Transgenic Cotton Expressing dsAgCYP6CY3 Significantly Delays the Growth and Development of Aphis gossypii by Inhibiting Its Glycolysis and TCA Cycle
Abstract
1. Introduction
2. Results
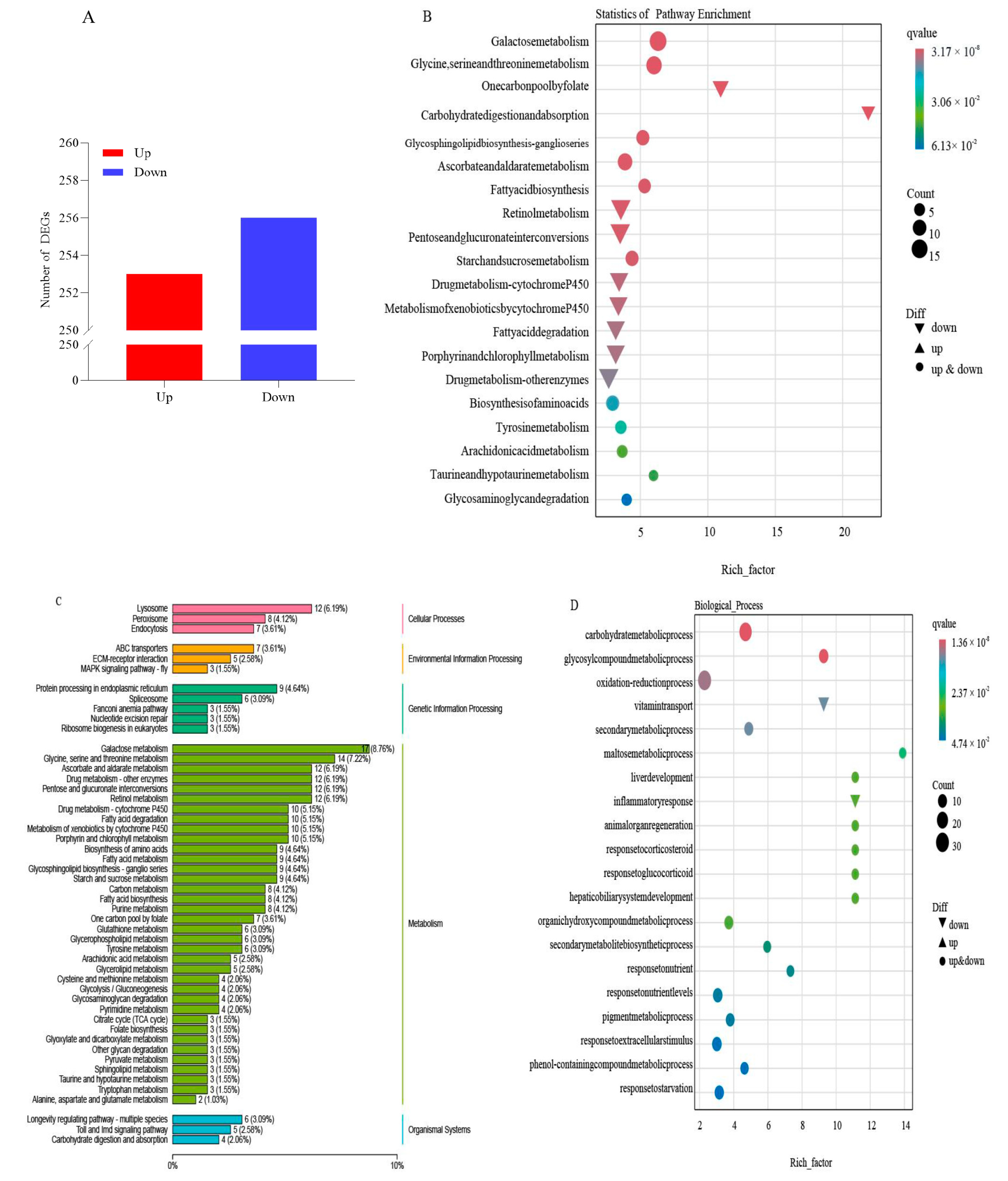
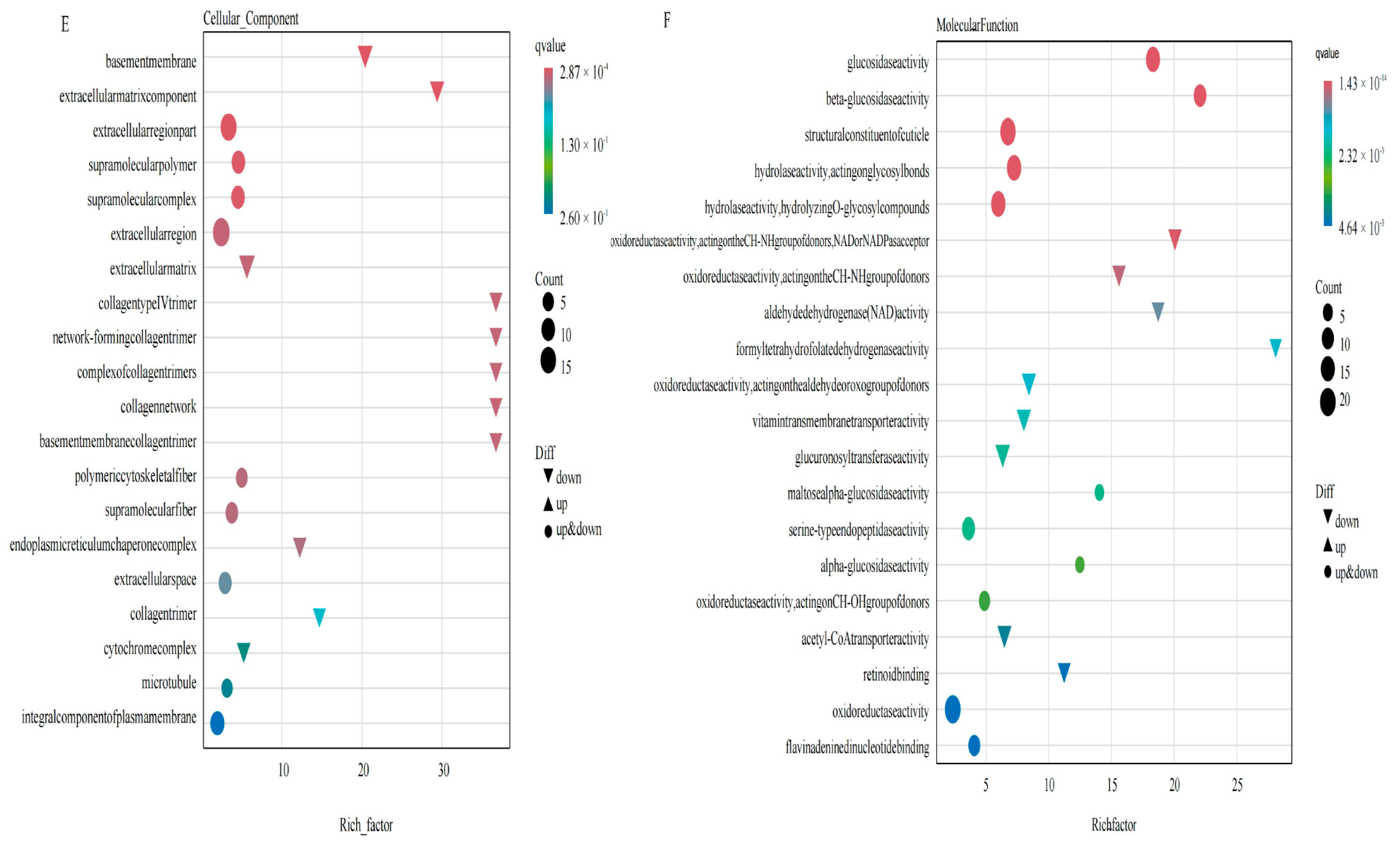
2.1. Effect of TG Cotton on Transcriptome of Cotton Aphid
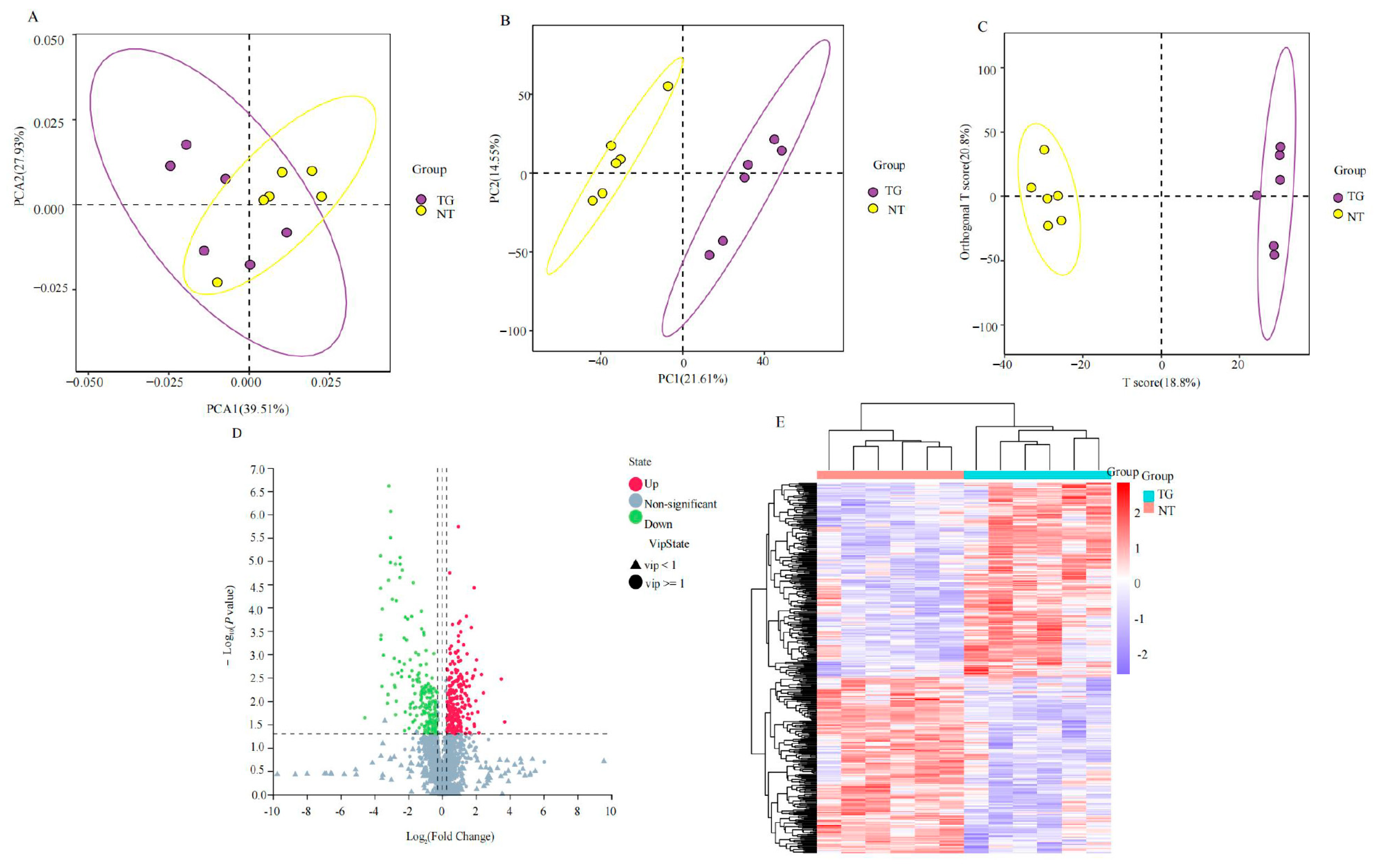
2.2. Effect of TG Cotton on Metabolome of the Cotton Aphid
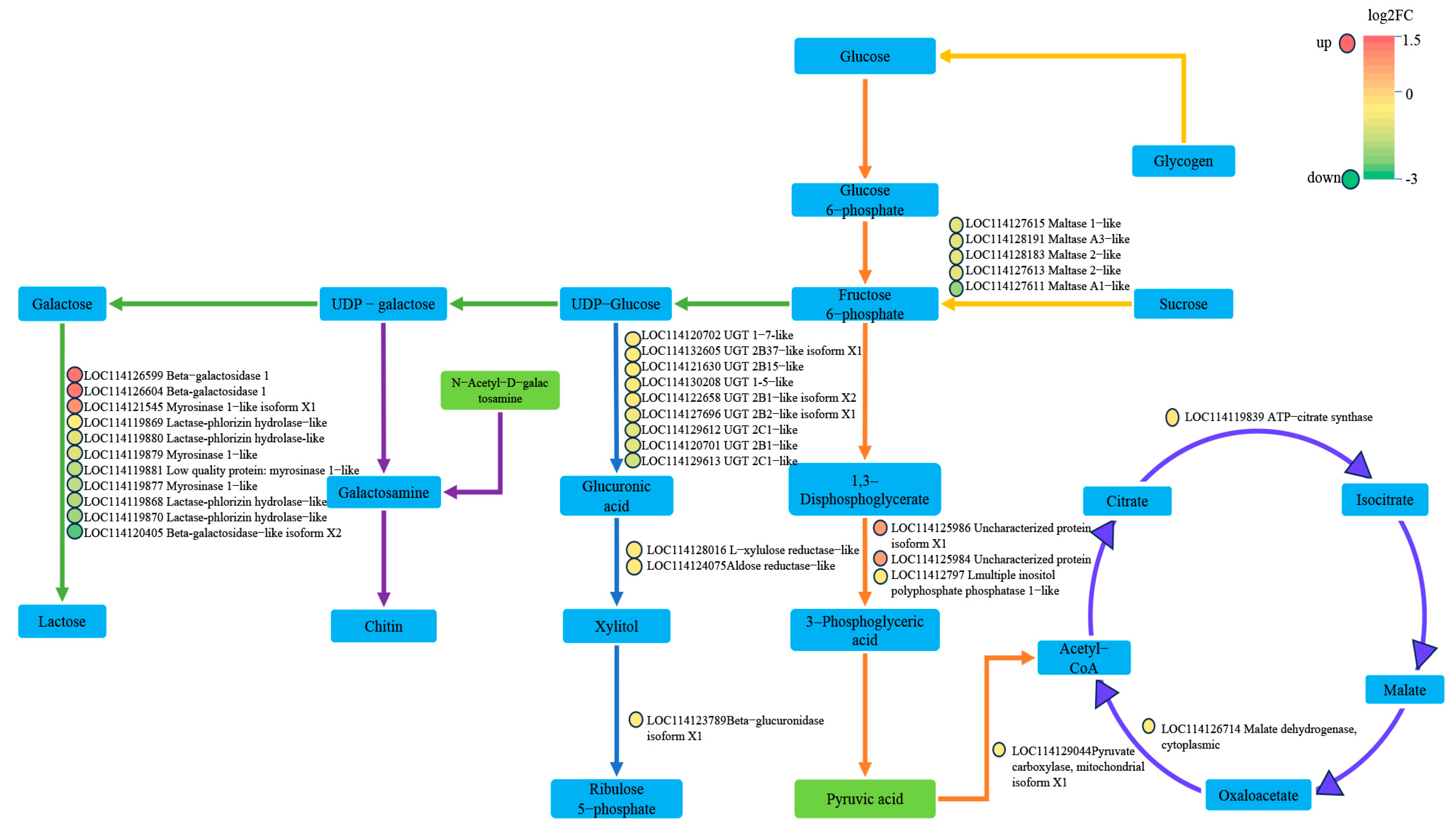
2.3. DEGs and DAMs Co-Enriched in Glycolysis and TCA Cycle Based on Transcriptomic and Metabolomic Analyses
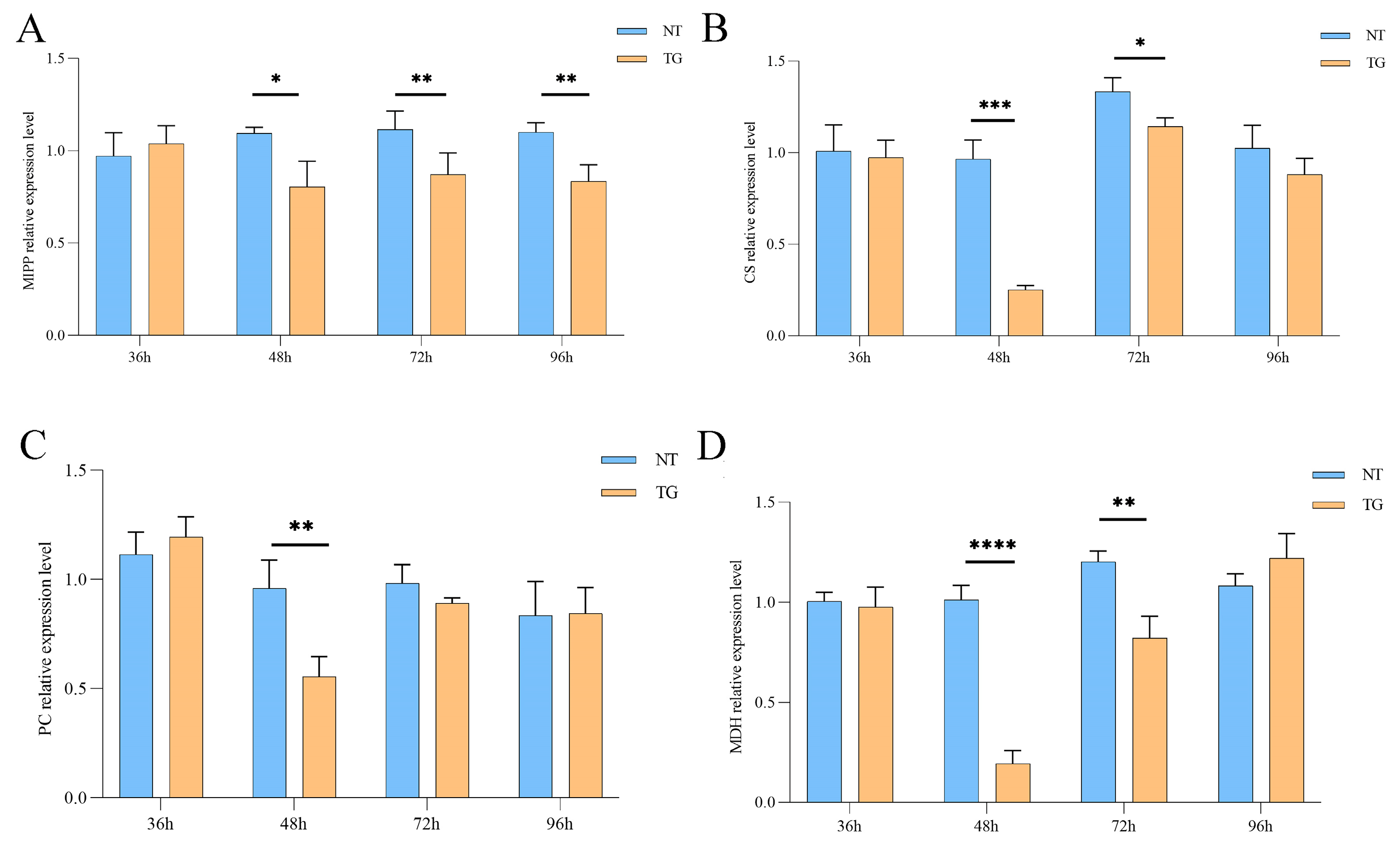
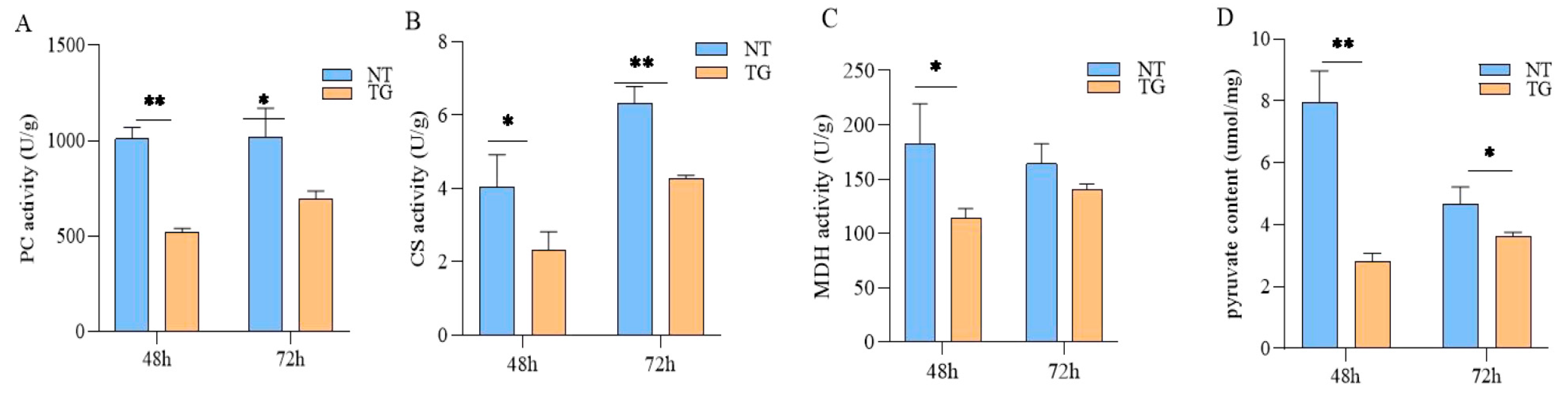
2.4. Effects on Glycolysis and TCA Cycle of Cotton Aphid Fed on TG Cotton
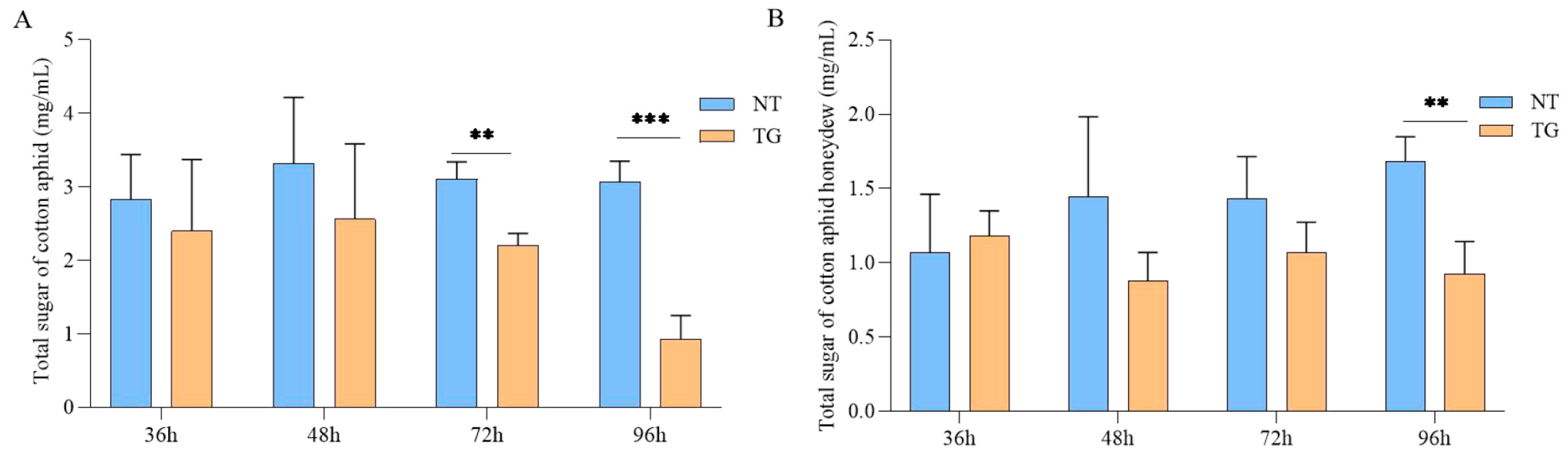
2.5. Change Profile of Total Sugar Content and Honeydew of Cotton Aphid Treated with TG Cotton
3. Discussion
4. Materials and Methods
4.1. Insects and Cotton Plants
4.2. RNA Sequencing (RNA-Seq) Data Analysis
4.3. Extraction of Cotton Aphid Metabolites
4.4. UHPLC-MS/MS Analysis and Data Analysis
4.5. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.6. Determination of Enzyme Activities and Pyruvate Content in Glycometabolism
4.7. Determination of Total Sugar Content
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakravarthy, V.S.; Reddy, T.P.; Reddy, V.D.; Rao, K.V. Current status of genetic engineering in cotton (Gossypium hirsutum L.): An assessment. Crit. Rev. Biotechnol. 2014, 34, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.; Zhao, J.; Xue, D.Y. Access and control of agro-biotechnology: Bt cotton, ecological change and risk in China. J. Peasant. Stud. 2009, 36, 345–364. [Google Scholar] [CrossRef]
- Xu, L.; Duan, X.; Lv, Y.; Zhang, X.; Nie, Z.; Xie, C.; Ni, Z.; Liang, R. Silencing of an aphid carboxylesterase gene by use of plant-mediated RNAi impairs Sitobion avenae tolerance of phoxim insecticides. Transgenic Res. 2014, 23, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Feng, G.L.; Yuan, J.G.; Gong, K.Y. Insecticide resistance of cotton aphid in North China. Insect Sci. 1994, 1, 242–251. [Google Scholar] [CrossRef]
