From Molecular to Radionuclide and Pharmacological Aspects in Transthyretin Cardiac Amyloidosis
Abstract
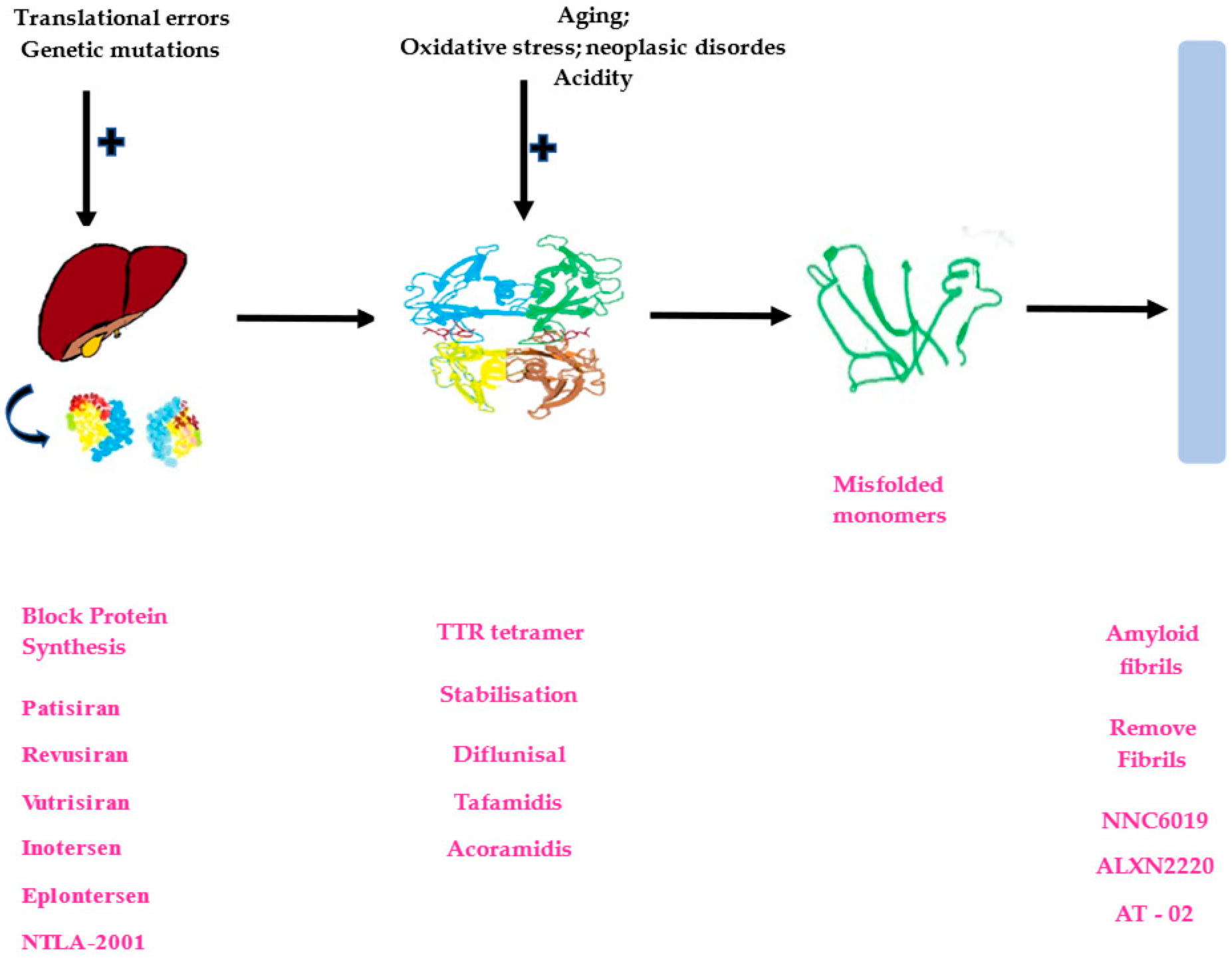
1. Introduction
2. Protein Misfolding and ATTR-CM
3. From Molecular to Radionuclide Aspects in ATTR-CM
| Tracer | Target and Original Application | CA Type | Advantage | Pitfalls and Limitations |
|---|---|---|---|---|
| 99mTc-PYP | Microcalcification (Bone scintigraphy) | ATTR>>AL | 98% sensitivity 96% specificity for ATTR-CM 1 H/CL ratio ≥ 1.5: 97% sensitivity 100% specificity for ATTR-CM | False positives in cases of acute MI, valvular/annular calcification, or due to extracardiac uptake. Additional 3 h imaging may be required if blood pool activity is noted. |
| 99mTc-DPD | Microcalcification (Bone scintigraphy) | ATTR>>AL | H/WB ratio > 0.091: 92% sensitivity and 88% specificity | Same as 99mTc-PYP. |
| 99mTc-HMDP | Microcalcification (Bone scintigraphy) | ATTR>>AL | Comparable to 99mTc-DPD | Same as 99mTc-PYP. |
| 11C-PIB | Amyloid (Brain imaging in Alzheimer dementia) | AL>>ATTR | Detects both AL-CA and ATTR-CM, ability to detect early disease. Can complement 99mTc-PYP scintigraphy | Short half-life (20 min) limits practicality, requiring onsite cyclotron for generation. Lack of large-sized studies to confirm efficacy. |
| 18F- Florbetapir/ Florbetaben/Flutebetamol/NaF | Amyloid (Brain imaging in Alzheimer dementia) | AL>>ATTR | Can diagnose both AL-CA and ATTR-CM. Allows for early detection and aids therapy response assessment | Lack of large-sized studies to confirm efficacy. |
4. From Molecular to Pharmacological Approaches in ATTR-CM
- TTR knockdown: gene-silencing therapy (synthetic oligonucleotides) and gene-editing therapy (CRISPR-Cas9),
- TTR stabilizers, and
- TTR depleters: monoclonal antibodies.
