An Innovative Method of Improving an Extract of Andrographis paniculata from Leaves: Its Anticancer Effect Involves the Cell Endoplasmic Reticulum
Abstract
:1. Introduction
- (1)
- The use of different alcohol solvents, which vary according to their hydrocarbon group length, to dissolve ALE and PA.
- (2)
- The improvement of the effectiveness of ALE and PA by varying some dissolution parameters.
- (3)
- The effect of ALE and PA on cancer cell lines to assess their potential role against cancer; for this purpose, a human neuroblastoma line (SH-SY5Y), a human breast cancer line (MCF-7), and a non-tumor line of human umbilical vein endothelial cells (Huvec), used as the control line, will be analyzed.
2. Results and Discussion
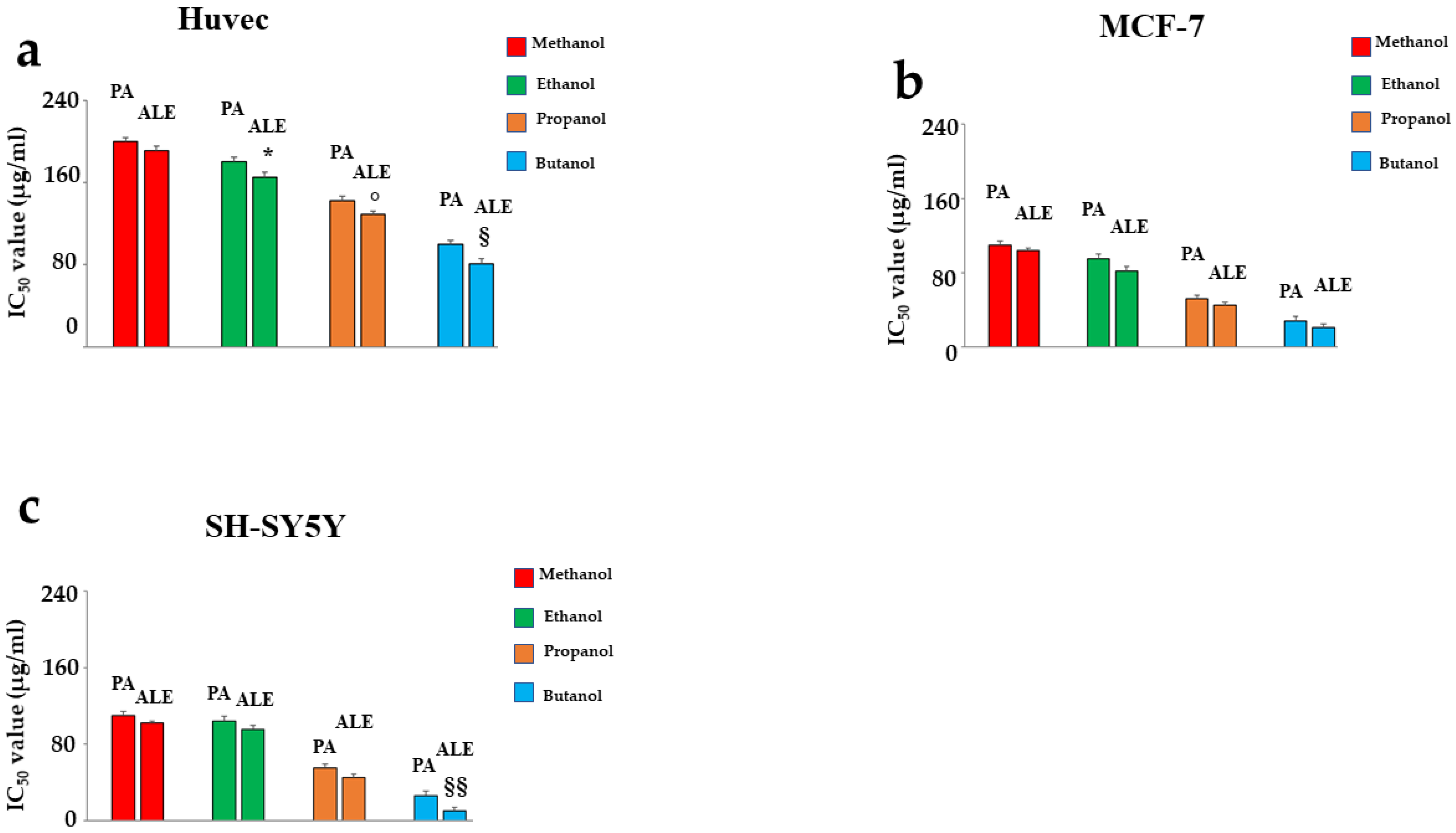
2.1. Effects of ALE and PA Dissolved in Alcoholic Solvents
- (a)
- when the compounds were dissolved in 1-butanol, they exhibited significantly higher toxicity than all the other solvents.
- (b)
- ALE extract (in any solvent) has always been more toxic than PA.
- (c)
- In non-cancer cells, only the highest concentrations of ALE and PA exhibited toxicity of no more than 30%. On the contrary, cancer lines reported significantly higher toxicity following treatment with ALE and PA.
- (d)
- Neurons were found to be more sensitive than breast cancer cells, and in fact, ALE and PA were more toxic.

