Delivery of Mesenchymal Stem Cells during Hypothermic Machine Perfusion in a Translational Kidney Perfusion Study
Abstract
1. Introduction
2. Results
2.1. Mesenchymal Stem Cells in HMP in Rodent Kidneys
2.2. Mesenchymal Stem Cells in a Porcine HMP Model
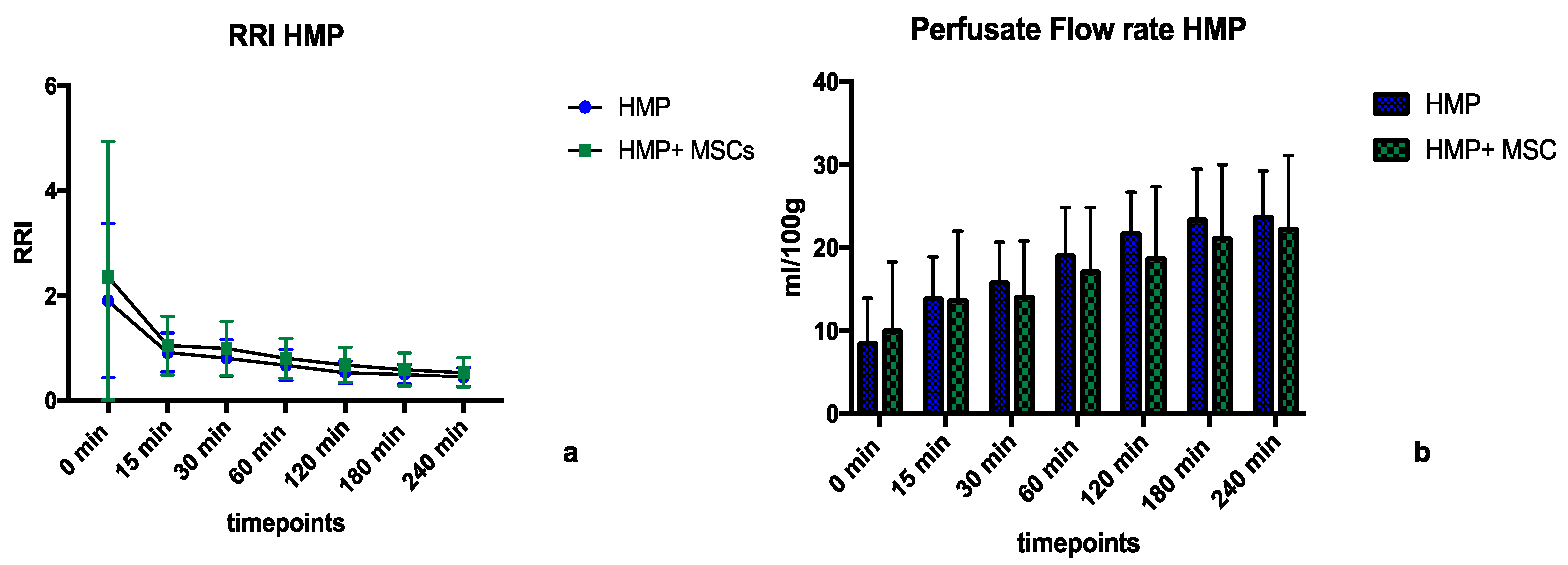
2.2.1. Perfusion Parameters during HMP
