Metal Toxicity: Effects on Energy Metabolism in Fish
Abstract
:1. Introduction
2. Metals in the Environment
3. Metal Toxicity
- (1)
- They interact competitively with the elements which are necessary for proper functioning of the body (competition for binding sites, e.g., on transporter proteins, enzymes, nucleic acids, neurotransmitters, sugars, ATP, glutathione);
- (2)
- They block the functional groups of significant biologically active compounds, e.g., -SH, -OH, -NH2, -COOH, and -S-S;
- (3)
- They change the structure of biomolecules and biological membranes;
- (4)
- They limit the pool of available bioanions (e.g., phosphates);
- (5)
- They interfere with the synthesis of ATP;
- (6)
- They form insoluble salts in biological fluids;
- (7)
- They participate in redox reactions that generate free radicals.
- (1)
- Oxidative stress and lipid peroxidation (reactive oxygen species (ROS) increase, membrane damage, and oxidation of biomolecules);
- (2)
- DNA damage (mutagenicity, cell death, impaired DNA replication);
- (3)
- Enzyme disfunction and metabolic disorders (cell mechanism disturbances, metabolic disruptions, organ and tissue pathology);
- (4)
- Endocrine disruption (hormonal production disturbances, alteration in reproductive parameters).
4. Energetic and Metabolic Effects of Metal Toxicity
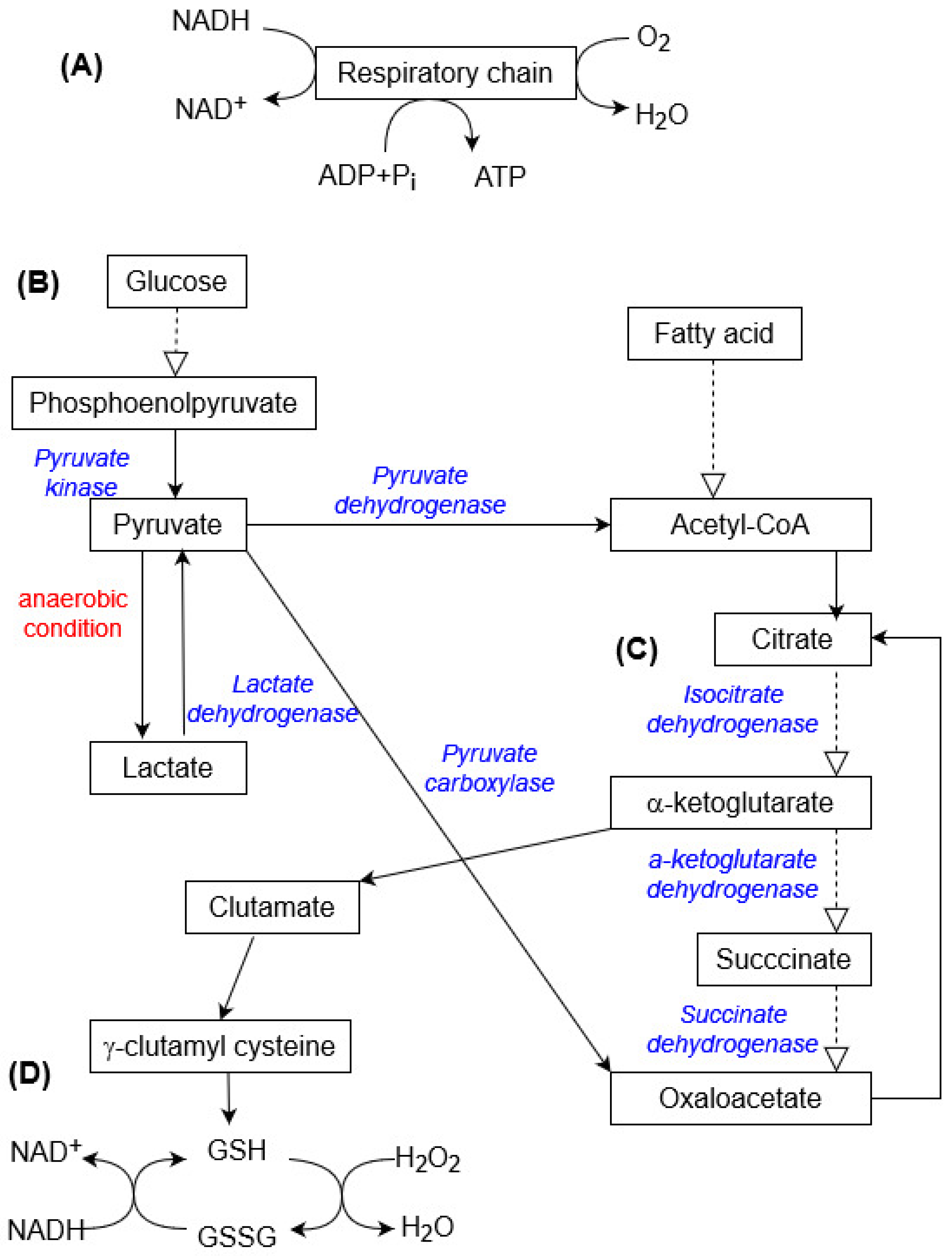
4.1. Oxygen Consumption and ATP Production
4.2. ATP Use
4.3. ATP and ROS Production
- -
- The levels of adenosine are determined primarily from the dephosphorylation of its immediate precursor, adenosine monophosphate (AMP); on the other hand, phosphorylation plays an integral role in the activation of antioxidant enzymes;
- -
- Under normal conditions, adenosine is phosphorylated by adenosine kinase to AMP and subsequently to ATP to restore the nucleotide pool, but adenosine is also produced from the hydrolysis of S-adenosylhomocysteine by S-adenosylhomocysteine hydrolase;
- -
- Adenosine receptors can be regulated by oxidative stress, and their activation leads to an increase in the activities of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase, along with a reduction in malondialdehyde (a marker of lipid peroxidation);
- -
- Adenosine also contributes to sedation, bradycardia, vasorelaxation, the inhibition of lipolysis, and the regulation of the immune system.
4.4. Mitochondria Are Target Organelles
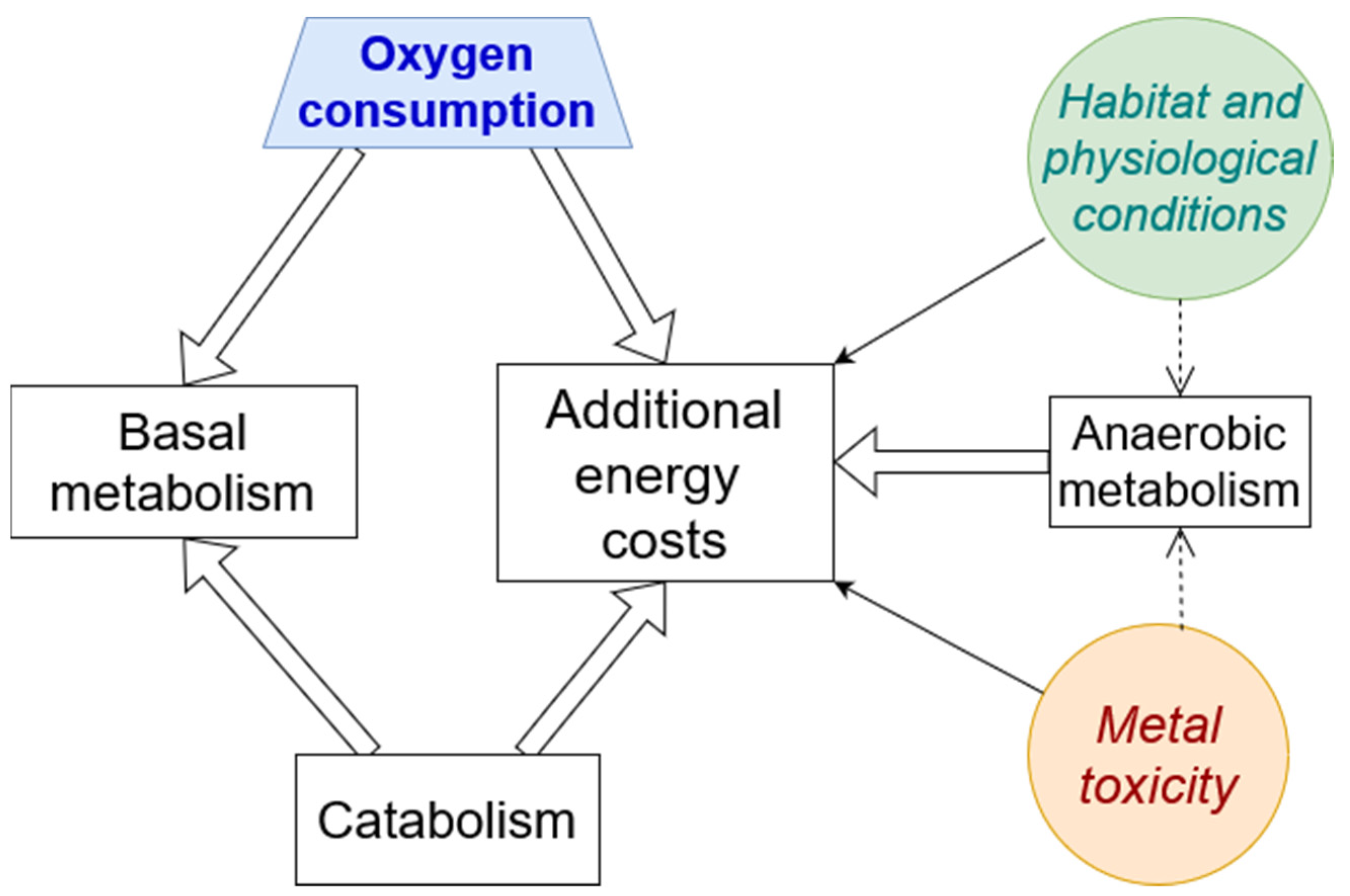
4.5. Catabolism, Hypoxia, and Anaerobic Metabolism
5. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; V. 3: Environmental Toxicology; pp. 133–164. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Megorskii, V.V.; Gashkina, N.A.; Kudryavtseva, L.P. Water pollution effect on population health in an industrial northern region. Water Res. 2010, 37, 194–203. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. N. Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhang, Y.; Wu, Z.; Yang, R.; Chen, X.; Yang, J.; Zhu, L. Health risk assessment of heavy metals in freshwater fish in the central and eastern North China. Ecotoxicol. Environ. Saf. 2018, 157, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Surenbaatar, U.; Lee, S.; Kwon, J.-Y.; Lim, H.; Kim, J.-J.; Kim, Y.-H.; Hong, Y.-S. Bioaccumulation of Lead, Cadmium, and Arsenic in a Mining Area and Its Associated Health Effects. Toxics 2023, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Lall, S.P.; Kaushik, S.J. Nutrition and Metabolism of Minerals in Fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.M. An introduction to metals in fish physiology and toxicology: Basic principles. In Homeostasis and Toxicology of Essential Metals; Wood, C.M., Farrel, A.P., Brauner, C.J., Eds.; Academic Press: London, UK, 2012; pp. 1–53. [Google Scholar] [CrossRef]
- Chabot, D.; McKenzie, D.J.; Craig, J.F. Metabolic rate in fishes: Definitions, methods and significance for conservation physiology. J. Fish Biol. 2016, 88, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, C.; Enberg, K.; Mangel, M. Modelling and interpreting fish bioenergetics—A role for behaviour, life history traits, and survival trade-offs. J. Fish Biol. 2016, 88, 389–402. [Google Scholar] [CrossRef]
- van der Oost, R.; McKenzie, D.J.; Verweij, F.; Satumalay, C.; van der Molen, N.; Winter, M.J.; Chipman, J.K. Identifying adverse outcome pathways (AOP) for Amsterdam city fish by integrated field monitoring. Environ. Toxicol. Pharmacol. 2020, 74, 103301. [Google Scholar] [CrossRef]
- Samuelsson, L.M.; Björlenius, B.; Förlin, L.; Larsson, D.G.J. Reproducible 1H NMR-based metabolomic responses in fish exposed to different sewage effluents in two separate studies. Environ. Sci. Technol. 2011, 45, 1703–1710. [Google Scholar] [CrossRef]
- Gashkina, N.A. Essential elements in the organs and tissues of fish depending on the freshwater toxicity and physiological state. Geochem. Int. 2017, 55, 927–934. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Gashkina, N.A.; Dinu, M.I. Enrichment of Surface Water by Elements: Effects of Air Pollution, Acidification and Eutrophication. Environ. Process. 2016, 3, 39–58. [Google Scholar] [CrossRef]
