Cancer-Targeting Applications of Cell-Penetrating Peptides
Abstract
1. Introduction
2. Cell-Penetrating Peptides with Cancer-Targeting Applications
2.1. PEP-010: A Bifunctional Peptide Targeting Caspase-9/PP2A Interaction to Induce Apoptosis
2.2. ATX-101: A CPP Targeting Proliferating Cell Nuclear Antigen (PCNA) for Enhanced Cancer Therapy
2.3. AVB-620: A Novel Fluorescent Peptide Dye for In Vivo Malignant Tissue Visualization
2.4. Z12 and ZEBRA-Derived CPPs: Advancing Cancer Immunotherapy with Enhanced Vaccine Efficacy
2.5. pVEC: A Versatile Non-Endocytic CPP for Targeted Delivery of Therapeutic Biomolecules
2.6. Pep-1: Exploiting Membrane Composition for Enhanced Selectivity in Targeting Cancer Cells
2.7. MAP: Amplifying Cytotoxicity and Antiproliferative Effects in Cancer Therapy Through Drug Conjugation
2.8. p28: A Dual-Action CPP Targeting Wild-Type and Mutant p53 for Comprehensive Cancer Therapy
2.9. SAP and SAP(E): Precision Drug Delivery Platforms for Targeted Cancer Therapy with Minimal Toxicity
2.10. Bac1-24: A Multifunctional Platform for Targeted Macromolecular Therapies in Solid Tumors
2.11. BIM-SAHBA and SAHBD: Overcoming Apoptosis Resistance in Cancer via Targeted BH3- and MCL-1 Inhibition
2.12. ALRN-6924: A Stapled Peptide Restoring p53 Function for Targeted Cancer Therapy
2.13. P1pal-7: A Versatile Pepducin Targeting PAR1 for Cancer Therapies
2.14. EN1-iPeps: Homeodomain-Derived CPPs Targeting Oncogenic Transcription Factors for Selective Cancer Therapy
2.15. Vectocell®/DPVs: Innovative Peptide Vectors for Targeted Drug Delivery and Combatting Multidrug Resistance in Cancer
2.16. CPPecp: Targeting Tumor Cell Migration and Angiogenesis for Comprehensive Cancer Therapy
2.17. Melittin and Its Derivatives: Harnessing Venom-Derived Peptides for Targeted Cancer Therapy and Drug Delivery
2.18. Lycosin-I and R-Lycosin-I: Optimizing Venom-Derived Peptides for Enhanced Anticancer Efficacy
2.19. Pardaxins: Amphipathic Peptides Targeting Membrane Disruption and Mitochondrial Dysfunction in Cancer Therapy
2.20. BT1718: A Bicyclic Peptide Toxin Conjugate Targeting MT1-MMP for Precision Cancer Therapy
2.21. 177Lu-DOTA0-Tyr3-Octreotate: A Game-Changer in Peptide Receptor Radionuclide Therapy for Neuroendocrine Tumors


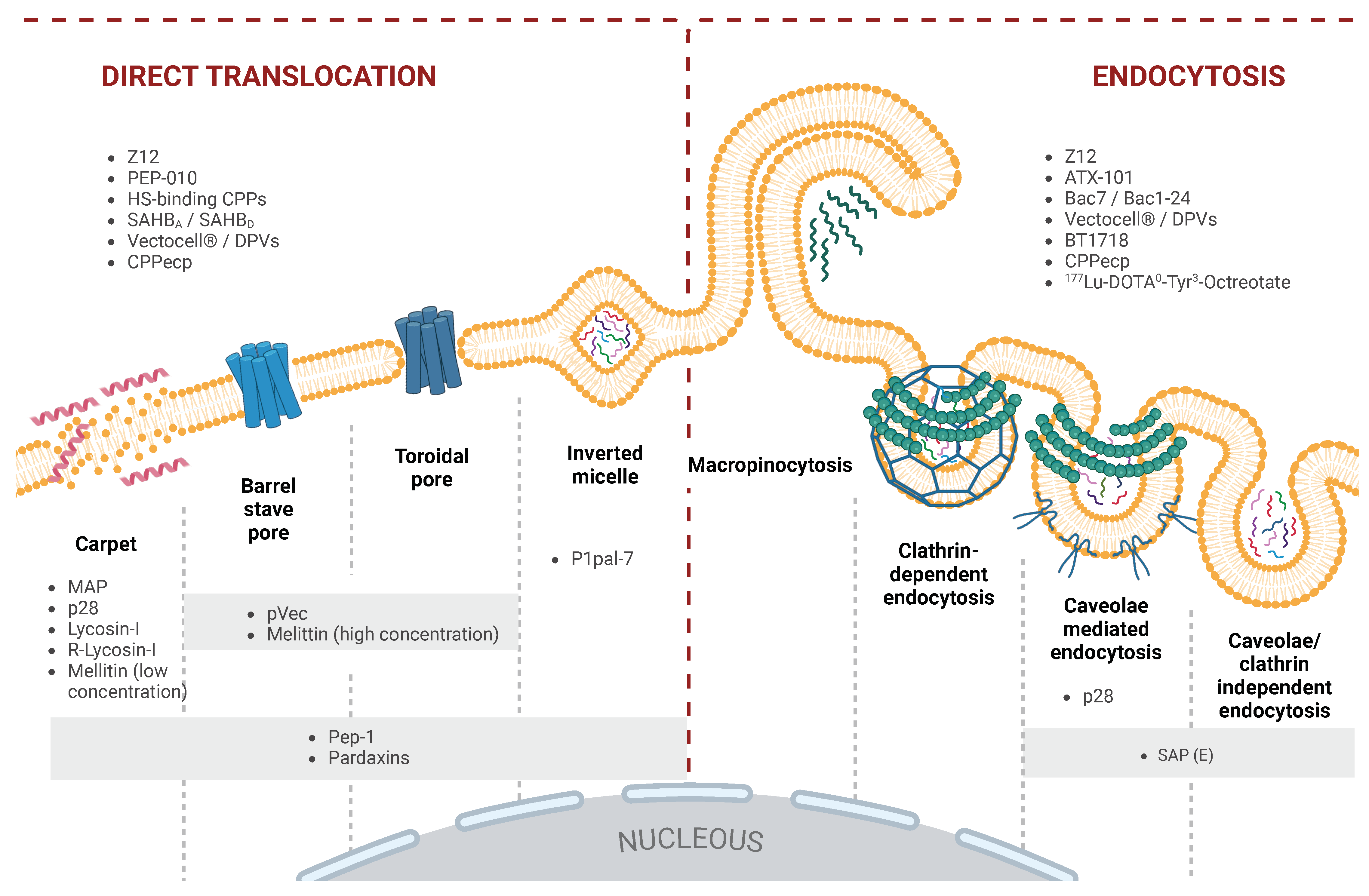
3. Overview of Cell Translocation Mechanisms of CPPs
3.1. Direct Translocation
3.1.1. Inverted Micelle Formation
3.1.2. Direct Translocation via Pore Formation
3.1.3. Carpet Model
3.2. Endocytosis as a Pathway for the Cellular Uptake of CPPs
3.2.1. Macropinocytosis
3.2.2. Clathrin-Mediated Endocytosis (CME)
3.2.3. Caveolae-Mediated Endocytosis (CvME)
3.2.4. Clathrin- and Caveolae-Independent Endocytosis
4. Discussion
4.1. Dual Role of CPPs as Therapeutic Agents and Precision Delivery Systems in Oncology
4.2. Structural Diversity and Mechanisms of Cellular Uptake
4.3. ADMET Challenges
4.4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADMET | Absortion, Distribution, Metabolism, Excretion and Toxicity |
| AML | Acute Myeloid Leukemia |
| AMP | Antimicrobial Peptide |
| BTC | Bicycle Toxin Conjugate |
| CME | Clathrin-Mediated Endocytosis |
| CPP | Cell-Penetrating Peptide |
| cCPP | Cationic Cell-Penetrating Peptide |
| CvME | Caveolae-Mediated Endocytosis |
| DPV | Diatos Peptide Vector |
| ELP | Elastin-Like Polypeptide |
| EN1 | Engrailed 1 |
| ER+ | Estrogen Receptor-Positive |
| FRET | Fluorescence Resonance Energy Transfer |
| GAG | Glycosaminoglican |
| GEP-NET | Gastroenteropancreatic Neuroendocrine Tumor |
| GPCR | G Protein-Coupled Receptor |
| HS | Heparan Sulfate |
| HTH | Helix-turn-helix |
| iPep | Interfering Peptide |
| MAP | Model Amphipathic Peptide |
| MDR | Multidrug Resistance |
| MMP | Matrix Metalloproteinase |
| MT1-MMP | Membrane Type 1 Matrix Metalloproteinase |
| NET | Neuroendocrine Tumor |
| OSCC | Oral Squamous Cell Carcinoma |
| PAR1 | Protease-Activated Receptor 1 |
| PCNA | Proliferating Cell Nuclear Antigen |
| PDX | Patient-Derived Xenograft |
| PGE2 | Prostaglandin E2 |
| PNA | Peptide Nucleic Acid |
| PP2A | Protein Phosphatase 2A |
| PRRT | Peptide Receptor Radionuclide Therapy |
| PS | Phosphatidylserine |
| ROS | Reactive Oxygen Species |
| saCPP | Secondary Amphipathic Cell-Penetrating Peptide |
| SAR | Structure-Activity Relationship |
| SSTR2 | Type 2 Somatostatin Receptor |
| TAM | Tumor-Associated Macrophage |
| TNBC | Triple-Negative Breast Cancer |
| WT | Wild-Type |
References
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Loewenstein, P.M. Autonomous Functional Domains of Chemically Synthesized Human lmmunodeficiency Virus Tat Trans-Activator Protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Mäe, M.; Langel, U. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr. Opin. Pharmacol. 2006, 6, 509–514. [Google Scholar] [CrossRef]
- Järver, P.; Mäger, I.; Langel, U. In vivo biodistribution and efficacy of peptide mediated delivery. Trends Pharmacol. Sci. 2010, 31, 528–535. [Google Scholar] [CrossRef]
- Taylor, B.N.; Mehta, R.R.; Yamada, T.; Lekmine, F.; Christov, K.; Chakrabarty, A.M.; Green, A.; Bratescu, L.; Shilkaitis, A.; Beattie, C.W.; et al. Noncationic Peptides Obtained From Azurin Preferentially Enter Cancer Cells. Cancer Res. 2009, 69, 537–546. [Google Scholar] [CrossRef]
- Johansson, H.J.; El-Andaloussi, S.; Holm, T.; Mäe, M.; Jänes, J.; Maimets, T.; Langel, U. Characterization of a Novel Cytotoxic Cell-penetrating Peptide Derived From p14ARF Protein. Mol. Ther. 2008, 16, 115–123. [Google Scholar] [CrossRef]
- Moreno-Vargas, L.M.; Prada-Gracia, D. Exploring the Chemical Features and Biomedical Relevance of Cell-Penetrating Peptides. (Submitted).
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef]
- Lundin, P.; Johansson, H.; Guterstam, P.; Holm, T.; Hansen, M.; Langel, U.; Andaloussi, S.E. Distinct Uptake Routes of Cell-Penetrating Peptide Conjugates. Bioconjug. Chem. 2008, 19, 2535–2542. [Google Scholar] [CrossRef]
- Madani, F.; Lindberg, S.; Langel, U.; Futaki, S.; Gräslund, A. Mechanisms of Cellular Uptake of Cell-Penetrating Peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef]
- Jacobson, K.; Mouritsen, O.G.; Anderson, R.G.W. Lipid rafts: At a crossroad between cell biology and physics. Nat. Cell Biol. 2007, 9, 7–14. [Google Scholar] [CrossRef]