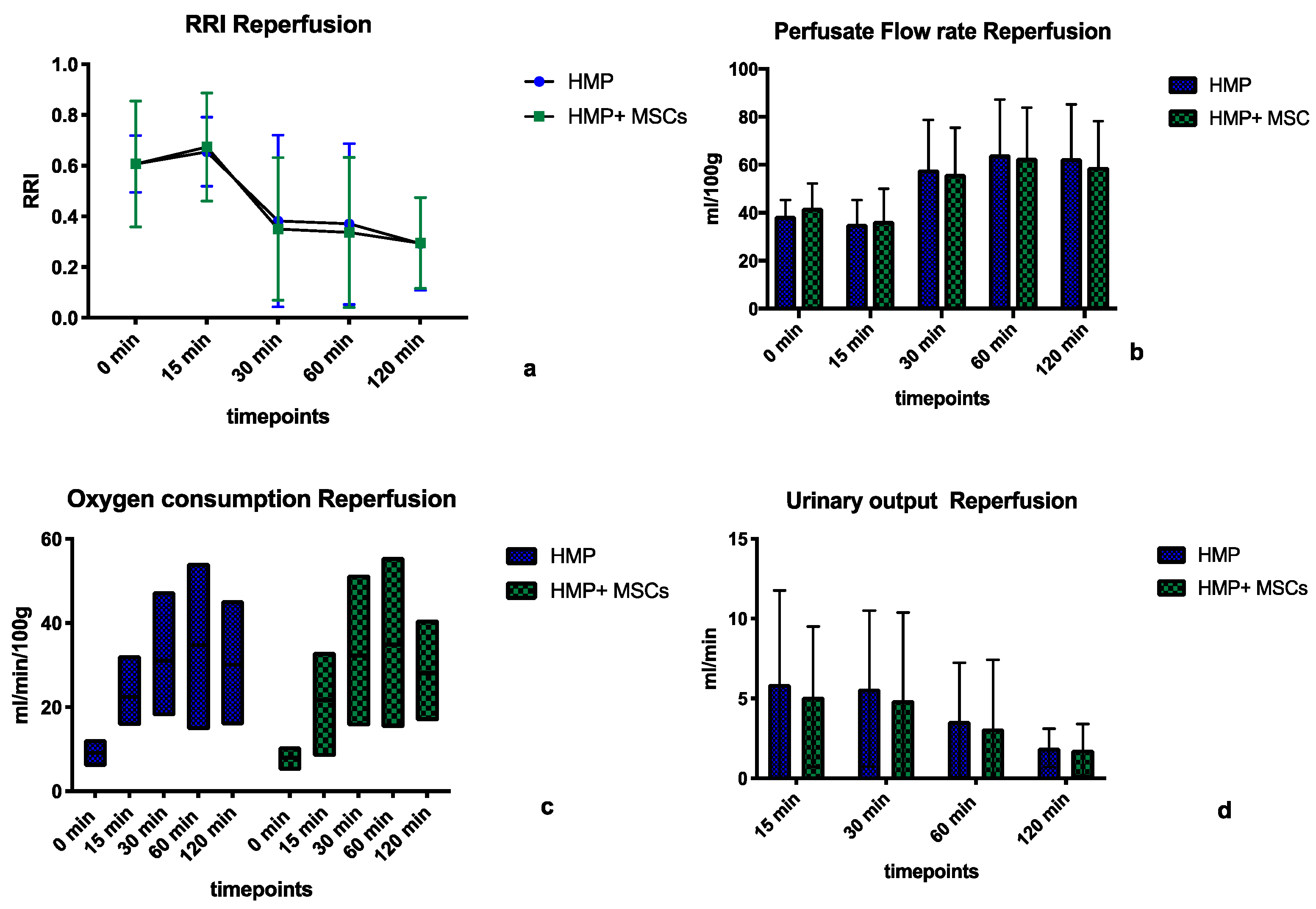
2.2.2. Perfusion Parameters during Normothermic Reperfusion
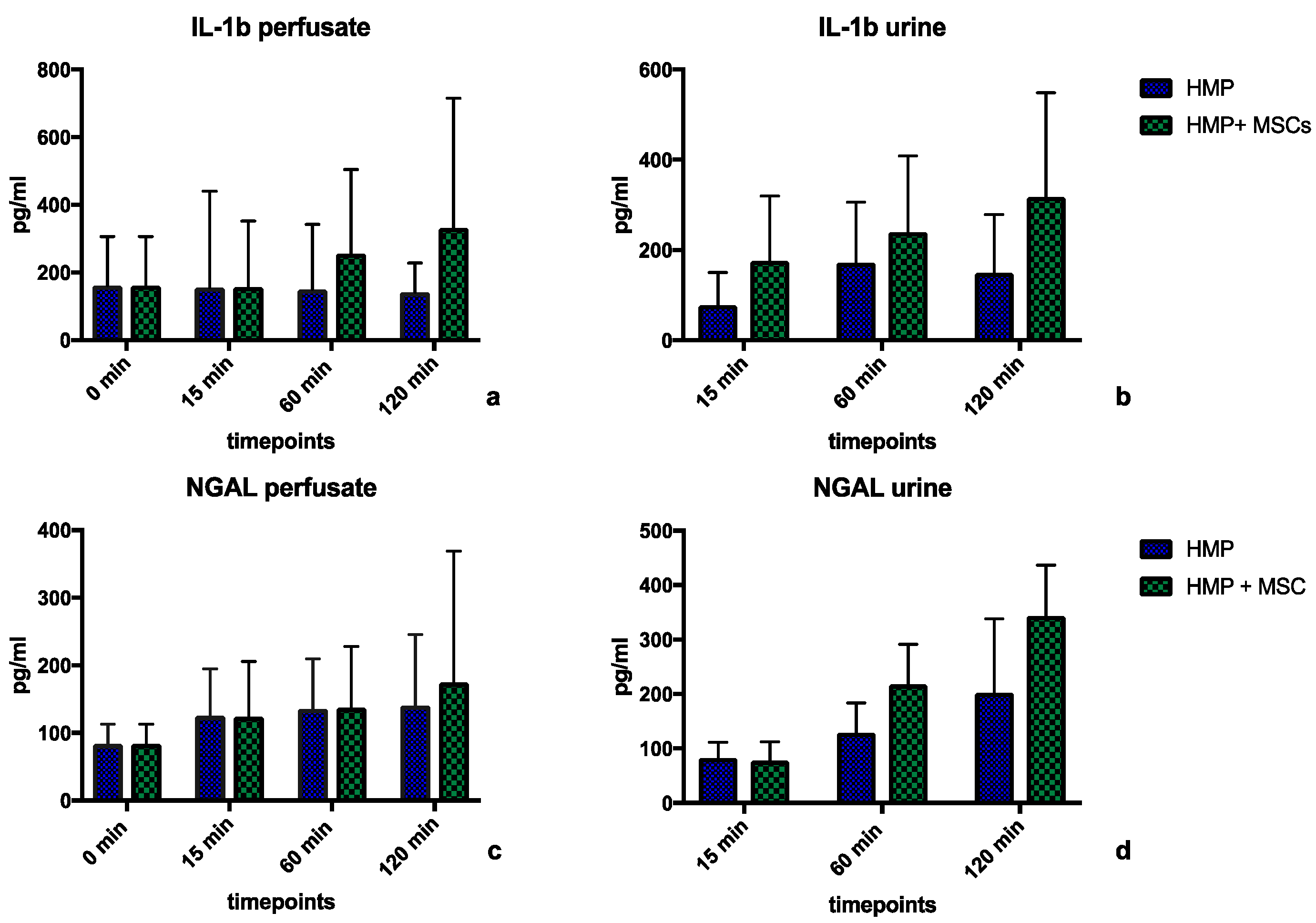
2.2.3. Perfusate Analyses during Normothermic Reperfusion
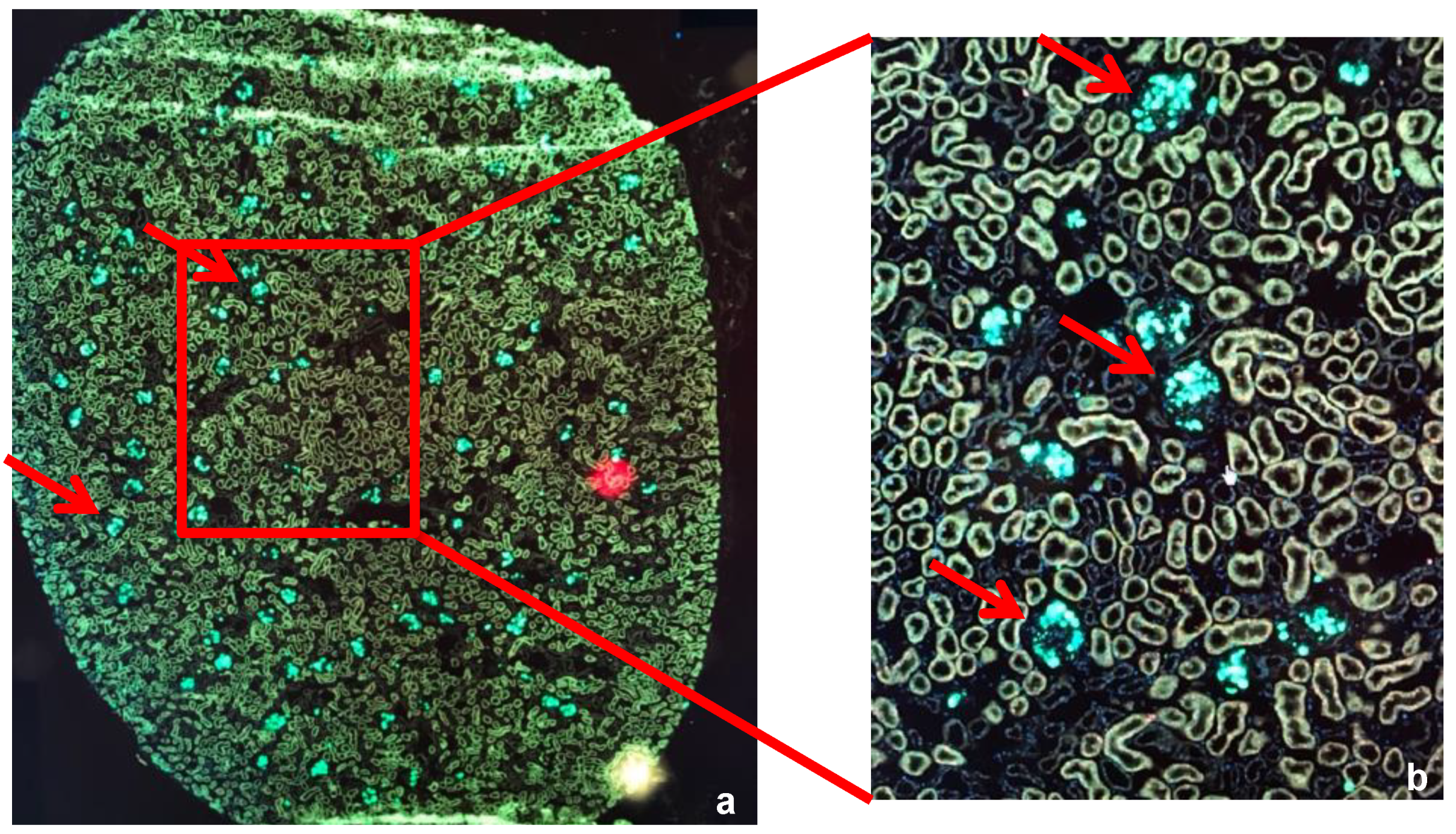
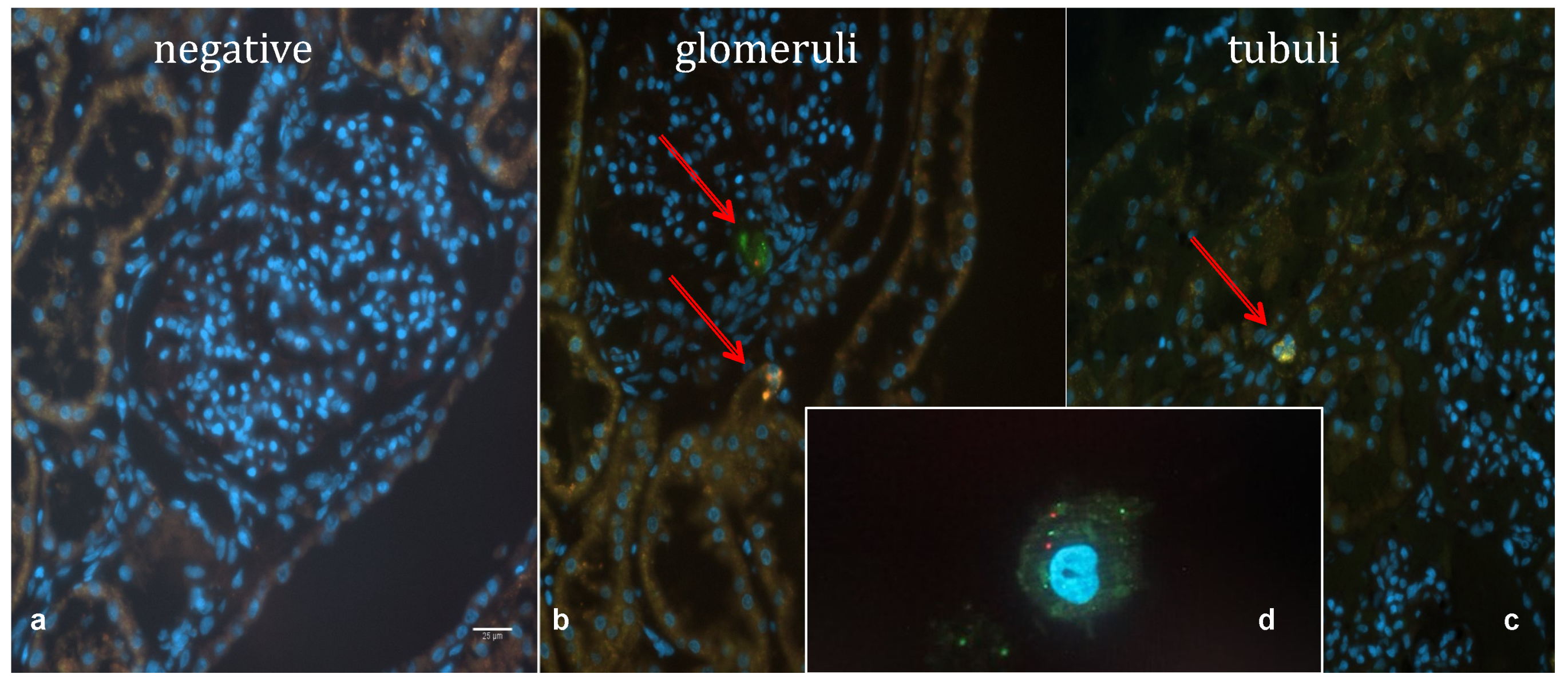
2.2.4. Widefield Microscopy Visualization of MSCs