- Lu, Y.H.; Liang, G.M.; Zhang, Y.J.; Yang, X.M. Advances in the management of insect pests of cotton in China since the 21st century. J. Appl. Entomol. 2020, 57, 477–490. [Google Scholar]
- Ma, K.S.; Li, F.; Liu, Y.; Liang, P.Z.; Chen, X.W.; Gao, X.W. Identification of microRNAs and their response to the stress of plant allelochemicals in Aphis gossypii (Hemiptera: Aphididae). BMC Mol. Biol. 2017, 18, 5. [Google Scholar] [CrossRef]
- Carletto, J.; Martin, T.; Masutti, F.V.; Brévault, T. Insecticide resistance traits differ among and within host races in Aphis gossypii. Pest. Manag. 2010, 66, 301–307. [Google Scholar] [CrossRef]
- Gray, S.M.; Moyer, J.W.; Kennedy, G.G.; Campbell, C.L. Virus-suppression and aphid resistance effects on spatial and temporal spread of watermelon mosaic virus 2. Phytopathology 1986, 76, 11. [Google Scholar] [CrossRef]
- Berlandier, F.A. Aphids on the World’s crops. An information and identification guide. Aust. J. Entomol. 2000, 39, 354–355. [Google Scholar]
- Rebijith, K.B.; Asokan, R.; Kumar, N.K.; Krishna, V.; Chaitanya, B.N.; Ramamurthy, V.V. DNA barcoding and elucidation of cryptic aphid species (Hemiptera: Aphididae) in India. Bull. Entomol. Res. 2013, 103, 601–610. [Google Scholar] [CrossRef]
- Ewart, W.H.; Metcalf, R.L.; Tollenaar, W.; Kos, M. Vet LEM and Harvey JA, Honeydew as a food source for natural enemies: Making the best of a bad meal? Biol. Control 2008, 45, 176–184. [Google Scholar]
- Fernández de Bobadilla, M.; Ramírez, N.M.; Calvo-Agudo, M.; Dicke, M.; Tena, A. Honeydew management to promote biological control. Curr. Opin. Insect Sci. 2024, 61, 101151. [Google Scholar] [CrossRef]
