Bioactive Phenolics of Hyoscyamus muticus L. Subsp. Falezlez: A Molecular and Biochemical Approach to Antioxidant and Urease Inhibitory Activities
Abstract
:1. Introduction
2. Results
2.1. Survey and Measurement of Phenolic Compounds
2.2. Antioxidant Activity
2.3. Urease Inhibition
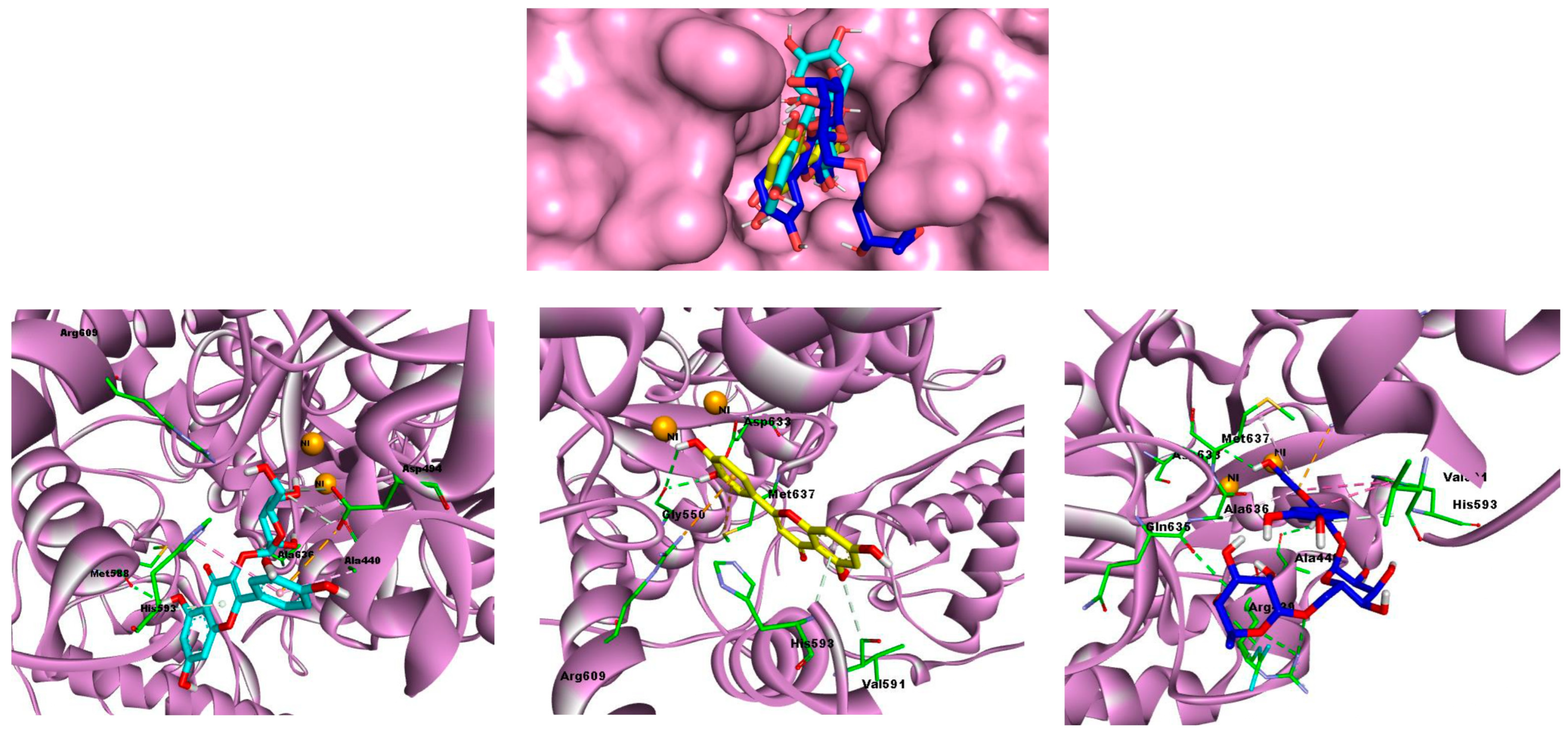
2.4. Molecular Docking Studies
3. Discussion
4. Materials and Methods
4.1. Botanical Specimens
4.2. Technical Setup and Chromatography Parameters
4.3. Assessment of Antioxidant Effects
4.4. Inhibitory Capacities Targeting Urease
4.5. Molecular Docking Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayari-Guentri, S.; Saad, S.; Ait Kettout, T.; Gaceb-Terrak, R.; Djemouai, N. Seeds of Hyoscyamus muticus L. Subsp. Falezlez: Morpho-anatomical Features, Phytochemical Investigation and Evidence for Antioxidant Activities. Chem. Biodivers. 2024, 21, e202401026. [Google Scholar] [CrossRef] [PubMed]
- Ayari-Guentri, S.; Djemouai, N.; Saad, S.; Karoune, S.; Gaceb-Terrak, R.; Rahmania, F. Hyoscyamus muticus L. Subsp. Falezlez Methanolic Extract: Phytochemical Composition and Biological Activities. Eur. J. Biol. Res. 2022, 12, 190–206. [Google Scholar]
- Almalki, M.A. In Vitro Antibacterial, Antifungal and Other Medical Properties of Endangered Medicinal Plant Seeds. Pharmacol. Pharm. 2017, 8, 189–204. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Zayed, R.; El-Shamy, H. Screening of Bioactive Lipids and Radical Scavenging Potential of Some Solanaceae Plants. Food Chem. 2007, 103, 885–890. [Google Scholar] [CrossRef]
- Mohammad, M.K.; Almasri, I.M.; Tawaha, K.; Issa, A.; Al-Nadaf, A.; Hudaib, M.; AlKhatib, H.S.; Abu-Gharbieh, E.; Bustanji, Y. Antioxidant, Antihyperuricemic and Xanthine Oxidase Inhibitory Activities of Hyoscyamus Reticulatus. Pharm. Biol. 2010, 48, 1376–1383. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Kadi, K.; Martín-García, A.I.; Yilmaz, M.A.; Akkal, S.; Boumegoura, A.; Alhomida, A.S.; Ola, M.S.; Ali, A. LC/MS-MS Analysis of Phenolic Compounds in Hyoscyamus Albus L. Extract: In Vitro Antidiabetic Activity, in Silico Molecular Docking, and in Vivo Investigation against STZ-Induced Diabetic Mice. Pharmaceuticals 2023, 16, 1015. [Google Scholar] [CrossRef]
- Albayrak, İ.; Demirci, T.; Baydar, N.G. Enhancement of in Vitro Production of Tropane Alkaloids and Phenolic Compounds in Hyoscyamus Niger by Culture Types and Elicitor Treatments. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 156, 72. [Google Scholar] [CrossRef]
- Konieczna, I.; Zarnowiec, P.; Kwinkowski, M.; Kolesinska, B.; Fraczyk, J.; Kaminski, Z.; Kaca, W. Bacterial Urease and Its Role in Long-Lasting Human Diseases. Curr. Protein Pept. Sci. 2012, 13, 789–806. [Google Scholar] [CrossRef]
- Kuwahara, H.; Miyamoto, Y.; Akaike, T.; Kubota, T.; Sawa, T.; Okamoto, S.; Maeda, H. Helicobacter Pylori Urease Suppresses Bactericidal Activity of Peroxynitrite via Carbon Dioxide Production. Infect. Immun. 2000, 68, 4378–4383. [Google Scholar] [CrossRef]