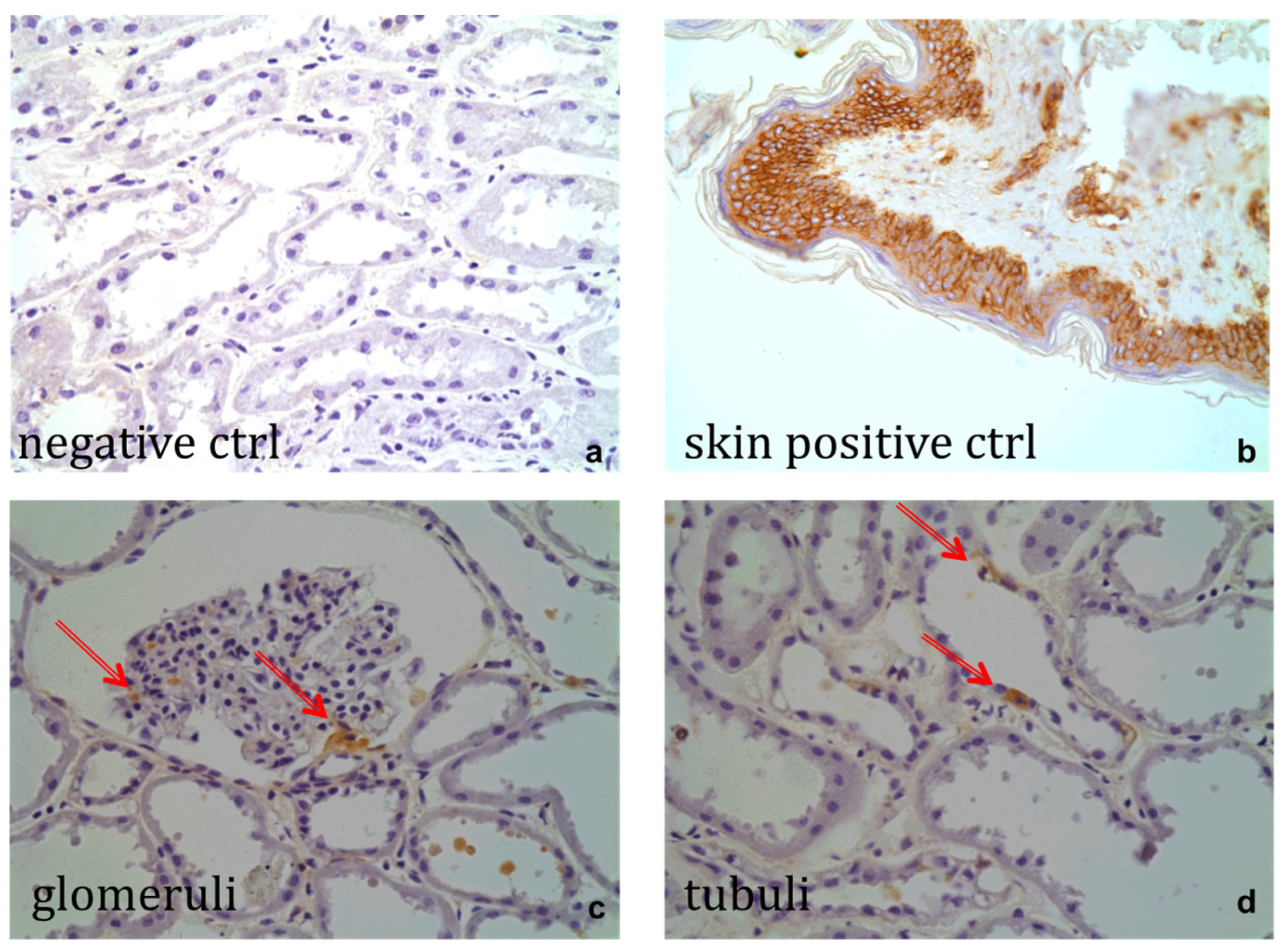
2.2.5. Immunohistochemistry
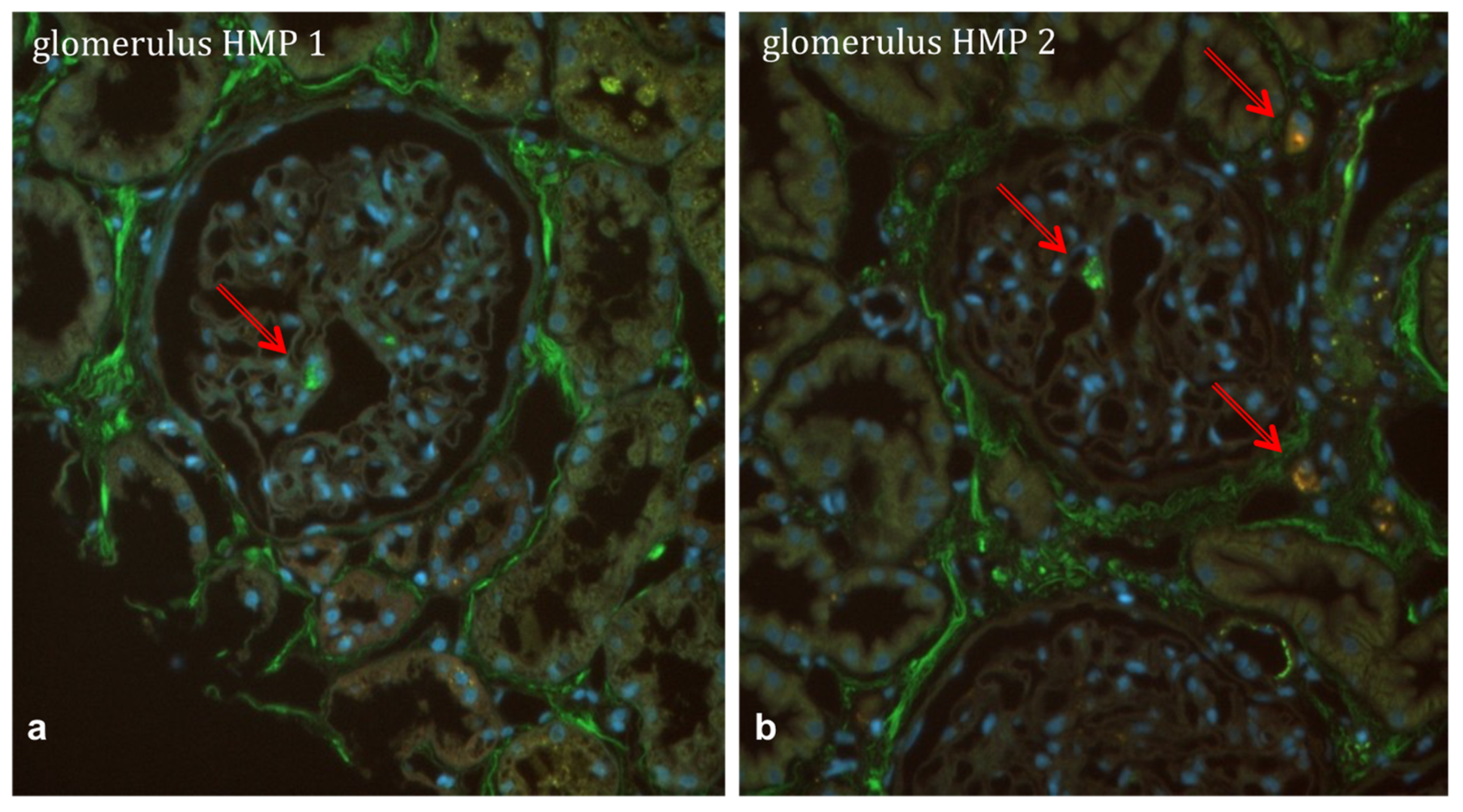
2.3. Mesenchymal Stem Cells in a Human HMP Model
3. Discussion
4. Materials and Methods
4.1. Study Groups
- Group rHMP + MSC: rodent kidneys undergoing 1 h of HMP + 3 × 106 MSCs (n = 3).
