Exploring the Link Between Infections and Primary Osteoarthritis: A Next-Generation Metagenomic Sequencing Approach
Abstract
1. Introduction
Objective
2. Results
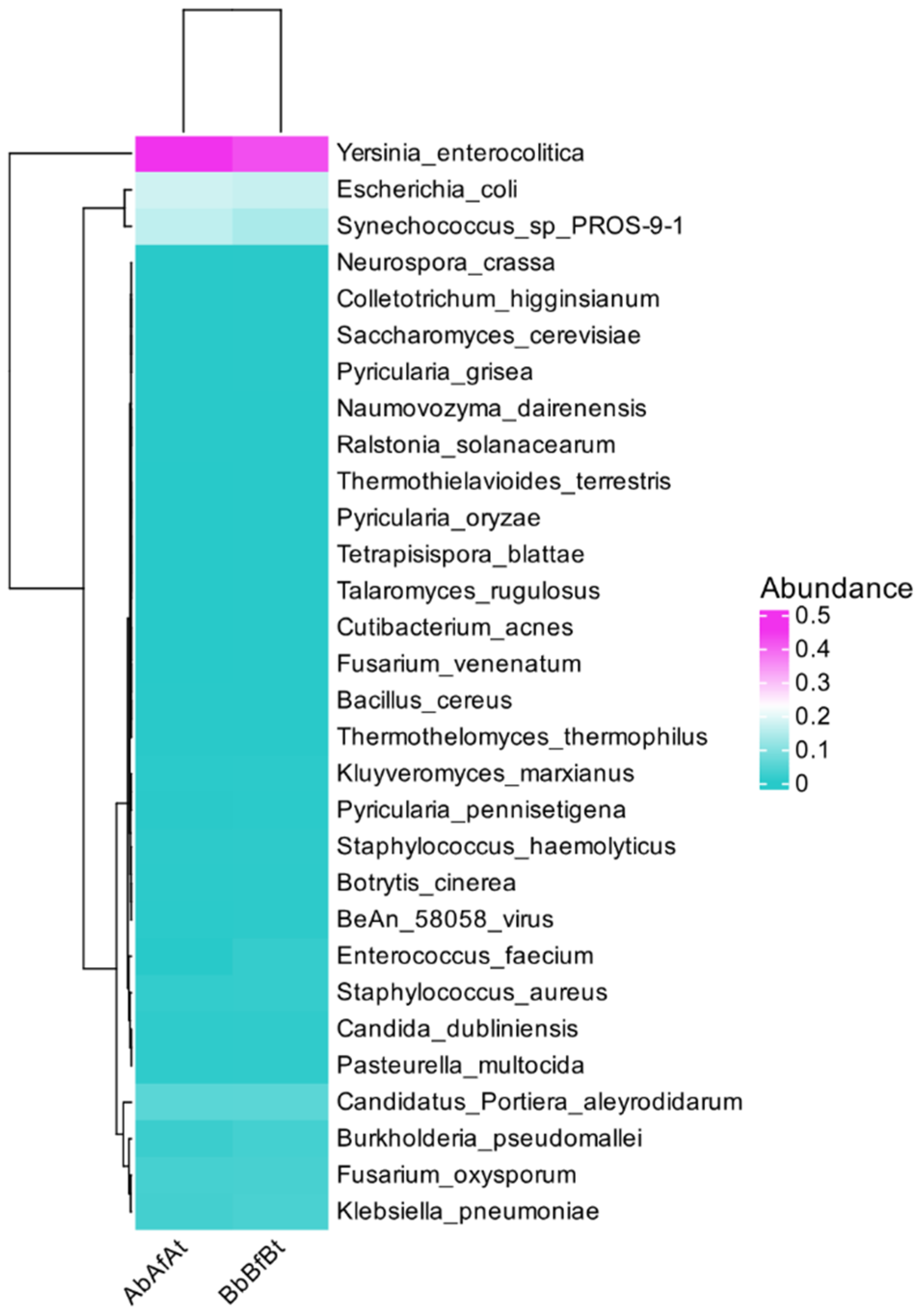
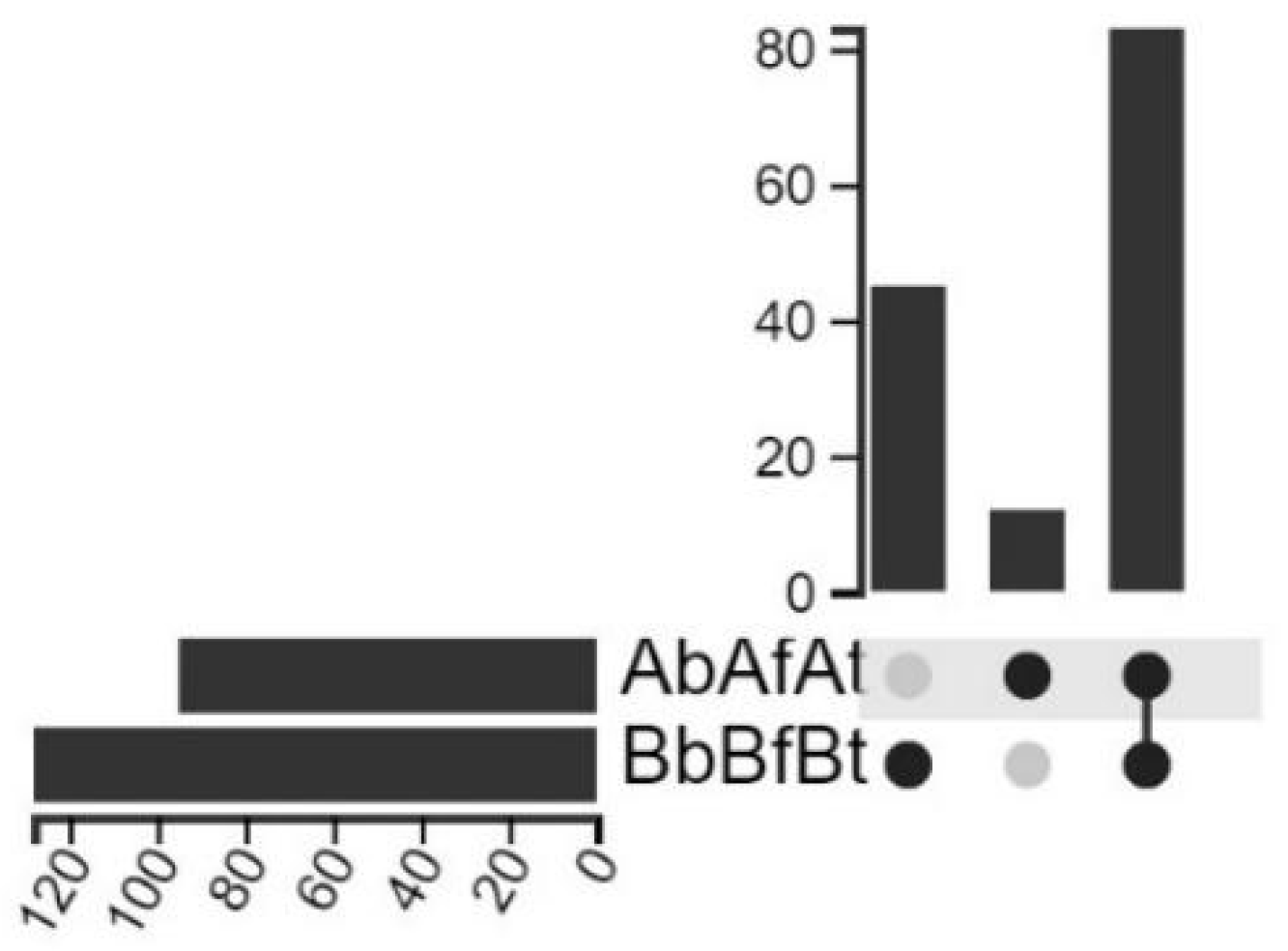