- Rai, R.; Saraswat, V.A.; Dhiman, R.K. Gut Microbiota: Its Role in Hepatic Encephalopathy. J. Clin. Exp. Hepatol. 2015, 5, S29–S36. [Google Scholar] [CrossRef]
- Sah, D.K.; Arjunan, A.; Lee, B.; Jung, Y. Do Reactive Oxygen Species and H. Pylori Infection: A Comprehensive Review of Their Roles in Gastric Cancer Development. Antioxidants 2023, 12, 1712. [Google Scholar] [CrossRef]
- Allam, E.A.H. Urolithiasis Unveiled: Pathophysiology, Stone Dynamics, Types, and Inhibitory Mechanisms: A Review. Afr. J. Urol. 2024, 30, 34. [Google Scholar] [CrossRef]
- Razi, A.; Ghiaei, A.; Dolatabadi, F.K.; Haghighi, R. Unraveling the Association of Bacteria and Urinary Stones in Patients with Urolithiasis: An Update Review Article. Front Med (Lausanne) 2024, 11, 1401808. [Google Scholar] [CrossRef] [PubMed]
- Back, A.; Tupper, K.Y.; Bai, T.; Chiranand, P.; Goldenberg, F.D.; Frank, J.I.; Brorson, J.R. Ammonia-Induced Brain Swelling and Neurotoxicity in an Organotypic Slice Model. Neurol. Res. 2011, 33, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.; Kim, B.C.; Cho, K.A.; Song, J. The Cerebral Effect of Ammonia in Brain Aging: Blood–Brain Barrier Breakdown, Mitochondrial Dysfunction, and Neuroinflammation. J. Clin. Med. 2021, 10, 2773. [Google Scholar] [CrossRef]
- York, N.E.; Borofsky, M.S.; Lingeman, J.E. Risks Associated with Drug Treatments for Kidney Stones. Expert. Opin. Drug Saf. 2015, 14, 1865–1877. [Google Scholar] [CrossRef]
- Lafay, S.; Morand, C.; Manach, C.; Besson, C.; Scalbert, A. Absorption and Metabolism of Caffeic Acid and Chlorogenic Acid in the Small Intestine of Rats. Br. J. Nutr. 2006, 96, 39–46. [Google Scholar] [CrossRef]
- Rego, Y.F.; Queiroz, M.P.; Brito, T.O.; Carvalho, P.G.; de Queiroz, V.T.; de Fátima, Â.; Macedo Jr, F. A Review on the Development of Urease Inhibitors as Antimicrobial Agents against Pathogenic Bacteria. J. Adv. Res. 2018, 13, 69–100. [Google Scholar] [CrossRef]
- Rezvani, M. Oxidative Stress-Induced Gastrointestinal Diseases: Biology and Nanomedicines—A Review. BioChem 2024, 4, 189–216. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From Imbalance to Impairment: The Central Role of Reactive Oxygen Species in Oxidative Stress-Induced Disorders and Therapeutic Exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Čižmárová, B.; Hubková, B.; Tomečková, V.; Birková, A. Flavonoids as Promising Natural Compounds in the Prevention and Treatment of Selected Skin Diseases. Int. J. Mol. Sci. 2023, 24, 6324. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Akhtar, S.; Sestili, P.; Riaz, M.; Ismail, A.; Labbe, R.G. Antioxidant, Antimicrobial and Urease Inhibitory Activities of Phenolics-rich Pomegranate Peel Hydro-alcoholic Extracts. J. Food Biochem. 2016, 40, 550–558. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Tong, B.; Wang, D.; Tian, X.; Liu, J.; Chen, J.; Xiao, X.; Wang, S. Rhizobacterial Compositions and Their Relationships with Soil Properties and Medicinal Bioactive Ingredients in Cinnamomum Migao. Front. Microbiol. 2023, 14, 1078886. [Google Scholar] [CrossRef] [PubMed]