4.1. TTR Knockdown
4.2. TTR Stabilizers
4.3. TTR Depleters
4.4. Combination Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Picken, M.M. The Pathology of Amyloidosis in Classification: A Review. Acta Haematol. 2020, 143, 322–334. [Google Scholar] [CrossRef]
- Falk, R.H.; Alexander, K.M.; Liao, R.; Dorbala, S. AL (Light-Chain) Cardiac Amyloidosis: A Review of Diagnosis and Therapy. J. Am. Coll. Cardiol. 2016, 68, 1323–1341. [Google Scholar] [CrossRef]
- Wisniowsk, B.; Wechalekar, A. Confirming the Diagnosis of Amyloidosis. Acta Haematol. 2020, 143, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Wille, H.; Dorosh, L.; Amidian, S.; Schmitt-Ulms, G.; Stepanova, M. Combining molecular dynamics simulations and experimental analyses in protein misfolding. Adv. Protein Chem. Struct. Biol. 2019, 118, 33–110. [Google Scholar] [PubMed]
- Khan, M. Interplay of protein misfolding pathway and unfolded-protein response in acute promyelocytic leukemia. Expert Rev. Proteom. 2010, 7, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Castilla, J.; Saá, P.; Morales, R.; Abid, K.; Maundrell, K.; Soto, C. Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Methods Enzymol. 2006, 412, 3–21. [Google Scholar] [PubMed]
- Leighton, P.L.; Allison, W.T. Protein Misfolding in Prion and Prion-Like Diseases: Reconsidering a Required Role for Protein Loss-of-Function. J. Alzheimer’s Dis. 2016, 54, 3–29. [Google Scholar] [CrossRef]
- Cuanalo-Contreras, K.; Mukherjee, A.; Soto, C. Role of protein misfolding and proteostasis deficiency in protein misfolding diseases and aging. Int. J. Cell Biol. 2013, 2013, 638083. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, S.; Dong, H.; Liu, Y.; Liu, C.; Zhang, X. Advanced Techniques for Detecting Protein Misfolding and Aggregation in Cellular Environments. Chem. Rev. 2023, 123, 12254–12311. [Google Scholar] [CrossRef]
- Lyubchenko, Y.L.; Kim, B.H.; Krasnoslobodtsev, A.V.; Yu, J. Nanoimaging for protein misfolding diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 526–543. [Google Scholar] [CrossRef] [PubMed]
- Sepehrvand, N.; Youngson, E.; Fine, N.; Venner, C.P.; Paterson, I.; Bakal, J.; Westerhout, C.; Mcalister, F.A.; Kaul, P.; Ezekowitz, J.A. The Incidence and Prevalence of Cardiac Amyloidosis in a Large Community-Based Cohort in Alberta, Canada. J. Card. Fail. 2022, 28, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Yokochi, T. Transthyretin cardiac amyloidosis: An update on diagnosis and treatment. ESC Heart Fail. 2019, 6, 1128–1139. [Google Scholar] [CrossRef]
- Rubin, J.; Maurer, M.S. Cardiac Amyloidosis: Overlooked, Underappreciated, and Treatable. Annu. Rev. Med. 2020, 7, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Zahra, F. Transthyretin Amyloid Cardiomyopathy (ATTR-CM). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ruberg, F.L.; Maurer, M.S. Cardiac Amyloidosis Due to Transthyretin Protein: A Review. JAMA 2024, 331, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Porcari, A.; Fontana, M.; Gillmore, J.D. Transthyretin cardiac amyloidosis. Cardiovasc. Res. 2023, 118, 3517–3535. [Google Scholar] [CrossRef]
- Kyle, R.A.; Larson, D.R.; Kurtin, P.J.; Kumar, S.; Cerhan, J.R.; Therneau, T.M.; Rajkumar, S.V.; Vachon, C.M.; Dispenzieri, A. Incidence of AL Amyloidosis in Olmsted County, Minnesota, 1990 through 2015. Mayo Clin. Proc. 2019, 4, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Merlini, G. How I treat AL amyloidosis. Blood 2022, 139, 2918–2930. [Google Scholar] [CrossRef] [PubMed]
- De Michieli, L.; Sinigiani, G.; De Gaspari, M.; Branca, A.; Rizzo, S.; Basso, C.; Trentin, L.; Iliceto, S.; Perazzolo Marra, M.; Cipriani, A.; et al. Light-chain cardiac amyloidosis for the non-expert: Pearls and pitfalls. Intern. Emerg. Med. 2023, 18, 1879–1886. [Google Scholar] [CrossRef]
- Jung, M.H.; Chang, S.; Han, E.J.; Youn, J.C. Multimodal Imaging and Biomarkers in Cardiac Amyloidosis. Diagnostics 2022, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Çavuşoğlu, Y.; Özpelit, E.; Çelik, A.; İkitimur, B.; Kayıkçıoğlu, M.; Tokgözoğlu, L.; Tüfekçioğlu, O.; Yılmaz, M.B. Cardiac amyloidosis: Recent advances in the diagnosis and therapy. Turk. Kardiyol. Dern. Ars. 2019, 47 (Suppl. S2), 1–34. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, K.; Isono, K.; Ohya, Y.; Shiraki, N.; Tasaki, M.; Inomata, Y.; Ueda, M.; Era, T.; Kume, S.; Ando, Y.; et al. Characterization of heterozygous ATTR Tyr114Cys amyloidosis-specific induced pluripotent stem cells. Heliyon 2024, 10, e24590. [Google Scholar] [CrossRef] [PubMed]
- Brito, D.; Albrecht, F.C.; de Arenaza, D.P.; Bart, N.; Better, N.; Carvajal-Juarez, I.; Conceição, I.; Damy, T.; Dorbala, S.; Fidalgo, J.C.; et al. World Heart Federation Consensus on Transthyretin Amyloidosis Cardiomyopathy (ATTR-CM). Glob Heart. 2023, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Bart, N.K.; Thomas, L.; Korczyk, D.; Atherton, J.J.; Stewart, G.J.; Fatkin, D. Amyloid Cardiomyopathy. Heart Lung Circ. 2020, 29, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Arghavani, P.; Badiei, A.; Ghadami, S.A.; Habibi-Rezaei, M.; Moosavi-Movahedi, F.; Delphi, L.; Moosavi-Movahedi, A.A. Inhibiting mTTR Aggregation/Fibrillation by a Chaperone-like Hydrophobic Amino Acid-Conjugated SPION. J. Phys. Chem. B 2022, 126, 1640–1654. [Google Scholar] [CrossRef]