- Lin, R.; Yang, M.; Yao, B. The phylogenetic and evolutionary analyses of detoxification gene families in Aphidinae species. PLoS ONE 2022, 17, e0263462. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.S.; Rider, D.S.; Walsh, T.K.; De Vos, M.; Gordon, K.H.; Ponnala, L.; Macmil, S.L.; Roe, B.A.; Jander, G. Comparative analysis of detoxification enzymes in Acyrthosiphon pisum and Myzus persicae. Insect Mol. Biol. 2010, 19, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Rane, R.V.; Walsh, T.K.; Pearce, S.L.; Jermiin, L.S.; Gordon, K.H.; Richards, S.; Oakeshott, J.G. Are feeding preferences and insecticide resistance associated with the size of detoxifying enzyme families in insect herbivores? Curr. Opin. Insect Sci. 2016, 13, 70–76. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Feng, Q. Fat body biology in the last decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Dou, W.; Li, C.R.; Wang, J.J. CYP314A1-dependent 20-hydroxyecdysone biosynthesis is involved in regulating the development of pupal diapause and energy metabolism in the Chinese citrus fruit fly, Bactrocera minax. Pest Manag. Sci. 2022, 78, 3384–3393. [Google Scholar] [CrossRef]
- Guan, L.; Wang, X.; Wan, S.; Wang, Y.; Zhang, X.; Wang, S.; Li, C.; Tang, B. The role of TcCYP6K1 and TcCYP9F2 in influencing trehalose metabolism under high-CO2 stress in Tribolium castaneum (Coleoptera). Insects 2024, 15, 502. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhu, X.; Wang, C.; Wang, L.; Zhang, K.; Li, D.; Ji, J.; Niu, L.; Luo, J.; Cui, J. Silencing of cytochrome P450 gene AgoCYP6CY19 reduces the tolerance to host plant in cotton- and cucumber-specialized aphids, Aphis gossypii. J. Agric. Food Chem. 2022, 70, 12408–12417. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Functional analysis of cytochrome P450 genes linked with acetamiprid resistance in melon aphid, Aphis gossypii. Pestic. Biochem. Phys. 2020, 170, 104687. [Google Scholar] [CrossRef]