- Bilger, W.; Johnsen, T.; Schreiber, U. UV-excited Chlorophyll Fluorescence as a Tool for the Assessment of UV-protection by the Epidermis of Plants. J. Exp. Bot. 2001, 52, 2007–2014. [Google Scholar] [CrossRef]
- Ramadan, R.M.; Youssef, F.S.; Fouad, E.A.; Orabi, A.; Khalifa, M.M. The Pharmacological Impact of Astragalus Membranaceus against Coccidial and Bacterial Infection in Vitro. Egypt. Pharm. J. 2023, 22, 324–335. [Google Scholar] [CrossRef]
- Elsharkawy, E.R.; Ed-dra, A.; Abdallah, E.M.; Ali, A.M.H. Antioxidant, Antimicrobial and Antifeedant Activity of Phenolic Compounds Accumulated in Hyoscyamus muticus L. Afr. J. Biotechnol. 2018, 17, 311–321. [Google Scholar]
- Lekmine, S.; Boussekine, S.; Kadi, K.; Martín-García, A.I.; Kheddouma, A.; Nagaz, K.; Bensouici, C. A Comparative Study on Chemical Profile and Biological Activities of Aerial Parts (Stems, Flowers, Leaves, Pods and Seeds) of Astragalus Gombiformis. Biocatal. Agric. Biotechnol. 2020, 27, 101668. [Google Scholar] [CrossRef]
- Ayari-Guentri, S.; Djemouai, N.; Gaceb-Terrak, R.; Rahmania, F. Chemical Composition and Antioxidant Activity of Hyoscyamus muticus L. Subsp. Falezlez (Coss.) Maire from Algeria. J. Essent. Oil Bear. Plants 2017, 20, 1370–1379. [Google Scholar] [CrossRef]
- Pero, R.W.; Lund, H.; Leanderson, T. Antioxidant Metabolism Induced by Quinic Acid. Increased Urinary Excretion of Tryptophan and Nicotinamide. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 335–346. [Google Scholar] [CrossRef]
- Chuda, Y.; Ono, H.; Ohnishi-Kameyama, M.; Nagata, T.; Tsushida, T. Structural Identification of Two Antioxidant Quinic Acid Derivatives from Garland (Chrysanthemum Coronarium L.). J. Agric. Food Chem. 1996, 44, 2037–2039. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sharma, P. Cinnamic Acid Derivatives: A New Chapter of Various Pharmacological Activities. J. Chem. Pharm. Res. 2011, 3, 403. [Google Scholar]
- Musalia, L.M.; Anandan, S.; Sastry, V.R.B.; Katiyar, R.C.; Agrawal, D.K. Effect of Replacement of Groundnut Cake with Urea-Treated Neem (Azadirachta Indica A. Juss) Seed Kernel Cake on Nutrient Utilisation in Lambs. Asian-Australas. J. Anim. Sci. 2002, 15, 1273–1277. [Google Scholar] [CrossRef]
- Shah, N.A.; Khan, M.R.; Sattar, S.; Ahmad, B.; Mirza, B. HPLC-DAD Analysis, Antioxidant Potential and Anti-Urease Activity of Asparagus Gracilis Collected from District Islamabad. BMC Complement. Altern. Med. 2014, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Opekun, A.R.; Abudayyeh, S.; Graham, D.Y. Effect of Different Organic Acids (Citric, Malic and Ascorbic) on Intragastric Urease Activity. Aliment. Pharmacol. Ther. 2005, 21, 1145–1148. [Google Scholar] [CrossRef]
- Chelleng, N.; Puzari, M.; Chetia, P.; Tamuly, C. Phenolic Compounds of Zanthoxylum Armatum DC as Potential Inhibitors of Urease and SARS-CoV2 Using Molecular Docking Approach and with Simulation Study. Nat. Prod. Res. 2023, 37, 1993–1997. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Švajdlenka, E. Biological Evaluation and Molecular Docking of Protocatechuic Acid from Hibiscus Sabdariffa L. as a Potent Urease Inhibitor by an ESI-MS Based Method. Molecules 2017, 22, 1696. [Google Scholar] [CrossRef]