- Witteles, R.M.; Bokhari, S.; Damy, T.; Elliott, P.M.; Falk, R.H.; Fine, N.M.; Gospodinova, M.; Obici, L.; Rapezzi, C.; Garcia-Pavia, P. Screening for Transthyretin Amyloid Cardiomyopathy in Everyday Practice. JACC Heart Fail. 2019, 7, 709–716. [Google Scholar] [CrossRef]
- Uversky, V.N. Protein intrinsic disorder and structure-function continuum. Prog. Mol. Biol. Transl. Sci. 2019, 166, 1–17. [Google Scholar] [PubMed]
- Rehman, I.; Farooq, M.; Botelho, S. Biochemistry, Secondary Protein Structure. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Nishimura, C.; Kikuchi, T. Non-Native Structures of Apomyoglobin and Apoleghemoglobin in Folding Intermediates Related to the Protein Misfolding. Molecules 2023, 28, 3970. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Louros, N.; Schymkowitz, J.; Rousseau, F. Mechanisms and pathology of protein misfolding and aggregation. Nat. Rev. Mol. Cell Biol. 2023, 12, 912–933. [Google Scholar] [CrossRef] [PubMed]
- Xu, H. Non-Equilibrium Protein Folding and Activation by ATP-Driven Chaperones. Biomolecules 2022, 12, 832. [Google Scholar] [CrossRef]
- Zito, E.; Lescure, A.; Borgese, N. Chemical chaperones in metabolic fitness beyond protein folding. Trends Endocrinol. Metab. 2024, 35, 572–575. [Google Scholar] [CrossRef]
- Kawagoe, S.; Ishimori, K.; Saio, T. Structural and Kinetic Views of Molecular Chaperones in Multidomain Protein Folding. Int. J. Mol. Sci. 2022, 23, 2485. [Google Scholar] [CrossRef]
- Sami, N.; Rahman, S.; Kumar, V.; Zaidi, S.; Islam, A.; Ali, S.; Ahmad, F.; Hassan, M.I. Protein aggregation, misfolding and consequential human neurodegenerative diseases. Int. J. Neurosci. 2017, 127, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Krshnan, L.; van de Weijer, M.L.; Carvalho, P. Endoplasmic Reticulum-Associated Protein Degradation. Cold Spring Harb. Perspect. Biol. 2022, 14, a041247. [Google Scholar] [CrossRef] [PubMed]
- Prodromou, C.; Aran-Guiu, X.; Oberoi, J.; Perna, L.; Chapple, J.P.; van der Spuy, J. HSP70-HSP90 Chaperone Networking in Protein-Misfolding Disease. Subcell. Biochem. 2023, 101, 389–425. [Google Scholar]
- Nademi, S.; Dickhout, J.G. Protein misfolding in endoplasmic reticulum stress with applications to renal diseases. Adv. Protein Chem. Struct. Biol. 2019, 118, 217–247. [Google Scholar]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.N.; Khan, R.H. Protein misfolding and related human diseases: A comprehensive review of toxicity, proteins involved, and current therapeutic strategies. Int. J. Biol. Macromol. 2022, 223 Pt A, 143–160. [Google Scholar] [CrossRef]
- Ju, Y.J.; Lee, H.W.; Choi, J.W.; Choi, M.S. The Role of Protein S-Nitrosylation in Protein Misfolding-Associated Diseases. Life 2021, 11, 705. [Google Scholar] [CrossRef]
- Coyne, L.P.; Chen, X.J. Consequences of inner mitochondrial membrane protein misfolding. Mitochondrion 2019, 49, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Al Mamun, A.; Rahman, M.A.; Behl, T.; Perveen, A.; Hafeez, A.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Ashraf, G.M. Emerging Proof of Protein Misfolding and Interactions in Multifactorial Alzheimer’s Disease. Curr. Top. Med. Chem. 2020, 20, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.M.; Roberts, C.J. Protein misfolding and aggregation research: Some thoughts on improving quality and utility. Biotechnol. Prog. 2013, 29, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Gaczynska, M.; Osmulski, P.A. Targeting Protein-Protein Interactions in the Ubiquitin-Proteasome Pathway. Adv. Protein Chem. Struct. Biol. 2018, 110, 123–165. [Google Scholar] [PubMed]
- Kabir, M.T.; Uddin, M.S.; Abdeen, A.; Ashraf, G.M.; Perveen, A.; Hafeez, A.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Evidence Linking Protein Misfolding to Quality Control in Progressive Neurodegenerative Diseases. Curr. Top. Med. Chem. 2020, 20, 2025–2043. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Saikia, B.; Gogoi, C.R.; Baruah, A. Advances in the understanding of protein misfolding and aggregation through molecular dynamics simulation. Prog. Biophys. Mol. Biol. 2022, 175, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.V.; Poulsen, E.G.; Rebula, C.A.; Hartmann-Petersen, R. Protein quality control in the nucleus. Biomolecules 2014, 4, 646–661. [Google Scholar] [CrossRef]
- Soto, C.; Estrada, L.D. Protein misfolding and neurodegeneration. Arch. Neurol. 2008, 65, 184–189. [Google Scholar] [CrossRef]
- Pande, M.; Srivastava, R. Molecular and clinical insights into protein misfolding and associated amyloidosis. Eur. J. Med. Chem. 2019, 184, 111753. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Oh, C.K.; Zhang, X.; Lipton, S.A. Protein S-nitrosylation and oxidation contribute to protein misfolding in neurodegeneration. Free Radic. Biol. Med. 2021, 172, 562–577. [Google Scholar] [CrossRef]
- Nakamura, M.; Ando, Y. Amyloidosis and oxidative stress. Rinsho Byori 2003, 51, 140–145. [Google Scholar] [PubMed]
