Hypoxia Promotes Osteoclast Differentiation by Weakening USP18-Mediated Suppression on the NF-κB Signaling Pathway
Abstract
:1. Introduction
2. Results
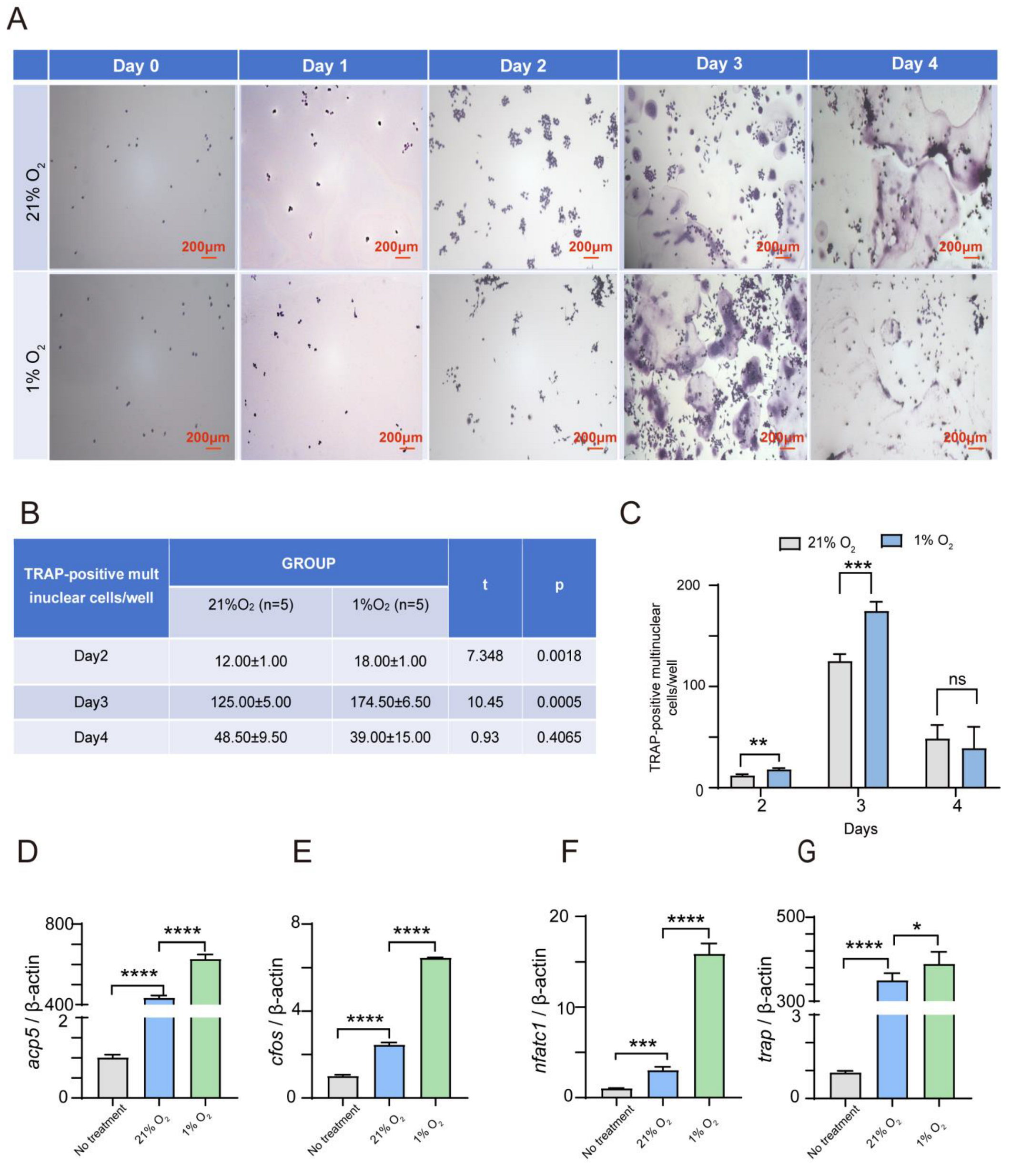
2.1. Hypoxia Significantly Enhances the Osteoclasts’ Differentiation of Both RAW264.7 and BMDM Cells