2.2. Comparison of IC50 of ALE and PA Dissolved in Several Solvents
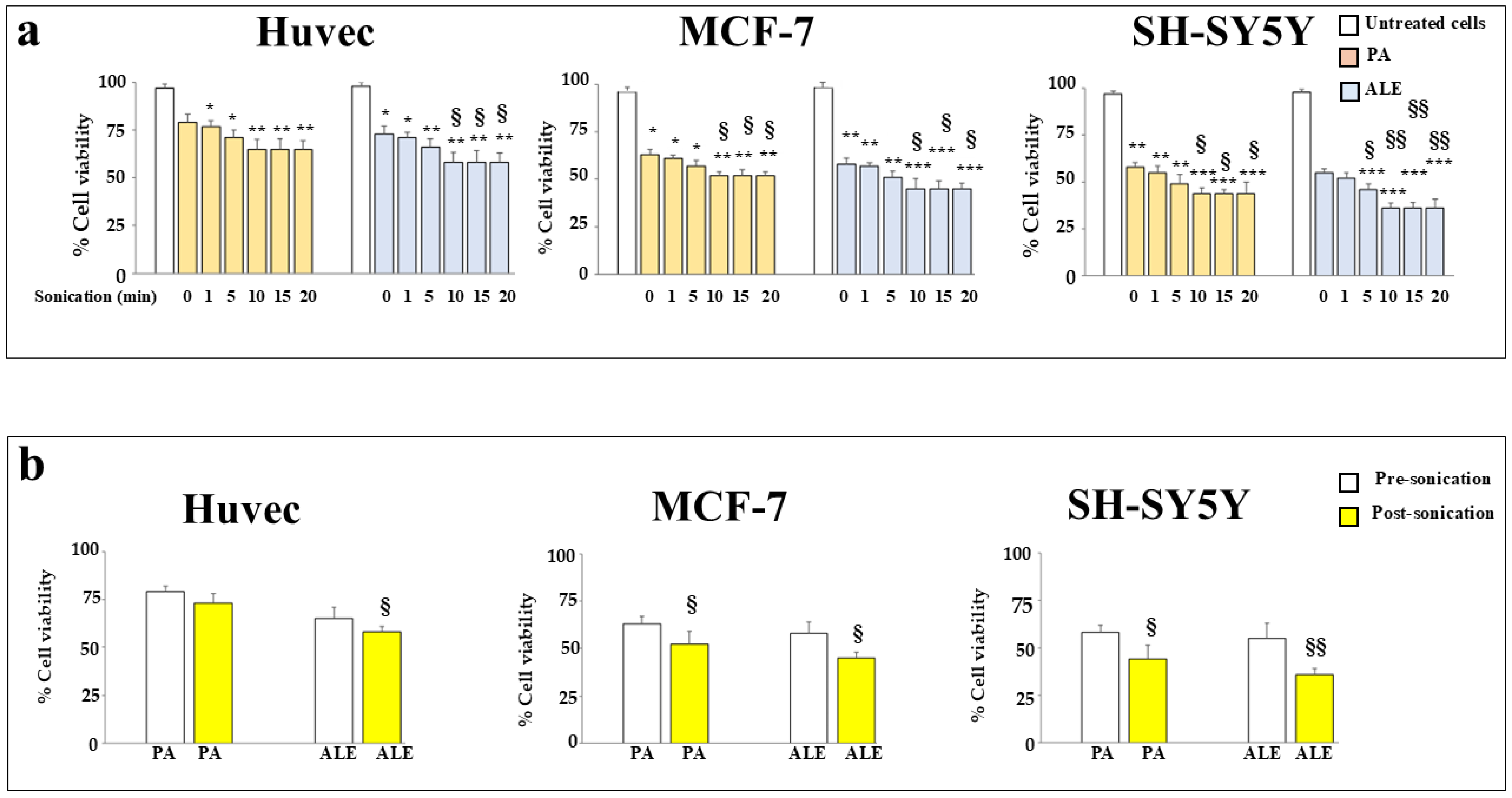
2.3. Sonication Improves the Anticancer Effects of the Extracts
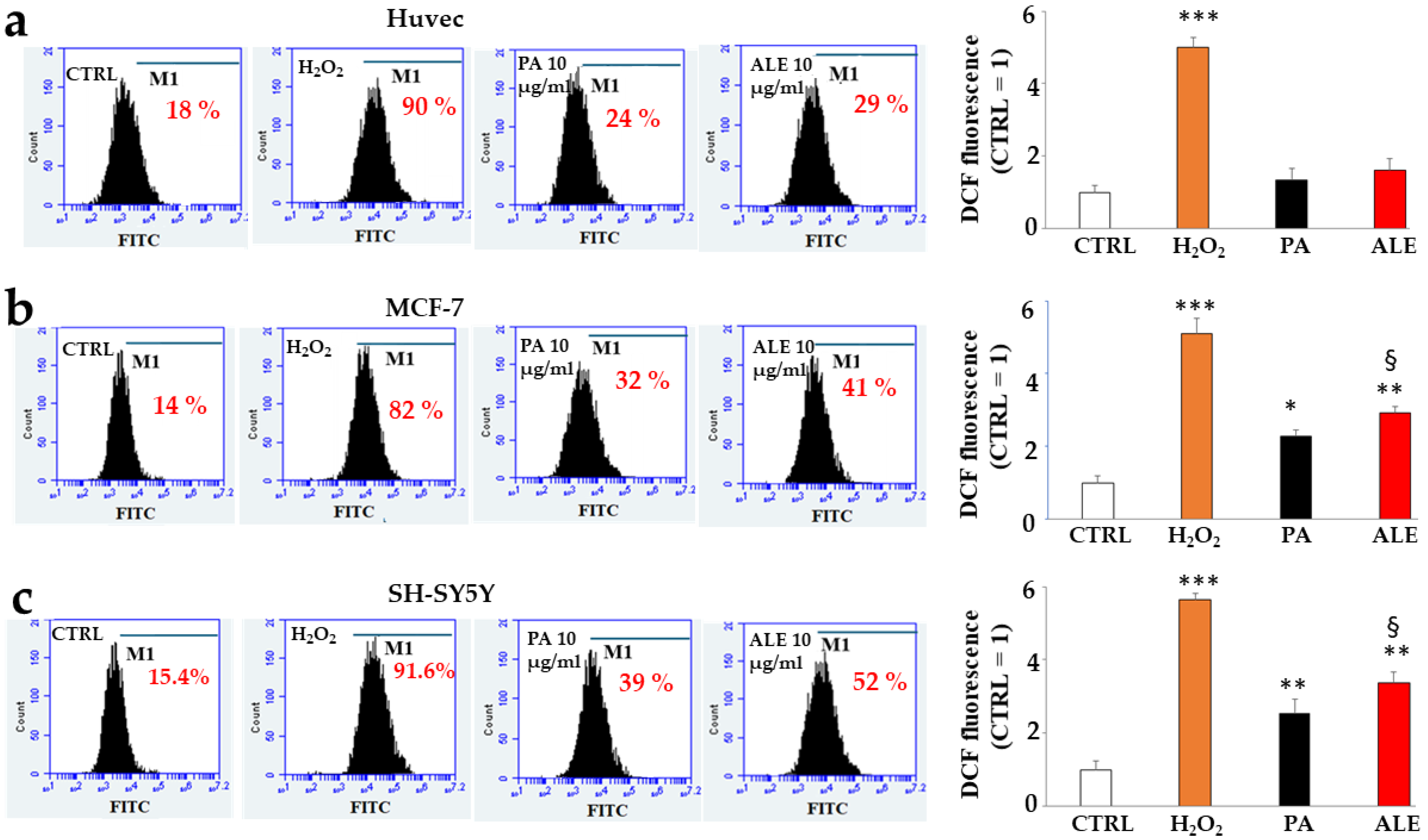
2.4. Antioxidant Potential of PA and ALE
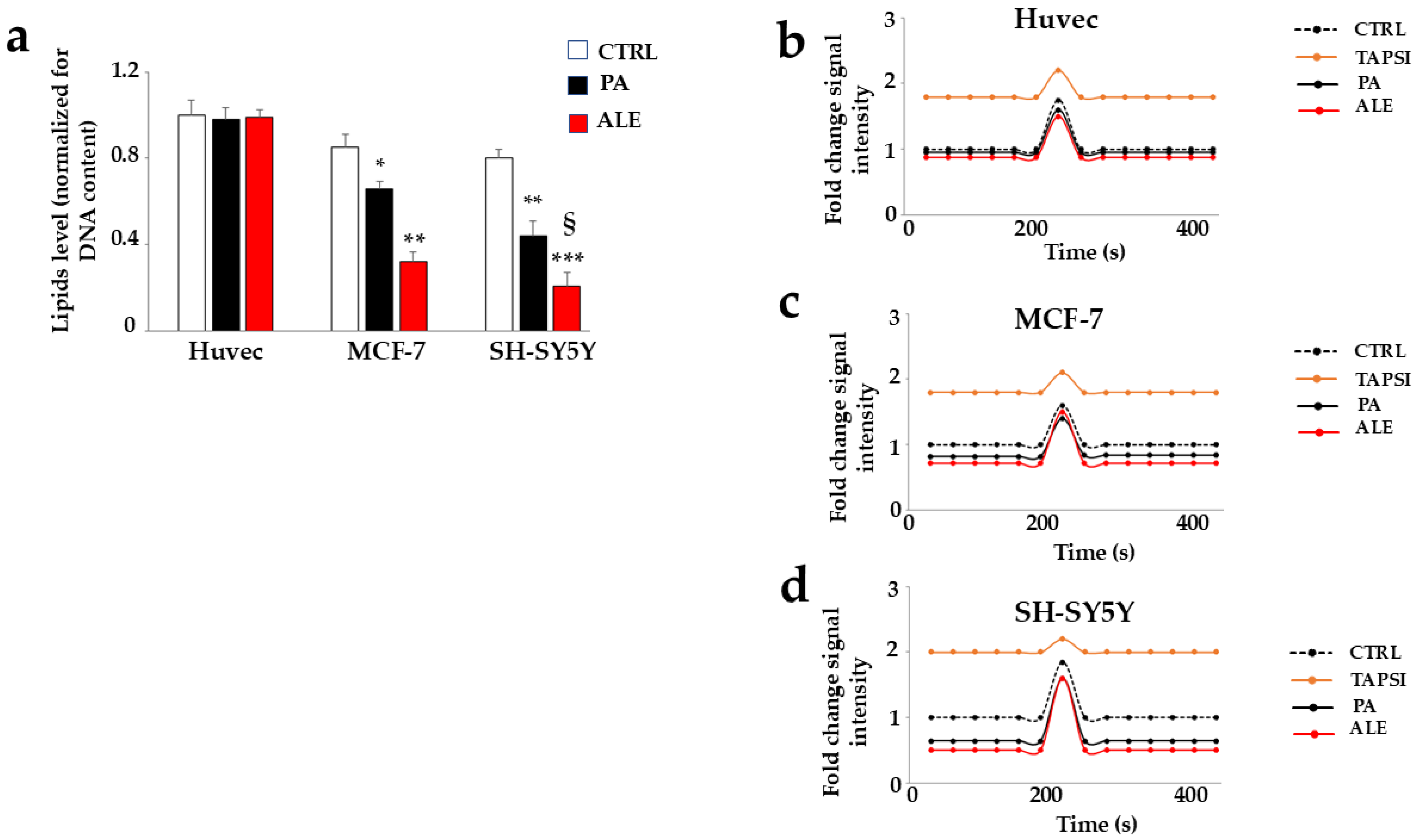
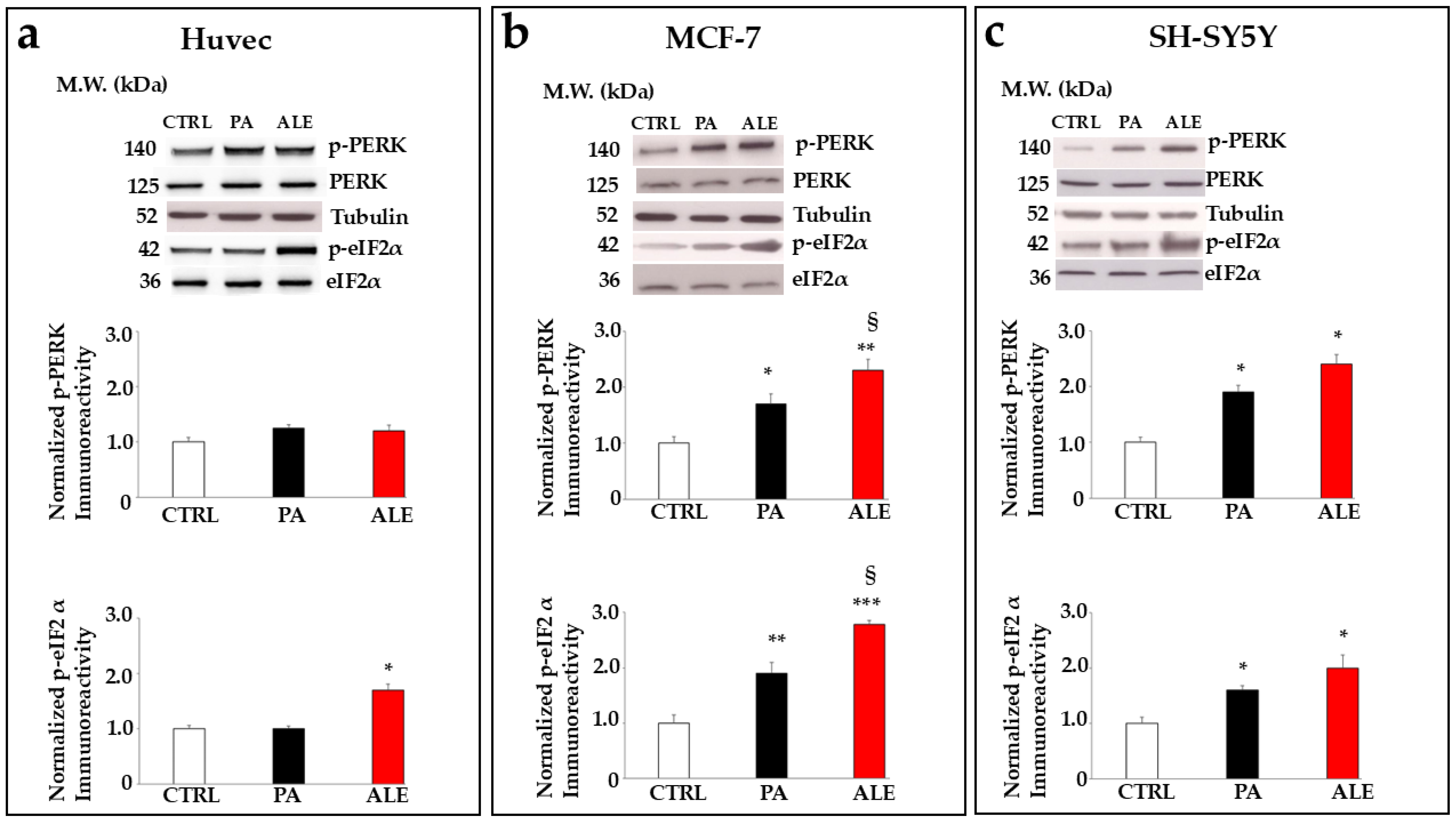
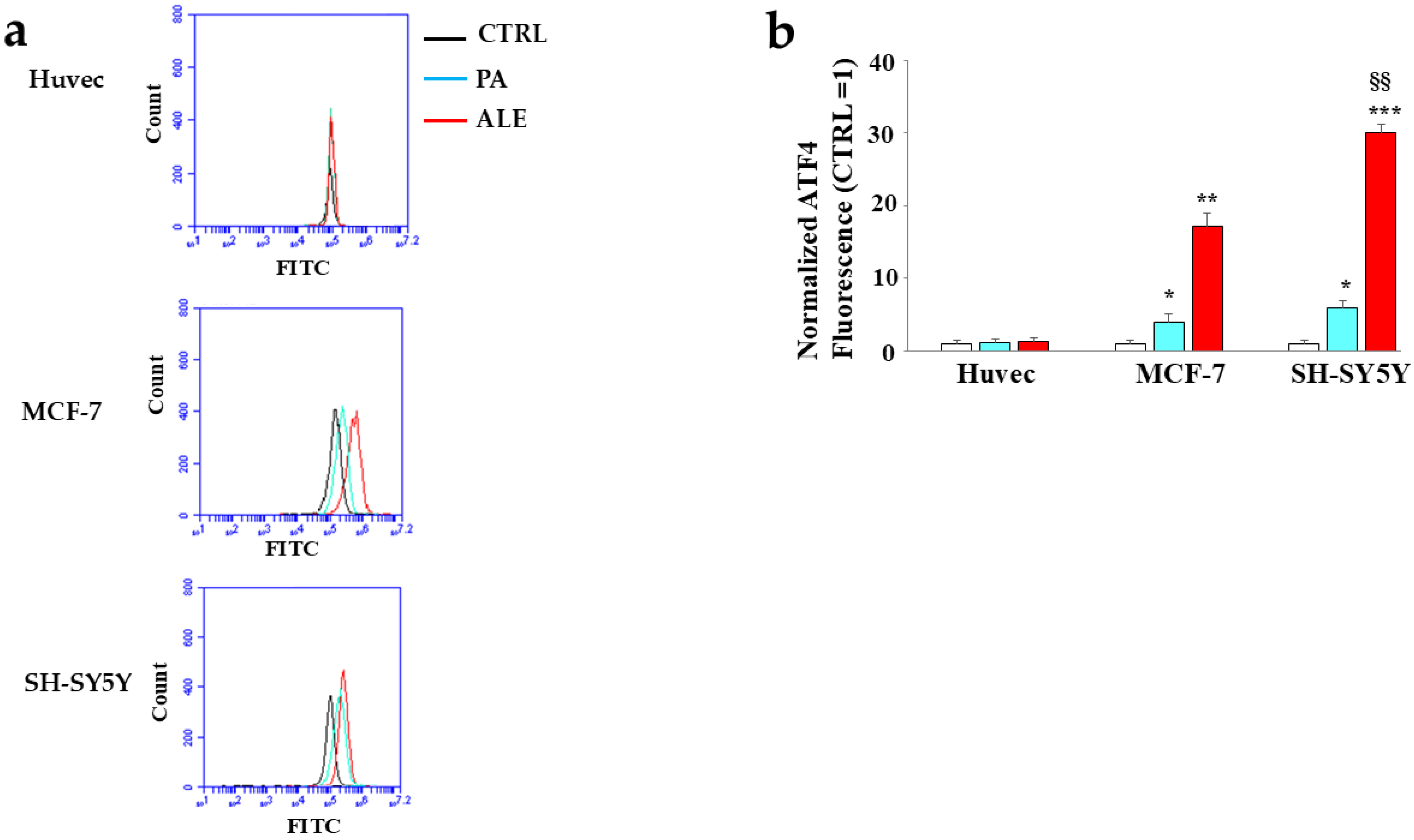
2.5. Involvement of Endoplasmic Reticulum
2.6. PA and ALE Involve PERK Sensor of UPR
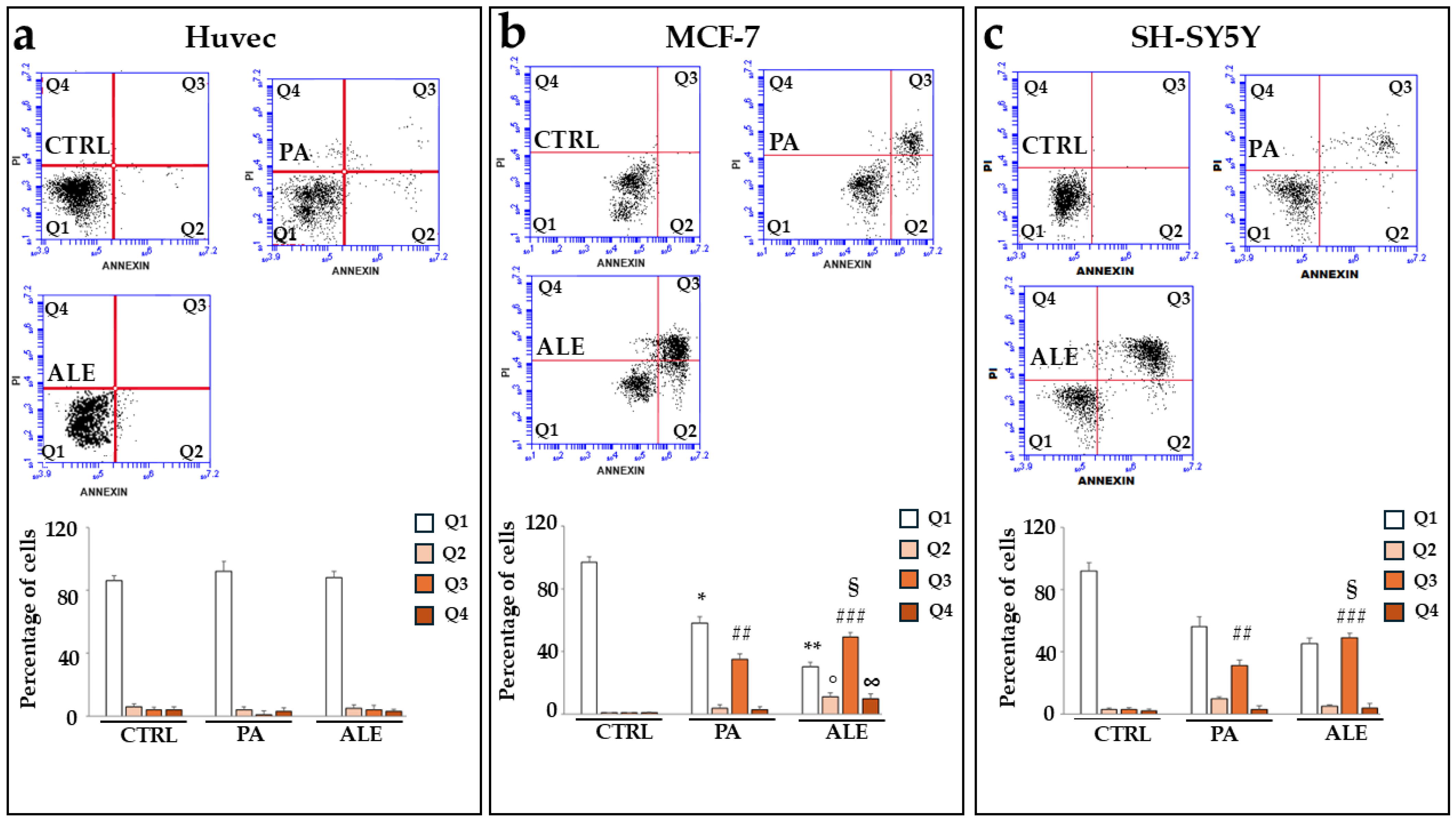
2.7. UPR Activation, Induced by PA and ALE, Promotes Cell Apoptotic Death
3. Materials and Methods
3.1. Plant Material
- (1)
- andrographolide 49.7%
- (2)
- 14 deoxyandrographolide 0.2%
- (3)
- neoandrographolide 0.2%
3.2. Cell Cultures
3.3. Measurement of Cell Viability
3.4. Reducing Power Assay
3.5. Ferrous Ion (Fe2+) Chelating Activity Assay
3.6. ORAC Assay
3.7. ROS Accumulation Measurement
3.8. Lipid Measurement: Spectrometric Quantification
3.9. Intracellular Calcium Measurements
3.10. Cell Lysis and Immunoblot Analysis
3.11. Measurement of ATF-4 Expression Through Immuno-Cytofluorometry
3.12. Annexin V Staining
4. Conclusions
- (1)
- ALE and PA dissolve better in butanol, rather than methanol, ethanol, and propanol. Butanol has a higher number of -CH2 groups, which makes the solvent more hydrophobic and facilitates the dissolution of andrographolide contained in different amounts.
- (2)
- After ALE and PA were dissolved, they were subjected to a sonication cycle of 10′, responsible for better dissolution and greater effect. This additional treatment, used on A. paniculata extracts for the first time, has made a unique and innovative ALE and PA.
- (3)