- Group pHMP: porcine kidneys after 25 min of WIT, 24 h CSS, followed by 4 h of HMP and 2 h of normothermic reperfusion with autologous porcine whole blood (n = 6).
- Group pHMP + MSC: porcine kidneys after 25 min of WIT, 24 h CSS, followed by 4 h of HMP + 1–5 × 106 human MSCs and 2 h of normothermic reperfusion with autologous porcine whole blood (n = 6).
- Group hHMP + MSC: human kidneys after 24 h of CSS and 4 h of HMP + 1 × 106 (n = 1), 3 × 106 (n = 1), or 5 × 106 human MSCs (n = 1).
4.2. Mesenchymal Stem Cells
4.2.1. Rat Mesenchymal Stem Cells
- CD29: PE anti-mouse/rat CD29 (clone: HMβ1-1, Bio Legend Cat. 102207);
- CD34: Alexa Fluor 647 anti-mouse CD34 (clone: ICO115, Novus Biologicals Cat. NBP2-33076AF647);
- CD44: RPE mouse anti-rat CD44 (clone OX-50, Bio Rad Cat. MCA643PE);
- CD45: Alexa Fluor 647 anti-rat CD45 (clone: OX-1, BioLegend Cat. 202212);
- CD90: PerCP anti-rat CD90/mouse, CD90.1 (Thy-1.1; clone: OX-7, BioLegend Cat. 202512; Lot: B171081).