- Wei, Y.J. Cloning and Expression of Cytochrome P450 CYP6CY3 of Aphis gossypii Glover and Its Silencing by RNAi; Xinjiang University: Ürümqi, China, 2017. [Google Scholar]
- Zhang, L.; Wei, Y.; Wei, L.; Liu, X.; Liu, N. Effects of transgenic cotton lines expressing dsAgCYP6CY3-P1 on the growth and detoxification ability of Aphis gossypii Glover. Pest Manag. Sci. 2023, 79, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Kola, V.S.; Renuka, P.; Madhav, M.S.; Mangrauthia, S.K. Key enzymes and proteins of crop insects as candidate for RNAi based gene silencing. Front. Physiol. 2015, 6, 119. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Z.; Huang, S.; Chen, Z.; Sun, Y.; Duan, P.; Ma, Y.; Xia, L. RNAi-mediated plant protection against aphids. Pest Manag. Sci. 2016, 72, 1090–1098. [Google Scholar] [CrossRef]
- Zhang, X.T.; Liu, X.N.; Ma, J.; Zhao, J. Silencing of cytochrome P450 CYP6B6 gene of cotton bollworm (Helicoverpa armigera) by RNAi. Bull. Entomol. Res. 2013, 103, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Guan, K.L. Interplay between YAP/TAZ and Metabolism. Cell Metab. 2018, 28, 196–206. [Google Scholar] [CrossRef]
- Li, F.L.; Yu, Y.X.; Guo, M.M.; Lin, Y.; Jiang, Y.H.; Qu, M.; Sun, X.J.; Li, Z.X.; Zhai, Y.X.; Tan, Z.J. Integrated analysis of physiological, transcriptomics and metabolomics provides insights into detoxification disruption of PFOA exposure in Mytilus edulis. Ecotoxicol. Environ. Saf. 2021, 214, 112081. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Schena, M.; Shalon, D.; Davis, R.W.; Brown, P.O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 2016, 270, 467–470. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Han, S. Advances in metabolomics for understanding complex biological systems. Front. Mol. Biosci. 2019, 6, 58. [Google Scholar]
- Koppaka, V.; Thompson, D.C.; Nichenametla, S.N. Pyruvate metabolism and its role in cellular energy production. Biochem. Soc. T. 2012, 40, 108–112. [Google Scholar]