- ALE and PA have shown an anti-tumoral effect on cancer cells, which is not present in healthy cells. This effect altered the physiology of the endoplasmic reticulum, which promoted cell apoptotic death. For this reason, A. paniculata could be used and exploited in pharmacological therapy against certain neoplasms. However, it should be remembered that the results were obtained in isolated cancer cells, which do not enjoy the homeostasis generated by a whole tissue or organ. In the organism, an anti-tumoral drug fails to distinguish between cancer and non-cancer cells, constituting the biggest limit of cancer therapy. It would be appropriate, therefore, to test ALE and PA in vivo or in clinical practice to increase information on the phytotherapeutic role of A. paniculata.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mishra, S.K.; Sangwan, N.S.; Sangwan, R.S. Andrographis paniculata (Kalmegh): A Review. Pharmacogn. Rev. 2007, 1, 283–298. [Google Scholar]
- Hossain, M.S.; Urbi, Z.; Sule, A.; Rahman, K.M.H. Andrographis paniculata (Burm. f.) Wall. Ex Nees: A review of ethnobotany, phytochemistry, and pharmacology. Sci. World J. 2014, 2014, 274905. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, B.; Bajpai, V. Andrographis paniculata (Burm.f.) Nees: Traditional uses, phytochemistry, pharmacological properties, and quality control/quality assurance. J. Ethnopharmacol. 2021, 275, 114054. [Google Scholar] [CrossRef] [PubMed]
- Bhaisare, S.; Pathak, S.; Ajankar, V.V. Physiological Activities of the King of Bitters (Andrographis paniculata): A Review. Cureus 2023, 15, e43515. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.W.; Lin, B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2017, 34, 1227–1238. [Google Scholar] [CrossRef]
- Pholphana, N.; Panomvanab, D.; Rangkadiloka, N.; Suriyoa, T.; Puranajotib, P.; Ungtrakuld, T.; Pongpund, W.; Thaeopatthab, S.; Songvutb, P.; Satayavivada, J. Andrographis paniculata: Dissolution investigation and pharmacokinetic studies of four major active diterpenoids after multiple oral dose administration in healthy Thai volunteers. J. Ethnopharmacol. 2016, 194, 513–521. [Google Scholar] [CrossRef]