- Webster, T.M.U.; Bury, N.; van Aerle, R.; Santos, E.M. Global transcriptome profiling reveals molecular mechanisms of metal tolerance in a chronically exposed wild population of brown trout. Environ. Sci. Technol. 2013, 47, 8869–8877. [Google Scholar] [CrossRef]
- Couture, P.; Pyle, G. Live fast and die young: Metal effects on condition and physiology of wild yellow perch from along two metal contamination gradients. Hum. Ecol. Risk. Assess. 2008, 14, 73–96. [Google Scholar] [CrossRef]
- Abril, S.I.M.; Dalmolin, C.; Costa, P.G.; Bianchini, A. Expression of genes related to metal metabolism in the freshwater fish Hyphessobrycon luetkenii living in a historically contaminated area associated with copper mining. Environ. Toxicol. Pharmacol. 2018, 60, 146–156. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Gashkina, N.A.; Sharov, A.N.; Vandysh, O.I.; Kudryavtseva, L.P. Anthropogenic transformations of the Arctic ecosystem of Lake Imandra: Tendencies for recovery after long period of pollution. Water Res. 2009, 36, 296–309. [Google Scholar] [CrossRef]
- Gashkina, N.A.; Moiseenko, T.I.; Kudryavtseva, L.P. Fish response of metal bioaccumulation to reduced toxic load on long-term contaminated Lake Imandra. Ecotoxicol. Environ. Saf. 2020, 191, 110205. [Google Scholar] [CrossRef]
- Senoro, D.B.; Plasus, M.M.G.; Gorospe, A.F.B.; Nolos, R.C.; Baaco, A.T.; Lin, C. Metals and Metalloid Concentrations in Fish, Its Spatial Distribution in PPC, Philippines and the Attributable Risks. Toxics 2023, 11, 621. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Moiseenko, T.I.; Gashkina, N.A.; Tatsiy, Y.G. Modeling and Predicting the Environment State in the Impact Area of a Copper–Nickel Plant: A Balanced Model of the Transformations of Atmospheric Deposition at the Catchment and in Lake. Geochem. Int. 2023, 61, 768–779. [Google Scholar] [CrossRef]
- Długaszek, M. Studies on relationships between essential and toxic elements in selected body fluids, cells and tissues. Chem. Biol. Interact. 2019, 297, 57–66. [Google Scholar] [CrossRef]
- Paschoalini, A.L.; Bazzoli, N. Heavy metals affecting Neotropical freshwater fish: A review of the last 10 years of research. Aquat. Toxicol. 2021, 237, 105906. [Google Scholar] [CrossRef]
- Naz, S.; Chatha, A.M.M.; Téllez-Isaías, G.; Ullah, S.; Ullah, Q.; Khan, M.Z.; Shah, M.K.; Abbas, G.; Kiran, A.; Mushtaq, R.; et al. A Comprehensive Review on Metallic Trace Elements Toxicity in Fishes and Potential Remedial Measures. Water 2023, 15, 3017. [Google Scholar] [CrossRef]
- Emon, F.J.; Rohani, F.; Sumaiya, N.; Jannat, M.F.T.; Akter, Y.; Shahjahan; Kari, Z.A.; Tahiluddin, A.B.; Goh, K.W. Bioaccumulation and Bioremediation of Heavy Metals in Fishes—A Review. Toxics 2023, 11, 510. [Google Scholar] [CrossRef]