- Fiore, M.; Cambieri, C.; Libonati, L.; Moret, F.; D’Andrea, E.; Di Certo, M.G.; Passananti, C.; Gabanella, F.; Corbi, N.; Garibaldi, M.; et al. Oxidative Stress in Transthyretin-Mediated Amyloidosis: An Exploratory Study. Antioxidants 2024, 13, 998. [Google Scholar] [CrossRef]
- Ueda, M. Transthyretin: Its function and amyloid formation. Neurochem. Int. 2022, 155, 105313. [Google Scholar] [CrossRef]
- Mirioglu, S.; Uludag, O.; Hurdogan, O.; Kumru, G.; Berke, I.; Doumas, S.A.; Frangou, E.; Gul, A. AA Amyloidosis: A Contemporary View. Curr. Rheumatol. Rep. 2024, 26, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Kouadir, M.; Yang, Y. NALP3 inflammasome activation in protein misfolding diseases. Life Sci. 2015, 135, 9–14. [Google Scholar] [CrossRef]
- Nilsson, K.P.; Ikenberg, K.; Aslund, A.; Fransson, S.; Konradsson, P.; Röcken, C.; Moch, H.; Aguzzi, A. Structural typing of systemic amyloidoses by luminescent-conjugated polymer spectroscopy. Am. J. Pathol. 2010, 176, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Sekijima, Y. Transthyretin (ATTR) amyloidosis: Clinical spectrum, molecular pathogenesis and disease-modifying treatments. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1036–1043. [Google Scholar] [CrossRef]
- Williams, M.A.C.; Shankar, B.; Vaishnav, J.; Ranek, M.J. Current and potential therapeutic strategies for transthyretin cardiac amyloidosis. Front. Drug Discov. 2022, 2, 1015545. [Google Scholar] [CrossRef]
- Jain, H.; Reddy, M.M.R.K.; Dey, R.C.; Jain, J.; Shakhatreh, Z.; Manandhar, S.; Neupane, P.; Waleed, M.S.; Yadav, R.; Sah, B.K.; et al. Exploring Transthyretin Amyloid Cardiomyopathy: A Comprehensive Review of the Disease and Upcoming Treatments. Curr. Probl. Cardiol. 2024, 49 Pt B, 102057. [Google Scholar] [CrossRef]
- Aimo, A.; Rapezzi, C.; Perfetto, F.; Cappelli, F.; Palladini, G.; Obici, L.; Merlini, G.; Di Bella, G.; Serenelli, M.; Zampieri, M.; et al. Quality of life assessment in amyloid transthyretin (ATTR) amyloidosis. Eur. J. Clin. Investig. 2021, 51, e13598. [Google Scholar] [CrossRef]
- Siddiqi, O.K.; Ruberg, F.L. Cardiac amyloidosis: An update on pathophysiology, diagnosis, and treatment. Trends Cardiovasc. Med. 2018, 28, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Treviño-Herrera, A.B.; Bustamante-Vargas, A.P.; Lisker-Cervantes, A.; Ríos, Y.; Valles Valles, D.; Villanueva-Mendoza, C.; González-Duarte, A.; Concha-Del-Río, L.E. Vitreous involvement as initial presentation of hereditary transthyretin amyloidosis related to the rare TTR Ile107Met (p.Ile127Met) pathogenic variant. Ophthalmic Genet. 2022, 43, 413–419. [Google Scholar] [CrossRef]
- Danni, W.; Wei, C. Molecular mechanisms and emerging therapies in wild-type transthyretin amyloid cardiomyopathy. Heart Fail. Rev. 2024, 29, 511–521. [Google Scholar]
- Gonzalez-Duarte, A.; Ulloa-Aguirre, A. A Brief Journey through Protein Misfolding in Transthyretin Amyloidosis (ATTR Amyloidosis). Int. J. Mol. Sci. 2021, 22, 13158. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K. The interaction of zinc with the multi-functional plasma thyroid hormone distributor protein, transthyretin: Evolutionary and cross-species comparative aspects. Biometals 2021, 34, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.P.; Ho, P.C.; Tsai, K.J. TTR (transthyretin) leads the autophagy disaster relief team against TARDBP/TDP-43 proteinopathy. Autophagy 2023, 19, 2403–2405. [Google Scholar] [CrossRef]
- Maurer, M.S.; Bokhari, S.; Damy, T.; Dorbala, S.; Drachman, B.M.; Fontana, M.; Grogan, M.; Kristen, A.V.; Lousada, I.; Nativi-Nicolau, J.; et al. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circ. Heart Fail. 2019, 12, e006075. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, A.; Fontana, M.; Gillmore, J.D. RNA Targeting and Gene Editing Strategies for Transthyretin Amyloidosis. BioDrugs 2023, 37, 127–142. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef]
- Pinheiro, F.; Varejão, N.; Esperante, S.; Santos, J.; Velázquez-Campoy, A.; Reverter, D.; Pallarès, I.; Ventura, S. A potent aggregation inhibitor for the treatment of familial leptomeningeal amyloidosis. FEBS J. 2021, 288, 310–324. [Google Scholar] [CrossRef]
- Mangrolia, P.; Murphy, R.M. Retinol-Binding Protein Interferes with Transthyretin-Mediated beta-Amyloid Aggregation Inhibition. Biochemistry 2018, 57, 5029–5040. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Saraiva, M.J. Transthyretin: A multifaceted protein. Biomol. Concepts 2014, 5, 45–54. [Google Scholar] [CrossRef]
- Sekijima, Y.; Nakamura, K. Hereditary Transthyretin Amyloidosis. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2021. [Google Scholar]
- Chander, M.J.; Dalal, J.; Chopra, V.K.; Narasimhan, C.; Kerkar, P.; Oomman, A.; Fcsi, S.R.; Sharma, A.R.; Dougall, P.; Simon, S.; et al. Suspecting and diagnosing transthyretin amyloid cardiomyopathy (ATTR-CM) in India: An Indian expert consensus. Indian Heart J. 2022, 74, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, D.; Bonfioli, G.B.; Aimo, A.; Adamo, M.; Canepa, M.; Inciardi, R.M.; Lombardi, C.M.; Nardi, M.; Pagnesi, M.; Riccardi, M.; et al. Treating amyloid transthyretin cardiomyopathy: Lessons learned from clinical trials. Front. Cardiovasc. Med. 2023, 10, 1154594. [Google Scholar] [CrossRef] [PubMed]