- Thingale, A.D.; Shaikh, K.S.; Channekar, P.R.; Galgatte, U.C.; Chaudhari, P.D.; Bothiraja, C. Enhanced hepatoprotective activity of andrographolide complexed with a biomaterial. Drug Deliv. 2014, 22, 117–124. [Google Scholar] [CrossRef]
- Loureiro Damasceno, J.P.; Silva da Rosa, H.; Silva de Araújo, L.; Jacometti Cardoso Furtado, N.A. Andrographis paniculata Formulations: Impact on Diterpene Lactone Oral Bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 19–30. [Google Scholar] [CrossRef]
- Soo, H.L.; Quah, S.Y.; Sulaiman, I.; Sagineedu, S.R.; Lim, J.C.W.; Stanslas, J. Advances and challenges in developing andrographolide and its analogs as cancer therapeutic agents. Drug Discov. Today 2019, 24, 1890–1898. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, S.; Shukla, A.; Oh, S.H.; Kim, H.M. Andrographolide and analogs in cancer prevention. Front. Biosci. (Elite Ed.) 2015, 7, 255–266. [Google Scholar] [PubMed]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015, 10, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Oppedisano, F.; Macrì, R.; et al. Environmental and Nutritional “Stressors” and Oligodendrocyte Dysfunction: Role of Mitochondrial and Endoplasmatic Reticulum Impairment. Biomedicines 2020, 8, 553. [Google Scholar] [CrossRef]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef]
- Kopp, M.C.; Larburu, N.; Durairaj, V.; Adams, C.J.; Ali, M. UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat. Struct. Mol. Biol. 2019, 26, 1053–1062. [Google Scholar] [CrossRef]
- Wortel, I.M.N.; van der Meer, L.T.; Kilberg, M.S.; van Leeuwen, F.N. Surviving Stress: Modulation of ATF4-Mediated Stress Responses in Normal and Malignant Cells. Trends Endocrinol. Metab. 2017, 28, 794–806. [Google Scholar] [CrossRef]