- Shevchenko, A.; Simons, K. Lipidomics: Coming to grips with lipid diversity. Nat. Rev. Mol. Cell Biol. 2010, 11, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, M.; Takeuchi, T.; Nakase, I.; Jones, A.T.; Futaki, S. Cellular Internalization and Distribution of Arginine-Rich Peptides as a Function of Extracellular Peptide Concentration, Serum, and Plasma Membrane Associated Proteoglycans. Bioconjug. Chem. 2008, 19, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.Y.; Delaroche, D.; Burlina, F.; Alves, I.D.; Chassaing, G.; Sagan, S. Translocation and Endocytosis for Cell-penetrating Peptide Internalization. J. Biol. Chem. 2009, 284, 33957–33965. [Google Scholar] [CrossRef] [PubMed]
- Tünnemann, G.; Ter-Avetisyan, G.; Martin, R.M.; Stöckl, M.; Herrmann, A.; Cardoso, M.C. Live-cell analysis of cell penetration ability and toxicity of oligo-arginines. J. Pept. Sci. 2008, 14, 469–476. [Google Scholar] [CrossRef]
- Di, L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef]
- Gammon, S.T.; Villalobos, V.M.; Prior, J.L.; Sharma, V.; Piwnica-Worms, D. Quantitative Analysis of Permeation Peptide Complexes Labeled with Technetium-99m: Chiral and Sequence-Specific Effects on Net Cell Uptake. Bioconjug. Chem. 2003, 14, 368–376. [Google Scholar] [CrossRef]
- Adhikari, A.; Bhattarai, B.R.; Aryal, A.; Thapa, N.; KC, P.; Adhikari, A.; Maharjan, S.; Chanda, P.B.; Regmi, B.P.; Parajuli, N. Reprogramming natural proteins using unnatural amino acids. RSC Adv. 2021, 11, 38126–38145. [Google Scholar] [CrossRef]
- Kato, T.; Yamashita, H.; Misawa, T.; Nishida, K.; Kurihara, M.; Tanaka, M.; Demizu, Y.; Oba, M. Plasmid DNA delivery by arginine-rich cell-penetrating peptides containing unnatural amino acids. Bioorg. Med. Chem. 2016, 24, 2681–2687. [Google Scholar] [CrossRef]
- Salehi, D.; Mozaffari, S.; Zoghebi, K.; Lohan, S.; Mandal, D.; Tiwari, R.K.; Parang, K. Amphiphilic Cell-Penetrating Peptides Containing Natural and Unnatural Amino Acids as Drug Delivery Agents. Cells 2022, 11, 1156. [Google Scholar] [CrossRef]
- Bitler, B.; Schroeder, J. Anti-Cancer Therapies that Utilize Cell Penetrating Peptides. Recent Patents Anti-Cancer Drug Discov. 2010, 5, 99–108. [Google Scholar] [CrossRef]
- Christian, Y.; Redkar, A.S.; Kumar, N.; Jancy, S.V.; Chandrasekharan, A.; Santhoshkumar, T.R.; Ramakrishnan, V. Structural regression modelling of peptide based drug delivery vectors for targeted anti-cancer therapy. Drug Deliv. Transl. Res. 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arrouss, I.; Nemati, F.; Roncal, F.; Wislez, M.; Dorgham, K.; Vallerand, D.; Rabbe, N.; Karboul, N.; Carlotti, F.; Bravo, J.; et al. Specific Targeting of Caspase-9/PP2A Interaction as Potential New Anti-Cancer Therapy. PLoS ONE 2013, 8, e60816. [Google Scholar] [CrossRef] [PubMed]
- Fominaya, J.; Bravo, J.; Decaudin, D.; Brossa, J.Y.; Nemati, F.; Rebollo, A. Enhanced serum proteolysis resistance of cell-penetrating peptides. Ther. Deliv. 2015, 6, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Arrouss, I.; Decaudin, D.; Choquet, S.; Azar, N.; Parizot, C.; Zini, J.; Nemati, F.; Rebollo, A. Cell Penetrating Peptides as a Therapeutic Strategy in Chronic Lymphocytic Leukemia. Protein Pept. Lett. 2015, 22, 539–546. [Google Scholar] [CrossRef]
- Dorgham, K.; Murail, S.; Tuffery, P.; Savier, E.; Bravo, J.; Rebollo, A. Binding and Kinetic Analysis of Human Protein Phosphatase PP2A Interactions with Caspase 9 Protein and the Interfering Peptide C9h. Pharmaceutics 2022, 14, 2055. [Google Scholar] [CrossRef]
- Germini, D.; Farhat, R.; Dadon, L.; Lacroix, A.; Nemati, F.; Rebollo, A.; Decaudin, D.; Wiels, J.; Brenner, C. A translational study for biomarker identification of PEP-010, a pro-apoptotic peptide restoring apoptosis in cancer models. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2024, 1871, 167492. [Google Scholar] [CrossRef]
- Lebel-Binay, S.; Nemati, F.; Dominguez-Berrocal, L.; Fleury, J.; Naguez, A.; Decaudin, D.; Rebollo, A. Abstract 3904: PEP-010, a cell penetrating & interfering peptide as a new therapeutic approach in breast cancer. Cancer Res. 2018, 78, 3904. [Google Scholar] [CrossRef]
- Lacroix, A.; Farhat, R.; Robert, A.; Brenner, C.; Wiels, J.; Germini, D. The first-in-class pro-apoptotic peptide PEP-010 is effective in monotherapy and in combination with paclitaxel on resistant ovarian adenocarcinoma cell models. Front. Pharmacol. 2024, 15, 1444973. [Google Scholar] [CrossRef]
- Müller, R.; Misund, K.; Holien, T.; Bachke, S.; Gilljam, K.M.; Våtsveen, T.K.; Rø, T.B.; Bellacchio, E.; Sundan, A.; Otterlei, M. Targeting Proliferating Cell Nuclear Antigen and Its Protein Interactions Induces Apoptosis in Multiple Myeloma Cells. PLoS ONE 2013, 8, e70430. [Google Scholar] [CrossRef]
- Gilljam, K.M.; Feyzi, E.; Aas, P.A.; Sousa, M.M.; Müller, R.; Vågbø, C.B.; Catterall, T.C.; Liabakk, N.B.; Slupphaug, G.; Drabløs, F.; et al. Identification of a novel, widespread, and functionally important PCNA-binding motif. J. Cell Biol. 2009, 186, 645–654. [Google Scholar] [CrossRef]
- Gravina, G.L.; Colapietro, A.; Mancini, A.; Rossetti, A.; Martellucci, S.; Ventura, L.; Franco, M.D.; Marampon, F.; Mattei, V.; Biordi, L.A.; et al. ATX-101, a Peptide Targeting PCNA, Has Antitumor Efficacy Alone or in Combination with Radiotherapy in Murine Models of Human Glioblastoma. Cancers 2022, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Lemech, C.R.; Kichenadasse, G.; Marschner, J.P.; Alevizopoulos, K.; Otterlei, M.; Millward, M. ATX-101, a cell-penetrating protein targeting PCNA, can be safely administered as intravenous infusion in patients and shows clinical activity in a Phase 1 study. Oncogene 2023, 42, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Miampamba, M.; Liu, J.; Harootunian, A.; Gale, A.J.; Baird, S.; Chen, S.L.; Nguyen, Q.T.; Tsien, R.Y.; González, J.E. Sensitive in vivo Visualization of Breast Cancer Using Ratiometric Protease-activatable Fluorescent Imaging Agent, AVB-620. Theranostics 2017, 7, 3369–3386. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, Y.; Xu, K.; Dai, Z. Current trends and key considerations in the clinical translation of targeted fluorescent probes for intraoperative navigation. Aggregate 2021, 2, e23. [Google Scholar] [CrossRef]
- Woo, Y.; Chaurasiya, S.; O’Leary, M.; Han, E.; Fong, Y. Fluorescent imaging for cancer therapy and cancer gene therapy. Mol. Ther.-Oncolytics 2021, 23, 231–238. [Google Scholar] [CrossRef]
