Combination of sST2/LVMI Ratio and Modified MELD Scores Predicts Mortality in End-Stage Heart Failure
Abstract
1. Introduction
2. Results

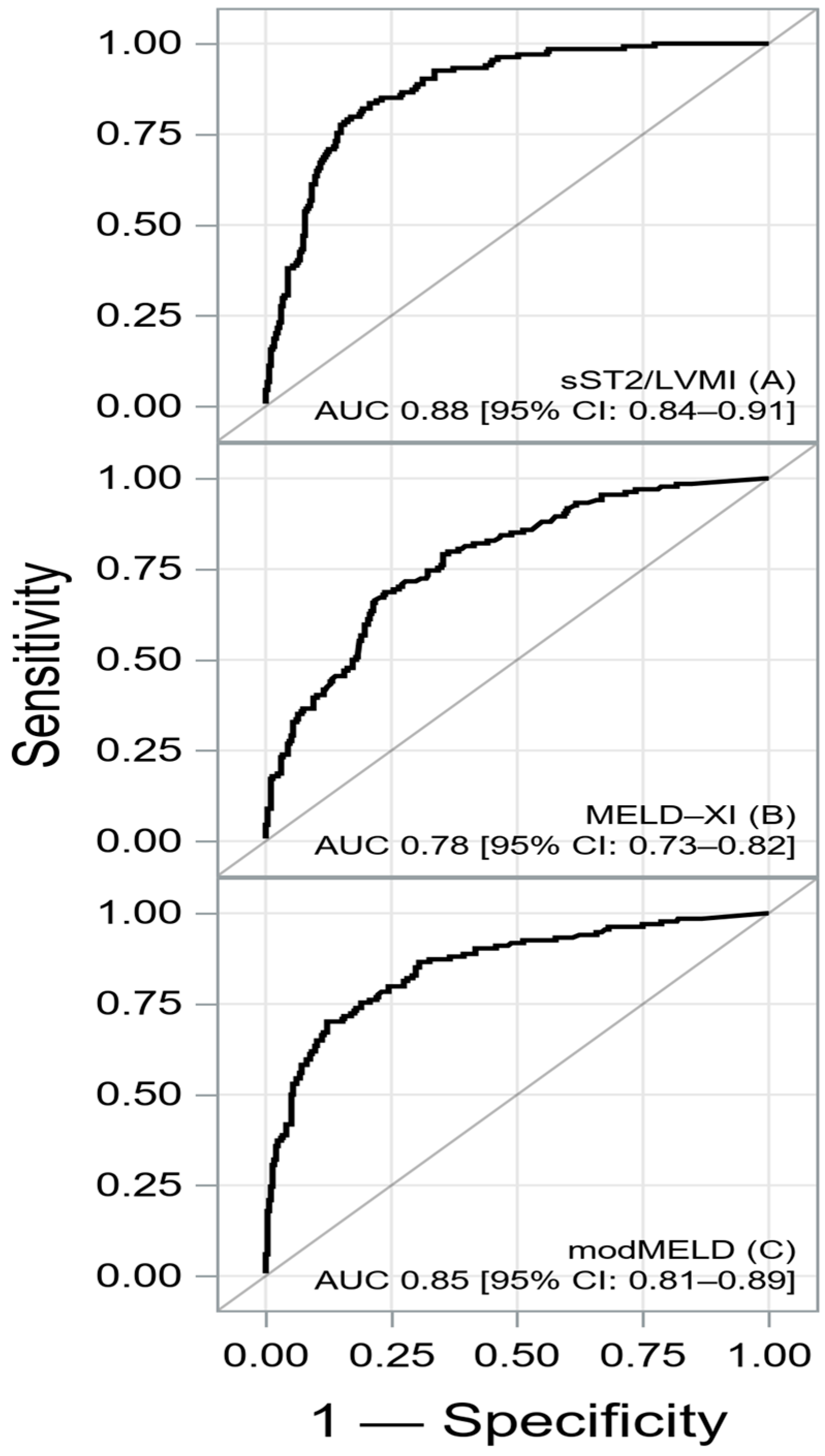
| AUC [±95 CI] | Cut-Off | Sensitivity [±95 CI] | Specificity [±95 CI] | Accuracy | |
|---|---|---|---|---|---|
| sST2/LVMI | 0.88 [0.84–0.91] | ≥0.306 | 0.84 [0.76–0.89] | 0.79 [0.74–0.84] | 0.81 [0.77–0.84] |
| MELD-XI | 0.78 [0.73–0.83] | ≥13.96 | 0.69 [0.60–0.76] | 0.76 [0.71–0.81] | 0.74 [0.69–0.78] |
| ModMELD | 0.85 [0.81–0.89] | ≥12.55 | 0.70 [0.62–0.78] | 0.88 [0.84–0.91] | 0.82 [0.78–0.86] |
| sST2/LVMI-MELDXI | 0.90 [0.87–0.93] | ≥5.07 | 0.80 [0.72–0.86] | 0.85 [0.80–0.89] | 0.83 [0.79–0.87] |
| sST2/LVMI-modMELD | 0.92 [0.89–0.95] | ≥4.04 | 0.81 [0.74–0.88] | 0.92 [0.88–0.94] | 0.88 [0.85–0.91] |
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Study Population
4.2. Echocardiography
4.3. Analyzed Biomarkers and Scores
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Nessler, J.; Rostoff, P. Prognostic scores in advanced heart failure: Where are we now and where are we going? Pol. Arch. Intern. Med. 2017, 127, 235–237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferrero, P.; Iacovoni, A.; D’Elia, E.; Vaduganathan, M.; Gavazzi, A.; Senni, M. Prognostic scores in heart failure—Critical appraisal and practical use. Int. J. Cardiol. 2015, 188, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, W.; Szyguła-Jurkiewicz, B.; Siedlecki, Ł.; Gąsior, M. Prognostic scales in advanced heart failure. Pol. J. Thorac. Cardiovasc. Surg. 2018, 15, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Sciatti, E.; Merlo, A.; Scangiuzzi, C.; Limonta, R.; Gori, M.; D’Elia, E.; Aimo, A.; Vergaro, G.; Emdin, M.; Senni, M. Prognostic Value of sST2 in Heart Failure. J. Clin. Med. 2023, 12, 3970. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, M.; Fu, M.; Cui, X.; Lian, Z.; Xin, H.; Zhou, J.; Ge, J. Increased ratio of sST2/LVMI predicted cardiovascular mortality and heart failure rehospitalization in heart failure with reduced ejection fraction patients: A prospective cohort study. BMC Cardiovasc. Disord. 2021, 21, 396. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Kato, T.S.; Shulman, B.P.; Takayama, H.; Farr, M.; Jorde, U.P.; Mancini, D.M.; Naka, Y.; Schulze, P.C. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: Use of the Model of End-stage Liver Disease (MELD) and MELD eXcluding INR (MELD-XI) scoring system. J. Heart Lung Transplant. 2012, 31, 601–610. [Google Scholar] [CrossRef]