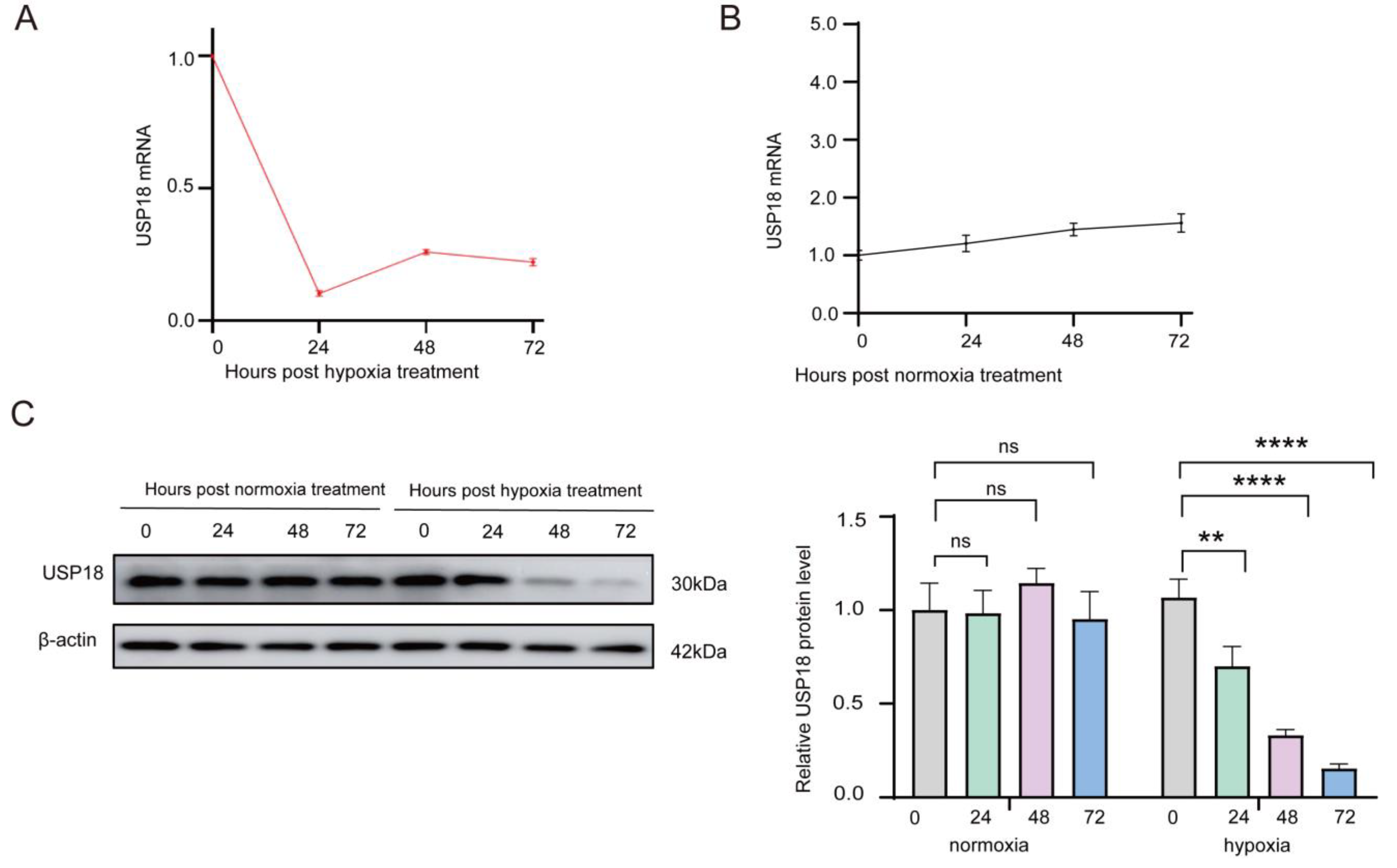
2.2. Hypoxia Dramatically Suppresses USP18 Expression in Osteoclast Differentiation
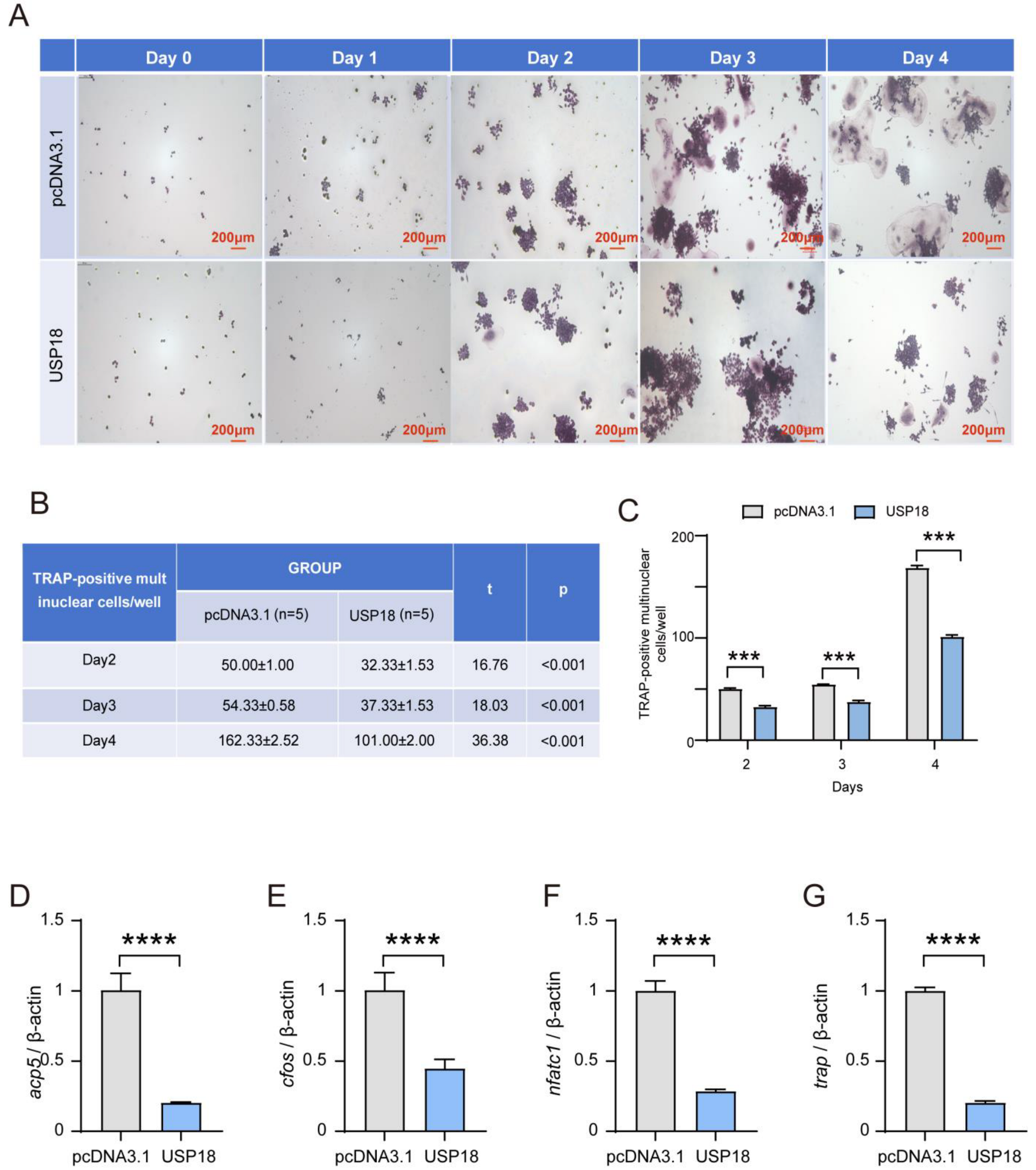
2.3. USP18 Negatively Modulates Osteoclast Differentiation
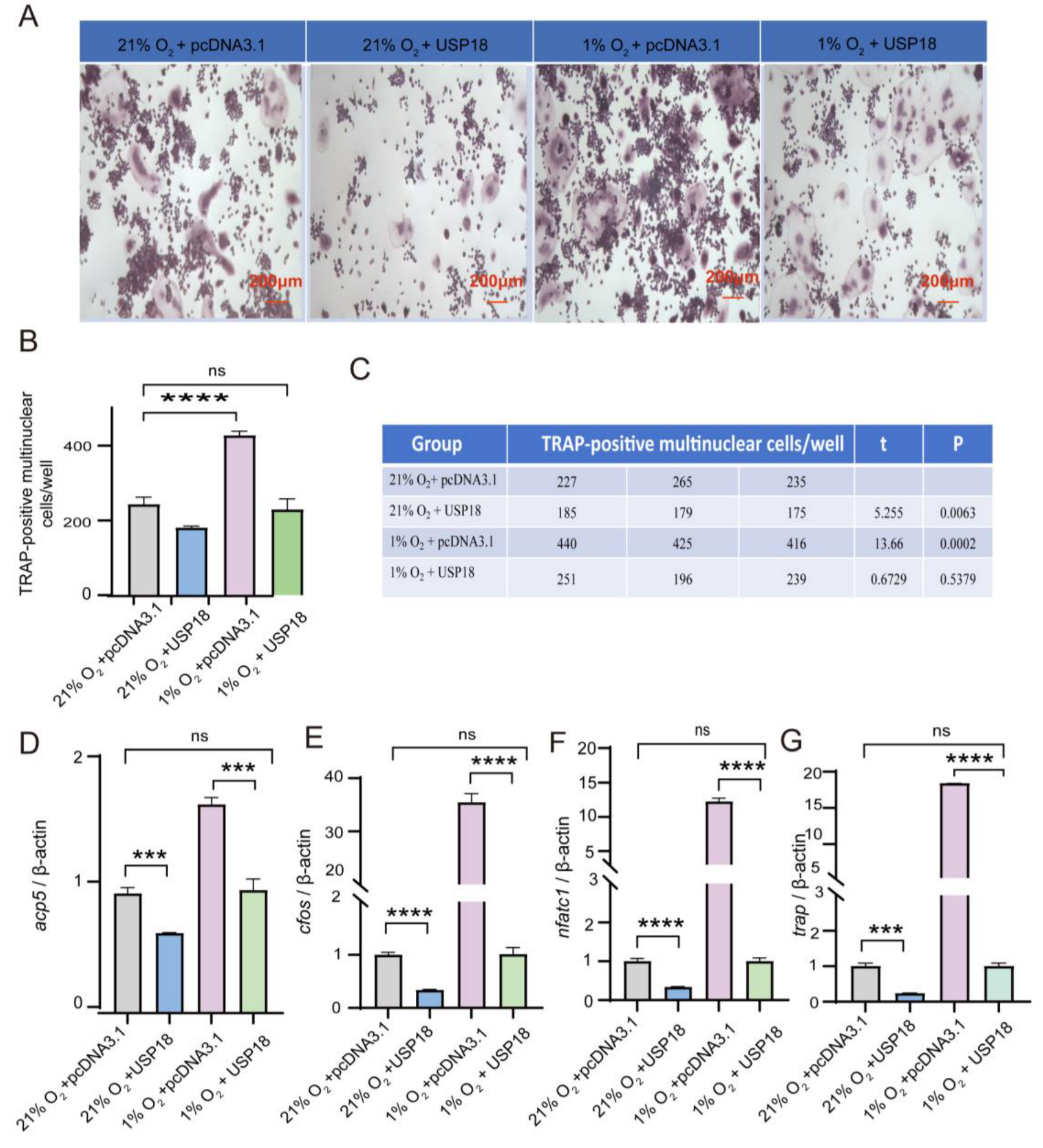
2.4. Overexpressing USP18 Rescues Hypoxia-Enhanced Osteoclast Differentiation
