Giavaresi, G.; Contartese, D.; Bigi, A.; Boanini, E.; Parrilli, A.; Lolli, R.; Gasbarrini, A.; Barbanti Brodano, G.; Fini, M. Effect of strontium substituted ß-TCP associated to mesenchymal stem cells from bone marrow and adipose tissue on spinal fusion in healthy and ovariectomized rat. J. Cell Physiol. 2019, 234, 20046–20056. [Google Scholar] [CrossRef]
- Liu, Y.; Ming, L.; Luo, H.; Liu, W.; Zhang, Y.; Liu, H.; Jin, Y. Integration of a calcined bovine bone and BMSC-sheet 3D scaffold and the promotion of bone regeneration in large defects. Biomaterials 2013, 34, 9998–10006. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Shenoy, S.J.; Nair, R.P.; Varma, H.K.; John, A. Strontium Hydroxyapatite scaffolds engineered with stem cells aid osteointegration and osteogenesis in osteoporotic sheep model. Colloids Surf. B Biointerfaces 2018, 163, 346–354. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, P.; Xue, S.; Xu, Y.; Tan, J.; Liu, G. Adipose-derived stem cells alleviate osteoporosis by enhancing osteogenesis and inhibiting adipogenesis in a rabbit model. Cytotherapy 2014, 16, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.K.; Lee, D.; Kim, S.J.; Han, G.H.; Lee, D.; Sheen, S.H.; Sohn, S. Injection of a PMMA-doped MSC spheroid gel for the treatment of painful osteoporotic vertebral compression fractures. Bioeng. Transl. Med. 2023, 8, e10577. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Z.; Zhao, L.; Liu, Y.; Su, Y.; Gong, X.; Liu, F.; Zhang, L. The heterogeneity of mesenchymal stem cells: An important issue to be addressed in cell therapy. Stem Cell Res. Ther. 2023, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Soontararak, S.; Chow, L.; Johnson, V.; Coy, J.; Wheat, W.; Regan, D.; Dow, S. Mesenchymal Stem Cells (MSC) Derived from Induced Pluripotent Stem Cells (iPSC) Equivalent to Adipose-Derived MSC in Promoting Intestinal Healing and Microbiome Normalization in Mouse Inflammatory Bowel Disease Model. Stem Cells Transl. Med. 2018, 7, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kim, H.; Kim, J.; Kim, S.; Park, T.S.; Kim, T.M. Extracellular vesicles from induced pluripotent stem cell-derived mesenchymal stem cells enhance the recovery of acute kidney injury. Cytotherapy 2024, 26, 51–62. [Google Scholar] [CrossRef]