- McCommis, K.S.; Hodges, W.T.; Bricker, D.K.; Wisidagama, D.R.; Compan, V.; Remedi, M.S.; Thummel, C.S.; Finck, B.N. An ancestral role for the mitochondrial pyruvate carrier in glucose-stimulated insulin secretion. Mol. Metab. 2016, 5, 602–614. [Google Scholar] [CrossRef] [PubMed]
- Vigueira, P.A.; McCommis, K.S.; Schweitzer, G.G.; Remedi, M.S.; Chambers, K.T.; Fu, X.; McDonald, W.G.; Cole, S.L.; Colca, J.R.; Kletzien, R.F.; et al. Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell Rep. 2014, 7, 2042–2053. [Google Scholar] [CrossRef]
- Bricker, D.K.; Taylor, E.B.; Schell, J.C.; Orsak, T.; Boutron, A.; Chen, Y.C.; Cox, J.E.; Cardon, C.M.; Van Vranken, J.G.; Dephoure, N.; et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 2012, 337, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Vrbacký, M.; Drahota, Z.; Mrácek, T.; Vojtísková, A.; Jesina, P.; Stopka, P.; Houstek, J. Respiratory chain components involved in the glycerophosphate dehydrogenase-dependent ROS production by brown adipose tissue mitochondria. Biochim. Biophys. Acta—Bioenerg. 2007, 1767, 989–997. [Google Scholar] [CrossRef][Green Version]
- Olembo, N.K.; Pearson, D.J. Changes in the contents of intermediates of proline and carbohydrate metabolism in flight muscle of the tsetse fly Glossina morsitans and the fleshfly Sarcophaga tibialis. Insect Biochem. 1982, 12, 657–662. [Google Scholar] [CrossRef]
- Wang, T.; Geng, S.L.; Guan, Y.M.; Xu, W.H. Deacetylation of metabolic enzymes by Sirt2 modulates pyruvate homeostasis to extend insect lifespan. Aging 2018, 10, 1053–1072. [Google Scholar] [CrossRef] [PubMed]
- Salmina, A.B.; Kuvacheva, N.V.; Morgun, A.V.; Komleva, Y.K.; Pozhilenkova, E.A.; Lopatina, O.L.; Gorina, Y.V.; Taranushenko, T.E.; Petrova, L.L. Glycolysis-mediated control of blood-brain barrier development and function. Int. J. Biochem. Cell Biol. 2015, 64, 174–184. [Google Scholar] [CrossRef]