- Banerjee, A.; Ahmed, H.; Yang, P.; Czinn, S.J.; Blanchard, T.G. Endoplasmic reticulum stress and IRE-1 signaling cause apoptosis in colon cancer cells in response to andrographolide treatment. Oncotarget 2016, 7, 41432–41444. [Google Scholar] [CrossRef]
- Hong, H.; Cao, W.; Wang, Q.; Liu, C.; Huang, C. Synergistic antitumor effect of Andrographolide and cisplatin through ROS-mediated ER stress and STAT3 inhibition in colon cancer. Med. Oncol. 2022, 39, 101. [Google Scholar] [CrossRef]
- De Guzman, M.R.; Yap Ang, M.B.M.; Huang, S.-H.; Hu, F.-C.; Chiao, Y.-H.; Tsai, H.-A.; Lee, K.-R. Cosolvent-Driven Interfacial Polymerization for Superior Separation Performance of Polyurea-Based Pervaporation Membrane. Polymers 2021, 13, 1179. [Google Scholar] [CrossRef]
- Plou, F.-J.; Cruces, M.A.; Ferrer, M.; Fuentes, G.; Pastor, E.; Bernabé, M.; Christensen, M.; Comelles, F.; Parra, J.L.; Ballesteros, A.J. Enzymatic acylation of di- and trisaccharides with fatty acids: Choosing the appropriate enzyme, support and solvent. J. Biotechnol. 2002, 96, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Patra, J.K.; Bonesi, M.; Das, S.; Nath, R.; Das Talukdar, A.; Das, G.; Loizzo, M.R. Anti-Cancer Agent: The Labdane Diterpenoid-Andrographolide. Plants 2023, 12, 1969. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.A.; Sridevi, K.; Kumar, N.V.; Nanduri, S.; Rajagopal, S.J. Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 2004, 92, 291–295. [Google Scholar] [CrossRef]
- Haq, S.A.; Paudwal, G.; Banjare, N.; Andrabi, N.I.; Wazir, P.; Nandi, U.; Ahmed, Z.; Gupta, P.N. Sustained release polymer and surfactant based solid dispersion of andrographolide exhibited improved solubility, dissolution, pharmacokinetics, and pharmacological activity. Int. J. Pharm. 2024, 651, 123786. [Google Scholar]