- Tahara, N.; Lairez, O.; Endo, J.; Okada, A.; Ueda, M.; Ishii, T.; Kitano, Y.; Lee, H.E.; Russo, E.; Kubo, T. 99mTechnetium-pyrophosphate scintigraphy: A practical guide for early diagnosis of transthyretin amyloid cardiomyopathy. ESC Heart Fail. 2022, 9, 251–262. [Google Scholar] [CrossRef]
- Nativi-Nicolau, J.; Judge, D.P.; Hoffman, J.E.; Gundapaneni, B.; Keohane, D.; Sultan, M.B.; Grogan, M. Natural history and progression of transthyretin amyloid cardiomyopathy: Insights from ATTR-ACT. ESC Heart Fail. 2021, 8, 3875–3884. [Google Scholar] [CrossRef] [PubMed]
- Hendren, N.S.; De Lemos, J.A.; Berry, J.D.; Kozlitina, J.; Saelices, L.; Ji, A.X.; Shao, Z.; Liu, C.F.; Garg, S.; Farr, M.A.; et al. Circulating transthyretin and retinol binding protein 4 levels among middle-age V122I TTR carriers in the general population. Amyloid 2024, 31, 124–131. [Google Scholar] [CrossRef]
- Klaassen, S.H.C.; Lemmink, H.H.; Bijzet, J.; Glaudemans, A.W.J.M.; Bos, R.; Plattel, W.; van den Berg, M.P.; Slart, R.H.J.A.; Nienhuis, H.L.A.; van Veldhuisen, D.J.; et al. Late onset cardiomyopathy as presenting sign of ATTR A45G amyloidosis caused by a novel TTR mutation (p.A65G). Cardiovasc. Pathol. 2017, 29, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Thimm, A.; Oubari, S.; Hoffmann, J.; Carpinteiro, A.; Papathanasiou, M.; Luedike, P.; Kessler, L.; Rischpler, C.; Röcken, C.; Diebold, I.; et al. A novel TTR mutation (p.Ala65Val) underlying late-onset hereditary transthyretin (ATTRv) amyloidosis with mixed cardiac and neuropathic phenotype: A case report. BMC Neurol. 2022, 22, 469. [Google Scholar] [CrossRef]
- Valastyan, J.S.; Lindquist, S. Mechanisms of protein-folding diseases at a glance. Dis. Model. Mech. 2014, 7, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lewkowicz, E.; Gursky, O. Dynamic protein structures in normal function and pathologic misfolding in systemic amyloidosis. Biophys. Chem. 2022, 280, 106699. [Google Scholar] [CrossRef] [PubMed]
- Merlini, G.; Seldin, D.C.; Gertz, M.A. Amyloidosis: Pathogenesis and new therapeutic options. J. Clin. Oncol. 2011, 29, 1924–1933. [Google Scholar] [CrossRef]
- Subedi, S.; Sasidharan, S.; Nag, N.; Saudagar, P.; Tripathi, T. Amyloid Cross-Seeding: Mechanism, Implication, and Inhibition. Molecules 2022, 27, 1776. [Google Scholar] [CrossRef] [PubMed]
- Westermark, G.T.; Fändrich, M.; Westermark, P. AA amyloidosis: Pathogenesis and targeted therapy. Annu. Rev. Pathol. 2015, 10, 321–344. [Google Scholar] [CrossRef] [PubMed]
- D’Aguanno, V.; Ralli, M.; Artico, M.; Russo, F.Y.; Scarpa, A.; Fiore, M.; Tirassa, P.; Severini, C.; de Vincentiis, M.; Greco, A. Systemic Amyloidosis: A Contemporary Overview. Clin. Rev. Allergy Immunol. 2020, 59, 304–322. [Google Scholar] [CrossRef] [PubMed]
- Limbocker, R.; Cremades, N.; Cascella, R.; Tessier, P.M.; Vendruscolo, M.; Chiti, F. Characterization of Pairs of Toxic and Nontoxic Misfolded Protein Oligomers Elucidates the Structural Determinants of Oligomer Toxicity in Protein Misfolding Diseases. Acc. Chem. Res. 2023, 56, 1395–1405. [Google Scholar] [CrossRef]
- Rinauro, D.J.; Chiti, F.; Vendruscolo, M.; Limbocker, R. Misfolded protein oligomers: Mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases. Mol. Neurodegener. 2024, 19, 20. [Google Scholar] [CrossRef]
- Voulgarelis, M.; Mitroulis, I.; Tzioufas, A.G. Amyloidosis; Spinger: Berlin/Heidelberg, Germany, 2019; pp. 297–311. [Google Scholar]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 1 of 2—Evidence Base and Standardized Methods of Imaging. Circ. Cardiovasc. Imaging 2021, 14, e000029. [Google Scholar] [PubMed]
- Khor, Y.M.; Cuddy, S.; Falk, R.H.; Dorbala, S. Multimodality Imaging in the Evaluation and Management of Cardiac Amyloidosis. Semin. Nucl. Med. 2020, 50, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; Cuddy, S.; Falk, R.H. How to Image Cardiac Amyloidosis: A Practical Approach. JACC Cardiovasc. Imaging 2020, 13, 1368–1383. [Google Scholar] [CrossRef]
- Emdin, M.; Vergaro, G.; Aimo, A.; Fontana, M. Cardiac Amyloidosis: Diagnosis and Treatment; Springer Nature: Cham, Switzerland, 2024. [Google Scholar]
- Elgazzar, A.H. (Ed.) The Pathophysiologic Basis of Nuclear Medicine; Springer International Publishing: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Paeng, J.C.; Choi, J.Y. Nuclear Imaging for Cardiac Amyloidosis: Bone Scan, SPECT/CT, and Amyloid-Targeting PET. Nucl. Med. Mol. Imaging 2021, 55, 61–70. [Google Scholar] [CrossRef]
- Stan, C.; Mititelu, R.; Adam, R.D.; Jurcuţ, R. Awareness of Nuclear Medicine Physicians in Romania Regarding the Diagnostic of Cardiac Amyloidosis-A Survey-Based Study. Diagnostics 2022, 12, 556. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Strobel, K.; Kampen, W.U.; Kuwert, T.; van der Bruggen, W.; Mohan, H.K.; Gnanasegaran, G.; Delgado-Bolton, R.; Weber, W.A.; Beheshti, M.; et al. The EANM practice guidelines for bone scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1723–1738. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, R.M.; Kansal, P.; Holly, T.A. Cases from a busy nuclear cardiology laboratory: Potential pitfalls in the interpretation of cardiac scintigraphy for ATTR cardiac amyloidosis. J. Nucl. Cardiol. 2021, 28, 653–660. [Google Scholar] [CrossRef]