- García-Sánchez, D.; Fernández, D.; Rodríguez-Rey, J.C.; Pérez-Campo, F.M. Enhancing survival, engraftment, and osteogenic potential of mesenchymal stem cells. World J. Stem Cells 2019, 11, 748–763. [Google Scholar] [CrossRef]
- Sheyn, D.; Kallai, I.; Tawackoli, W.; Cohn Yakubovich, D.; Oh, A.; Su, S.; Da, X.; Lavi, A.; Kimelman-Bleich, N.; Zilberman, Y.; et al. Gene-modified adult stem cells regenerate vertebral bone defect in a rat model. Mol. Pharm. 2011, 8, 1592–1601. [Google Scholar] [CrossRef]

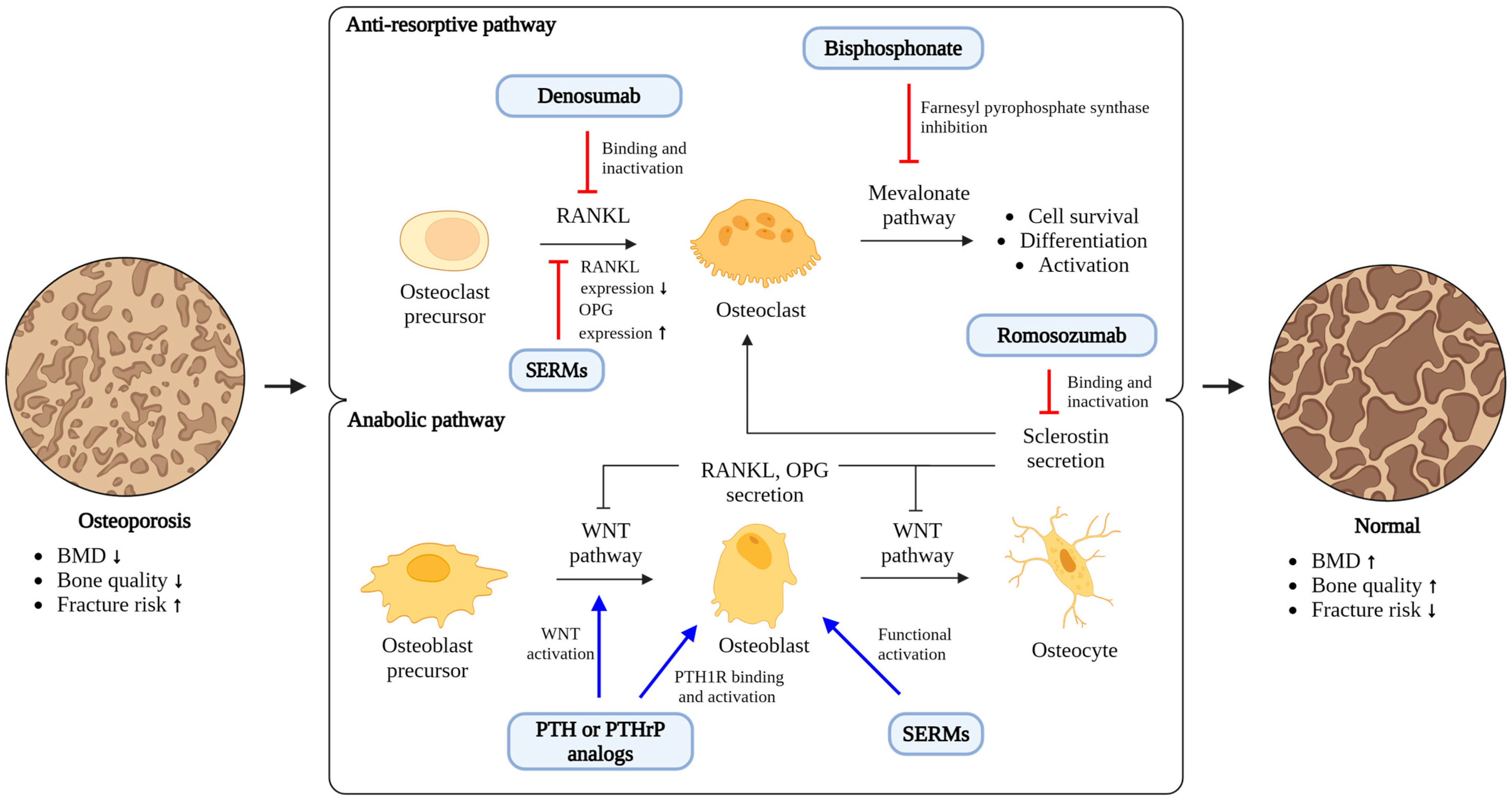
| Anti-Osteoporotic Agents | Anti-Fracture Efficacy | Impacts on OVCF Healing | ||
|---|---|---|---|---|
| Vertebral | Non-Vertebral | Hip | ||
| Anti-resorptive agents | ||||
| - Alendronate | + | + | + | No significant clinical outcomes |
| - Risedronate | + | + | + | |
| - Ibandronate | + | + ** | NE * | |
| - Zoledronate | + | + | + | |
| - Raloxifene (SERM) | + | NE * | NE * | No clinical studies |
| - Denosumab | + | + | + | Limited clinical studies |
| Anabolic agents | ||||
| - Teriparatide | + | + | NE * | Improvement of vertebral body collapse and kyphotic angle **** |
| - Romosozumab | + | + *** | + *** | No clinical studies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Lee, Y.; Liu, Y.; Yu, Y.; Han, I. Stem Cell and Regenerative Therapies for the Treatment of Osteoporotic Vertebral Compression Fractures. Int. J. Mol. Sci. 2024, 25, 4979. https://doi.org/10.3390/ijms25094979
Zhang S, Lee Y, Liu Y, Yu Y, Han I. Stem Cell and Regenerative Therapies for the Treatment of Osteoporotic Vertebral Compression Fractures. International Journal of Molecular Sciences. 2024; 25(9):4979. https://doi.org/10.3390/ijms25094979
Chicago/Turabian StyleZhang, Songzi, Yunhwan Lee, Yanting Liu, Yerin Yu, and Inbo Han. 2024. "Stem Cell and Regenerative Therapies for the Treatment of Osteoporotic Vertebral Compression Fractures" International Journal of Molecular Sciences 25, no. 9: 4979. https://doi.org/10.3390/ijms25094979
APA StyleZhang, S., Lee, Y., Liu, Y., Yu, Y., & Han, I. (2024). Stem Cell and Regenerative Therapies for the Treatment of Osteoporotic Vertebral Compression Fractures. International Journal of Molecular Sciences, 25(9), 4979. https://doi.org/10.3390/ijms25094979