- Szygula-Jurkiewicz, B.; Szczurek, W.; Skrzypek, M.; Zakliczyński, M.; Siedlecki, Ł.; Przybyłowski, P.; Zembala, M.; Gąsior, M. One-year survival of ambulatory patients with end-stage heart failure: The analysis of prognostic factors. Pol. Arch. Intern. Med. 2017, 127, 254–260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matilla, L.; Ibarrola, J.; Arrieta, V.; Garcia-Peña, A.; Martinez-Martinez, E.; Sádaba, R.; Alvarez, V.; Navarro, A.; Fernández-Celis, A.; Gainza, A.; et al. Soluble ST2 promotes oxidative stress and inflammation in cardiac fibroblasts: An in vitro and in vivo study in aortic stenosis. Clin. Sci. 2019, 133, 1537–1548. [Google Scholar] [CrossRef]
- McCarthy, C.P.; Januzzi, J.L., Jr. Soluble ST2 in Heart Failure. Heart Fail. Clin. 2018, 14, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Chien, K.L.; Hsu, H.C.; Su, T.C.; Chen, M.F.; Lee, Y.T. Left ventricular mass and risk of cardiovascular events and all-cause death among ethnic Chinese--the Chin-Shan Community Cardiovascular Cohort study. Int. J. Cardiol. 2011, 149, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, W.; Szyguła-Jurkiewicz, B.; Zakliczyński, M.; Król, B.; Gąsior, M.; Zembala, M. Prognostic utility of the N terminal prohormone of brain natriuretic peptide and the modified Model for End Stage Liver Disease in patients with end stage heart failure. Pol. Arch. Intern. Med. 2018, 128, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, W.; Gąsior, M.; Romuk, E.; Skrzypek, M.; Szyguła-Jurkiewicz, B. Usefulness of combining prognostic scores to predict survival in patients with advanced heart failure. J. Heart Lung Transplant. 2019, 38, 1224–1227. [Google Scholar] [CrossRef] [PubMed]
- Gotou, M.; Suzuki, A.; Shiga, T.; Kikuchi, N.; Hagiwara, N. Implication of modified MELD scores for postdischarge prognosis in hospitalized patients with heart failure. Heart Vessels 2023, 38, 535–542. [Google Scholar] [CrossRef]
- Kim, M.S.; Kato, T.S.; Farr, M.; Wu, C.; Givens, R.C.; Collado, E.; Mancini, D.M.; Schulze, P.C. Hepatic dysfunction in ambulatory patients with heart failure: Application of the MELD scoring system for outcome prediction. J. Am. Coll. Cardiol. 2013, 61, 2253–2261. [Google Scholar] [CrossRef]