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Bacchi-Reggiani, L.; et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99m Tc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, S.; Cerqueira, M.D. Tc-99m-PYP imaging for cardiac amyloidosis: Defining the best protocol before the flood gates burst. J. Nucl. Cardiol. 2020, 27, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 14, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Scully, P.R.; Patel, K.P.; Treibel, T.A.; Thornton, G.D.; Hughes, R.K.; Chadalavada, S.; Katsoulis, M.; Hartman, N.; Fontana, M.; Pugliese, F.; et al. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. Eur. Heart J. 2020, 41, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Munteanu, A.; Mititelu, R.; Onciul, S.; Deleanu, D.; Iliescu, V.A.; Popescu, B.A.; Jurcut, R. Severe Aortic Stenosis and ATTRwt Amyloidosis—Beware in the Aging: A Case Report and Review of the Literature. Clin. Interv. Aging 2020, 15, 1863–1872. [Google Scholar] [CrossRef]
- Cuddy, S.; Dorbala, S.; Di Carli, M.F. Imaging of cardiac amyloidosis: Will this become a unique application for dual-isotope imaging? J. Nucl. Cardiol. 2020, 27, 38–40. [Google Scholar] [CrossRef]
- Asif, T.; Gomez, J.; Singh, V.; Doukky, R.; Nedeltcheva, A.; Malhotra, S. Comparison of planar with tomographic pyrophosphate scintigraphy for transthyretin cardiac amyloidosis: Perils and pitfalls. J. Nucl. Cardiol. 2021, 28, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.U.; Hawkins, P.N.; Cappelli, F.; Perfetto, F.; Zampieri, M.; Argiro, A.; Petrie, A.; Law, S.; Porcari, A.; Razvi, Y.; et al. Tc-99m labelled bone scintigraphy in suspected cardiac amyloidosis. Eur. Heart J. 2023, 44, 2187–2198. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Nebhwani, M.; Chaibekava, K.; Achten, A.; Oerlemans, M.I.F.J.; Michels, M.; van der Meer, P.; Nienhuis, H.L.A.; Weerts, J.; van Empel, V.; Rocca, H.B.; et al. Detection of cardiac amyloidosis on routine bone scintigraphy: An important gatekeeper role for the nuclear medicine physician. Int. J. Cardiovasc. Imaging 2024, 40, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.A.; Peiró-Aventin, B.; Biagioni, G.; Tini, G.; Saturi, G.; Kronberger, C.; Achten, A.; Dobner, S.; Te Rijdt, W.P.; Gasperetti, A.; et al. Evaluation of the 2021 ESC recommendations for family screening in hereditary transthyretin cardiac amyloidosis. Eur. J. Heart Fail. 2024, 26, 2025–2034. [Google Scholar] [PubMed]
- Ionescu, T.M.; Ciocoiu, M.; Lupușoru, R.V.; Grierosu, I.; Sascău, R.A.; Jalloul, W.; Iacob, R.; Stolniceanu, C.R.; Clement, A.; Stătescu, A.M.; et al. Role of Diphosphonates Bone Scintigraphy in Correlation with Biomarkers for a Personalized Approach to ATTR Cardiac Amyloidosis in North-Eastern Romania. Diagnostics 2022, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Shiri, I.; Balzer, S.; Baj, G.; Bernhard, B.; Hundertmark, M.; Bakula, A.; Nakase, M.; Tomii, D.; Barbati, G.; Dobner, S.; et al. Multi-modality artificial intelligence-based transthyretin amyloid cardiomyopathy detection in patients with severe aortic stenosis. Eur. J. Nucl. Med. Mol. Imaging 2024. [Google Scholar] [CrossRef]
- Navarro-Saez, M.D.C.; Feijoo-Massó, C.; Berenguer Sánchez, A.; Parra Parente, T.; Guillamon Toran, L.; Marcano-Fernández, F.; Camara-Cabrera, J.; Bravo Ferrer, Z.D.C.; Comet Monte, R.; Calvet Calvo, X. Early Diagnosis of Amyloidosis and Cardiac Involvement through Carpal Tunnel Surgery and Predictive Factors. J. Clin. Med. 2024, 13, 4328. [Google Scholar] [CrossRef]
- Castaño, A.; DeLuca, A.; Weinberg, R.; Pozniakoff, T.; Blaner, W.S.; Pirmohamed, A.; Bettencourt, B.; Gollob, J.; Karsten, V.; Vest, J.A.; et al. Serial scanning with technetium pyrophosphate (99mTc-PYP) in advanced ATTR cardiac amyloidosis. J. Nucl. Cardiol. 2016, 23, 1355–1363. [Google Scholar] [CrossRef]
- Papathanasiou, M.; Kessler, L.; Bengel, F.M.; Jakstaite, A.M.; Kersting, D.; Varasteh, Z.; Luedike, P.; Carpinteiro, A.; Herrmann, K.; Rassaf, T.; et al. Regression of Myocardial 99mTc-DPD Uptake After Tafamidis Treatment of Cardiac Transthyretin Amyloidosis. J. Nucl. Med. 2023, 64, 1083–1086. [Google Scholar] [CrossRef]
- Bokhari, S.; Shahzad, R.; Castaño, A.; Maurer, M.S. Nuclear imaging modalities for cardiac amyloidosis. J. Nucl. Cardiol. 2014, 21, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Suh, H.Y.; Park, S.; Oh, S.; Kwak, S.G.; Kim, H.M.; Koh, Y.; Park, J.B.; Kim, H.K.; Cho, H.J.; et al. Compound Positron Emission Tomography in Patients with AL Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2020, 75, 380–390. [Google Scholar] [CrossRef]