- Unkart, J.T.; Chen, S.L.; Wapnir, I.L.; González, J.E.; Harootunian, A.; Wallace, A.M. Intraoperative Tumor Detection Using a Ratiometric Activatable Fluorescent Peptide: A First-in-Human Phase 1 Study. Ann. Surg. Oncol. 2017, 24, 3167–3173. [Google Scholar] [CrossRef]
- Derouazi, M.; Berardino-Besson, W.D.; Belnoue, E.; Hoepner, S.; Walther, R.; Benkhoucha, M.; Teta, P.; Dufour, Y.; Maroun, C.Y.; Salazar, A.M.; et al. Novel Cell-Penetrating Peptide-Based Vaccine Induces Robust CD4+ and CD8+ T Cell–Mediated Antitumor Immunity. Cancer Res. 2015, 75, 3020–3031. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, W.; Gao, Z.; Yang, S.; Zhou, G.; Chang, Y.; Ma, Y.; Liang, X.; Shao, J.; Chang, H. Antigenicity and immunogenicity of recombinant proteins comprising African swine fever virus proteins p30 and p54 fused to a cell-penetrating peptide. Int. Immunopharmacol. 2021, 101, 108251. [Google Scholar] [CrossRef]
- Belnoue, E.; Berardino-Besson, W.D.; Gaertner, H.; Carboni, S.; Dunand-Sauthier, I.; Cerini, F.; Suso-Inderberg, E.M.; Wälchli, S.; König, S.; Salazar, A.M.; et al. Enhancing Antitumor Immune Responses by Optimized Combinations of Cell-penetrating Peptide-based Vaccines and Adjuvants. Mol. Ther. 2016, 24, 1675–1685. [Google Scholar] [CrossRef]
- Grau, M.; Walker, P.R.; Derouazi, M. Mechanistic insights into the efficacy of cell penetrating peptide-based cancer vaccines. Cell. Mol. Life Sci. 2018, 75, 2887–2896. [Google Scholar] [CrossRef]
- Belnoue, E.; Mayol, J.F.; Carboni, S.; Besson, W.D.B.; Dupuychaffray, E.; Nelde, A.; Stevanovic, S.; Santiago-Raber, M.L.; Walker, P.R.; Derouazi, M. Targeting self and neo-epitopes with a modular self-adjuvanting cancer vaccine. JCI Insight 2019, 4, e127305. [Google Scholar] [CrossRef]
- Elmquist, A.; Lindgren, M.; Bartfai, T.; Langel, U. VE-Cadherin-Derived Cell-Penetrating Peptide, pVEC, with Carrier Functions. Exp. Cell Res. 2001, 269, 237–244. [Google Scholar] [CrossRef]
- Elmquist, A.; Hansen, M.; Langel, U. Structure–activity relationship study of the cell-penetrating peptide pVEC. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 721–729. [Google Scholar] [CrossRef]
- Myrberg, H.; Zhang, L.; Mäe, M.; Langel, U. Design of a Tumor-Homing Cell-Penetrating Peptide. Bioconjug. Chem. 2008, 19, 70–75. [Google Scholar] [CrossRef]
- Kersemans, V.; Cornelissen, B. Targeting the Tumour: Cell Penetrating Peptides for Molecular Imaging and Radiotherapy. Pharmaceuticals 2010, 3, 600–620. [Google Scholar] [CrossRef]
- Regberg, J.; Srimanee, A.; Langel, U. Applications of Cell-Penetrating Peptides for Tumor Targeting and Future Cancer Therapies. Pharmaceuticals 2012, 5, 991–1007. [Google Scholar] [CrossRef]
- Deshayes, S.; Plénat, T.; Charnet, P.; Divita, G.; Molle, G.; Heitz, F. Formation of transmembrane ionic channels of primary amphipathic cell-penetrating peptides. Consequences on the mechanism of cell penetration. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1846–1851. [Google Scholar] [CrossRef][Green Version]
- Kurzawa, L.; Pellerano, M.; Morris, M.C. PEP and CADY-mediated delivery of fluorescent peptides and proteins into living cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 2274–2285. [Google Scholar] [CrossRef]
- Henriques, S.T.; Costa, J.; Castanho, M.A.R.B. Translocation of β-Galactosidase? Mediated by the Cell-Penetrating Peptide Pep-1 into Lipid Vesicles and Human HeLa Cells Is Driven by Membrane Electrostatic Potential. Biochemistry 2005, 44, 10189–10198. [Google Scholar] [CrossRef]
- Henriques, S.T.; Castanho, M.A. Environmental factors that enhance the action of the cell penetrating peptide pep-1 A spectroscopic study using lipidic vesicles. Biochim. Biophys. Acta (BBA)-Biomembr. 2005, 1669, 75–86. [Google Scholar] [CrossRef]
- Sharonov, A.; Hochstrasser, R.M. Single-Molecule Imaging of the Association of the Cell-Penetrating Peptide Pep-1 to Model Membranes. Biochemistry 2007, 46, 7963–7972. [Google Scholar] [CrossRef] [PubMed]
- Henriques, S.T.; Castanho, M.A.R.B. Translocation or membrane disintegration? Implication of peptide–membrane interactions in pep-1 activity. J. Pept. Sci. 2008, 14, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur. J. Pharmacol. 2009, 625, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Almarwani, B.; Phambu, E.N.; Alexander, C.; Nguyen, H.A.T.; Phambu, N.; Sunda-Meya, A. Vesicles mimicking normal and cancer cell membranes exhibit differential responses to the cell-penetrating peptide Pep-1. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, Y.; Feng, Q.; Chen, Y. Tryptophan as a Probe to Study the Anticancer Mechanism of Action and Specificity of α-Helical Anticancer Peptides. Molecules 2014, 19, 12224–12241. [Google Scholar] [CrossRef]
- Papo, N.; Shai, Y. New Lytic Peptides Based on the d,l-Amphipathic Helix Motif Preferentially Kill Tumor Cells Compared to Normal Cells. Biochemistry 2003, 42, 9346–9354. [Google Scholar] [CrossRef]
- Ding, B.; Chen, Z. Molecular Interactions between Cell Penetrating Peptide Pep-1 and Model Cell Membranes. J. Phys. Chem. B 2012, 116, 2545–2552. [Google Scholar] [CrossRef]
- Wang, T.; Wang, C.; Zheng, S.; Qu, G.; Feng, Z.; Shang, J.; Cheng, Y.; He, N. Insight into the Mechanism of Internalization of the Cell-Penetrating Carrier Peptide Pep-1 by Conformational Analysis. J. Biomed. Nanotechnol. 2020, 16, 1135–1143. [Google Scholar] [CrossRef]
- Gallo, G. Making Proteins into Drugs: Assisted Delivery of Proteins and Peptides into Living Neurons. Methods Cell Biol. 2003, 71, 325–338. [Google Scholar] [CrossRef]
- Pandey, A.V.; Mellon, S.H.; Miller, W.L. Protein Phosphatase 2A and Phosphoprotein SET Regulate Androgen Production by P450c17*. J. Biol. Chem. 2003, 278, 2837–2844. [Google Scholar] [CrossRef]
- Lee, S.J.; Kang, H.K.; Choi, Y.J.; Eum, W.S.; Park, J.; Choi, S.Y.; Kwon, H.Y. PEP-1-paraoxonase 1 fusion protein prevents cytokine-induced cell destruction and impaired insulin secretion in rat insulinoma cells. BMB Rep. 2018, 51, 538–543. [Google Scholar] [CrossRef]
- Gehler, S.; Shaw, A.E.; Sarmiere, P.D.; Bamburg, J.R.; Letourneau, P.C. Brain-Derived Neurotrophic Factor Regulation of Retinal Growth Cone Filopodial Dynamics Is Mediated through Actin Depolymerizing Factor/Cofilin. J. Neurosci. 2004, 24, 10741–10749. [Google Scholar] [CrossRef]
- Garnon, J.; Lachance, C.; Marco, S.D.; Hel, Z.; Marion, D.; Ruiz, M.C.; Newkirk, M.M.; Khandjian, E.W.; Radzioch, D. Fragile X-related Protein FXR1P Regulates Proinflammatory Cytokine Tumor Necrosis Factor Expression at the Post-transcriptional Level*. J. Biol. Chem. 2005, 280, 5750–5763. [Google Scholar] [CrossRef]