- Shimizu, K.; Matsuoka, Y. Regulation of glycolytic flux and overflow metabolism depending on the source of energy generation for energy demand. Biotechnol. Adv. 2019, 37, 284–305. [Google Scholar] [CrossRef]
- Feng, J.; Li, J.; Wu, L.; Yu, Q.; Ji, J.; Wu, J.; Dai, W.; Guo, C. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 126. [Google Scholar] [CrossRef]
- Soto-Heredero, G.; Gómez de Las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef]
- Zheng, L.; Tan, M.; Yan, S.; Jiang, D. Cadmium exposure-triggered growth retardation in Hyphantria cunea larvae involves disturbances in food utilization and energy metabolism. Ecotoxicol. Environ. Saf. 2023, 256, 114886. [Google Scholar] [CrossRef]
- Yu, H.Z.; Zhang, Q.; Lu, Z.J.; Deng, M.J. Validamycin treatment significantly inhibits the glycometabolism and chitin synthesis in the common cutworm, Spodoptera litura. Insect Sci. 2022, 29, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Zou, H.; Zou, H.; Jing, T.; Li, X.; Yan, G.; Geng, N.; Zhang, B.; Zhang, Z.; Zhang, S.; et al. 3-Bromopyruvate-induced glycolysis inhibition impacts larval growth and development and carbohydrate homeostasis in fall webworm, Hyphantria cunea Drury. Pestic. Biochem. Physiol. 2021, 179, 104961. [Google Scholar] [CrossRef] [PubMed]
- Rigoulet, M.; Bouchez, C.; Paumard, P.; Ransac, S.; Cuvellier, S.; Duvezin-Caubet, S.; Mazat, J.; Devin, A. Cell energy metabolism: An update. Biochim. Biophys. Acta—Bioenerg. 2020, 1861, 148276. [Google Scholar] [CrossRef]
- Shoshan, M.C. 3-Bromopyruvate: Targets and outcomes. J. Bioenerg. Biomembr. 2012, 44, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK—Sensing energy while talking to other signaling pathways. Cell Metab. 2014, 20, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Kishton, R.J.; Barnes, C.E.; Nichols, A.G.; Cohen, S.; Gerriets, V.A.; Siska, P.J.; Macintyre, A.N.; Goraksha-Hicks, P.; de Cubas, A.A.; Liu, T.; et al. AMPK is essential to balance glycolysis and mitochondrial metabolism to control T-ALL cell stress and survival. Cell Metab. 2016, 23, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.H. Hormonal control of tick development and reproduction. Parasitology 2004, 129, S127–S143. [Google Scholar] [CrossRef]