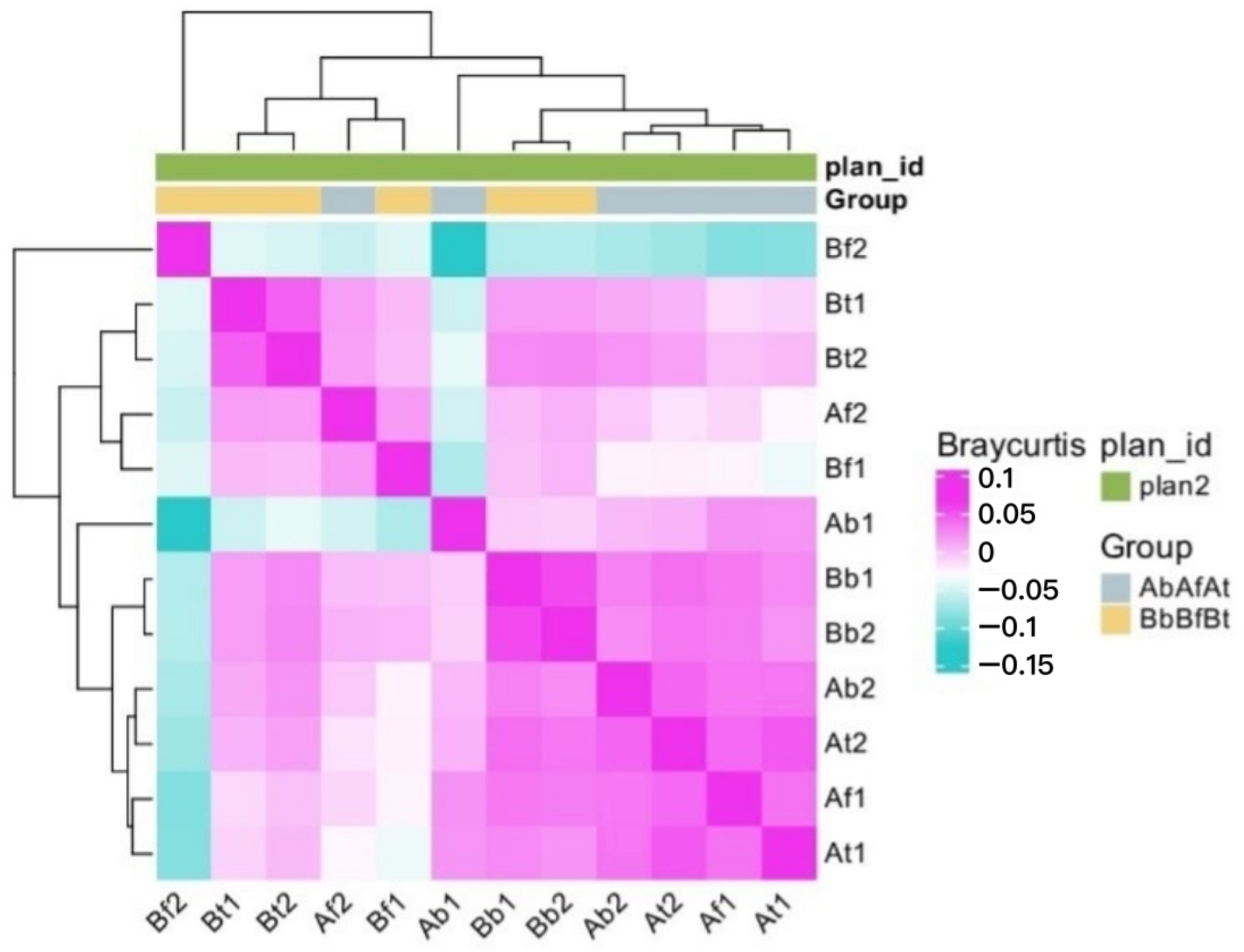
2.1. Taxonomic Distribution
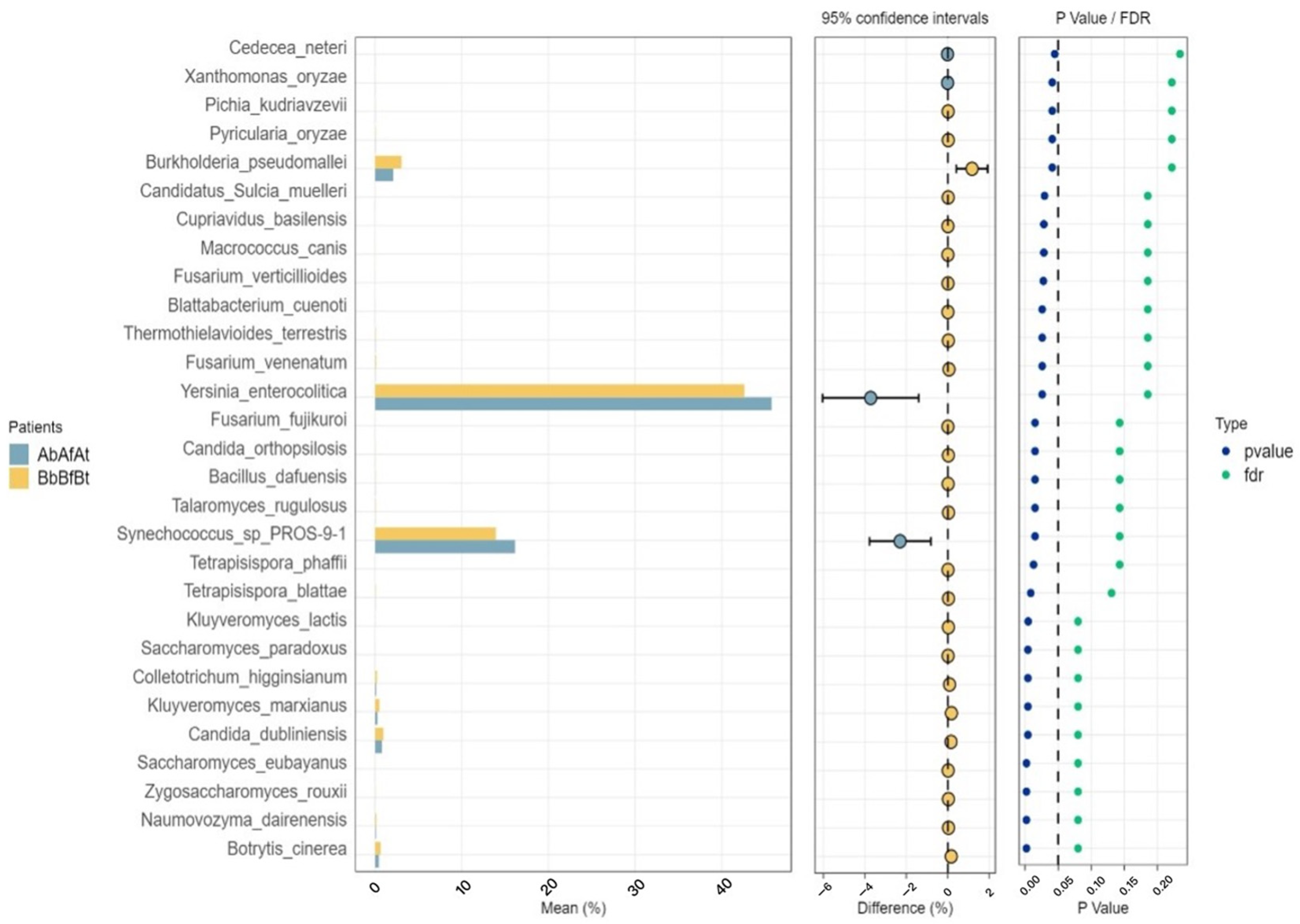
2.2. Microbial Communities
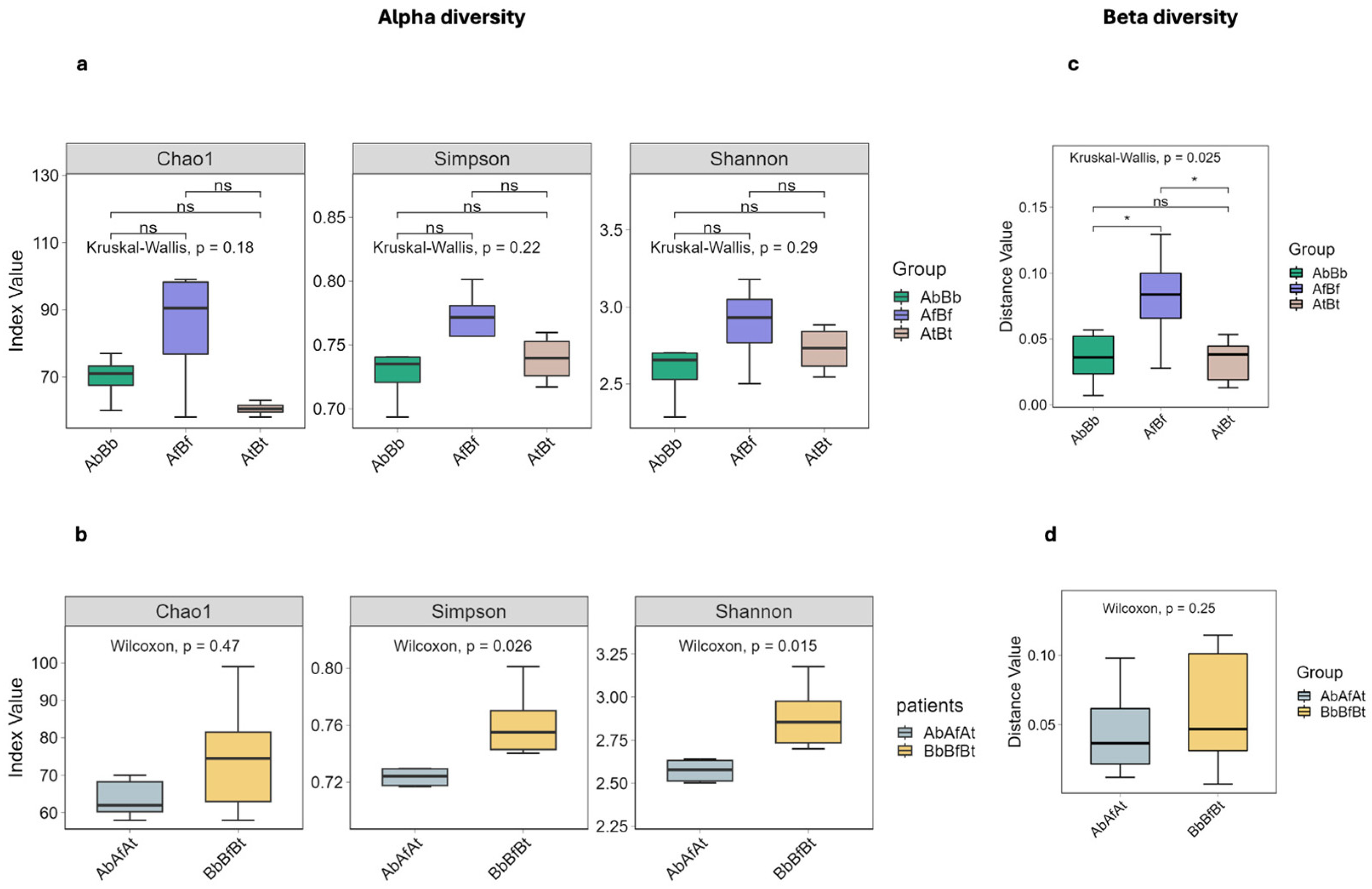
2.3. Taxonomic Diversity
3. Discussion
The Limitations of the Study
4. Materials and Methods
4.1. The Patients
- -
- No medical history of symptoms suggestive of acute arthritis, including reactive post-infectious arthritis;
- -
- No medical history of musculoskeletal trauma;
- -
- No autoimmune or metabolic inflammatory joint disease: rheumatoid arthritis, morbid obesity, gout, diabetes, or psoriasis;
- -
- No active infection elsewhere in the body;
- -
- No active participation in sports that chronically stress the knee joint;
- -
- No neurodegenerative diseases;
- -
- Below the age of 70 years.