- Bardag-Gorce, F.; Riley, N.; Nguyen, V.; Montgomery, R.; French, B.; Li, J.; Leeuwen, F.v.; Lungo, W.; McPhaul, L.; French, S. The mechanism of cytokeratin aggresome formation: The role of mutant ubiquitin (UBB+1). Exp. Mol. Pathol. 2003, 74, 160–167. [Google Scholar] [CrossRef]
- Gros, E.; Deshayes, S.; Morris, M.C.; Aldrian-Herrada, G.; Depollier, J.; Heitz, F.; Divita, G. A non-covalent peptide-based strategy for protein and peptide nucleic acid transduction. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 384–393. [Google Scholar] [CrossRef]
- Chang, J.C.; Chang, H.S.; Yeh, C.Y.; Chang, H.J.; Cheng, W.L.; Lin, T.T.; Liu, C.S.; Chen, S.T. Regulation of mitochondrial fusion and mitophagy by intra-tumoral delivery of membrane-fused mitochondria or Midiv-1 enhances sensitivity to doxorubicin in triple-negative breast cancer. Biomed. Pharmacother. 2022, 153, 113484. [Google Scholar] [CrossRef]
- Jannoo, R.; Xia, Z.; Row, P.E.; Kanamarlapudi, V. Targeting of the Interleukin-13 Receptor (IL-13R)α2 Expressing Prostate Cancer by a Novel Hybrid Lytic Peptide. Biomolecules 2023, 13, 356. [Google Scholar] [CrossRef]
- Jannoo, R.; Walker, W.; Kanamarlapudi, V. Targeting and Sensitization of Breast Cancer Cells to Killing with a Novel Interleukin-13 Receptor α2-Specific Hybrid Cytolytic Peptide. Cancers 2023, 15, 2772. [Google Scholar] [CrossRef]
- Guo, X.; Wu, G.; Wang, H.; Chen, L. Pep-1&borneol–Bifunctionalized Carmustine-Loaded Micelles Enhance Anti-Glioma Efficacy Through Tumor-Targeting and BBB-Penetrating. J. Pharm. Sci. 2019, 108, 1726–1735. [Google Scholar] [CrossRef]
- Hällbrink, M.; Florén, A.; Elmquist, A.; Pooga, M.; Bartfai, T.; Langel, U. Cargo delivery kinetics of cell-penetrating peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2001, 1515, 101–109. [Google Scholar] [CrossRef]
- Palm-Apergi, C.; Lorents, A.; Padari, K.; Pooga, M.; Hällbrink, M. The membrane repair response masks membrane disturbances caused by cell-penetrating peptide uptake. FASEB J. 2009, 23, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Alves, C.; Duarte, D.; Costa, A.; Sarmento, B.; Almeida, A.J.; Gomes, P.; Vale, N. Model Amphipathic Peptide Coupled with Tacrine to Improve Its Antiproliferative Activity. Int. J. Mol. Sci. 2021, 22, 242. [Google Scholar] [CrossRef] [PubMed]
- Scheller, A.; Oehlke, J.; Wiesner, B.; Dathe, M.; Krause, E.; Beyermann, M.; Melzig, M.; Bienert, M. Structural requirements for cellular uptake of α-helical amphipathic peptides. J. Pept. Sci. 1999, 5, 185–194. [Google Scholar] [CrossRef]
- Fialho, A.M.; Bernardes, N.; Chakrabarty, A.M. Exploring the anticancer potential of the bacterial protein azurin. AIMS Microbiol. 2016, 2, 292–303. [Google Scholar] [CrossRef]
- Mehta, R.R.; Yamada, T.; Taylor, B.N.; Christov, K.; King, M.L.; Majumdar, D.; Lekmine, F.; Tiruppathi, C.; Shilkaitis, A.; Bratescu, L.; et al. A cell penetrating peptide derived from azurin inhibits angiogenesis and tumor growth by inhibiting phosphorylation of VEGFR-2, FAK and Akt. Angiogenesis 2011, 14, 355–369. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Khazaei, M.; Avan, A.; Hasanian, S.M.; Cho, W.C.; Soleimanpour, S. p28 Bacterial Peptide, as an Anticancer Agent. Front. Oncol. 2020, 10, 1303. [Google Scholar] [CrossRef]
- Garizo, A.R.; Castro, F.; Martins, C.; Almeida, A.; Dias, T.P.; Fernardes, F.; Barrias, C.C.; Bernardes, N.; Fialho, A.M.; Sarmento, B. p28-functionalized PLGA nanoparticles loaded with gefitinib reduce tumor burden and metastases formation on lung cancer. J. Control Release 2021, 337, 329–342. [Google Scholar] [CrossRef]
- Mander, S.; Gorman, G.S.; Coward, L.U.; Christov, K.; Green, A.; Gupta, T.K.D.; Yamada, T. The brain-penetrant cell-cycle inhibitor p28 sensitizes brain metastases to DNA-damaging agents. Neuro-Oncol. Adv. 2023, 5, vdad042. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Movaqar, A.; Asgharzadeh, F.; Derakhshan, M.; Ghazvini, K.; Hasanian, S.M.; Avan, A.; Mostafapour, A.; Khazaei, M.; Soleimanpour, S. Anticancer activity of Pseudomonas aeruginosa derived peptide with iRGD in colon cancer therapy. Iran. J. Basic Med. Sci. 2023, 26, 768–776. [Google Scholar] [CrossRef]
- Fernández-Carneado, J.; Kogan, M.J.; Pujals, S.; Giralt, E. Amphipathic peptides and drug delivery. Pept. Sci. 2004, 76, 196–203. [Google Scholar] [CrossRef]
- Martín, I.; Teixidó, M.; Giralt, E. Design, Synthesis and Characterization of a New Anionic Cell-Penetrating Peptide: SAP(E). ChemBioChem 2011, 12, 896–903. [Google Scholar] [CrossRef]
- Franz, J.; Lelle, M.; Peneva, K.; Bonn, M.; Weidner, T. SAP(E)—A cell-penetrating polyproline helix at lipid interfaces. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 2028–2034. [Google Scholar] [CrossRef]
- Lelle, M.; Frick, S.U.; Steinbrink, K.; Peneva, K. Novel cleavable cell-penetrating peptide–drug conjugates: Synthesis and characterization. J. Pept. Sci. 2014, 20, 323–333. [Google Scholar] [CrossRef]
- Nadas, J.; Sun, D. Anthracyclines as effective anticancer drugs. Expert Opin. Drug Discov. 2006, 1, 549–568. [Google Scholar] [CrossRef]
- Frank, R.; Gennaro, R.; Schneider, K.; Przybylski, M.; Romeo, D. Amino acid sequences of two proline-rich bactenecins. Antimicrobial peptides of bovine neutrophils. J. Biol. Chem. 1990, 265, 18871–18874. [Google Scholar] [CrossRef]
- Sadler, K.; Eom, K.D.; Yang, J.L.; Dimitrova, Y.; Tam, J.P. Translocating Proline-Rich Peptides from the Antimicrobial Peptide Bactenecin 7. Biochemistry 2002, 41, 14150–14157. [Google Scholar] [CrossRef]
- Bidwell, G.L.; Davis, A.N.; Raucher, D. Targeting a c-Myc inhibitory polypeptide to specific intracellular compartments using cell penetrating peptides. J. Control Release 2009, 135, 2–10. [Google Scholar] [CrossRef]
- Massodi, I.; Moktan, S.; Rawat, A.; Bidwell, G.L.; Raucher, D. Inhibition of ovarian cancer cell proliferation by a cell cycle inhibitory peptide fused to a thermally responsive polypeptide carrier. Int. J. Cancer 2010, 126, 533–544. [Google Scholar] [CrossRef]
- LaBelle, J.L.; Katz, S.G.; Bird, G.H.; Gavathiotis, E.; Stewart, M.L.; Lawrence, C.; Fisher, J.K.; Godes, M.; Pitter, K.; Kung, A.L.; et al. A stapled BIM peptide overcomes apoptotic resistance in hematologic cancers. J. Clin. Investig. 2012, 122, 2018–2031. [Google Scholar] [CrossRef]