- Rosengren, S.; Skibsted Clemmensen, T.; Tolbod, L.; Granstam, S.O.; Eiskjær, H.; Wikström, G.; Vedin, O.; Kero, T.; Lubberink, M.; Harms, H.J.; et al. Diagnostic Accuracy of [11C]PIB Positron Emission Tomography for Detection of Cardiac Amyloidosis. J. Am. Coll. Cardiol. Img. 2020, 13, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Uppal, D.; Wang, Y.C.; Xu, X.; Kokkinidis, D.G.; Travin, M.I.; Tauras, J.M. Nuclear Imaging for the Diagnosis of Cardiac Amyloidosis in 2021. Diagnostics 2021, 11, 996. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ha, S.; Kim, Y.I. M Cardiac amyloidosis imaging with amyloid positron emission tomography: A systematic review and meta-analysis. J. Nucl. Cardiol. 2020, 27, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Manwani, R.; Page, J.; Lane, T.; Burniston, M.; Skillen, A.; Lachmann, H.J.; Gillmore, J.D.; Fontana, M.; Whelan, C.; Hawkins, P.N.; et al. A pilot study demonstrating cardiac uptake with 18F-florbetapir PET in AL amyloidosis patients with cardiac involvement. J. Protein Fold. Disord. 2018, 25, 247–252. [Google Scholar] [CrossRef]
- Law, P.; Wang, W.Y.; Moore, P.T.; Mollee, P.N.; Ng, A.C. Cardiac Amyloid Imaging with 18F-Florbetaben PET: A Pilot Study. J. Nucl. Med. 2016, 57, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Bukhari, S.; Eisele, Y.S.; Soman, P. Molecular Imaging of Cardiac Amyloidosis. J. Nucl. Med. 2020, 61, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Abulizi, M.; Cottereau, A.S.; Guellich, A.; Vandeventer, S.; Galat, A.; Van Der Gucht, A.; Plante-Bordeneuve, V.; Dubois-Randé, J.L.; Bodez, D.; Rosso, J.; et al. Early-phase myocardial uptake intensity of 99mTc-HMDP vs 99mTc-DPD in patients with hereditary transthyretin-related cardiac amyloidosis. J. Nucl. Cardiol. 2018, 25, 217–222. [Google Scholar] [CrossRef]
- Zamecnik, P.C.; Stephenson, M.L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc. Natl. Acad. Sci. USA 1978, 75, 280–284. [Google Scholar] [CrossRef]
- Friedrich, M.; Aigner, A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs 2022, 36, 549–571. [Google Scholar] [CrossRef]
- Hammond, S.M.; Aartsma-Rus, A.; Alves, S.; Borgos, S.E.; Buijsen, R.A.M.; Collin, R.W.J.; Covello, G.; Denti, M.A.; Desviat, L.R.; Echevarría, L.; et al. Delivery of oligonucleotide-based therapeutics: Challenges and opportunities. EMBO Mol. Med. 2021, 13, e13243. [Google Scholar] [CrossRef] [PubMed]
- Minamisawa, M.; Claggett, B.; Adams, D.; Kristen, A.V.; Merlini, G.; Slama, M.S.; Dispenzieri, A.; Shah, A.M.; Falk, R.H.; Karsten, V.; et al. Association of Patisiran, an RNA Interference Therapeutic, with Regional Left Ventricular Myocardial Strain in Hereditary Transthyretin Amyloidosis: The APOLLO Study. JAMA Cardiol. 2019, 4, 466–472. [Google Scholar] [CrossRef]
- Tingen, H.S.A.; Tubben, A.; Bijzet, J.; van den Berg, M.P.; van der Meer, P.; Houwerzijl, E.J.; Muntinghe, F.L.H.; van der Zwaag, P.A.; Glaudemans, A.W.J.M.; Oerlemans, M.I.F.J.; et al. Cardiac [99mTc]Tc-hydroxydiphosphonate uptake on bone scintigraphy in patients with hereditary transthyretin amyloidosis: An early follow-up marker? Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Kale, P.; Fontana, M.; Berk, J.L.; Grogan, M.; Gustafsson, F.; Hung, R.R.; Gottlieb, R.L.; Damy, T.; González-Duarte, A.; et al. Patisiran Treatment in Patients with Transthyretin Cardiac Amyloidosis. N. Engl. J. Med. 2023, 389, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Kale, P.; Maurer, M.S.; Fontana, M.; Grogan, M.; Fernandes, F.; Palecek, T.; Taylor, M.; Hung, A.R.R.; González-Duarte, A.; Poulsenet, S.; et al. Exploratory abalyses from the AOLLO-B, a phase 3 study of patisiran in patients with ATTR amyloidosis with cardiomyopathy. In Proceedings of the Heart Failure Society of America (HFSA) Annual Scientific Meeting, Washington, DC, USA, 30 September–3 October 2022. [Google Scholar]
- Judge, D.P.; Kristen, A.V.; Grogan, M.; Maurer, M.S.; Falk, R.H.; Hanna, M.; Gillmore, J.; Garg, P.; Vaishnaw, A.K.; Harrop, J.; et al. Phase 3 Multicenter Study of Revusiran in Patients with Hereditary Transthyretin-Mediated (hATTR) Amyloidosis with Cardiomyopathy (ENDEAVOUR). Cardiovasc. Drugs Ther. 2020, 34, 357–370. [Google Scholar] [CrossRef]
- Adams, D.; Tournev, I.L.; Taylor, M.S.; Coelho, T.; Planté-Bordeneuve, V.; Berk, J.L.; González-Duarte, A.; Gillmore, J.D.; Low, S.C.; Sekijima, Y.; et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: A randomized clinical trial. Amyloid 2023, 30, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Gillmore, J.D.; Kale, P.; Berk, J.L.; Maurer, M.S.; Conceição, I.; DiCarli, M.; Solomon, S.; Chen, C.; Arum, S.; et al. HELIOS-A: 18-Month Exploratory Cardiac Results from the Phase 3 Study of Vutrisiran in Patients with Hereditary Transthyretin-Mediated Amyloidosis. Eur. Heart J. Suppl. 2022, 24 (Suppl. K), suac121.654. [Google Scholar] [CrossRef]
- Fontana, M.; Berk, J.; Gillmore, J.D.; Witteles, R.M.; Grogan, M.; Drachman, B.; Damy, T.; Garcia-Pavia, P.; Taubel, J.; Solomon, S.D.; et al. Vutrisiran in Patients with Transthyretin Amyloidosis with Cardiomyopathy. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Planté-Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L.; et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tanowitz, M.; Hettrick, L.; Revenko, A.; Kinberger, G.A.; Prakash, T.P.; Seth, P.P. Asialoglycoprotein receptor 1 mediates productive uptake of N-acetylgalactosamine-conjugated and unconjugated phosphorothioate antisense oligonucleotides into liver hepatocytes. Nucleic Acids Res. 2017, 45, 12388–12400. [Google Scholar] [CrossRef]