4.2. Samples
4.3. DNA Isolation and Library Construction
4.4. Sequencing
4.5. Quality Control of Sequencing Reads and Removal of Host Reads
4.6. Assembly and Gene Prediction
4.7. Taxonomic Annotation
4.8. Diversities
4.8.1. Alpha Diversity
4.8.2. Beta Diversity
4.9. Differential Species Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, Regional, and National Burden of Osteoarthritis, 1990–2020 and Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Motta, F.; Barone, E.; Sica, A.; Selmi, C. Inflammaging and osteoarthritis. Clin. Rev. Allergy Immunol. 2023, 64, 222–238. [Google Scholar] [CrossRef]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Howell, D.S. Pathogenesis of osteoarthritis. Am. J. Med. 1986, 80, 24–28. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Cho, M.L.; Choi, H.Y.; Yoon, C.S.; Jhun, J.Y.; Oh, H.J.; Kim, H.Y. The catabolic pathway mediated by toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006, 54, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- van Lent, P.L.E.M.; Blom, A.B.; Schelbergen, R.F.P.; Slöetjes, A.; Lafeber, F.P.J.G.; Lems, W.F.; Cats, H.; Vogl, T.; Roth, J.; van den Berg, W.B. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012, 64, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Kanda, V.; Bush-Joseph, C.; Verma, N.; Chubinskaya, S.; Mikecz, K.; Glant, T.T.; Malfait, A.M.; Crow, M.K.; Spear, G.T.; et al. Synovial fluid from patients with early osteoarthritis modulates fibroblast-like synoviocyte responses to toll-like receptor 4 and toll-like receptor 2 ligands via soluble CD14. Arthritis Rheum. 2012, 64, 2268–2277. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Thiemermann, C. Role of metabolic endotoxemia in systemic inflammation and potential interventions. Front. Immunol. 2020, 11, 594150. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.O.; Cho, M.L.; Kang, C.M.; Jhun, J.Y.; Park, J.S.; Oh, H.J.; Min, J.K.; Park, S.H.; Kim, H.Y. Toll-like receptor 2 and 4 combination engagement upregulate IL-15 synergistically in human rheumatoid synovial fibroblasts. Immunol. Lett. 2007, 109, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Dubey, D.; Kumar, S.; Rawat, A.; Guleria, A.; Kumari, R.; Ahmed, S.; Singh, R.; Misra, R.; Kumar, D. NMR-based metabolomics revealed the underlying inflammatory pathology in reactive arthritis synovial joints. J. Proteome Res. 2021, 20, 5088–5102. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, H. History of reactive arthritis. Historical milestones and future. Z. Rheumatol. 2022, 81, 692–698. [Google Scholar] [CrossRef]
- Marchese, L.; Contartese, D.; Giavaresi, G.; Di Sarno, L.; Salamanna, F. The complex interplay between the gut microbiome and osteoarthritis: A systematic review on potential correlations and therapeutic approaches. Int. J. Mol. Sci. 2023, 25, 143. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Miller, S.A. Clinical metagenomics. Nat. Rev. Genet. 2019, 20, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, R.H.; Bathoorn, E.; Chlebowicz, M.A.; Couto, N.; Ferdous, M.; García-Cobos, S.; Kooistra-Smid, A.M.D.; Raangs, E.C.; Rosema, S.; Veloo, A.C.M.; et al. Application of next generation sequencing in clinical microbiology and infection prevention. J. Biotechnol. 2017, 243, 16–24. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, S.; Guo, W.; Peng, L.; Zhao, F.; Wang, L.; Fan, G.; Zhu, Y.; Xu, D.; Liu, G.; et al. Sequencing Introduced False Positive Rare Taxa Lead to Biased Microbial Community Diversity, Assembly, and Interaction Interpretation in Amplicon Studies. Environ. Microbiome 2022, 17, 43. [Google Scholar] [CrossRef]