- Stewart, M.L.; Fire, E.; Keating, A.E.; Walensky, L.D. The MCL-1 BH3 Helix is an Exclusive MCL-1 inhibitor and Apoptosis Sensitizer. Nat. Chem. Biol. 2010, 6, 595–601. [Google Scholar] [CrossRef]
- Lee, S.; Braun, C.R.; Bird, G.H.; Walensky, L.D. Chapter Two Photoreactive Stapled Peptides to Identify and Characterize BCL-2 Family Interaction Sites by Mass Spectrometry. Methods Enzymol. 2014, 544, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Hadji, A.; Schmitt, G.K.; Schnorenberg, M.R.; Roach, L.; Hickey, C.M.; Leak, L.B.; Tirrell, M.V.; LaBelle, J.L. Preferential targeting of MCL-1 by a hydrocarbon-stapled BIM BH3 peptide. Oncotarget 2019, 10, 6219–6233. [Google Scholar] [CrossRef] [PubMed]
- Ingelshed, K.; Melssen, M.M.; Kannan, P.; Chandramohan, A.; Partridge, A.W.; Jiang, L.; Wermeling, F.; Lane, D.P.; Nestor, M.; Spiegelberg, D. MDM2/MDMX inhibition by Sulanemadlin synergizes with anti-Programmed Death 1 immunotherapy in wild-type p53 tumors. iScience 2024, 27, 109862. [Google Scholar] [CrossRef] [PubMed]
- Guerlavais, V.; Sawyer, T.K.; Carvajal, L.; Chang, Y.S.; Graves, B.; Ren, J.G.; Sutton, D.; Olson, K.A.; Packman, K.; Darlak, K.; et al. Discovery of Sulanemadlin (ALRN-6924), the First Cell-Permeating, Stabilized α-Helical Peptide in Clinical Development. J. Med. Chem. 2023, 66, 9401–9417. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.N.; Patel, M.R.; Bauer, T.M.; Goel, S.; Falchook, G.S.; Shapiro, G.I.; Chung, K.Y.; Infante, J.R.; Conry, R.M.; Rabinowits, G.; et al. Phase 1 Trial of ALRN-6924, a Dual Inhibitor of MDMX and MDM2, in Patients with Solid Tumors and Lymphomas Bearing Wild-type TP53Phase 1 Trial of ALRN-6924, a Dual MDMX/MDM2 Inhibitor. Clin. Cancer Res. 2021, 27, 5236–5247. [Google Scholar] [CrossRef]
- Pairawan, S.; Zhao, M.; Yuca, E.; Annis, A.; Evans, K.; Sutton, D.; Carvajal, L.; Ren, J.G.; Santiago, S.; Guerlavais, V.; et al. First in class dual MDM2/MDMX inhibitor ALRN-6924 enhances antitumor efficacy of chemotherapy in TP53 wild-type hormone receptor-positive breast cancer models. Breast Cancer Res. 2021, 23, 29. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Somaiah, N.; DuBois, S.; Dumbrava, E.E.I.; Shapiro, G.; Patel, M.; Goel, S.; Bauer, T.; Pinchasik, D.; Annis, A.; et al. 475P A phase IIa clinical trial combining ALRN-6924 and palbociclib for the treatment of patients with tumours harboring wild-type p53 and MDM2 amplification or MDM2/CDK4 co-amplification. Ann. Oncol. 2019, 30, v179–v180. [Google Scholar] [CrossRef]
- Covic, L.; Gresser, A.L.; Talavera, J.; Swift, S.; Kuliopulos, A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc. Natl. Acad. Sci. USA 2002, 99, 643–648. [Google Scholar] [CrossRef]
- Covic, L.; Misra, M.; Badar, J.; Singh, C.; Kuliopulos, A. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat. Med. 2002, 8, 1161–1165. [Google Scholar] [CrossRef]
- Cisowski, J.; O’Callaghan, K.; Kuliopulos, A.; Yang, J.; Nguyen, N.; Deng, Q.; Yang, E.; Fogel, M.; Tressel, S.; Foley, C.; et al. Targeting Protease-Activated Receptor-1 with Cell-Penetrating Pepducins in Lung Cancer. Am. J. Pathol. 2011, 179, 513–523. [Google Scholar] [CrossRef]
- Zhang, P.; Gruber, A.; Kasuda, S.; Kimmelstiel, C.; O’Callaghan, K.; Cox, D.H.; Bohm, A.; Baleja, J.D.; Covic, L.; Kuliopulos, A. Suppression of Arterial Thrombosis Without Affecting Hemostatic Parameters With a Cell-Penetrating PAR1 Pepducin. Circulation 2012, 126, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Covic, L.; Kuliopulos, A. Protease-Activated Receptor 1 as Therapeutic Target in Breast, Lung, and Ovarian Cancer: Pepducin Approach. Int. J. Mol. Sci. 2018, 19, 2237. [Google Scholar] [CrossRef] [PubMed]
- Boire, A.; Covic, L.; Agarwal, A.; Jacques, S.; Sherifi, S.; Kuliopulos, A. PAR1 Is a Matrix Metalloprotease-1 Receptor that Promotes Invasion and Tumorigenesis of Breast Cancer Cells. Cell 2005, 120, 303–313. [Google Scholar] [CrossRef]
- Tressel, S.L.; Koukos, G.; Tchernychev, B.; Jacques, S.L.; Covic, L.; Kuliopulos, A. Pharmacology, Biodistribution, and Efficacy of GPCR-Based Pepducins in Disease Models. Methods Mol. Biol. 2011, 683, 259–275. [Google Scholar] [CrossRef]
- Michael, E.; Covic, L.; Kuliopulos, A. Cell Penetrating Peptides, Methods and Protocols: Lipopeptide Pepducins as Therapeutic Agents. Methods Mol. Biol. 2021, 2383, 307–333. [Google Scholar] [CrossRef]
- Brouillette, R.L.; Besserer-Offroy, E.; Mona, C.E.; Chartier, M.; Lavenus, S.; Sousbie, M.; Belleville, K.; Longpré, J.M.; Marsault, E.; Grandbois, M.; et al. Cell-penetrating pepducins targeting the neurotensin receptor type 1 relieve pain. Pharmacol. Res. 2020, 155, 104750. [Google Scholar] [CrossRef]
- Kuliopulos, A.; Gurbel, P.A.; Rade, J.J.; Kimmelstiel, C.D.; Turner, S.E.; Bliden, K.P.; Fletcher, E.K.; Cox, D.H.; Covic, L.; Investigators, o.b.o.t.T.P. PAR1 (Protease-Activated Receptor 1) Pepducin Therapy Targeting Myocardial Necrosis in Coronary Artery Disease and Acute Coronary Syndrome Patients Undergoing Cardiac Catheterization. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2990–3003. [Google Scholar] [CrossRef]
- Affolter, M.; Slattery, M.; Mann, R.S. A Lexicon for Homeodomain-DNA Recognition. Cell 2008, 133, 1133–1135. [Google Scholar] [CrossRef]
- Chu, S.W.; Noyes, M.B.; Christensen, R.G.; Pierce, B.G.; Zhu, L.J.; Weng, Z.; Stormo, G.D.; Wolfe, S.A. Exploring the DNA-recognition potential of homeodomains. Genome Res. 2012, 22, 1889–1898. [Google Scholar] [CrossRef]
- Beltran, A.S.; Graves, L.M.; Blancafort, P. Novel role of Engrailed 1 as a prosurvival transcription factor in basal-like breast cancer and engineering of interference peptides block its oncogenic function. Oncogene 2014, 33, 4767–4777. [Google Scholar] [CrossRef]
- Gandhi, N.S.; Blancafort, P.; Mancera, R.L. Atomistic molecular dynamics simulations of bioactive engrailed 1 interference peptides (EN1-iPeps). Oncotarget 2018, 9, 22383–22397. [Google Scholar] [CrossRef] [PubMed]
- Sorolla, A.; Ho, D.; Wang, E.; Evans, C.W.; Ormonde, C.F.G.; Rashwan, R.; Singh, R.; Iyer, K.S.; Blancafort, P. Sensitizing basal-like breast cancer to chemotherapy using nanoparticles conjugated with interference peptide. Nanoscale 2016, 8, 9343–9353. [Google Scholar] [CrossRef] [PubMed]