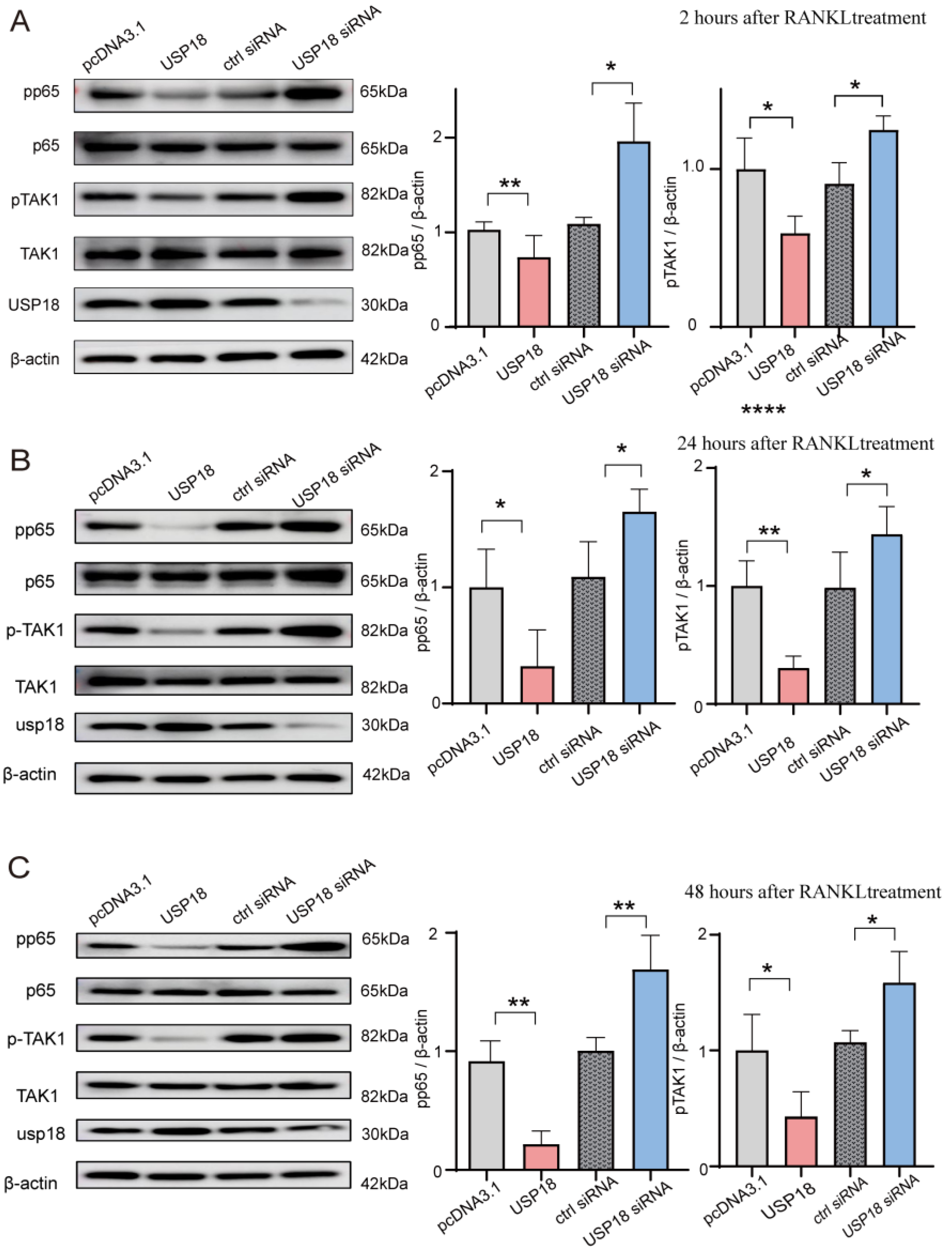
2.5. USP18 Inhibits Osteoclast Differentiation by Suppressing the NF-κB Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
- cFos, 5′-cgggtttcaacgccgacta-3′
- 5′-ttggcactagagacggacaga-3′;
- Acp5, 5′-cactcccaccctgagatttgt-3′
- 5′-catcgtctgcacggttctg-3′;
- Nfatc1, 5′-cagtgtgaccgaagatacctgg-3′
- 5′-tcgagacttgatagggacccc-3′;
- Trap, 5′-tggtccaggagcttaactgc-3′
- 5′-gtcaggagtgggagccatatg-3′;
- Usp18, 5′-caggagtccctgatttgcgt-3′
- 5′-caaggcatcctccagggttt-3′;
- β-actin, 5′-tctgctggaaggtggacagt-3′
- 5′-cctctatgccaacacagtgc-3′.