- Han, S. Osteoarthritis year in review 2022: Biology. Osteoarthr. Cartil. 2022, 30, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Takahashi, N.; Terabe, K.; Ohashi, Y.; Kishimoto, K.; Yokota, Y.; Suzuki, M.; Kojima, T.; Imagama, S. Activation of transient receptor potential vanilloid 4 protects articular cartilage against inflammatory responses via CaMKK/AMPK/NF-κB signaling pathway. Sci. Rep. 2021, 11, 15508. [Google Scholar] [CrossRef]
- Kasperkiewicz, K.; Świerzko, A.S.; Przybyła, M.; Szemraj, J.; Barski, J.; Skurnik, M.; Kałużyński, A.; Cedzyński, M. The role of Yersinia enterocolitica O:3 lipopolysaccharide in collagen-induced arthritis. J. Immunol. Res. 2020, 2020, 7439506. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.E.; Sebastian, A.; Murugesh, D.K.; Hum, N.R.; McCool, J.L.; Hsia, A.W.; Christiansen, B.A.; Loots, G.G. LPS-induced inflammation prior to injury exacerbates the development of post-traumatic osteoarthritis in mice. J. Bone Miner. Res. 2020, 35, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.; Yang, J.I.; Park, S.; Chun, J.S. Lipopolysaccharide binding protein and CD14, cofactors of toll-like receptors, are essential for low-grade inflammation-induced exacerbation of cartilage damage in mouse models of posttraumatic osteoarthritis. Arthritis Rheumatol. 2021, 73, 1451–1460. [Google Scholar] [CrossRef]
- Guillier, L.; Fravalo, P.; Leclercq, A.; Thébault, A.; Kooh, P.; Cadavez, V.; Gonzales-Barron, U. Risk factors for sporadic Yersinia enterocolitica infections: A systematic review and meta-analysis. Microb. Risk Anal. 2021, 17, 100141. [Google Scholar] [CrossRef]
- Larsen, J.H. Yersinia enterocolitica infections and rheumatic diseases. Scand. J. Rheumatol. 1980, 9, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Granfors, K.; Jalkanen, S.; von Essen, R.; Lahesmaa-Rantala, R.; Isomäki, O.; Pekkola-Heino, K.; Merilahti-Palo, R.; Saario, R.; Isomäki, H.; Toivanen, A. Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N. Engl. J. Med. 1989, 320, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Granfors, K.; Merilahti-Palo, R.; Luukkainen, R.; Möttönen, T.; Lahesmaa, R.; Probst, P.; Märker-Hermann, E.; Toivanen, P. Persistence of Yersinia antigens in peripheral blood cells from patients with Yersinia enterocolitica O:3 infection with or without reactive arthritis. Arthritis Rheum. 1998, 41, 855–862. [Google Scholar] [CrossRef]
- Wuorela, M.; Jalkanen, S.; Toivanen, P.; Granfors, K. Yersinia lipopolysaccharide is modified by human monocytes. Infect. Immun. 1993, 61, 5261–5270. [Google Scholar] [CrossRef]
- Di Genaro, M.S.; Muñoz, E.; Aguilera, C.; de Guzmán, A.M. Yersinia enterocolitica O:8 and O:5 lipopolysaccharide arthritogenicity in hamsters. Rheumatology 2000, 39, 73–78. [Google Scholar] [CrossRef][Green Version]
- Toivanen, A.; Merilahti-Palo, R.; Gripenberg, C.; Lahesmaa-Rantala, R.; Söderström, K.O.; Jaakkola, U.M. Yersinia-associated arthritis in the rat: Experimental model for human reactive arthritis? Acta Pathol. Microbiol. Immunol. Scand. C 1986, 94, 261–269. [Google Scholar] [CrossRef]