- Wood, C.M.; Farrell, A.P.; Brauner, C.J. Fish Physiology: Homeostasis and Toxicology of Non-Essential Metals; Academic Press: San Diego, CA, USA, 2012; Volume 31B, p. 507. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M. Handbook on the Toxicology of Metals; Academic Press: New York, NY, USA, 2015; p. 1385. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Towards a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Else, P.L. Basal metabolic rate: History, composition, regulation, and usefulness. Physiol. Biochem. Zool. 2004, 77, 869–876. [Google Scholar] [CrossRef]
- Chabot, D.; Steffensen, J.F.; Farrell, A.P. The determination of standard metabolic rate in fishes. J. Fish Biol. 2016, 88, 81–121. [Google Scholar] [CrossRef]
- Rolfe, D.F.S.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef]
- Jonckheere, A.I.; Smeitink, J.A.M.; Rodenburg, R.J.T. Mitochondrial ATP synthase: Architecture, function and pathology. J. Inherit. Metab. Dis. 2012, 35, 211–225. [Google Scholar] [CrossRef]
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef]
- Arnst, N.; Redolfi, N.; Lia, A.; Bedetta, M.; Greotti, E.; Pizzo, P. Mitochondrial Ca2+ Signaling and Bioenergetics in Alzheimer’s Disease. Biomedicines 2022, 10, 3025. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 4th ed.; W.H. Freeman and Company: New York, NY, USA, 2004; p. 1119. [Google Scholar] [CrossRef]
- Richards, J.G.; Sardella, B.A.; Schulte, P.M. Regulation of pyruvate dehydrogenase in the common killifish, Fundulus heteroclitus, during hypoxia exposure. Am. J. Physiol. 2008, 295, R979–R990. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of Glycolysis by the Hypoxia-Inducible Factor (HIF): Implications for Cellular Physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef]
- Wang, X.; An, P.; Gu, Z.; Luo, Y.; Luo, J. Mitochondrial Metal Ion Transport in Cell Metabolism and Disease. Int. J. Mol. Sci. 2021, 22, 7525. [Google Scholar] [CrossRef]
- Griffith, M.B. Toxicological perspective on the osmoregulation and ionoregulation physiology of major ions by freshwater animals: Teleost fish, crustacea, aquatic insects, and Mollusca. Environ. Toxicol. Chem. 2017, 36, 576–600. [Google Scholar] [CrossRef]
- Richards, J.G.; Wang, Y.S.; Brauner, C.J.; Gonzalez, R.J.; Patrick, M.L.; Schulte, P.M.; Choppari-Gomes, A.R.; Almeida-Val, V.M.; Val, A.L. Metabolic and ionoregulatory responses of the Amazonian cichlid, Astronotus ocellatus, to severe hypoxia. J. Comp. Physiol. B 2007, 177, 361–374. [Google Scholar] [CrossRef]