4.3. Immunoblot and Antibodies
4.4. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
4.5. Cell Transfection
- siUsp18-1 GGACGCAAAGCCTCTGAAA;
- siUsp18-2 CAATCTGGAACCTGACTAA;
- siUsp18-3 CCTATGGGAACCACAGATA.
4.6. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.M.; Li, N.; Li, K.; Li, X.Y.; Zhang, P.; Xuan, Y.J.; Cheng, X.G. Discordance in Diagnosis of Osteoporosis by Quantitative Computed Tomography and Dual-Energy X-Ray Absorptiometry in Chinese Elderly Men. J. Orthop. Transl. 2019, 18, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Cao, M.M.; Li, Y.J.; Dai, G.C.; Lu, P.P.; Zhang, M.; Bai, L.Y.; Chen, X.X.; Zhang, C.; Shi, L.; et al. The Regulative Effect and Repercussion of Probiotics and Prebiotics on Osteoporosis: Involvement of Brain-Gut-Bone Axis. Crit. Rev. Food Sci. Nutr. 2023, 63, 7510–7528. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Ilyas, S.; Lee, J.; Lee, D. Emerging Roles of Natural Compounds in Osteoporosis: Regulation, Molecular Mechanisms and Bone Regeneration. Pharmaceuticals 2024, 17, 984. [Google Scholar] [CrossRef]
- Stegen, S.; Stockmans, I.; Moermans, K.; Thienpont, B.; Maxwell, P.H.; Carmeliet, P.; Carmeliet, G. Osteocytic Oxygen Sensing Controls Bone Mass Through Epigenetic Regulation of Sclerostin. Nat. Commun. 2018, 9, 2557. [Google Scholar] [CrossRef]
- The Lancet Diabetes & Endocrinology. Osteoporosis: Overlooked in Men for Too Long. Lancet Diabetes Endocrinol. 2021, 9, 1. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Cao, M.M.; Li, Y.J.; Chen, X.X.; Yu, Q.; Rui, Y.F. A Narrative Review of the Moderating Effects and Repercussion of Exercise Intervention on Osteoporosis: Ingenious Involvement of Gut Microbiota and Its Metabolites. J. Transl. Med. 2022, 20, 490. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Z.; Wang, Y.; Xu, W.; Chen, H.; Xu, J.; Luo, S.; Zhang, Y.; Zhao, D.; Hu, J. The Efficacy and Safety of Denosumab in Postmenopausal Women with Osteoporosis Previously Treated with Bisphosphonates: A Review. J. Orthop. Transl. 2020, 22, 7–13. [Google Scholar] [CrossRef]
- Brown, J.P. Long-Term Treatment of Postmenopausal Osteoporosis. Endocrinol. Metab. 2021, 36, 544–552. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Bone Cell Communication Factors Provide a New Therapeutic Strategy for Osteoporosis. Chonnam Med. J. 2020, 56, 94–98. [Google Scholar] [CrossRef]
- Zepeda, A.B.; Pessoa, A., Jr.; Castillo, R.L.; Figueroa, C.A.; Pulgar, V.M.; Farías, J.G. Cellular and Molecular Mechanisms in the Hypoxic Tissue: Role of HIF-1 and ROS. Cell Biochem. Funct. 2013, 31, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Keeley, T.P.; Mann, G.E. Defining Physiological Normoxia for Improved Translation of Cell Physiology to Animal Models and Humans. Physiol. Rev. 2019, 99, 161–234. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.S.; Rameshwar, P.; Chang, V.; Bandari, P. Oxygen Saturation in the Bone Marrow of Healthy Volunteers. Blood 2002, 99, 394. [Google Scholar] [CrossRef] [PubMed]
- Arnett, T.R.; Gibbons, D.C.; Utting, J.C.; Orriss, I.R.; Hoebertz, A.; Rosendaal, M.; Meghji, S. Hypoxia is a Major Stimulator of Osteoclast Formation and Bone Resorption. J. Cell. Physiol. 2003, 196, 2–8. [Google Scholar] [CrossRef]
- Tando, T.; Sato, Y.; Miyamoto, K.; Morita, M.; Kobayashi, T.; Funayama, A.; Kanaji, A.; Hao, W.; Watanabe, R.; Oike, T.; et al. Hif1α is Required for Osteoclast Activation and Bone Loss in Male Osteoporosis. Biochem. Biophys. Res. Commun. 2016, 470, 391–396. [Google Scholar] [CrossRef]