- de los Toyos, J.R.; Menéndez, P.; Sampedro, A.; Hardisson, C. Yersinia enterocolitica serotype 0:3-induced arthritis in mice: Microbiological and histopathological information. APMIS 1992, 100, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Saebø, A.; Lassen, J. Yersinia enterocolitica: An inducer of chronic inflammation. Int. J. Tissue React. 1994, 16, 51–57. [Google Scholar]
- Liu, Y.X.; Zhong, H.; Le, K.J.; Cui, M. Bloodstream infection caused by Yersinia enterocolitica in a host with ankylosing spondylitis: A case report and literature review. Ann. Palliat. Med. 2021, 10, 5780–5785. [Google Scholar] [CrossRef]
- Tuompo, R.; Lääveri, T.; Hannu, T.; Pakkanen, S.H.; Kirveskari, J.; Leirisalo-Repo, M.; Kantele, A. Reactive arthritis and other musculoskeletal symptoms associated with acquisition of diarrhoeagenic Escherichia coli (DEC). Ann. Rheum. Dis. 2020, 79, 605–611. [Google Scholar] [CrossRef]
- Newkirk, M.M.; Zbar, A.; Baron, M.; Manges, A.R. Distinct bacterial colonization patterns of Escherichia coli subtypes associate with rheumatoid factor status in early inflammatory arthritis. Rheumatology 2010, 49, 1311–1316. [Google Scholar] [CrossRef]
- Shafiee, D.; Salpynov, Z.; Gusmanov, A.; Khuanbai, Y.; Mukhatayev, Z.; Kunz, J. Enteric infection-associated reactive arthritis: A systematic review and meta-analysis. J. Clin. Med. 2024, 13, 3433. [Google Scholar] [CrossRef] [PubMed]
- Noyori, K.; Okamoto, R.; Takagi, T.; Hyodo, A.; Suzuki, K.; Koshino, T. Experimental induction of arthritis in rats immunized with Escherichia coli 0: 14 lipopolysaccharide. J. Rheumatol. 1994, 21, 484–488. [Google Scholar] [PubMed]
- Callieri, C. Synechococcus plasticity under environmental changes. FEMS Microbiol. Lett. 2017, 364, fnx229. [Google Scholar] [CrossRef]
- Wiśniewska, K.A.; Śliwińska-Wilczewska, S.; Lewandowska, A.U. Airborne microalgal and cyanobacterial diversity and composition during rain events in the southern Baltic Sea region. Sci. Rep. 2022, 12, 2029. [Google Scholar] [CrossRef] [PubMed]
- Facciponte, D.N.; Bough, M.W.; Seidler, D.; Carroll, J.L.; Ashare, A.; Andrew, A.S.; Tsongalis, G.J.; Vaickus, L.J.; Henegan, P.L.; Butt, T.H.; et al. Identifying aerosolized cyanobacteria in the human respiratory tract: A proposed mechanism for cyanotoxin-associated diseases. Sci. Total Environ. 2018, 645, 1003–1013. [Google Scholar] [CrossRef]
- Jiang, Z.F.; Xia, F.; Johnson, K.W.; Bartom, E.; Tuteja, J.H.; Stevens, R.; Grossman, R.L.; Brumin, M.; White, K.P.; Ghanim, M. Genome sequences of the primary endosymbiont “Candidatus Portiera aleyrodidarum” in the whitefly Bemisia tabaci B and Q biotypes. J. Bacteriol. 2012, 194, 6678–6679. [Google Scholar] [CrossRef] [PubMed]
- Maymon, M.; Sela, N.; Shpatz, U.; Galpaz, N.; Freeman, S. The origin and current situation of Fusarium oxysporum f. sp. cubense tropical race 4 in Israel and the Middle East. Sci. Rep. 2020, 10, 1590. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, C.I.; Lee, J.H.; Lee, W.J.; Huh, K.; Cho, S.Y.; Chung, D.R.; Peck, K.R. Clinical features and outcomes of invasive fusariosis: A case series in a single center with literature review. Infect. Chemother. 2023, 55, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Tortorano, A.M.; Prigitano, A.; Esposto, M.C.; Arsic Arsenijevic, V.; Kolarovic, J.; Ivanovic, D.; Paripovic, L.; Klingspor, L.; Nordøy, I.; Hamal, P.; et al. ECMM Working Group European confederation of medical mycology (ECMM) epidemiological survey on invasive infections due to Fusarium species in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.D.; Garcia, E.C. Burkholderia pseudomallei. Trends Microbiol. 2024, 32, 105–106. [Google Scholar] [CrossRef]