- Farhat, E.; Cheng, H.; Romestaing, C.; Pamenter, M.; Weber, J.-M. Goldfish Response to Chronic Hypoxia: Mitochondrial Respiration, Fuel Preference and Energy Metabolism. Metabolites 2021, 11, 187. [Google Scholar] [CrossRef]
- Wilkie, M.P.; Pamenter, M.E.; Alkabie, S.; Carapic, D.; Shin, D.S.; Buck, L.T. Evidence of anoxia-induced channel arrest in the brain of the goldfish (Carassius auratus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 355–362. [Google Scholar] [CrossRef]
- Monteiro, S.M.; Mancera, J.M.; Fontaínhas-Fernandes, A.; Sousa, M. Copper induced alterations of biochemical parameters in the gill and plasma of Oreochromis niloticus. Comp. Biochem. Physiol. C Pharmacol. Toxicol. 2005, 141, 375–383. [Google Scholar] [CrossRef]
- da Silva, A.O.F.; Martineza, C.B.R. Acute effects of cadmium on osmoregulation of the freshwater teleost Prochilodus lineatus: Enzymes activity and plasma ions. Aquat. Toxicol. 2014, 156, 161–168. [Google Scholar] [CrossRef]
- Magnoni, L.J.; Eding, E.; Leguen, I.; Prunet, P.; Geurden, I.; Ozório, R.O.A.; Schrama, J.W. Hypoxia, but not an electrolyte-imbalanced diet, reduces feed intake, growth and oxygen consumption in rainbow trout (Oncorhynchus mykiss). Sci. Rep. 2018, 8, 4965. [Google Scholar] [CrossRef]
- Gashkina, N.A.; Moiseenko, T.I. Influence of Thermal Pollution on the Physiological Conditions and Bioaccumulation of Metals, Metalloids, and Trace Metals in Whitefish (Coregonus lavaretus L.). Int. J. Mol. Sci. 2020, 12, 4343. [Google Scholar] [CrossRef]
- Gashkina, N.A.; Moiseenko, T.I.; Shuman, L.A.; Koroleva, I.M. Biological responses of whitefish (Coregonus lavaretus L.) to reduced toxic impact: Metal accumulation, haematological, immunological, and histopathological alterations. Ecotoxicol. Environ. Saf. 2022, 239, 113659. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Rumkumar, V.; Hallam, D.M.; Nue, Z. Adenosine, oxidative stress and cytoprotection. Jpn. J. Pharmacol. 2001, 86, 265–274. [Google Scholar] [CrossRef]
- Jones, O.A.H.; Dondero, F.; Viarengo, A.; Griffin, J.L. Metabolic profiling of Mytilus galloprovincialis and its potential applications for pollution assessment. Mar. Ecol. Prog. Ser. 2008, 369, 169–179. [Google Scholar] [CrossRef]
- Spann, N.; Aldridge, D.C.; Griffin, J.L.; Jones, O.A. Size-dependent effects of low level cadmium and zinc exposure on the metabolome of the Asian clam, Corbicula fluminea. Aquat. Toxicol. 2011, 105, 589–599. [Google Scholar] [CrossRef]
- Rana, S.V. Metals and apoptosis: Recent developments. J. Trace Elem. Med. Biol. 2008, 22, 262–284. [Google Scholar] [CrossRef]