- Brent, M.B.; Emmanuel, T.; Simonsen, U.; Brüel, A.; Thomsen, J.S. Hypobaric Hypoxia Deteriorates Bone Mass and Strength in Mice. Bone 2022, 154, 116203. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Huang, W.; Liang, J.; Chen, Y. The Intricate Interplay Between HIFs, ROS, and the Ubiquitin System in the Tumor Hypoxic Microenvironment. Pharmacol. Ther. 2022, 240, 108303. [Google Scholar] [CrossRef]
- Kubaichuk, K.; Kietzmann, T. Involvement of E3 Ligases and Deubiquitinases in the Control of HIF-α Subunit Abundance. Cells 2019, 8, 598. [Google Scholar] [CrossRef]
- Mennerich, D.; Kubaichuk, K.; Kietzmann, T. DUBs., Hypoxia, and Cancer. Trends Cancer 2019, 5, 632–653. [Google Scholar] [CrossRef]
- Clague, M.J.; Urbé, S.; Komander, D. Breaking the Chains: Deubiquitylating Enzyme Specificity Begets Function. Nat. Rev. Mol. Cell Biol. 2019, 20, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.I.; Goswami, R.; Brown, S.L.; Costanzo, K.; Shores, T.; Allan, S.; Odah, R.; Mohan, R.D. Deubiquitinases in Neurodegeneration. Cells 2022, 11, 556. [Google Scholar] [CrossRef]
- Deng, L.; Meng, T.; Chen, L.; Wei, W.; Wang, P. The Role of Ubiquitination in Tumorigenesis and Targeted Drug Discovery. Signal Transduct. Target. Ther. 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Reverter, D. Molecular Mechanisms of DUBs Regulation in Signaling and Disease. Int. J. Mol. Sci. 2021, 22, 986. [Google Scholar] [CrossRef]
- Guo, Y.C.; Zhang, S.W.; Yuan, Q. Deubiquitinating Enzymes and Bone Remodeling. Stem Cells Int. 2018, 2018, 3712083. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, X.; Hu, M.; Yang, S.; Li, X.; Han, R.; Zhou, J.; Li, D.; Liu, D. CYLD Inhibits Osteoclastogenesis to Ameliorate Alveolar Bone Loss in Mice with Periodontitis. J. Cell. Physiol. 2023, 238, 1036–1045. [Google Scholar] [CrossRef]
- Jin, W.; Chang, M.; Paul, E.M.; Babu, G.; Lee, A.J.; Reiley, W.; Wright, A.; Zhang, M.; You, J.; Sun, S.-C. Deubiquitinating Enzyme CYLD Negatively Regulates RANK Signaling and Osteoclastogenesis in Mice. J. Clin. Investig. 2008, 118, 1858–1866. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Park, K.H.; Oh, H.; Kim, J.-M.; Shin, D.Y.; Lee, J.M.; Lee, J.W.; Singh, A.; Lee, K.-Y.; Hu, D.; et al. CHMP5 Controls Bone Turnover Rates by Dampening NF-κB Activity in Osteoclasts. J. Exp. Med. 2015, 212, 1283–1301. [Google Scholar] [CrossRef]
- Yim, H.Y.; Park, C.; Lee, Y.D.; Arimoto, K.-I.; Jeon, R.; Baek, S.H.; Zhang, D.-E.; Kim, H.-H.; Kim, K.I. Elevated Response to Type I IFN Enhances RANKL-Mediated Osteoclastogenesis in Usp18-Knockout Mice. J. Immunol. 2016, 196, 3887–3895. [Google Scholar] [CrossRef]
- Li, C.; Qiu, M.; Chang, L.; Qi, J.; Zhang, L.; Ryffel, B.; Deng, L. The Osteoprotective Role of USP26 in Coordinating Bone Formation and Resorption. Cell Death Differ. 2022, 29, 1123–1136. [Google Scholar] [CrossRef]
- Li, Q.; Wang, M.; Xue, H.; Liu, W.; Guo, Y.; Xu, R.; Shao, B.; Yuan, Q. Ubiquitin-Specific Protease 34 Inhibits Osteoclast Differentiation by Regulating NF-κB Signaling. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2020, 35, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Bae, W.J.; Yi, J.K.; Kim, G.T.; Kim, E.C. Anti-Inflammatory and Anti-Osteoclastogenic Effects of Zinc Finger Protein A20 Overexpression in Human Periodontal Ligament Cells. J. Periodontal Res. 2016, 51, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.G.; Boone, D.L.; Chai, S.; Libby, S.L.; Chien, M.; Lodolce, J.P.; Ma, A. Failure to Regulate TNF-induced NF-kappaB and Cell Death Responses in A20-Deficient Mice. Science 2000, 289, 2350–2354. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.M.; Sanchez, S.; Cui, D.-C.; Dang, R.-J.; Wang, X.-Y.; Lin, Q.-X.; Wang, Y.; Wang, C.; Chen, D.-F.; et al. Deubiquitinase MYSM1 Is Essential for Normal Bone Formation and Mesenchymal Stem Cell Differentiation. Sci. Rep. 2016, 6, 22211. [Google Scholar] [CrossRef]