- Schefold, J.C.; Filippatos, G.; Hasenfuss, G.; Anker, S.D.; von Haehling, S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat. Rev. Nephrol. 2016, 12, 610–623. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, C.; Zhang, Q.; Hu, Z.; Ji, K.; Qian, L. Association between fibrinogen-to-albumin ratio and prognosis of patients with heart failure. Eur. J. Clin. Investig. 2023, 53, e14049. [Google Scholar] [CrossRef]
- Anjum, F.; Gilani, M.; Latif, M.; Sattar, A.; Ashraf, H.; Rafaqat, S. The Role of Coagulation in Heart Failure: A Literature Review. Curr. Heart Fail. Rep. 2024, 21, 277–291. [Google Scholar] [CrossRef]
- Jiang, P.; Gao, Z.; Zhao, W.; Song, Y.; Tang, X.F.; Xu, J.J.; Wang, H.H.; Jiang, L.; Chen, J.; Qiao, S.B.; et al. Relationship between fibrinogen levels and cardiovascular events in patients receiving percutaneous coronary intervention: A large single-center study. Chin. Med. J. 2019, 132, 914–921. [Google Scholar] [CrossRef]
- Vilar, R.; Fish, R.J.; Casini, A.; Neerman-Arbez, M. Fibrin(ogen) in human disease: Both friend and foe. Haematologica 2020, 105, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Cugno, M.; Mari, D.; Meroni, P.L.; Gronda, E.; Vicari, F.; Frigerio, M.; Coppola, R.; Bottasso, B.; Borghi, M.O.; Gregorini, L. Haemostatic and inflammatory biomarkers in advanced chronic heart failure: Role of oral anticoagulants and successful heart transplantation. Br. J. Haematol. 2004, 126, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhao, Y.; He, Y. Fibrinogen Level Predicts Outcomes in Critically Ill Patients with Acute Exacerbation of Chronic Heart Failure. Dis. Markers 2021, 2021, 6639393. [Google Scholar] [CrossRef]
- Yang, S.; Pi, J.; Ma, W.; Gu, W.; Zhang, H.; Xu, A.; Liu, Y.; Shi, T.; Yang, F.; Chen, L. Prognostic value of the fibrinogen-to-albumin ratio (FAR) in patients with chronic heart failure across the different ejection fraction spectrum. Libyan J. Med. 2024, 19, 2309757. [Google Scholar] [CrossRef]
- Green, D.; Foiles, N.; Chan, C.; Schreiner, P.J.; Liu, K. Elevated fbrinogen levels and subsequent subclinical atherosclerosis: The CARDIA Study. Atherosclerosis 2009, 202, 623–631. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, H.; Zhu, M.; Huang, Y. The inverted U-shaped association between blood fibrinogen and rehospitalization risk in patients with heart failure. Sci. Rep. 2024, 14, 15060. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Hernandez, M.; Cheungpasitporn, W.; Kashani, K.B.; Riaz, I.; Rangaswami, J.; Herzog, E.; Guglin, M.; Krittanawong, C. Hyponatremia in Heart Failure: Pathogenesis and Management. Curr. Cardiol. Rev. 2019, 15, 252–261. [Google Scholar] [CrossRef]