- Amtul, Z.; Siddiqui, R.A.; Choudhary, M.I. Chemistry and Mechanism of Urease Inhibition. Curr. Med. Chem. 2002, 9, 1323–1348. [Google Scholar] [CrossRef]
- Suenaga, S.; Takano, Y.; Saito, T. Unraveling Binding Mechanism and Stability of Urease Inhibitors: A QM/MM MD Study. Molecules 2023, 28, 2697. [Google Scholar] [CrossRef]
- Lekmine, S.; Boussekine, S.; Akkal, S.; Martín-García, A.I.; Boumegoura, A.; Kadi, K.; Djeghim, H.; Mekersi, N.; Bendjedid, S.; Bensouici, C.; et al. Investigation of Photoprotective, Anti-Inflammatory, Antioxidant Capacities and LC–ESI–MS Phenolic Profile of Astragalus Gombiformis Pomel. Foods 2021, 10, 1937. [Google Scholar] [CrossRef]
- Lekmine, S.; Bendjedid, S.; Benslama, O.; Martín-García, A.I.; Boussekine, S.; Kadi, K.; Akkal, S.; Nieto, G.; Sami, R.; Al-Mushhin, A.A.M. Ultrasound-Assisted Extraction, LC–MS/MS Analysis, Anticholinesterase, and Antioxidant Activities of Valuable Natural Metabolites from Astragalus Armatus Willd.: In Silico Molecular Docking and In Vitro Enzymatic Studies. Antioxidants 2022, 11, 2000. [Google Scholar] [CrossRef] [PubMed]
- Benslama, O.; Lekmine, S.; Mansouri, N. Phytochemical Constituents of Astragalus Monspessulanus and Integrative Analysis for Its Antioxidant, Photoprotective, and Antityrosinase Activities: Experimental and Computational Investigation. Eur. J. Integr. Med. 2023, 60, 102247. [Google Scholar] [CrossRef]
- Bendjedid, S.; Lekmine, S.; Tadjine, A.; Djelloul, R.; Bensouici, C. Analysis of Phytochemical Constituents, Antibacterial, Antioxidant, Photoprotective Activities and Cytotoxic Effect of Leaves Extracts and Fractions of Aloe Vera. Biocatal. Agric. Biotechnol. 2021, 33, 101991. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Smara, M.; Khalladi, R.; Moulai-Mostefa, N.; Madi, K.; Mansour, D.; Lekmine, S.; Benslama, O.; Tahraoui, H.; Zhang, J.; Amrane, A. Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling. Processes 2024, 12, 621. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Fayez, S.; Zengin, G.; Selvi, S.; Uba, A.I.; Mollica, A.; Bouyahya, A.; Ponniya, S.K.M.; Nilofar; Lekmine, S. Chemical Exploration of Different Extracts from Phytolacca Americana Leaves and Their Potential Utilization for Global Health Problems: In Silico and Network Pharmacology Validation. J. Biomol. Struct. Dyn. 2024, 1–21. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Kadi, K.; Ignacio Martín-García, A.; Shamsul Ola, M.; Abdullah Yilmaz, M.; Ali, A. Therapeutic Potential of Hyoscyamus Niger-Derived Compounds: Targeting Ovarian Cancer through Antioxidant Activity and EGFR Tyrosine Kinase Inhibition. J. King Saud. Univ. Sci. 2024, 36, 103103. [Google Scholar] [CrossRef]