- Ling, L.; Raikhel, A.S. Cross-talk of insulin-like peptides, juvenile hormone, and 20-hydroxyecdysone in regulation of metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2021, 118, e2023470118. [Google Scholar] [CrossRef] [PubMed]
- Terrettaz, J.; Assimacopoulos-Jeannet, F.; Jeanrenaud, B. Inhibition of hepatic glucose production by insulin in vivo in rats: Contribution of glycolysis. Am. J. Physiol. Physiol. Metab. 1986, 250 Pt 1, E346–E351. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Khan, S.A.; Lange, A.J. Regulation of glycolysis-role of insulin. Exp. Gerontol. 2005, 40, 894–899. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Yang, Q.; Li, Y.; Wu, J.; Xu, X. Identification of the neuropeptide gene family and feeding regulation by neuropeptide Y in Mythimna separata (Lepidoptera: Noctuidae). Int. J. Biol. Macromol. 2023, 224, 676–687. [Google Scholar] [CrossRef]
- Li, M.M.; Yang, Q.; Chen, L.H.; Li, Y.Y.; Wu, J.X.; Xu, X.L. Effect of short neuropeptide F signaling on larval feeding in Mythimna separata. Insect Sci. 2024, 31, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wan, S.; Lin, C.; Zhou, C.; Hu, L.; Deng, C.; Zhang, L. Integration of metabolome and transcriptome reveals the relationship of benzenoid-phenylpropanoid pigment and aroma in purple tea flowers. Front. Plant Sci. 2021, 12, 762330. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. MetaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Zou, H.; Zou, H.; Li, X.; Qiu, Q.; Geng, N.; Zhang, B.; Yan, G.; Zhang, Z.; Zhang, S.; Yao, B.; et al. Metformin-induced AMPK activation suppresses larval growth and molting probably by disrupting 20E synthesis and glycometabolism in fall webworm, Hyphantria cunea Drury. Pestic. Biochem. Phys. 2022, 183, 105083. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Sequences (5′−3′) | Purpose |