- Omar, H.R.; Charnigo, R.; Guglin, M. Prognostic Significance of Discharge Hyponatremia in Heart Failure Patients with Normal Admission Sodium (from the ESCAPE Trial). Am. J. Cardiol. 2017, 120, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Rossi, J.S.; Cotts, W.; Shin, D.D.; Hellkamp, A.S.; Piña, I.L.; Fonarow, G.C.; DeMarco, T.; Pauly, D.F.; Rogers, J.; et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch. Intern. Med. 2007, 167, 1998–2005. [Google Scholar] [CrossRef]
- Nadar, S.K.; Shaikh, M.M. Biomarkers in Routine Heart Failure Clinical Care. Card. Fail. Rev. 2019, 5, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, F.; Packer, M.; Coats, A.J.; Fowler, M.B.; Krum, H.; Mohacsi, P.; Rouleau, J.L.; Tendera, M.; Castaigne, A.; Anker, S.D.; et al. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: A substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation 2004, 110, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Senni, M.; Lopez-Sendon, J.; Cohen-Solal, A.; Ponikowski, P.; Nkulikiyinka, R.; Freitas, C.; Vlajnic, V.M.; Roessig, L.; Pieske, B. Vericiguat and NT- -proBNP in patients with heart failure with reduced ejection fraction: Analyses from the VICTORIA trial. ESC Heart Fail. 2022, 9, 3791–3803. [Google Scholar] [CrossRef]
- Kim, H.N.; Januzzi, J.L., Jr. Natriuretic peptide testing in heart failure. Circulation 2011, 123, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- Doehner, W.; Anker, S.D.; Butler, J.; Zannad, F.; Filippatos, G.; Ferreira, J.P.; Salsali, A.; Kaempfer, C.; Brueckmann, M.; Pocock, S.J.; et al. Uric acid and sodium-glucose cotransporter-2 inhibition with empagliflozin in heart failure with reduced ejection fraction: The EMPEROR-reduced trial. Eur. Heart J. 2022, 43, 3435–3446. [Google Scholar] [CrossRef]
- Miao, L.; Guo, M.; Pan, D.; Chen, P.; Chen, Z.; Gao, J.; Yu, Y.; Shi, D.; Du, J. Serum Uric Acid and Risk of Chronic Heart Failure: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 785327. [Google Scholar] [CrossRef]
- Packer, M. Uric acid is a biomarker of oxidative stress in the failing heart: Lessons learned from trials with allopurinol and SGLT2 inhibitors. J. Card. Fail. 2020, 26, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Rosticci, M.; Parini, A.; Baronio, C.; D’Addato, S.; Borghi, C. Serum uric acid is inversely proportional to estimated stroke volume and cardiac output in a large sample of pharmacologically untreated subjects: Data from the brisighella heart study. Intern. Emerg. Med. 2014, 9, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Park, S.K.; Lee, I.K.; Johnson, R.J. Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 2005, 16, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Sharaf El Din, U.A.A.; Salem, M.M.; Abdulazim, D.O. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J. Adv. Res. 2017, 8, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Tanaka, A.; Node, K.; Kobayashi, Y. Uric acid and cardiovascular disease: A clinical review. J. Cardiol. 2021, 78, 51–57. [Google Scholar] [CrossRef]
- Doehner, W.; Landmesser, U. Xanthine oxidase and uric acid in cardiovascular disease: Clinical impact and therapeutic options. Semin. Nephrol. 2011, 31, 433–440. [Google Scholar] [CrossRef]
- Kumrić, M.; Borovac, J.A.; Kurir, T.T.; Božić, J. Clinical Implications of Uric Acid in Heart Failure: A Comprehensive Review. Life 2021, 11, 53. [Google Scholar] [CrossRef]
- Yu, M.A.; Sánchez-Lozada, L.G.; Johnson, R.J.; Kang, D.H. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J. Hypertens. 2010, 28, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef] [PubMed]
- Heuman, D.M.; Mihas, A.A.; Habib, A.; Gilles, H.S.; Stravitz, R.T.; Sanyal, A.J.; Fisher, R.A. MELD-XI: A rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transplant. 2007, 13, 30–37. [Google Scholar] [CrossRef]

| Overal n = 429 | Survival n = 295 | Nonsurvival n = 134 | p | |
|---|---|---|---|---|
| Age, years | 56. 0 (50.0–60.0) | 56 (50.0–60.0) | 55 (50.0–60.0) | 0.6835 |
| Male, n (%) | 376 (87.6) | 263 (89.2) | 113 (84.3) | 0.1593 |
| Ischemic etiology of HF (%) HF, n (%) | 280 (65.3) | 191 (64.7) | 89 (66.4) | 0.736 |
| BMI, kg/m2 | 25.9 (23.1–29.4) | 26.1 (23.0–29.7) | 25.6 (23.2–28.2) | 0.1094 |
| Hypertension, n (%) | 208 (48.5) | 146 (49.5) | 62 (46.3) | 0.5359 |
| AF, n (%) | 223 (52) | 155 (52.5) | 68 (50.7) | 0.73 |
| Type 2 diabetes, n (%) | 234 (54.5) | 152 (51.5) | 82 (61.2) | 0.0620 |
| WBC, ×109/L | 7.3 (6.1–8.7) | 7.3 (6.0–8.6) | 7.4 (6.2–8.7) | 0.4293 |
| Hemoglobin, mmol/L | 8.8 (8.2–9.6) | 8.8 (8.2–9.6) | 8.8 (8.1–9.7) | 0.902 |
| Uric acid, µmol/L | 441.0 (364.0–533.0) | 419.0 (343.0–498.0) | 502.0 (430.0–599.0) | <0.0001 * |
| Urea, µmol/L | 8.1 (6.0–12.2) | 7.7 (5.9–11.7) | 9.10 (6.3–12.8) | 0.0485 * |
| Creatinine, umol/L | 109.0 (95.0–127.0) | 103.0 (90.0–119.0) | 126.0 (108.0–138.0) | <0.0001 * |
| Sodium, mmol/L | 139.0 (136.0–140.0) | 139.0 (138.0–141.0) | 137.0 (135.0–139.0) | <0.0001 * |