- Eisenhofer, R.; Minich, J.J.; Marotz, C.; Cooper, A.; Knight, R.; Weyrich, L.S. Contamination in low microbial biomass microbiome studies: Issues and recommendations. Trends Microbiol. 2019, 27, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Chen, J.; Lin, X.; He, W.; Liang, H.; Wang, M.; Li, W.; Wu, Z.; Han, M.; Jin, X.; et al. Comparison of the DNBSEQ Platform and Illumina HiSeq 2000 for Bacterial Genome Assembly. Sci. Rep. 2024, 14, 1292. [Google Scholar] [CrossRef]
- Chrisman, B.; He, C.; Jung, J.Y.; Stockham, N.; Paskov, K.; Washington, P.; Wall, D.P. The human “contaminome”: Bacterial, viral, and computational contamination in whole genome sequences from 1000 families. Sci. Rep. 2022, 12, 9863. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Koren, S.; Rhie, A.; Rautiainen, M.; Bzikadze, A.V.; Mikheenko, A.; Vollger, M.R.; Altemose, N.; Uralsky, L.; Gershman, A.; et al. The complete sequence of a human genome. Science 2022, 376, 44–53. [Google Scholar] [CrossRef]

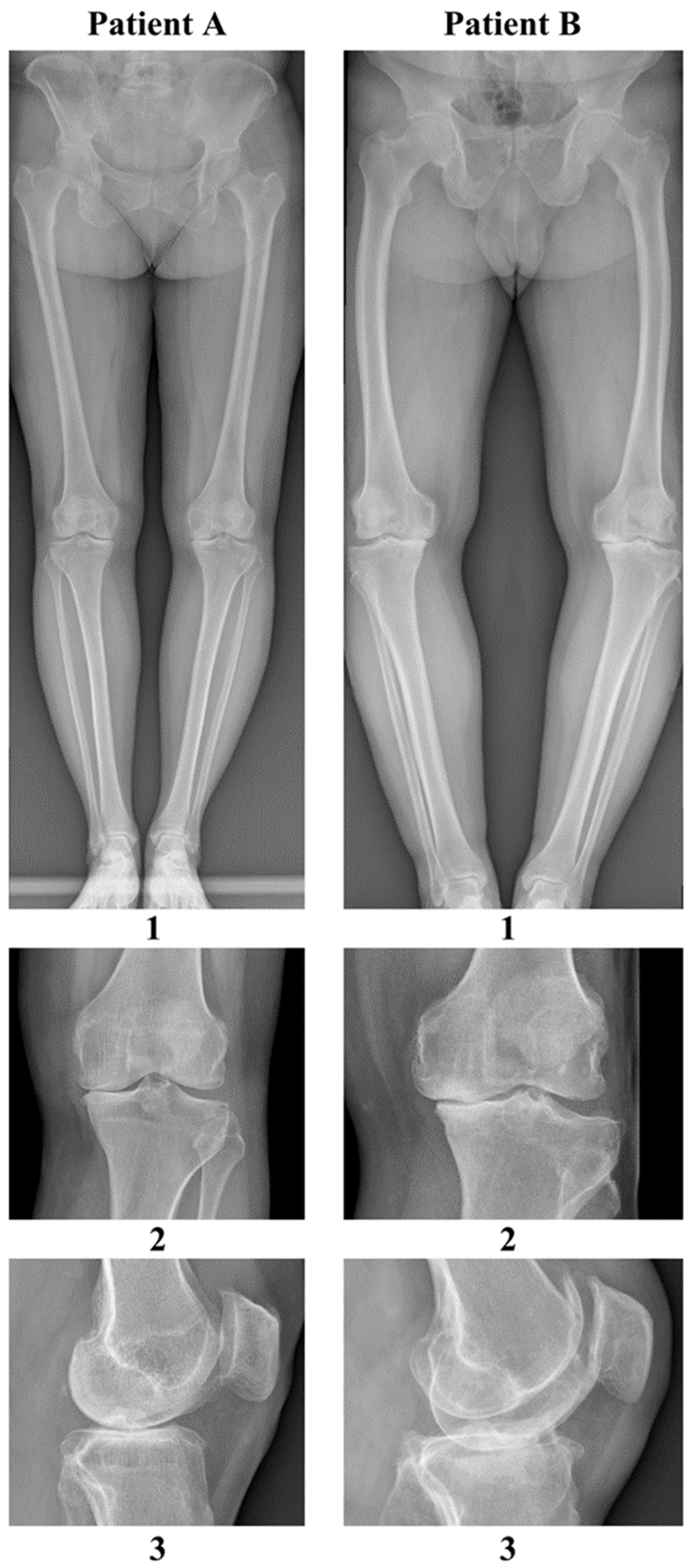
| Feature | Patient A | Patient B |
|---|---|---|
| Gender | female | male |
| Height | 165 cm | 170 cm |
| Weight | 77 kg | 96 kg |
| BMI | 28.0 kg/m2 | 33.6 kg/m2 |
| Blood type | A Rh (−) (no antibodies) | B Rh (+) (no antibodies) |
| Age | 69 | 61 |
| ASA scale | 2 | 2 |
| Occupation | office worker | farmer (farm work, contact with domesticated ruminants) |
| Chronic diseases | hypertension overweight restless legs syndrome, multilevel spondyloarthritis positive tuberculin skin test during childhood | hypertension, obesity class 1, recurrent urinary tract infections, during childhood |
| Chronic medications | ropinirole rosuvastatin lacidipine, valsartan mianserin, paracetamol + tramadol | amlodipine + telmisartan acetylsalicylic acid potassium aspartate magnesium aspartate |
| Previous surgeries and procedures | tonsillectomy (age 15) appendectomy (age 16) ovarian cyst removal (age 46) dental prosthetic implantation (age 64) decompression of stenosis in the lumbar spine (age 68) | right-sided inguinal hernia (age 58) |
| Allergies | none | household dust trimethoprim–sulfamethoxazole |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niecwietajewa, I.; Banasiewicz, J.; Zaremba-Wróblewski, G.; Majewska, A. Exploring the Link Between Infections and Primary Osteoarthritis: A Next-Generation Metagenomic Sequencing Approach. Int. J. Mol. Sci. 2025, 26, 20. https://doi.org/10.3390/ijms26010020
Niecwietajewa I, Banasiewicz J, Zaremba-Wróblewski G, Majewska A. Exploring the Link Between Infections and Primary Osteoarthritis: A Next-Generation Metagenomic Sequencing Approach. International Journal of Molecular Sciences. 2025; 26(1):20. https://doi.org/10.3390/ijms26010020
Chicago/Turabian StyleNiecwietajewa, Irina, Jakub Banasiewicz, Gabriel Zaremba-Wróblewski, and Anna Majewska. 2025. "Exploring the Link Between Infections and Primary Osteoarthritis: A Next-Generation Metagenomic Sequencing Approach" International Journal of Molecular Sciences 26, no. 1: 20. https://doi.org/10.3390/ijms26010020
APA StyleNiecwietajewa, I., Banasiewicz, J., Zaremba-Wróblewski, G., & Majewska, A. (2025). Exploring the Link Between Infections and Primary Osteoarthritis: A Next-Generation Metagenomic Sequencing Approach. International Journal of Molecular Sciences, 26(1), 20. https://doi.org/10.3390/ijms26010020