- Triki, Z.; Fergani, Z.; Lekmine, S.; Tahraoui, H.; Amrane, A.; Zamouche, M.; Kebir, M.; Assadi, A.A.; Khezami, L.; Zhang, J. Numerical Modelling and Performance Evaluation of Vacuum Membrane Distillation for Energy-Efficient Seawater Desalination: Towards Energy-Efficient Solutions. Water (Basel) 2023, 15, 3612. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Tahraoui, H.; Ola, M.S.; Laouani, A.; Kadi, K.; Martín-García, A.I.; Ali, A. Anti-Cholinergic Effects of the Phenolic Extract from the Astragalus Crenatus Plant: A Computational and Network Pharmacology Study. Pharmaceuticals 2024, 17. [Google Scholar] [CrossRef]
- Kunchandy, E.; Rao, M.N.A. Oxygen Radical Scavenging Activity of Curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Marco, G.J. A Rapid Method for Evaluation of Antioxidants. J. Am. Oil Chem. Soc. 1968, 45, 594–598. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl Radical Scavenging Activity of Compatible Solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szłyk, E. Determination of Antioxidant Capacities of Vegetable Oils by Ferric-Ion Spectrophotometric Methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef]
- Özyürek, M.; Güngör, N.; Baki, S.; Güçlü, K.; Apak, R. Development of a Silver Nanoparticle-Based Method for the Antioxidant Capacity Measurement of Polyphenols. Anal. Chem. 2012, 84, 8052–8059. [Google Scholar] [CrossRef]
- Gul, S.; Maab, S.; Rafiq, H.; Alam, A.; Rehman, M.U.; Assad, M.; AlAsmari, A.F.; Alasmari, F.; Ibrahim, M.; Khan, M. Exploring Bis-Schiff Bases with Thiobarbiturate Scaffold: In Vitro Urease Inhibition, Antioxidant Properties, and In Silico Studies. Russ. J. Bioorg Chem. 2024, 50, 1627–1638. [Google Scholar] [CrossRef]
- Mansouri, N.; Benslama, O.; Arhab, R. Homology Modeling, Docking and Molecular Dynamics Studies of Some Secondary Metabolites of Actinomycetes as Biocontrol Agents against the 3HNR Enzyme of the Phytopathogenic Fungus Alternaria Alternata. J. Biomol. Struct. Dyn. 2023, 41, 871–883. [Google Scholar] [CrossRef]
- Djeghim, H.; Bellil, I.; Benslama, O.; Lekmine, S.; Temim, E.; Boufendi, H.; Postigo, I.; Sánchez, P.; Khelifi, D. Effects of Genetic Diversity on the Allergenicity of Peanut (Arachis Hypogaea) Proteins: Identification of the Hypoallergenic Accessions Using BALB/c Mice Model and in Silico Analysis of Ara h 3 Allergen Cross-Reactivity. J. Proteom. 2024, 306, 105264. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Kadi, K.; Brik, A.; Djeffali, O.; Ounissi, M.; Slimani, M.; Ola, M.S.; Eldahshan, O.A.; Martín-García, A.I. Preliminary Investigation of Astragalus Arpilobus Subsp. Hauarensis: LC-MS/MS Chemical Profiling, In Vitro Evaluation of Antioxidant, Anti-Inflammatory Properties, Cytotoxicity, and In Silico Analysis against COX-2. Antioxidants 2024, 13, 654. [Google Scholar] [CrossRef]
- Boussekine, S.; Lekmine, S.; Gasmi, S.; Benkhedir, A.; Saker, H.; Lidoughi, A. The PROTECTIVE EFFECT OF SELENIUM ON DIABETIC NEPHROPATHY IN WISTAR RATS. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e5960. [Google Scholar] [CrossRef]
- Toumi, S.; Lekmine, S.; Touzout, N.; Moussa, H.; Elboughdiri, N.; Boudraa, R.; Benslama, O.; Kebir, M.; Danish, S.; Zhang, J. Harnessing Deep Learning for Real-Time Water Quality Assessment: A Sustainable Solution. Water 2024, 16, 3380. [Google Scholar] [CrossRef]