| Total bilirubin, µmol/L | 18,7 (12.6–24.4) | 16.9 (11.7–22.8) | 22.9 (15.9–32.3) | <0.0001 * |
| Albumin, g/L | 42.0 (37.0–45.0) | 43.0 (41.0–46.0) | 37.0 (35.0–41.0) | <0.0001 * |
| ALP, U/L | 79.0 (61.0–102.0) | 77.0 (57.0–101.0) | 83.5 (65.0–109.0) | 0.0447 * |
| GGTP, U/L | 73.0 (35.0–124.0) | 62.0 (33.0–112.0) | 91.0 (48.0–144.0) | 0.0001 * |
| Fibrinogen, mg/dL | 380.0 (308.0–459.00) | 369.0 (292.0–441.0) | 412.5 (341.0–537.0) | <0.0001 * |
| hs-CRP, mg/L | 4.2 (1.8–7.0) | 3.0 (1.5–5.6) | 6.0 (3.8–8.5) | <0.0001 * |
| NT-proBNP, pg/mL | 4095.0 (1955.0–7080.0) | 3564.0 (1761.0–6682.0) | 5526.0 (2517.0–7645.0) | 0.0006 * |
| sST2, ng/mL | 43.2 (33.9–77.9) | 35.6 (29.8–45.4) | 89.3 (70.2–101.3) | <0.0001 * |
| VO2max, mL/kg/min | 11.1 (10.2–11.9) | 11.0 (10.1–11.8) | 11.2 (10.3–12.0) | 0.0618 |
| CI, L/min/m2 | 1.9 (1.7–2.0) | 1.9 (1.7–1.9) | 1.9 (1.7–2.1) | 0.7124 |
| LVEDd, mm | 74.0 (69.0–79.0) | 73.0 (68.0–78.0) | 75.5 (70.0–82.0) | 0.0014 * |
| IVSd, mm | 10.0 (9.00–11.0) | 10.0 (9.0–11.0) | 10.0 (9.0–11.0) | 0.2246 |
| PWTd, mm | 10.0 (9.0–11.0) | 10.0 (9.0–11.0) | 10.0 (9.0–11.0) | 0.2405 |
| LVEF, % | 18.0 (15.0–20.0) | 18.0 (15.0–21.0) | 18.0 (15.0–20.0) | 0.1898 |
| LVMI, g/m2 | 176.7 (151.3–205.9) | 174.0 (149.9–199.2) | 189.5 (160.5–226.5) | 0.0002 * |
| Therapy on admission, n (%) | ||||
| ACEI/ARB, n (%) | 398 (92.8) | 272 (92.2) | 126 (94) | 0.4983 |
| B-blockers, n (%) | 400 (93.2) | 273 (92.5) | 127 (94.8) | 0.3931 |
| Loop diuretics per os, n (%) | 429 (100) | 295 (100.0) | 134 (100.0) | 1.00 |
| MRA, n (%) | 406 (94.6) | 282 (95.6) | 124 (92.5) | 0.1928 |
| Empagliflozin/dapagliflozin, n (%) | 223 (52) | 155 (52.5) | 68 (50.7) | 0.73 |
| ICD/CRT-D, n (%) | 429 (100) | 295 (100.0) | 134 (100.0) | 1.00 |
| Statins, n (%) | 307 (71.6) | 214 (72.5) | 93 (69.4) | 0.5041 |
| VKA/NOAC, n (%) | 223 (52) | 155 (52.5) | 68 (50.7) | 0.73 |
| Other parameters | ||||
| ModMELD | 10.2 (8.0–13.2) | 9.0 (7.5–11.1) | 14.2 (11.7–16.9) | <0.0001 * |
| MELD-XI | 12. 8 (10.9–15.1) | 12.0 (10.5–13.9) | 14.8 (13.3–17.1) | <0.0001 * |
| sST2/LVMI | 0.26 (0.19–0.40) | 0.22 (0.16–0.29) | 0.45 (0.34–0.58) | <0.0001 * |
| sST2/LVMI-MELD-XI | 4.5 (3.8–5.6) | 4.1 (3.6–4.7) | 5.9 (5.3–6.8) | <0.0001 * |
| sST2/LVMI-modMELD | 3.4 (2.7–4.5) | 2.9 (2.4–3.5) | 4.9 (4.2–5.7) | <0.0001 * |
| Univariable Data | Multivariable Data Model 1 | Multivariable Data Model 2 | ||||
|---|---|---|---|---|---|---|
| Parameter | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p |