- Coupade, C.d.; Fittipaldi, A.; Chagnas, V.; Michel, M.; Carlier, S.; Tasciotti, E.; Darmon, A.; Ravel, D.; Kearsey, J.; Giacca, M.; et al. Novel human-derived cell-penetrating peptides for specific subcellular delivery of therapeutic biomolecules. Biochem. J. 2005, 390, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Losic, F.; Quinonero, J.; Dubois, V.; Alluis, B.; Dechambre, M.; Michel, M.; Cailler, F.; Fernandez, A.M.; Trouet, A.; Kearsey, J. Improved Therapeutic Efficacy of Doxorubicin through Conjugation with a Novel Peptide Drug Delivery Technology (Vectocell). J. Med. Chem. 2006, 49, 6908–6916. [Google Scholar] [CrossRef]
- Chen, C.J.; Tsai, K.C.; Kuo, P.H.; Chang, P.L.; Wang, W.C.; Chuang, Y.J.; Chang, M.D.T. A Heparan Sulfate-Binding Cell Penetrating Peptide for Tumor Targeting and Migration Inhibition. BioMed Res. Int. 2015, 2015, 237969. [Google Scholar] [CrossRef]
- Rady, I.; Siddiqui, I.A.; Rady, M.; Mukhtar, H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017, 402, 16–31. [Google Scholar] [CrossRef]
- Rajabnejad, S.H.; Mokhtarzadeh, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Razavi, B.M. Targeted delivery of melittin to cancer cells by AS1411 anti-nucleolin aptamer. Drug Dev. Ind. Pharm. 2018, 44, 982–987. [Google Scholar] [CrossRef]
- Ceremuga, M.; Stela, M.; Janik, E.; Gorniak, L.; Synowiec, E.; Sliwinski, T.; Sitarek, P.; Saluk-Bijak, J.; Bijak, M. Melittin—A Natural Peptide from Bee Venom Which Induces Apoptosis in Human Leukaemia Cells. Biomolecules 2020, 10, 247. [Google Scholar] [CrossRef]
- Duffy, C.; Sorolla, A.; Wang, E.; Golden, E.; Woodward, E.; Davern, K.; Ho, D.; Johnstone, E.; Pfleger, K.; Redfern, A.; et al. Honeybee venom and melittin suppress growth factor receptor activation in HER2-enriched and triple-negative breast cancer. NPJ Precis. Oncol. 2020, 4, 24. [Google Scholar] [CrossRef]
- Salomone, F.; Cardarelli, F.; Luca, M.D.; Boccardi, C.; Nifosì, R.; Bardi, G.; Bari, L.D.; Serresi, M.; Beltram, F. A novel chimeric cell-penetrating peptide with membrane-disruptive properties for efficient endosomal escape. J. Control Release 2012, 163, 293–303. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, H.; Bae, Y.; Shin, K.; Kang, S.; Kim, H.; Oh, J.; Bae, H. Targeting of M2-like tumor-associated macrophages with a melittin-based pro-apoptotic peptide. J. Immunother. Cancer 2019, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.A.; Quinn, J.M.; Roach, S.T.; Palisoul, M.; Nguyen, M.; Noia, H.; Guo, L.; Fazal, J.; Mutch, D.G.; Wickline, S.A.; et al. p5RHH nanoparticle-mediated delivery of AXL siRNA inhibits metastasis of ovarian and uterine cancer cells in mouse xenografts. Sci. Rep. 2019, 9, 4762. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deng, M.; Xiang, J.; Ma, H.; Hu, W.; Zhao, Y.; Li, D.W.C.; Liang, S. A Novel Spider Peptide Toxin Suppresses Tumor Growth Through Dual Signaling Pathways. Curr. Mol. Med. 2012, 12, 1350–1360. [Google Scholar] [CrossRef]
- Tan, H.; Luo, W.; Wei, L.; Chen, B.; Li, W.; Xiao, L.; Manzhos, S.; Liu, Z.; Liang, S. Quantifying the Distribution of the Stoichiometric Composition of Anticancer Peptide Lycosin-I on the Lipid Membrane with Single Molecule Spectroscopy. J. Phys. Chem. B 2016, 120, 3081–3088. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ding, X.; Meng, S.; Liu, C.; Wang, H.; Xia, L.; Liu, Z.; Liang, S. Antimicrobial Potential of Lycosin-I, a Cationic and Amphiphilic Peptide from the Venom of the Spider Lycosa singorensis. Curr. Mol. Med. 2013, 13, 900–910. [Google Scholar] [CrossRef]
- Shen, H.; Xie, Y.; Ye, S.; He, K.; Yi, L.; Cui, R. Spider peptide toxin lycosin-I induces apoptosis and inhibits migration of prostate cancer cells. Exp. Biol. Med. 2018, 243, 725–735. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, J.; Yan, Y.; Chen, B.; Liu, B.; Jian, C.; Zhu, B.; Liang, S.; Zeng, Y.; Liu, Z. Arginine modification of lycosin-I to improve inhibitory activity against cancer cells. Org. Biomol. Chem. 2017, 15, 9379–9388. [Google Scholar] [CrossRef]
- Lazarovici, P.; Primor, N.; Loew, L.M. Purification and pore-forming activity of two hydrophobic polypeptides from the secretion of the Red Sea Moses sole (Pardachirus marmoratus). J. Biol. Chem. 1986, 261, 16704–16713. [Google Scholar] [CrossRef]
- Thompson, S.A.; Tachibana, K.; Nakanishi, K.; Kubota, I. Melittin-Like Peptides from the Shark-Repelling Defense Secretion of the Sole Pardachirus pavoninus. Science 1986, 233, 341–343. [Google Scholar] [CrossRef]
- Porcelli, F.; Buck, B.; Lee, D.K.; Hallock, K.J.; Ramamoorthy, A.; Veglia, G. Structure and Orientation of Pardaxin Determined by NMR Experiments in Model Membranes*. J. Biol. Chem. 2004, 279, 45815–45823. [Google Scholar] [CrossRef]
- Shai, Y. Pardaxin: Channel formation by a shark repellant peptide from fish. Toxicology 1994, 87, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Rádis-Baptista, G. Cell-Penetrating Peptides Derived from Animal Venoms and Toxins. Toxins 2021, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Lin, L.C.; Tzen, J.T.; Chen, J.Y. Pardaxin-induced apoptosis enhances antitumor activity in HeLa cells. Peptides 2011, 32, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Lee, J.F.; Chen, J.Y. Pardaxin, an Antimicrobial Peptide, Triggers Caspase-Dependent and ROS-Mediated Apoptosis in HT-1080 Cells. Mar. Drugs 2011, 9, 1995–2009. [Google Scholar] [CrossRef]
- Huang, T.C.; Chen, J.Y. Proteomic analysis reveals that pardaxin triggers apoptotic signaling pathways in human cervical carcinoma HeLa cells: Cross talk among the UPR, c-Jun and ROS. Carcinogenesis 2013, 34, 1833–1842. [Google Scholar] [CrossRef]
- Uen, W.C.; Choong, C.Y.; Tai, C.J.; Tai, C.J. Pardaxin Promoted Differentiation and Maturation of Leukemic Cells via Regulating TLR2/MyD88 Signal against Cell Proliferation. Evid.-Based Complement. Altern. Med. 2019, 2019, 7035087. [Google Scholar] [CrossRef]
- Chen, Y.P.; Shih, P.C.; Feng, C.W.; Wu, C.C.; Tsui, K.H.; Lin, Y.H.; Kuo, H.M.; Wen, Z.H. Pardaxin Activates Excessive Mitophagy and Mitochondria-Mediated Apoptosis in Human Ovarian Cancer by Inducing Reactive Oxygen Species. Antioxidants 2021, 10, 1883. [Google Scholar] [CrossRef]
- Han, Y.; Cui, Z.; Li, Y.H.; Hsu, W.H.; Lee, B.H. In Vitro and in Vivo Anticancer Activity of Pardaxin against Proliferation and Growth of Oral Squamous Cell Carcinoma. Mar. Drugs 2016, 14, 2. [Google Scholar] [CrossRef]
- Qu, B.; Yuan, J.; Liu, X.; Zhang, S.; Ma, X.; Lu, L. Anticancer activities of natural antimicrobial peptides from animals. Front. Microbiol. 2024, 14, 1321386. [Google Scholar] [CrossRef]