- Schwarz, T.; Sohn, C.; Kaiser, B.; Jensen, E.D.; Mansky, K.C. The 19S Proteasomal Lid Subunit POH1 Enhances the Transcriptional Activation by Mitf in Osteoclasts. J. Cell. Biochem. 2010, 109, 967–974. [Google Scholar] [CrossRef]
- Lian, C.; Wu, Z.; Gao, B.; Peng, Y.; Liang, A.; Xu, C.; Liu, L.; Qiu, X.; Huang, J.; Zhou, H.; et al. Melatonin Reversed Tumor Necrosis Factor-Alpha-Inhibited Osteogenesis of Human Mesenchymal Stem Cells by Stabilizing SMAD1 Protein. J. Pineal Res. 2016, 61, 317–327. [Google Scholar] [CrossRef]
- Zheng, H.L.; Xu, W.N.; Zhou, W.S.; Yang, R.-Z.; Chen, P.-B.; Liu, T.; Jiang, L.-S.; Jiang, S.-D. Beraprost Ameliorates Postmenopausal Osteoporosis by Regulating Nedd4-Induced Runx2 Ubiquitination. Cell Death Dis. 2021, 12, 497. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Cao, Y.; Gu, Y.; Zhang, L. Selective Compounds Enhance Osteoblastic Activity by Targeting HECT Domain of Ubiquitin ligase Smurf1. Oncotarget 2017, 8, 50521–50533. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Y.; Zhao, Z.; Lu, Y.; Shen, X.; Zhu, T.; Hou, M.; He, F.; Yang, H.; Zhang, Y.; et al. Bortezomib Rescues Ovariectomy-Induced Bone Loss via SMURF-Mediated Ubiquitination Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 9661200. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, W.; Jiao, G.; Chen, Y.; Liu, H. LncRNA SNHG1 Modulates p38 MAPK Pathway Through Nedd4 and Thus Inhibits Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Life Sci. 2019, 228, 208–214. [Google Scholar] [CrossRef]
- Liu, L.; Jin, R.; Duan, J.; Yang, L.; Cai, Z.; Zhu, W.; Nie, Y.; He, J.; Xia, C.; Gong, Q.; et al. Bioactive Iron Oxide Nanoparticles Suppress Osteoclastogenesis and Ovariectomy-Induced Bone Loss Through Regulating the TRAF6-p62-CYLD Signaling Complex. Acta Biomater. 2020, 103, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Tang, Y.D.; Zhai, J.; Zheng, C. The RING Finger Protein Family in Health and Disease. Signal. Transduct. Target Ther. 2022, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, R.; Xu, G.; Fan, P.; Luo, W.; Cai, C.; Ge, R.-L. Role of Ubiquitination in the Occurrence and Development of Osteoporosis (Review). Int. J. Mol. Med. 2024, 54, 68. [Google Scholar] [CrossRef] [PubMed]
- Győri, D.S.; Mócsai, A. Osteoclast Signal Transduction During Bone Metastasis Formation. Front. Cell Dev. Biol. 2020, 8, 507. [Google Scholar] [CrossRef]
- Utting, J.C.; Flanagan, A.M.; Brandao-Burch, A.; Orriss, I.R.; Arnett, T.R. Hypoxia Stimulates Osteoclast Formation from Human Peripheral Blood. Cell Biochem. Funct. 2010, 28, 374–380. [Google Scholar] [CrossRef]
- Muzylak, M.; Price, J.S.; Horton, M.A. Hypoxia Induces Giant Osteoclast Formation and Extensive Bone Resorption in the Cat. Calcif. Tissue Int. 2006, 79, 301–309. [Google Scholar] [CrossRef]
- Knowles, H.J.; Athanasou, N.A. Acute Hypoxia and Osteoclast Activity: A Balance Between Enhanced Resorption and Increased Apoptosis. J. Pathol. 2009, 218, 256–264. [Google Scholar] [CrossRef]
- Gorissen, B.; de Bruin, A.; Miranda-Bedate, A.; Korthagen, N.; Wolschrijn, C.; de Vries, T.J.; van Weeren, R.; Tryfonidou, M.A. Hypoxia Negatively Affects Senescence in Osteoclasts and Delays Osteoclastogenesis. J. Cell. Physiol. 2018, 234, 414–426. [Google Scholar] [CrossRef]