4.2.2. Human Mesenchymal Stem Cells
4.2.3. Double-Labelling of Human Mesenchymal Stem Cells
4.3. Organ Retrieval
4.3.1. Rat Kidneys
4.3.2. Porcine Kidneys
4.3.3. Human Kidneys
4.4. Perfusates
4.5. Machine Perfusion
4.5.1. HMP of Rodent Kidneys
4.5.2. HMP of Porcine and Human Kidneys
4.5.3. Whole-Blood Normothermic Reperfusion of Porcine Organs
4.6. Histological Analysis
4.7. Microscopy
4.8. Anti MHC-I Staining of Porcine Histology Sections
4.9. Analytical Methods
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moers, C.; Smits, J.M.; Maathuis, M.-H.J.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.H.; Squifflet, J.-P.; van Heurn, E.; et al. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Gu, G.; Wang, D.; Tai, Q.; Wu, L.; Ju, W.; Zhu, X.; Guo, Z.; He, X. Machine Perfusion versus Cold Storage of Kidneys Derived from Donation after Cardiac Death: A Meta-Analysis. PLoS ONE 2013, 8, e56368. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.M.; Morgan, R.D.; Knight, S.R.; Morris, P.J. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes. Br. J. Surg. 2013, 100, 991–1001. [Google Scholar] [CrossRef]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.D.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.-P.; van Heurn, E.; Monbaliu, D.; et al. Machine Perfusion versus Cold Storage for the Preservation of Kidneys Donated after Cardiac Death. Ann. Surg. 2010, 252, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Hamaoui, K.; Gowers, S.; Boutelle, M.; Cook, T.H.; Hanna, G.; Darzi, A.; Smith, R.; Dorling, A.; Papalois, V. Organ Pretreatment with Cytotopic Endothelial Localizing Peptides to Ameliorate Microvascular Thrombosis and Perfusion Deficits in Ex Vivo Renal Hemoreperfusion Models. Transplantation 2016, 100, e128–e139. [Google Scholar] [CrossRef] [PubMed]
- Hamaoui, K.; Aftab, A.; Gowers, S.; Boutelle, M.; Cook, T.; Rudd, D.; Dobson, G.P.; Papalois, V. An ex vivo comparison of adenosine and lidocaine solution and University of Wisconsin solution for hypothermic machine perfusion of porcine kidneys: Potential for development. J. Surg. Res. 2017, 208, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Roemeling-van Rhijn, M.; Weimar, W.; Hoogduijn, M.J. Mesenchymal stem cells. Curr. Opin. Organ Transplant. 2012, 17, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, F.; Remuzzi, G.; Perico, N. Mesenchymal stromal cells to promote kidney transplantation tolerance. Curr. Opin. Organ Transplant. 2014, 19, 47–53. [Google Scholar] [CrossRef] [PubMed]
- de Vries, D.K.; Schaapherder, A.F.M.; Reinders, M.E.J. Mesenchymal stromal cells in renal ischemia/reperfusion injury. Front. Immunol. 2012, 3, 162. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Duijvestein, M.; Vos, A.C.W.; Roelofs, H.; E Wildenberg, M.; Wendrich, B.B.; Verspaget, H.W.; Kooy-Winkelaar, E.M.C.; Koning, F.; Zwaginga, J.J.; Fidder, H.H.; et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: Results of a phase I study. Gut 2010, 59, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, F.; Perico, N.; Cortinovis, M.; Remuzzi, G. Mesenchymal stromal cells in renal transplantation: Opportunities and challenges. Nat. Rev. Nephrol. 2016, 12, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Franquesa, M.; Hoogduijn, M.J.; Reinders, M.E.; Eggenhofer, E.; Engela, A.U.; Mensah, F.K.; Torras, J.; Pileggi, A.; van Kooten, C.; Mahon, B.; et al. Mesenchymal Stem Cells in Solid Organ Transplantation (MiSOT) Fourth Meeting. Transplantation 2013, 96, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Reinders, M.E.; Fibbe, W.E.; Rabelink, T.J. Multipotent mesenchymal stromal cell therapy in renal disease and kidney transplantation. Nephrol. Dial. Transplant. 2010, 25, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Assis, A.C.M.; Carvalho, J.L.; Jacoby, B.A.; Ferreira, R.L.B.; Castanheira, P.; Diniz, S.O.F.; Cardoso, V.N.; Goes, A.M.; Ferreira, A.J. Time-dependent migration of systemically delivered bone marrow mesenchymal stem cells to the infarcted heart. Cell Transplant. 2010, 19, 219–230. [Google Scholar] [CrossRef]
- Hoogduijn, M.J.; Rhijn, M.R.-V.; Engela, A.U.; Korevaar, S.S.; Mensah, F.K.; Franquesa, M.; de Bruin, R.W.; Betjes, M.G.; Weimar, W.; Baan, C.C. Mesenchymal Stem Cells Induce an Inflammatory Response after Intravenous Infusion. Stem Cells Dev. 2013, 22, 2825–2835. [Google Scholar] [CrossRef]