- Camargo, M.M.P.; Fernandes, M.N.; Martinez, C.B.R. How aluminium expo-sure promotes osmoregulatory disturbances in the neotropical freshwater fishProchilus lineatus. Aquat. Toxicol. 2009, 94, 40–46. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Wu, R.-X.; Ji, J.-N.; Zhang, J.; Niu, S.-F.; Tang, B.-G.; Miao, B.-B.; Liang, Z.-B. Integrated Transcriptomics and Metabolomics Reveal Changes in Cell Homeostasis and Energy Metabolism in Trachinotus ovatus in Response to Acute Hypoxic Stress. Int. J. Mol. Sci. 2024, 25, 1054. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, M.; Wang, Q.; Yang, H. The Combined Use of Copper Sulfate and Trichlorfon Exerts Stronger Toxicity on the Liver of Zebrafish. Int. J. Mol. Sci. 2023, 24, 11203. [Google Scholar] [CrossRef]
- Rosabal, M.; Pierron, F.; Couture, P.; Baudrimont, M.; Hare, L.; Campbell, P.G. Subcellular partitioning of non-essential trace metals (Ag, As, Cd, Ni, Pb, and Tl) in livers of American (Anguilla rostrata) and European (Anguilla anguilla) yellow eels. Aquat. Toxicol. 2015, 160, 128–141. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, B.; Emmett, M.J.; Damle, M.; Sun, Z.; Feng, D.; Armour, S.M.; Remsberg, J.R.; Jager, J.; Soccio, R.E.; et al. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science 2015, 348, 1488–1492. [Google Scholar] [CrossRef]
- Saiz, N.; Herrera-Castillo, L.; Isorna, E.; Delgado, M.J.; Conde-Sieira, M.; Soengas, J.L.; de Pedro, N. REV-ERBα Agonist SR9009 Promotes a Negative Energy Balance in Goldfish. Int. J. Mol. Sci. 2022, 23, 2921. [Google Scholar] [CrossRef]
- Baumgarner, B.L.; Cooper, B.R. Evaluation of a tandem gas chromatography/time-of-flight mass spectrometry metabolomics platform as a single method to investigate the effect of starvation on whole-animal metabolism in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2012, 215, 1627–1632. [Google Scholar] [CrossRef]
- Kullgren, A.; Samuelsson, L.M.; Larsson, D.G.J.; Björnsson, B.T.; Bergman, E.J. A metabolomics approach to elucidate effects of food deprivation in juvenile rainbow trout (Oncorhynchus mykiss). Am. J. Physiol. Reg. 2010, 299, R1440–R1448. [Google Scholar] [CrossRef]
- Tuffnail, W.; Mills, G.A.; Cary, P.; Greenwood, R. An environmental 1H NMR metabolomic study of the exposure of the marine mussel Mytilus edulis to atrazine, lindane, hypoxia and starvation. Metabolomics 2009, 5, 33–43. [Google Scholar] [CrossRef]