- Pereira, S.V.; Colombo, F.B.; de Freitas, L.A.P. Ultrasound influence on the solubility of solid dispersions prepared for a poorly soluble drug. Ultrason. Sonochem. 2016, 29, 461–469. [Google Scholar] [CrossRef]
- Babu, V.R.; Areefulla, S.H.; Mallikarjun, V. Solubility and dissolution enhancement: An overview. J. Pharm. Res. 2010, 3, 141–145. [Google Scholar]
- Maiuolo, J.; Bonacci, S.; Bosco, F.; Guarnieri, L.; Ruga, S.; Leo, A.; Citraro, R.; Ragusa, S.; Palma, E.; Mollace, V.; et al. Two Olea europaea L. Extracts Reduce Harmful Effects in a Model of Neurotoxicity: Involvement of the Endoplasmic Reticulum. Planta Med. 2024, 90, 844–857. [Google Scholar] [CrossRef]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, a Natural Antioxidant: An Update. Antioxidants 2019, 8, 571. [Google Scholar] [CrossRef]
- Phetruen, T.; van Dam, B.; Chanarat, S. Andrographolide Induces ROS-Mediated Cytotoxicity, Lipid Peroxidation, and Compromised Cell Integrity in Saccharomyces cerevisiae. Antioxidants 2023, 12, 1765. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Lecci, R.M.; D’Antuono, I.; Cardinali, A.; Garbetta, A.; Linsalata, V.; Logrieco, A.F.; Leone, A. Antioxidant and Pro-Oxidant Capacities as Mechanisms of Photoprotection of Olive Polyphenols on UVA-Damaged Human Keratinocytes. Molecules 2021, 26, 2153. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.H.; Tsai, Y.T.; Huang, S.F.; Yu, T.C.; Kuo, P.C.; Chao, S.C.; Chou, M.F.; Tsai, C.S.; Lee, S.P. Effects of Andrographolide on Intracellular pH Regulation, Cellular Migration, and Apoptosis in Human Cervical Cancer Cells. Cancers 2020, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Czowski, B.J.; Romero-Moreno, R.; Trull, K.J.; White, K.A. Cancer and pH Dynamics: Transcriptional Regulation, Proteostasis, and the Need for New Molecular Tools. Cancers 2020, 12, 2760. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef]