- Wong, Y.H.; Lee, S.H. Short Fragmented Peptides from Pardachirus Marmoratus Exhibit Stronger Anticancer Activities in in silico Residue Replacement and Analyses. Curr. Drug Discov. Technol. 2024, 21, E220224227304. [Google Scholar] [CrossRef]
- Gowland, C.; Berry, P.; Errington, J.; Jeffrey, P.; Bennett, G.; Godfrey, L.; Pittman, M.; Niewiarowski, A.; Symeonides, S.N.; Veal, G.J. Development of a LC–MS/MS method for the quantification of toxic payload DM1 cleaved from BT1718 in a Phase I study. Bioanalysis 2021, 13, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Suojanen, J.; Salo, T.; Koivunen, E.; Sorsa, T.; Pirilä, E. A novel and selective membrane type-1 matrix metalloproteinase (MT1-MMP) inhibitor reduces cancer cell motility and tumor growth. Cancer Biol. Ther. 2009, 8, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Tanaka, N.; Sakamoto, T. MT1-MMP as a Key Regulator of Metastasis. Cells 2023, 12, 2187. [Google Scholar] [CrossRef]
- Valkema, R.; Pauwels, M.S.A.; Kvols, M.L.K.; Kwekkeboom, M.D.J.; Jamar, M.F.; de Jong, M.M.; Barone, P.R.; Walrand, M.S.; Kooij, P.P.P.; Bakker, M.W.H.; et al. Long-Term Follow-Up of Renal Function After Peptide Receptor Radiation Therapy with 90Y-DOTA0,Tyr3-Octreotide and 177Lu-DOTA0, Tyr3-Octreotate. J. Nucl. Med. 2005, 46, 83S–91S. [Google Scholar]
- Becx, M.N.; Minczeles, N.S.; Brabander, T.; Herder, W.W.d.; Nonnekens, J.; Hofland, J. A Clinical Guide to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE in Neuroendocrine Tumor Patients. Cancers 2022, 14, 5792. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Teunissen, J.J.; Bakker, W.H.; Kooij, P.P.; Herder, W.W.d.; Feelders, R.A.; Eijck, C.H.v.; Esser, J.P.; Kam, B.L.; Krenning, E.P. Radiolabeled Somatostatin Analog [177Lu-DOTA0,Tyr3]Octreotate in Patients With Endocrine Gastroenteropancreatic Tumors. J. Clin. Oncol. 2004, 23, 2754–2762. [Google Scholar] [CrossRef]
- Delker, A.; Ilhan, H.; Zach, C.; Brosch, J.; Gildehaus, F.J.; Lehner, S.; Bartenstein, P.; Böning, G. The Influence of Early Measurements Onto the Estimated Kidney Dose in [177Lu][DOTA0,Tyr3]Octreotate Peptide Receptor Radiotherapy of Neuroendocrine Tumors. Mol. Imaging Biol. 2015, 17, 726–734. [Google Scholar] [CrossRef]
- Kim, C.; Liu, S.V.; Subramaniam, D.S.; Torres, T.; Loda, M.; Esposito, G.; Giaccone, G. Phase I study of the 177Lu-DOTA0-Tyr3-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J. Immunother. Cancer 2020, 8, e000980. [Google Scholar] [CrossRef]
- Zwan, W.A.v.d.; Brabander, T.; Kam, B.L.R.; Teunissen, J.J.M.; Feelders, R.A.; Hofland, J.; Krenning, E.P.; Herder, W.W.d. Salvage peptide receptor radionuclide therapy with [177Lu-DOTA,Tyr3]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 704–717. [Google Scholar] [CrossRef]
- Kobayashi, N.; Takano, S.; Ito, K.; Sugiura, M.; Ogawa, M.; Takeda, Y.; Okubo, N.; Suzuki, A.; Tokuhisa, M.; Kaneta, T.; et al. Safety and efficacy of peptide receptor radionuclide therapy with 177Lu-DOTA0-Tyr3-octreotate in combination with amino acid solution infusion in Japanese patients with somatostatin receptor-positive, progressive neuroendocrine tumors. Ann. Nucl. Med. 2021, 35, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef]
- Gori, A.; Lodigiani, G.; Colombarolli, S.G.; Bergamaschi, G.; Vitali, A. Cell Penetrating Peptides: Classification, Mechanisms, Methods of Study, and Applications. ChemMedChem 2023, 18, e202300236. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhang, X.; Zhang, W.; Guo, S.; Jin, F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release 2014, 174, 126–136. [Google Scholar] [CrossRef]
- Eiríksdóttir, E.; Konate, K.; Langel, U.; Divita, G.; Deshayes, S. Secondary structure of cell-penetrating peptides controls membrane interaction and insertion. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 1119–1128. [Google Scholar] [CrossRef]
- Derossi, D.; Calvet, S.; Trembleau, A.; Brunissen, A.; Chassaing, G.; Prochiantz, A. Cell Internalization of the Third Helix of the Antennapedia Homeodomain Is Receptor-independent*. J. Biol. Chem. 1996, 271, 18188–18193. [Google Scholar] [CrossRef]
- Ruczynski, J.; Wierzbicki, P.M.; Kogut-Wierzbicka, M.; Mucha, P.; Siedlecka-Kroplewska, K.; Rekowski, P. Cell-penetrating peptides as a promising tool for delivery of various molecules into the cells. Folia Histochem. Cytobiol. 2014, 52, 257–269. [Google Scholar] [CrossRef]
- Duchardt, F.; Fotin-Mleczek, M.; Schwarz, H.; Fischer, R.; Brock, R. A Comprehensive Model for the Cellular Uptake of Cationic Cell-penetrating Peptides. Traffic 2007, 8, 848–866. [Google Scholar] [CrossRef]
- Ruseska, I.; Zimmer, A. Internalization mechanisms of cell-penetrating peptides. Beilstein J. Nanotechnol. 2020, 11, 101–123. [Google Scholar] [CrossRef]
- Bechara, C.; Sagan, S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef]
- Copolovici, D.M.; Langel, K.; Eriste, E.; Langel, U. Cell-Penetrating Peptides: Design, Synthesis, and Applications. ACS Nano 2014, 8, 1972–1994. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S.; Nakase, I.; Tadokoro, A.; Takeuchi, T.; Jones, A.T. Arginine-rich peptides and their internalization mechanisms. Biochem. Soc. Trans. 2007, 35, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.T. Macropinocytosis: Searching for an endocytic identity and role in the uptake of cell penetrating peptides. J. Cell. Mol. Med. 2007, 11, 670–684. [Google Scholar] [CrossRef]
- Haucke, V.; Kozlov, M.M. Membrane remodeling in clathrin-mediated endocytosis. J. Cell Sci. 2018, 131, jcs216812. [Google Scholar] [CrossRef]
- Machleidt, T.; Li, W.P.; Liu, P.; Anderson, R.G. Multiple Domains in Caveolin-1 Control Its Intracellular Traffic. J. Cell Biol. 2000, 148, 17–28. [Google Scholar] [CrossRef]
- Kiss, A.L.; Botos, E. Endocytosis via caveolae: Alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J. Cell. Mol. Med. 2009, 13, 1228–1237. [Google Scholar] [CrossRef]
- Pelkmans, L.; Püntener, D.; Helenius, A. Local Actin Polymerization and Dynamin Recruitment in SV40-Induced Internalization of Caveolae. Science 2002, 296, 535–539. [Google Scholar] [CrossRef]
| CPP | Type | Cancer Types | Function | Mechanism | Clinical Trial ID | References |
|---|---|---|---|---|---|---|
| PEP-010 | Cationic | Breast cancer | Restores apoptotic pathways | Disrupts the caspase-9/PP2A interaction, activating caspase-dependent apoptosis | NCT04733027 (Phase I) | [23,26,28] |