- Channar, P.A.; Saeed, A.; Albericio, F.; Larik, F.A.; Abbas, Q.; Hassan, M.; Raza, H.; Seo, S.-Y. Sulfonamide-Linked Ciprofloxacin, Sulfadiazine and Amantadine Derivatives as a Novel Class of Inhibitors of Jack Bean Urease; Synthesis, Kinetic Mechanism and Molecular Docking. Molecules 2017, 22, 1352. [Google Scholar] [CrossRef] [PubMed]
- Gherdaoui, D.; Yahoum, M.M.; Toumi, S.; Lekmine, S.; Lefnaoui, S.; Benslama, O.; Bouallouche, R.; Tahraoui, H.; Ola, M.S.; Ali, A. Elucidating Chiral Resolution of Aromatic Amino Acids Using Glycopeptide Selectors: A Combined Molecular Docking and Chromatographic Study. Int. J. Mol. Sci. 2024, 25, 9120. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.; Dahmoune, F.; Lekmine, S.; Mameri, A.; Tahraoui, H.; Hamid, S.; Benzitoune, N.; Moula, N.; Zhang, J.; Amrane, A. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Carthamus Caeruleus L. Rhizome: Integrating Central Composite Design, Gaussian Process Regression, and Multi-Objective Grey Wolf Optimization Approaches. Process Biochem. 2024, 147, 476–488. [Google Scholar] [CrossRef]
- Djellal, S.; Dahmoune, F.; Aoun, O.; Remini, H.; Belbahi, A.; Dairi, S.; Moussa, H.; Lakehal, M.; Kassouar, S.; Lakhdari, C. Optimization of Ultrasound-Assisted Extraction of Galactomannan from Carob Seeds “Ceratonia Siliqua L” and Evaluation of Their Functional Properties and in Vitro Anti-Inflammatory Activity. Sep. Sci. Technol. 2024, 59, 41–58. [Google Scholar] [CrossRef]
- Hamid, S.; Oukil, N.F.; Moussa, H.; Mahdjoub, M.M.; Djihad, N.; Berrabah, I.; Bouhenna, M.M.; Chebrouk, F.; Hentabli, M. Enhancing Basil Essential Oil Microencapsulation Using Pectin/Casein Biopolymers: Optimization through D-Optimal Design, Controlled Release Modeling, and Characterization. Int. J. Biol. Macromol. 2024, 265, 130948. [Google Scholar] [CrossRef]
- Moussa, H.; Hamid, S.; Mameri, A.; Lekmine, S.; Tahraoui, H.; Kebir, M.; Touzout, N.; Dahmoune, F.; Ola, M.S.; Zhang, J. From Green Chemistry to Healthy Environments: Silver Nanoparticles as a Dual Antioxidant and Antibacterial Agents for Advancing Biomedicine and Sustainable Wastewater Treatment. Bioengineering 2024, 11, 1205. [Google Scholar] [CrossRef]
- Rashid, M.; Rafique, H.; Roshan, S.; Shamas, S.; Iqbal, Z.; Ashraf, Z.; Abbas, Q.; Hassan, M.; Qureshi, Z.U.R.; Asad, M.H.H. Bin Enzyme Inhibitory Kinetics and Molecular Docking Studies of Halo-Substituted Mixed Ester/Amide-Based Derivatives as Jack Bean Urease Inhibitors. Biomed. Res. Int. 2020, 2020, 8867407. [Google Scholar] [CrossRef]
- Yahoum, M.M.; Toumi, S.; Hentabli, S.; Tahraoui, H.; Lefnaoui, S.; Hadjsadok, A.; Amrane, A.; Kebir, M.; Moula, N.; Assadi, A.A. Experimental Analysis and Neural Network Modeling of the Rheological Behavior of Xanthan Gum and Its Derivatives. Materials 2023, 16, 2565. [Google Scholar] [CrossRef]
- Ben Hadj Tahar, D.; Triki, Z.; Guendouz, M.; Tahraoui, H.; Zamouche, M.; Kebir, M.; Zhang, J.; Amrane, A. Characterization and Thermal Evaluation of a Novel Bio-Based Natural Insulation Material from Posidonia Oceanica Waste: A Sustainable Solution for Building Insulation in Algeria. ChemEngineering 2024, 8, 18. [Google Scholar] [CrossRef]