- Singh, S.; Paul, S.; Brás, N.F.; Kundu, C.N.; Karthikeyan, C.; Moorthy, N.S.H.N. Design, synthesis, and anticancer activity of some novel 1H-benzo[d]imidazole-5-carboxamide derivatives as fatty acid synthase inhibitors. Bioorg. Chem. 2023, 138, 106658. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, X.; Zhao, D.; Liu, H.; Hu, Y. Calcium homeostasis and cancer: Insights from endoplasmic reticulum-centered organelle communications. Trends Cell Biol. 2023, 33, 312–323. [Google Scholar] [CrossRef]
- Lin, J.H.; Li, H.; Zhang, Y.; Ron, D.; Walter, P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS ONE 2009, 4, e4170. [Google Scholar] [CrossRef]
- Wang, G.; Yang, Z.-Q.; Zhang, K. Endoplasmic reticulum stress response in cancer: Molecular mechanism and therapeutic potential. Am. J. Transl. Res. 2010, 2, 65–74. [Google Scholar]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Park, S.K.; Sanders, B.G.; Kline, K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic death receptor signaling. Breast Cancer Res. Treat. 2010, 124, 361–375. [Google Scholar] [CrossRef]
- Arena, P.; Miceli, N.; Marino, A.; Davì, F.; Cavò, E.; Spadaro, V.; Mondello, L.; Taviano, M.F. Comparative Study on Phenolic Profile and Biological Activities of the Aerial Parts of Sinapis pubescens L. subsp. pubescens (Brassicaceae) Wild from Sicily (Italy). Chem. Biodivers. 2023, 20, e202300309. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Bulotta, S.; Verderio, C.; Benfante, R.; Borgese, N. Selective activation of the transcription factor ATF6 mediates endoplasmic reticulum proliferation triggered by a membrane protein. Proc. Natl. Acad. Sci. USA 2011, 108, 7832–7837. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Macrì, R.; Bava, I.; Gliozzi, M.; Musolino, V.; Nucera, S.; Carresi, C.; Scicchitano, M.; Bosco, F.; Scarano, F.; et al. Myelin Disturbances Produced by Sub-Toxic Concentration of Heavy Metals: The Role of Oligodendrocyte Dysfunction. Int. J. Mol. Sci. 2019, 20, 4554. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiuolo, J.; Caminiti, R.; Mazza, V.; Oppedisano, F.; Nucera, S.; Serra, M.; Macrì, R.; Palma, E.; Eng, A.; Mollace, V.; et al. An Innovative Method of Improving an Extract of Andrographis paniculata from Leaves: Its Anticancer Effect Involves the Cell Endoplasmic Reticulum. Int. J. Mol. Sci. 2025, 26, 344. https://doi.org/10.3390/ijms26010344
Maiuolo J, Caminiti R, Mazza V, Oppedisano F, Nucera S, Serra M, Macrì R, Palma E, Eng A, Mollace V, et al. An Innovative Method of Improving an Extract of Andrographis paniculata from Leaves: Its Anticancer Effect Involves the Cell Endoplasmic Reticulum. International Journal of Molecular Sciences. 2025; 26(1):344. https://doi.org/10.3390/ijms26010344
Chicago/Turabian StyleMaiuolo, Jessica, Rosamaria Caminiti, Valeria Mazza, Francesca Oppedisano, Saverio Nucera, Maria Serra, Roberta Macrì, Ernesto Palma, Annie Eng, Vincenzo Mollace, and et al. 2025. "An Innovative Method of Improving an Extract of Andrographis paniculata from Leaves: Its Anticancer Effect Involves the Cell Endoplasmic Reticulum" International Journal of Molecular Sciences 26, no. 1: 344. https://doi.org/10.3390/ijms26010344
APA StyleMaiuolo, J., Caminiti, R., Mazza, V., Oppedisano, F., Nucera, S., Serra, M., Macrì, R., Palma, E., Eng, A., Mollace, V., & Muscoli, C. (2025). An Innovative Method of Improving an Extract of Andrographis paniculata from Leaves: Its Anticancer Effect Involves the Cell Endoplasmic Reticulum. International Journal of Molecular Sciences, 26(1), 344. https://doi.org/10.3390/ijms26010344