- Pool, M.; Eertman, T.; Parraga, J.S.; Hart, N.; Rhijn, M.R.-V.; Eijken, M.; Jespersen, B.; Reinders, M.; Hoogduijn, M.; Ploeg, R.; et al. Infusing Mesenchymal Stromal Cells into Porcine Kidneys during Normothermic Machine Perfusion: Intact MSCs Can Be Traced and Localised to Glomeruli. Int. J. Mol. Sci. 2019, 20, 3607. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.B.; Bussolati, B.; Bruno, S.; Fonsato, V.; Romanazzi, G.M.; Camussi, G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int. J. Mol. Med. 2004, 14, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Imberti, B.; Zoja, C.; Corna, D.; Tomasoni, S.; Abbate, M.; Rottoli, D.; Angioletti, S.; Benigni, A.; Perico, N.; et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Neyrinck, A.P.; Matthay, M.A.; Lee, J.W. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J. Biol. Chem. 2010, 285, 26211–26222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, J.; Pei, X.; Zhao, W. Systematic review and meta-analysis of mesenchymal stem/stromal cells therapy for impaired renal function in small animal models. Nephrology 2013, 18, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Reinders, M.E.J.; van Kooten, C.; Rabelink, T.J.; de Fijter, J.W. Mesenchymal Stromal Cell Therapy for Solid Organ Transplantation. Transplantation 2018, 102, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cai, J.; Jiao, X.; Yu, X.; Ding, X. Therapeutic potential of mesenchymal stem cells in acute kidney injury is affected by administration timing. Acta Biochim. Biophys. Sin. 2017, 49, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Erpicum, P.; Rowart, P.; Poma, L.; Krzesinski, J.-M.; Detry, O.; Jouret, F. Administration of Mesenchymal Stromal Cells before Renal Ischemia/Reperfusion Attenuates Kidney Injury and May Modulate Renal Lipid Metabolism in Rats. Sci. Rep. 2017, 7, 8687. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.I.G.; Moyon, B.; Coan, P.M.; Alfazema, N.; Venda, L.; Woollard, K.; Aitman, T. New Wistar Kyoto and spontaneously hypertensive rat transgenic models with ubiquitous expression of green fluorescent protein. Dis. Model. Mech. 2016, 9, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, S.; Pool, M.B.; Rozenberg, K.M.; Keller, A.K.; Moers, C.; Møldrup, U.; Møller, B.K.; Lignell, S.J.; Krag, S.; Sierra-Parraga, J.M.; et al. Mesenchymal stromal cell treatment of donor kidneys during ex vivo normothermic machine perfusion: A porcine renal autotransplantation study. Am. J. Transplant. 2021, 21, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Vallant, N.; Wolfhagen, N.; Sandhu, B.M.; Hamaoui, K.M.; Cook, T.F.; Pusey, C.M.; Papalois, V.M. A Comparison of Pulsatile Hypothermic and Normothermic Ex Vivo Machine Perfusion in a Porcine Kidney Model. Transplantation 2021, 105, 1760–1770. [Google Scholar] [CrossRef] [PubMed]
- Motegi, S.-I.; Ishikawa, O. Mesenchymal stem cells: The roles and functions in cutaneous wound healing and tumor growth. J. Dermatol. Sci. 2017, 86, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.M.; Shin, E.K.; Sung, J.H.; Moon, G.J.; Kim, E.H.; Cho, Y.H.; Park, H.D.; Bae, H.; Kim, J.; Bang, O.Y. Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci. Rep. 2018, 8, 1171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallant, N.; Wolfhagen, N.; Sandhu, B.; Hamaoui, K.; Papalois, V. Delivery of Mesenchymal Stem Cells during Hypothermic Machine Perfusion in a Translational Kidney Perfusion Study. Int. J. Mol. Sci. 2024, 25, 5038. https://doi.org/10.3390/ijms25095038
Vallant N, Wolfhagen N, Sandhu B, Hamaoui K, Papalois V. Delivery of Mesenchymal Stem Cells during Hypothermic Machine Perfusion in a Translational Kidney Perfusion Study. International Journal of Molecular Sciences. 2024; 25(9):5038. https://doi.org/10.3390/ijms25095038
Chicago/Turabian StyleVallant, Natalie, Nienke Wolfhagen, Bynvant Sandhu, Karim Hamaoui, and Vassilios Papalois. 2024. "Delivery of Mesenchymal Stem Cells during Hypothermic Machine Perfusion in a Translational Kidney Perfusion Study" International Journal of Molecular Sciences 25, no. 9: 5038. https://doi.org/10.3390/ijms25095038
APA StyleVallant, N., Wolfhagen, N., Sandhu, B., Hamaoui, K., & Papalois, V. (2024). Delivery of Mesenchymal Stem Cells during Hypothermic Machine Perfusion in a Translational Kidney Perfusion Study. International Journal of Molecular Sciences, 25(9), 5038. https://doi.org/10.3390/ijms25095038