| Analyst Number | Compound | Parent Ion (m/z) | MS2 (Collision Energy) | Chemical Formula | Type of Compound | H. muticus (µg dry/g Extract) |
|---|---|---|---|---|---|---|
| 1 | Quinic acid | 191.085 | 192.085 (23), 94 (22) | C7H12O6 | Phenolic acid | 63.12 ± 22.3 |
| 2 | Malic acid | 133.1115 | 134.1115 (15), 72 (18) | C4H6O5 | Organic acid | 7.9 ± 1.3 |
| 3 | tr-Aconitic acid | 172.985 | 173.985 (13), 130 (10) | C6H6O6 | Organic acid | N.D. |
| 4 | Gallic acid | 169.1125 | 170.1125 (15), 80 (26) | C7H6O5 | Phenolic acid | 125.25 ± 3.4 |
| 5 | Chlorogenic acid | 353.0191 | 354.0191 (18) | C16H18O9 | Phenolic acid | 17,108.3± 1.3 |
| 6 | Protocatechuic acid | 153.0109 | 154.0109 (17), 109 (27) | C7H6O4 | Phenolic acid | 1108.3± 1.2 |
| 7 | Tannic acid | 183.0124 | 184.0124 (23), 79 (35) | C76H52O46 | Phenolic acid | 977 ± 1.3 |
| 8 | tr-Caffeic acid | 179.0135 | 180.0135 (16), 135 (25), 90 (32) | C9H8O4 | Phenolic acid | 9.3 ± 1.9 |
| 9 | Vanillin | 151.1136 | 152.1136 (18), 93 (22) | C8H8O3 | Phenolic aldehyde | 96.7 ± 1.6 |
| 10 | p-Coumaric acid | 163.0119 | 164.0119 (16), 94 (32) | C9H8O3 | Phenolic acid | 875 ± 1.3 |
| 11 | Rosmarinic acid | 358.9161 | 359.9161 (18), 134 (43) | C18H16O8 | Phenolic acid | 125.2 ± 1.1 |
| 12 | Rutin | 609.1300 | 610.1300 (38), 272 (52), 302 (39) | C27H30O16 | Flavonoid (flavonol) | 269.25 ± 1.3 |
| 13 | Hesperidin | 611.1303 | 612.1303, 466 | C28H34O15 | Flavonoid (flavanone) | N.D. |
| 14 | Hyperoside | 463.1300 | 464.1300, 302 | C21H20O12 | Flavonoid (flavonol) | 523. ± 1.7 |
| 15 | 4-OH Benzoic acid | 137.093 | 138.093, 66 | C7H6O3 | Phenolic acid | N.D. |
| 16 | Salicylic acid | 137.093 | 138.093, 66, 76 | C7H6O3 | Phenolic acid | 56.3 ± 5.3 |
| 17 | Myricetin | 317.0179 | 318.0179, 152, 138 | C15H10O8 | Flavonoid (flavonol) | N.D. |
| 18 | Fisetin | 285.0135 | 286.0135, 122 | C15H10O6 | Flavonoid (flavonol) | N.D. |
| 19 | Coumarin | 147.0103 | 148.0103, 92, 78 | C9H6O2 | Aromatic lactone | 2.3± 2.3 |
| 20 | Quercetin | 300.9179 | 301.9179, 152, 122 | C15H10O7 | Flavonoid (flavonol) | 120. ± 6.3 |
| 21 | Naringenin | 271.0151 | 272.0152, 120, 108 | C15H12O5 | Flavonoid (flavanone) | N.D |
| 22 | Hesperetin | 301.0164 | 302.0165, 137, 109 | C16H14O6 | Flavonoid (flavanone) | 76. ± 2.3 |
| 23 | Luteolin | 285.0175 | 286.0176, 152, 134 | C15H10O6 | Flavonoid (flavone) | 122 ± 1.02 |
| 24 | Kaempferol | 285.0217 | 286.0218, 134, 152 | C15H10O6 | Flavonoid (flavonol) | 93. ± 4.2 |
| 25 | Apigenin | 269.0151 | 270.0152, 118 | C15H10O5 | Flavonoid (flavone) | 563 ± 2.3 |
| 26 | Rhamnetin | 315.0165 | 316.0166, 122, 301 | C16H12O7 | Flavonoid (flavonol) | N.D. |
| 27 | Chrysin | 253.0143 | 254.0144, 120, 108 | C15H10O4 | Flavonoid (flavone) | N.D. |
| Assay | H. muticus Extract (μg/mL) | BHT (μg/mL) | BHA (μg/mL) | Ascorbic Acid (μg/mL) |
|---|---|---|---|---|
| CUPRAC | 22.57 ± 1.2 | 7.75 ± 0.5 | 6.34 ± 0.4 | 7.05 ± 0.2 |