- Yu, A.L.; Chen, Y.C.; Tsai, C.H.; Wu, Y.A.; Su, M.Y.; Chou, C.H.; Shun, C.T.; Hsueh, H.W.; Juang, J.J.; Lee, M.J.; et al. Use of Technetium-99m-Pyrophosphate Single-Photon Emission Computed Tomography/Computed Tomography in Monitoring Therapeutic Changes of Eplontersen in Patients with Hereditary Transthyretin Amyloid Cardiomyopathy. J. Am. Heart Assoc. 2024, 13, e030512. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, F.V.; Link, W.; Ferreira, B.I. CRISPR/Cas9-mediated genome editing: From basic research to translational medicine. J. Cell Mol. Med. 2020, 24, 3766–3778. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Rettl, R.; Wollenweber, T.; Duca, F.; Binder, C.; Cherouny, B.; Dachs, T.M.; Camuz Ligios, L.; Schrutka, L.; Dalos, D.; Beitzke, D.; et al. Monitoring tafamidis treatment with quantitative SPECT/CT in transthyretin amyloid cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Tateishi, E.; Morita, Y.; Ohta-Ogo, K.; Izumi, C. Serial 99mTechnetium Pyrophosphate Scintigraphy and Multimodality Assessments After Different Doses of Tafamidis for Variant Transthyretin Cardiac Amyloidosis. Circ. Heart Fail. 2023, 16, e009595. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Fine, N.; Garcia-Pavia, P.; Klein, A.L.; Fernandes, F.; Weissman, N.J.; Maurer, M.S.; Boman, K.; Gundapaneni, B.; Sultan, M.B.; et al. Effect of Tafamidis on Cardiac Function in Patients with Transthyretin Amyloid Cardiomyopathy: A Post Hoc Analysis of the ATTR-ACT Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, G.; Pipilas, A.; Mussinelli, R.; Gopal, D.M.; Berk, J.L.; Connors, L.H.; Vellanki, N.; Hellawell, J.; Siddiqi, O.K.; Fox, J.; et al. Stabilization of Cardiac Function with Diflunisal in Transthyretin (ATTR) Cardiac Amyloidosis. J. Card. Fail. 2020, 26, 753–759. [Google Scholar] [CrossRef]
- Rosenblum, H.; Castano, A.; Alvarez, J.; Goldsmith, J.; Helmke, S.; Maurer, M.S. TTR (Transthyretin) Stabilizers Are Associated with Improved Survival in Patients with TTR Cardiac Amyloidosis. Circ. Heart Fail. 2018, 11, e004769. [Google Scholar] [CrossRef]
- Koyama, J.; Minamisawa, M.; Sekijima, Y.; Ikeda, S.I.; Kozuka, A.; Ebisawa, S.; Miura, T.; Motoki, H.; Okada, A.; Izawa, A.; et al. Left ventricular deformation and torsion assessed by speckle-tracking echocardiography in patients with mutated transthyretin-associated cardiac amyloidosis and the effect of diflunisal on myocardial function. Int. J. Cardiol. Heart Vasc. 2015, 9, 1–10. [Google Scholar] [CrossRef][Green Version]
- Fox, J.C.; Hellawell, J.L.; Rao, S.; O’Reilly, T.; Lumpkin, R.; Jernelius, J.; Gretler, D.; Sinha, U. First-in-Human Study of AG10, a Novel, Oral, Specific, Selective, and Potent Transthyretin Stabilizer for the Treatment of Transthyretin Amyloidosis: A Phase 1 Safety, Tolerability, Pharmacokinetic, and Pharmacodynamic Study in Healthy Adult Volunteers. Clin. Pharmacol. Drug Dev. 2020, 9, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Judge, D.P.; Cappelli, F.; Fontana, M.; Garcia-Pavia, P.; Gibbs, S.; Grogan, M.; Hanna, M.; Hoffman, J.; Masri, A. Efficacy and Safety of Acoramidis in Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2024, 390, 132–142. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Aus dem Siepen, F.; Donal, E.; Lairez, O.; van der Meer, P.; Kristen, A.V.; Mercuri, M.F.; Michalon, A.; Frost, R.J.A.; Grimm, J.; et al. Phase 1 Trial of Antibody NI006 for Depletion of Cardiac Transthyretin Amyloid. N. Engl. J. Med. 2023, 389, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.; Klein, M.; Guthrie, S.; Foster, J.S.; Williams, A.; Richey, T.; Balachandran, M.; Jackson, J.; Hancock, T.J.; Stuckey, A.; et al. The Peptide Fusion Immunoglobulin, AT-02, Exhibits Highly Potent Pan-Amyloid Reactivity And Immunomodulation. J. Card. Fail. 2024, 30, 210. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanciu, S.M.; Jurcut, R.; Dragoi Galrinho, R.; Stefani, C.; Miricescu, D.; Rusu, I.R.; Prisacariu, G.S.; Mititelu, R. From Molecular to Radionuclide and Pharmacological Aspects in Transthyretin Cardiac Amyloidosis. Int. J. Mol. Sci. 2025, 26, 146. https://doi.org/10.3390/ijms26010146
Stanciu SM, Jurcut R, Dragoi Galrinho R, Stefani C, Miricescu D, Rusu IR, Prisacariu GS, Mititelu R. From Molecular to Radionuclide and Pharmacological Aspects in Transthyretin Cardiac Amyloidosis. International Journal of Molecular Sciences. 2025; 26(1):146. https://doi.org/10.3390/ijms26010146
Chicago/Turabian StyleStanciu, Silviu Marcel, Ruxandra Jurcut, Ruxandra Dragoi Galrinho, Constantin Stefani, Daniela Miricescu, Ioana Ruxandra Rusu, Georgiana Sabina Prisacariu, and Raluca Mititelu. 2025. "From Molecular to Radionuclide and Pharmacological Aspects in Transthyretin Cardiac Amyloidosis" International Journal of Molecular Sciences 26, no. 1: 146. https://doi.org/10.3390/ijms26010146
APA StyleStanciu, S. M., Jurcut, R., Dragoi Galrinho, R., Stefani, C., Miricescu, D., Rusu, I. R., Prisacariu, G. S., & Mititelu, R. (2025). From Molecular to Radionuclide and Pharmacological Aspects in Transthyretin Cardiac Amyloidosis. International Journal of Molecular Sciences, 26(1), 146. https://doi.org/10.3390/ijms26010146