|---|---|---|
| MIPP gene F | AAATCTCAAACACAACTCGGG | qRT-PCR |
| MIPP gene R | ATGGCATTCGTAAAGTACGGC | qRT-PCR |
| PC gene F | CTGATGTAGTTGATGTTGCTGTTG | qRT-PCR |
| PC gene R | TTTCCTGATTTCATTGTTGTTGTA | qRT-PCR |
| CS gene F | TCACATTATGCGATACCAAGC | qRT-PCR |
| CS gene R | CAAGTTCCAATACACCAAGCC | qRT-PCR |
| MDH gene F | CCTCTTCATCCGGCATCCAACA | qRT-PCR |
| MDH gene R | CAAACACCTTCCAGAACACCCA | qRT-PCR |
| 18S rRNA F | CCGGAAAGATTGACAGATTGAG | qRT-PCR |
| 18S rRNA R | CAGGACAGAGTCTCGTTCGTTATC | qRT-PCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, W.; Li, T.; Li, Y.; Zhang, L.; Xie, J.; Liu, X. Transgenic Cotton Expressing dsAgCYP6CY3 Significantly Delays the Growth and Development of Aphis gossypii by Inhibiting Its Glycolysis and TCA Cycle. Int. J. Mol. Sci. 2025, 26, 264. https://doi.org/10.3390/ijms26010264
Kong W, Li T, Li Y, Zhang L, Xie J, Liu X. Transgenic Cotton Expressing dsAgCYP6CY3 Significantly Delays the Growth and Development of Aphis gossypii by Inhibiting Its Glycolysis and TCA Cycle. International Journal of Molecular Sciences. 2025; 26(1):264. https://doi.org/10.3390/ijms26010264
Chicago/Turabian StyleKong, Wenting, Tingting Li, Yuan Li, Lianjun Zhang, Jingang Xie, and Xiaoning Liu. 2025. "Transgenic Cotton Expressing dsAgCYP6CY3 Significantly Delays the Growth and Development of Aphis gossypii by Inhibiting Its Glycolysis and TCA Cycle" International Journal of Molecular Sciences 26, no. 1: 264. https://doi.org/10.3390/ijms26010264
APA StyleKong, W., Li, T., Li, Y., Zhang, L., Xie, J., & Liu, X. (2025). Transgenic Cotton Expressing dsAgCYP6CY3 Significantly Delays the Growth and Development of Aphis gossypii by Inhibiting Its Glycolysis and TCA Cycle. International Journal of Molecular Sciences, 26(1), 264. https://doi.org/10.3390/ijms26010264