- Ngo, H.T.T.; Nguyen, T.D.; Nguyen, T.T.H.; Le, T.T.; Nguyen, D.Q. Adverse Effects of Toxic Metal Pollution in Rivers on the Physiological Health of Fish. Toxics 2022, 10, 528. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Luo, Z.; Pan, Y.-X.; Zheng, J.-L.; Zhu, Q.-L.; Sun, L.-D.; Zhuo, M.-Q.; Hu, W. Differential induction of enzymes and genes involved in lipid metabolism in liver and visceral adipose tissue of juvenile yellow catfish Pelteobagrus fulvidraco exposed to copper. Aquat. Toxicol. 2013, 136–137, 72–78. [Google Scholar] [CrossRef]
- Costa, P.M.; Diniz, M.S.; Caeiro, S.; Lobo, J.; Martins, M.; Ferreira, A.M.; Caetano, M.; Vale, C.; DelValls, T.; Costa, M.H. Histological biomarkers in liver and gills of juvenile Solea senegalensis exposed to contaminated estuarine sediments: A weighted indices approach. Aquat. Toxicol. 2009, 92, 202–212. [Google Scholar] [CrossRef]
- Tzaneva, V.; Vadeboncoeur, C.; Ting, J.; Perry, S.F. Effects of hypoxia-induced gill remodelling on the innervation and distribution of ionocytes in the gill of goldfish, Carassius auratus. J. Comp. Neurol. 2014, 522, 118–130. [Google Scholar] [CrossRef]
- Turko, A.J.; Cisternino, B.; Wright, P.A. Calcified gill filaments increase respiratory function in fishes. Proc. R. Soc. B. 2020, 287, 20192796. [Google Scholar] [CrossRef]
- Wood, C.M.; Eom, J. The osmorespiratory compromise in the fish gill. Comp. Biochem. Physiol. A Physiol. 2021, 254, 110895. [Google Scholar] [CrossRef]
- Cameron, J.S.; DeWitt, J.P.; Ngo, T.T.; Yajnik, T.; Chan, S.; Chung, E.; Kang, E. Cardiac KATP channel alterations associated with acclimation to hypoxia in goldfish (Carassius auratus L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 554–564. [Google Scholar] [CrossRef]
- Williams, K.J.; Cassidy, A.A.; Verhille, C.E.; Lamarre, S.G.; MacCormack, T.J. Diel Cycling Hypoxia Enhances Hypoxia Tolerance in Rainbow Trout (Oncorhynchus mykiss): Evidence of Physiological and Metabolic Plasticity. J. Exp. Biol. 2019, 222, jeb206045. [Google Scholar] [CrossRef]
- Lardon, I.; Eyckmans, M.; Vu, T.N.; Laukens, K.; De Boeck, G.; Dommisse, R. 1H-NMR Study of the Metabolome of a Moderately Hypoxia-Tolerant Fish, the Common Carp (Cyprinus carpio). Metabolomics 2013, 9, 1216–1227. [Google Scholar] [CrossRef]
- de Magalhães, C.R.; Farinha, A.P.; Blackburn, G.; Whitfield, P.D.; Carrilho, R.; Schrama, D.; Cerqueira, M.; Rodrigues, P.M. Gilthead Seabream Liver Integrative Proteomics and Metabolomics Analysis Reveals Regulation by Different Prosurvival Pathways in the Metabolic Adaptation to Stress. Int. J. Mol. Sci. 2022, 23, 15395. [Google Scholar] [CrossRef]
- Hines, A.; Oladiran, G.S.; Bignell, J.P.; Stentiford, G.D.; Viant, M.R. Direct sampling of organisms from the field and knowledge of their phenotype: Key recommendations for environmental metabolomics. Environ. Sci. Technol. 2007, 41, 3375–3381. [Google Scholar] [CrossRef]