| CRP (+) | 1.018 [1.001–1.035] | 0.0353 | ||||
| Fibrinogen (+) | 1.004 [1.003–1.005] | <0.001 | 1.002 [1.001–1.004] | <0.001 | 1.002 [1.000–1.003] | 0.0099 |
| ALP (+) | 1.005 [1.000–1.010] | 0.0472 | ||||
| GGTP (+) | 1.006 [1.003–1.009] | <0.001 | ||||
| Uric acid (+) | 1.003 [1.002–1.004] | <0.001 | 1.001 [1.000–1.002] | 0.0426 | 1.001 [1.000–1.002] | 0.0489 |
| Urea (+) | 1.023 [0.996–1.051] | 0.0980 | ||||
| NT-proBNP (a) | 1.006 [1.003–1.009] | <0.001 | 1.004 [1.004–1.007] | 0.0081 | 1.005 [1.002–1.008] | 0.0020 |
| Sodium (−) | 1.164 [1.103–1.229] | <0.001 | 1.065 [1.004–1.130] | 0.0360 | ||
| sST2/LVMI-MELD-XI (+) | 2.718 [2.369–3.118] | <0.001 | 2.501 [2.168–2.886] | <0.001 | ||
| sST2/LVMI-modMELD (+) | 2.718 [2.389–3.092] | <0.001 | 2.552 [2.224–2.928] | <0.0001 | ||
| sST2/LVMI-modMELD, AUC [±95 CI] 1 | p | |
| modMELD, AUC [±95 CI] | 0.0214 [0.00251–0.0402] | 0.0263 |
| sST2/LVMI, AUC [±95 CI] | 0.0862 [0.0540–0.1183] | 0.0001 |
| sST2/LVMI-MELD-XI, AUC [±95 CI] 1 | p | |
| MELD-XI, AUC [±95 CI] | 0.0486 [0.0213–0.0760] | 0.0005 |
| sST2/LVMI AUC [±95 CI] | 0.0456 [0.0157–0.0755] | 0.0028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczurek-Wasilewicz, W.; Jurkiewicz, M.; Skrzypek, M.; Romuk, E.; Jóźwiak, J.; Gąsior, M.; Szyguła-Jurkiewicz, B. Combination of sST2/LVMI Ratio and Modified MELD Scores Predicts Mortality in End-Stage Heart Failure. Int. J. Mol. Sci. 2025, 26, 171. https://doi.org/10.3390/ijms26010171
Szczurek-Wasilewicz W, Jurkiewicz M, Skrzypek M, Romuk E, Jóźwiak J, Gąsior M, Szyguła-Jurkiewicz B. Combination of sST2/LVMI Ratio and Modified MELD Scores Predicts Mortality in End-Stage Heart Failure. International Journal of Molecular Sciences. 2025; 26(1):171. https://doi.org/10.3390/ijms26010171
Chicago/Turabian StyleSzczurek-Wasilewicz, Wioletta, Michał Jurkiewicz, Michał Skrzypek, Ewa Romuk, Jacek Jóźwiak, Mariusz Gąsior, and Bożena Szyguła-Jurkiewicz. 2025. "Combination of sST2/LVMI Ratio and Modified MELD Scores Predicts Mortality in End-Stage Heart Failure" International Journal of Molecular Sciences 26, no. 1: 171. https://doi.org/10.3390/ijms26010171
APA StyleSzczurek-Wasilewicz, W., Jurkiewicz, M., Skrzypek, M., Romuk, E., Jóźwiak, J., Gąsior, M., & Szyguła-Jurkiewicz, B. (2025). Combination of sST2/LVMI Ratio and Modified MELD Scores Predicts Mortality in End-Stage Heart Failure. International Journal of Molecular Sciences, 26(1), 171. https://doi.org/10.3390/ijms26010171