- Kang, D.; Jiang, H.; Wu, Q.; Fisher, P.B. Cloning and Characterization of Human Ubiquitin-Processing Protease-43 from Terminally Differentiated Human Melanoma Cells Using a Rapid Subtraction Hybridization Protocol RaSH. Gene 2001, 267, 233–242. [Google Scholar] [CrossRef]
- Malakhov, M.P.; Malakhova, O.A.; Kim, K.I.; Ritchie, K.J.; Zhang, D.-E. UBP43 (USP18) Specifically Removes ISG15 from Conjugated Proteins. J. Biol. Chem. 2002, 277, 9976–9981. [Google Scholar] [CrossRef]
- Kim, K.I.; Yan, M.; Malakhova, O.; Luo, J.-K.; Shen, M.-F.; Zou, W.; de la Torre, J.C.; Zhang, D.-E. Ube1L and Protein ISGylation Are Not Essential for Alpha/Beta Interferon Signaling. Mol. Cell. Biol. 2006, 26, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Malakhova, O.A.; Kim, K.I.; Luo, J.K.; Zou, W.; Kumar, K.G.S.; Fuchs, S.Y.; Shuai, K.; Zhang, D.-E. UBP43 is a Novel Regulator of Interferon Signaling Independent of Its ISG15 Isopeptidase Activity. EMBO J. 2006, 25, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xian, H.; Hu, J.; Tian, S.; Qin, Y.; Wang, R.-F.; Cui, J. USP18 Negatively Regulates NF-κB Signaling by Targeting TAK1 and NEMO for Deubiquitination Through Distinct Mechanisms. Sci. Rep. 2015, 5, 12738. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, J.L.; Baltimore, D. Two Pathways to NF-kappaB. Mol. Cell 2002, 10, 693–695. [Google Scholar] [CrossRef]
- Ahmad, S.; Abbas, M.; Ullah, M.F.; Aziz, M.H.; Beylerli, O.; Alam, M.A.; Syed, M.A.; Uddin, S.; Ahmad, A. Long Non-Coding RNAs Regulated NF-κB Signaling in Cancer Metastasis: Micromanaging by Not So Small Non-Coding RNAs. Semin. Cancer Biol. 2022, 85, 155–163. [Google Scholar] [CrossRef]
- Qu, R.; Chen, X.; Hu, J.; Fu, Y.; Peng, J.; Li, Y.; Chen, J.; Li, P.; Liu, L.; Cao, J.; et al. Ghrelin Protects Against Contact Dermatitis and Psoriasiform Skin Inflammation by Antagonizing TNF-α/NF-κB Signaling Pathways. Sci. Rep. 2019, 9, 1348. [Google Scholar] [CrossRef]
- Xing, L.; Bushnell, T.P.; Carlson, L.; Tai, Z.; Tondravi, M.; Siebenlist, U.; Young, F.; Boyce, B.F. NF-kappaB p50 and p52 EXPRESSION IS NOT REQUIRED for RANK-Expressing Osteoclast Progenitor Formation but Is Essential for RANK- and Cytokine-Mediated Osteoclastogenesis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2002, 17, 1200–1210. [Google Scholar] [CrossRef]
- Luo, G.; Li, F.; Li, X.; Wang, Z.-G.; Zhang, B. TNF-α and RANKL Promote Osteoclastogenesis by Upregulating RANK via the NF-κB Pathway. Mol. Med. Rep. 2018, 17, 6605–6611. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Li, B.; Chai, S.; Zhang, R.; Cai, C.; Ge, R. Hypoxia Promotes Osteoclast Differentiation by Weakening USP18-Mediated Suppression on the NF-κB Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 10. https://doi.org/10.3390/ijms26010010
Fan X, Li B, Chai S, Zhang R, Cai C, Ge R. Hypoxia Promotes Osteoclast Differentiation by Weakening USP18-Mediated Suppression on the NF-κB Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(1):10. https://doi.org/10.3390/ijms26010010
Chicago/Turabian StyleFan, Xiaoxia, Botong Li, Shengjun Chai, Rong Zhang, Chunmei Cai, and Rili Ge. 2025. "Hypoxia Promotes Osteoclast Differentiation by Weakening USP18-Mediated Suppression on the NF-κB Signaling Pathway" International Journal of Molecular Sciences 26, no. 1: 10. https://doi.org/10.3390/ijms26010010
APA StyleFan, X., Li, B., Chai, S., Zhang, R., Cai, C., & Ge, R. (2025). Hypoxia Promotes Osteoclast Differentiation by Weakening USP18-Mediated Suppression on the NF-κB Signaling Pathway. International Journal of Molecular Sciences, 26(1), 10. https://doi.org/10.3390/ijms26010010