- Wu, H.; Wang, W.-X. NMR-based metabolomic studies on the toxicological effects of cadmium and copper on green mussels Perna viridis. Aquat. Toxicol. 2010, 100, 339–345. [Google Scholar] [CrossRef]
- Magnoni, L.J.; Novais, S.C.; Eding, E.; Leguen, I.; Lemos, M.F.L.; Ozório, R.O.A.; Geurden, I.; Prunet, P.; Schrama, J.W. Acute Stress and an Electrolyte- Imbalanced Diet, but Not Chronic Hypoxia, Increase Oxidative Stress and Hamper Innate Immune Status in a Rainbow Trout (Oncorhynchus mykiss) Isogenic Line. Front. Physiol. 2019, 10, 453. [Google Scholar] [CrossRef]
- Souza, C.F.; Baldissera, M.D.; Zeppenfeld, C.C.; Descovi, S.; Stefani, L.M.; Baldisserotto, B.; da Silva, A.S. Oxidative stress mediated the inhibition of cerebral creatine kinase activity in silver catfish fed with aflatoxin B1-contaminated diet. Fish Physiol Biochem 2019, 45, 63–70. [Google Scholar] [CrossRef]
- Cho, H.J.; Cho, H.Y.; Park, J.-W.; Kwon, O.-S.; Lee, H.-S.; Huh, T.L.; Kang, B.S. NADP+-dependent cytosolic isocitrate dehydrogenase provides NADPH in the presence of cadmium due to the moderate chelating effect of glutathione. J Biol Inorg Chem. 2018, 23, 849–860. [Google Scholar] [CrossRef]
- Perumalsamy, N.; Arumugam, K. Enzymes Activity in Fish Exposed to Heavy Metals and the Electro-Plating Effluent at Sub-Lethal Concentrations. Water Qual. Expo. Health 2013, 5, 93–101. [Google Scholar] [CrossRef]
- Carvalhais, A.; Pereira, B.; Sabato, M.; Seixas, R.; Dolbeth, M.; Marques, A.; Guilherme, S.; Pereira, P.; Pacheco, M.; Mieiro, C. Mild Effects of Sunscreen Agents on a Marine Flatfish: Oxidative Stress, Energetic Profiles, Neurotoxicity and Behaviour in Response to Titanium Dioxide Nanoparticles and Oxybenzone. Int. J. Mol. Sci. 2021, 22, 1567. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gashkina, N.A. Metal Toxicity: Effects on Energy Metabolism in Fish. Int. J. Mol. Sci. 2024, 25, 5015. https://doi.org/10.3390/ijms25095015
Gashkina NA. Metal Toxicity: Effects on Energy Metabolism in Fish. International Journal of Molecular Sciences. 2024; 25(9):5015. https://doi.org/10.3390/ijms25095015
Chicago/Turabian StyleGashkina, Natalia A. 2024. "Metal Toxicity: Effects on Energy Metabolism in Fish" International Journal of Molecular Sciences 25, no. 9: 5015. https://doi.org/10.3390/ijms25095015
APA StyleGashkina, N. A. (2024). Metal Toxicity: Effects on Energy Metabolism in Fish. International Journal of Molecular Sciences, 25(9), 5015. https://doi.org/10.3390/ijms25095015