| ATX-101 | Cationic | Multyple myeloma/Sarcoma | Reaches the cell nucleus to enhance damage repair, cellular stress response, and the efficacy of several anticancer agents | Disrupts the PCNA/APIM-containing protein interaction | NCT05116683 (Phase II), NCT04814875 (Phase I/II), NCT01462786 (Phase I) | [30,31,32] |
| AVB-620 | Cationic | Breast cancer | Real-time tumor visualization during surgery | CPP conjugated with fluorophores Cy5/Cy7 for FRET, targeted to human breast cancer cells due to their MMP overexpression | NCT02391194 (Phase I), NCT03113825 (Phase II) | [37] |
| Z12 and ZEBRA-Derived CPPs | Cationic | Broad spectrum, including aggressive brain cancers | Component of cancer vaccines | Promotes immune responses against tumors when conjugated with multi-epitopic antigens | NCT04046445 (Phase I) | [38,39] |
| pVEC and PEGA | Cationic | Breast cancer | Targeted drug delivery vector | Selective non-endocytic translocating mechanism (pVEC) by targeting molecular markers on tumor cells when conjugated with homing peptides (PEGA) | [38,46,47] | |
| Pep-1 | Cationic | Broad spectrum | Targeted macromolecular carrier and drug delivery vector | High selective non-endocytic translocation through cancer cell membranes is primarily due to the high presence of acidic components | [55,56] | |
| MAP | Cationic | Broad spectrum | Bifunctional CPP that disrupts cancer cell membranes | Selective strong electrostatic interactions with negatively charged phospholipids | [73] | |
| p28 | Cationic | Multiple cancer types, including glioblastoma and hepatocellular carcinoma | Promotes cell-cycle arrest and apoptosis in tumor cells | Interacts with wild-type and mutant p53 proteins, inhibiting their ubiquitination and regulating their levels | NCT00914914 (Phase I), NCT01975116 (Phase I), NCT05359861 (Phase II), NCT06102525 (Phase I) | [77,78,79,80] |
| SAP and SAP(E) | Proline-rich amphipatic | Broad spectrum | Targeted drug delivery vector with minimal toxicity | Specific electrostatic interactions with negatively charged membrane components (SAP); internalization of aggregates in a non-clathrin or caveoline-mediated endocytosis (SAP(E)) | [84,85] | |
| Bac1-24 | Proline-rich amphipatic | Broad spectrum, particularly solid tumors | Targeted delivery agent of therapeutic proteins and peptides | Hydrophobic domains and specific electrostatic interactions with negatively charged phospholipids | [89] | |
| BIM-SAHBA | Stapled peptide | Hematologic cancers | Restores apoptosis in resistant cancer cells | Blocks the anti-apoptotic sequestration of BAX/BAK BH3 helices, mimicking the BH3 death domain | [90] | |
| SAHBD | Stapled peptide | Cancers where MCL-1 overexpression is a critical survival factor (myeloma, acute myeloid leukemia, melanoma, etc.) | Restores apoptosis in resistant cancer cells | Inhibits the MCL-1 anti-apoptotic activity, disrupting its interaction with pro-apoptotic proteins | [91] | |
| ALRN-6924 | Stapled peptide | Broad spectrum, including breast cancer and acute myeloid leukemia | Restores p53 function, reactivating apoptosis | Binds strongly to MDM2 and MDMX, inhibiting the p53 suppression | NCT02264613 (Phase I/II), NCT04022876 (Phase I), NCT03654716 (Phase I), NCT05622058 (Phase) | [97,98] |
| P1pal-7 | Pepducin | Breast, lung, and ovarian cancer | Reduces tumor growth and slows cancer progression. Anti-angiogenic agent | Interacts with PAR1, inhibiting its activation | [103,104] | |
| EN1-iPeps | Homeodomain-derived | Breast cancer | Triggers a selective apoptosis response | Inhibits the EN1 transcription factor in tumor cells where it is overexpressed | [111,113] | |
| Vectocell®/DPVs | HS Binding CPP | Broad spectrum | Targeted drug delivery agent (from small compounds to macromolecules) | Caveolae-mediated endocytosis of DPVs-Glycosaminoglycan clusters | [115] | |
| CPPecp | HS Binding CPP | Tumors with high HS expression, including colon cancer | Inhibits cancer cell migration and angiogenesis | Binding to overexpressed heparan sulfate on the surface of cancer cells | [116] | |
| Melittin and derivatives | Derived from animal venoms and toxins | Broad spectrum | Drug delivery vector and apoptosis inductors in tumor-associated macrophages | An amphipathic -helix structure enables interactions with the membrane, allowing the internalization of conjugated pro-apoptotic peptides | [92,119,120] | |
| Lycosin-I and R-lycosin-I | Derived from animal venoms and toxins | Broad spectrum | Induces apoptosis in cancer cells and inhibits cell proliferation | Activates the mitochondrial death pathway and upregulates p27 | [125,126,127,128] | |
| Pardaxins | Derived from animal venoms and toxins | Broad spectrum including aggressive cancers such as ovarian cancer and oral squamous cell carcinoma | Apoptosis inductor | Generation of ROS and mitochondrial membrane depolarization | [134,139] | |
| BT1718 | Cyclic | Solid and refractary tumors | Selective realese of cytotoxic agents | Binds to overexpressed MT1-MMP in tumors realising DM1, a cytotoxic payload | NCT03486730 (Phase I/II) | [142,144,145] |
| 177Lu-DOTA0-Tyr3-Octreotate (Lutathera®) | Cyclic | SSTR2-positive neuroendocrine tumors | Selective delivery of cytotoxic agent | This radioconjugate utilizes the somatostatin analog TATE to target SSTR2-positive neuroendocrine tumors, delivering a cytotoxic dose of radiation | NCT02125474 (Phase II), NCT02236910 (Phase II), NCT03325816 (Phase I/Phase II) | [146,150,151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Vargas, L.M.; Prada-Gracia, D. Cancer-Targeting Applications of Cell-Penetrating Peptides. Int. J. Mol. Sci. 2025, 26, 2. https://doi.org/10.3390/ijms26010002
Moreno-Vargas LM, Prada-Gracia D. Cancer-Targeting Applications of Cell-Penetrating Peptides. International Journal of Molecular Sciences. 2025; 26(1):2. https://doi.org/10.3390/ijms26010002
Chicago/Turabian StyleMoreno-Vargas, Liliana Marisol, and Diego Prada-Gracia. 2025. "Cancer-Targeting Applications of Cell-Penetrating Peptides" International Journal of Molecular Sciences 26, no. 1: 2. https://doi.org/10.3390/ijms26010002
APA StyleMoreno-Vargas, L. M., & Prada-Gracia, D. (2025). Cancer-Targeting Applications of Cell-Penetrating Peptides. International Journal of Molecular Sciences, 26(1), 2. https://doi.org/10.3390/ijms26010002