| Reducing power | 13.5 ± 2.3 | / | / | 6.36 ± 0.3 |
| β-carotene | 6.12 ± 1.8 | 9.21 ± 0.6 | 9.15 ± 0.4 | / |
| DMSO alkaline | 12 ± 1.2 | / | / | / |
| SNP | 6.5 ± 1.5 | / | / | 7.42 ± 0.1 |
| Phenanthroline | 23 ± 1.8 | 2.54± 0.8 | 2.35 ± 0.7 | 3.15 ± 0.5 |
| Hydroxyl radical | 39.95 ± 2.3 | / | / | 13.44 ± 0.7 |
| Urease (5 mg/mL) Inhibition (%) | C50 (µg/mL) | |
|---|---|---|
| H. muticus | 91.35 | 5.6 ± 1.20 |
| Thiourea | 96 | 2.6 ± 0.08 |
| Binding Energy (Kcal/mol) | Detailed Examination of Hydrogen Bonds (Distance Å) | Hydrophobic Bonding | Electrostatic Bonding | |
|---|---|---|---|---|
| Hyperoside | −7.9 | Asp494 (3.28), Ala636 (3.45), Met588 (2.22) | His593, Aala440 | Asp494 |
| Rutin | −7.6 | Arg439 (3.04), Arg439 (3.1) Arg439 (3.24), Gln635 (2.71), Ala440 (2.23), Asp633 (2.40) Val591 (3.78) | His593, His593, Ala636, Mer637 | Arg609 |
| Luteolin | −6.9 | His593 (3.69), Gly550 (3.06), Gly550 (2.10), Val519 (3.54) | - | Met637, Arg609 |
| Apigenine | −6.8 | Ala440 (2.01), Gly550 (2.16), Val591 (3.61) | Met637, His593 | Arg609 |
| Kaemferol | −6.8 | Ile807 (2.87), Gly562 (3.30), Ly559 (2.04), Lys559 (3.22) | Ile563, Lys559 | - |
| Hesperetin | −6.7 | His593 (3.33), Arg609 | Ala636, His409, His407, His545, His492, His519 | - |
| Chlorogenic acid | −6.7 | Ala636 (2.04), Gln635 (2.15), Arg439 (2.44), His593 (3.15), His492 (2.35) | Ala636 | Arg609 |
| Rosmarinic acid | −5.8 | Ala436 (2.40), Arg439 (3.27), His593 (3.05) | Ala440 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekmine, S.; Benslama, O.; Bensalah, B.; Touzout, N.; Moussa, H.; Tahraoui, H.; Ola, M.S.; Hafsa, H.; Zhang, J.; Amrane, A. Bioactive Phenolics of Hyoscyamus muticus L. Subsp. Falezlez: A Molecular and Biochemical Approach to Antioxidant and Urease Inhibitory Activities. Int. J. Mol. Sci. 2025, 26, 370. https://doi.org/10.3390/ijms26010370
Lekmine S, Benslama O, Bensalah B, Touzout N, Moussa H, Tahraoui H, Ola MS, Hafsa H, Zhang J, Amrane A. Bioactive Phenolics of Hyoscyamus muticus L. Subsp. Falezlez: A Molecular and Biochemical Approach to Antioxidant and Urease Inhibitory Activities. International Journal of Molecular Sciences. 2025; 26(1):370. https://doi.org/10.3390/ijms26010370
Chicago/Turabian StyleLekmine, Sabrina, Ouided Benslama, Bachir Bensalah, Nabil Touzout, Hamza Moussa, Hichem Tahraoui, Mohammad Shamsul Ola, Haroun Hafsa, Jie Zhang, and Abdeltif Amrane. 2025. "Bioactive Phenolics of Hyoscyamus muticus L. Subsp. Falezlez: A Molecular and Biochemical Approach to Antioxidant and Urease Inhibitory Activities" International Journal of Molecular Sciences 26, no. 1: 370. https://doi.org/10.3390/ijms26010370
APA StyleLekmine, S., Benslama, O., Bensalah, B., Touzout, N., Moussa, H., Tahraoui, H., Ola, M. S., Hafsa, H., Zhang, J., & Amrane, A. (2025). Bioactive Phenolics of Hyoscyamus muticus L. Subsp. Falezlez: A Molecular and Biochemical Approach to Antioxidant and Urease Inhibitory Activities. International Journal of Molecular Sciences, 26(1), 370. https://doi.org/10.3390/ijms26010370









