Chemoresistance in Pancreatic Cancer: The Role of Adipose-Derived Mesenchymal Stem Cells and Key Resistance Genes
Abstract
1. Introduction
2. Results
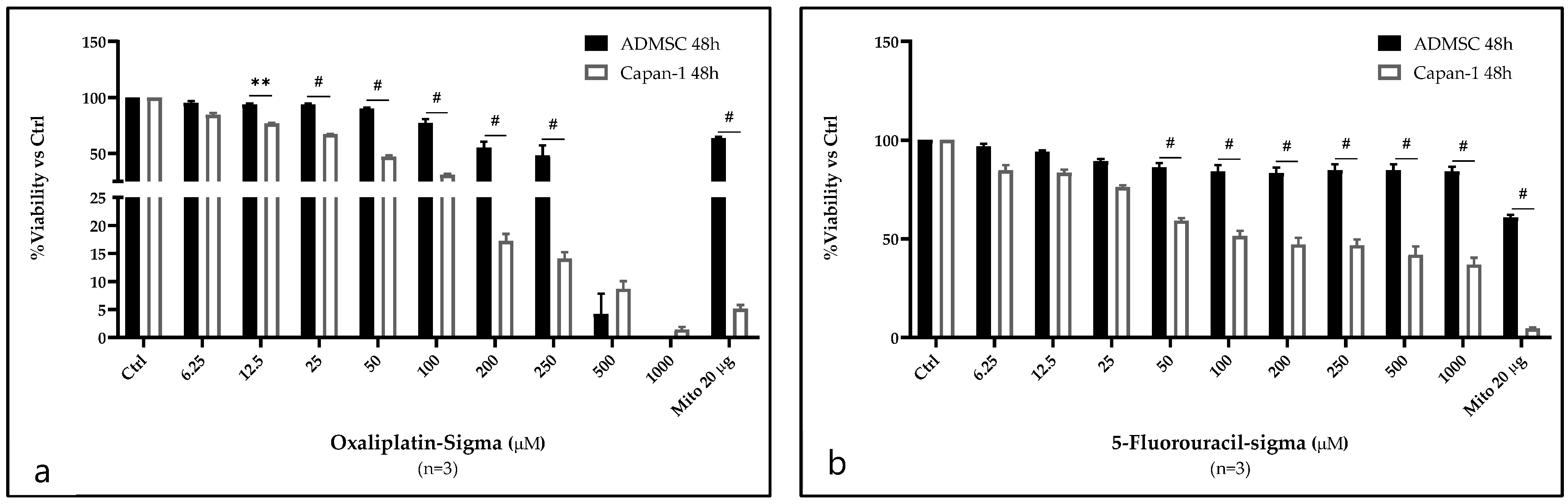
2.1. Analysis of OXP and 5-FU Cytotoxicity on Capan-1 Cells and ASCs
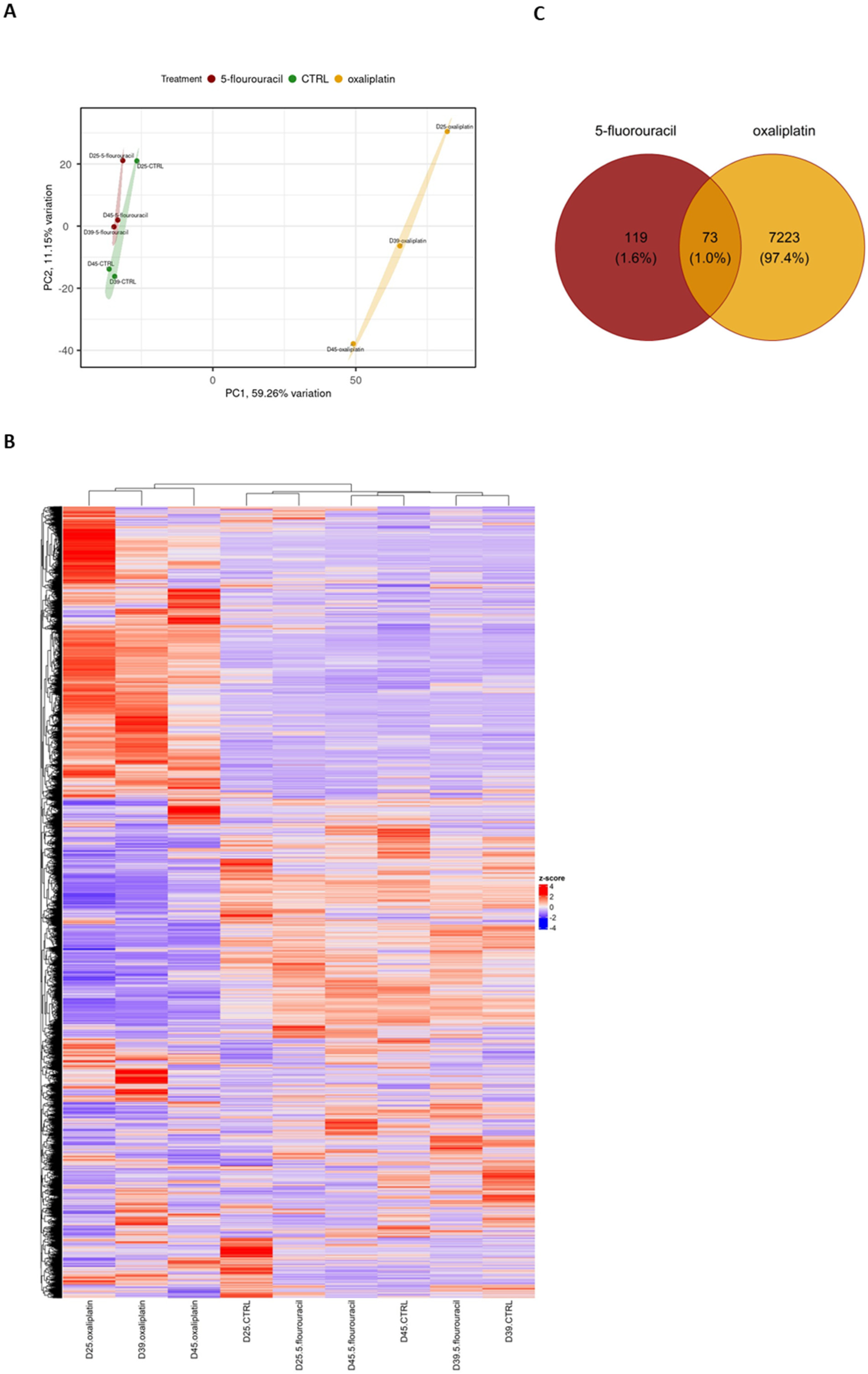
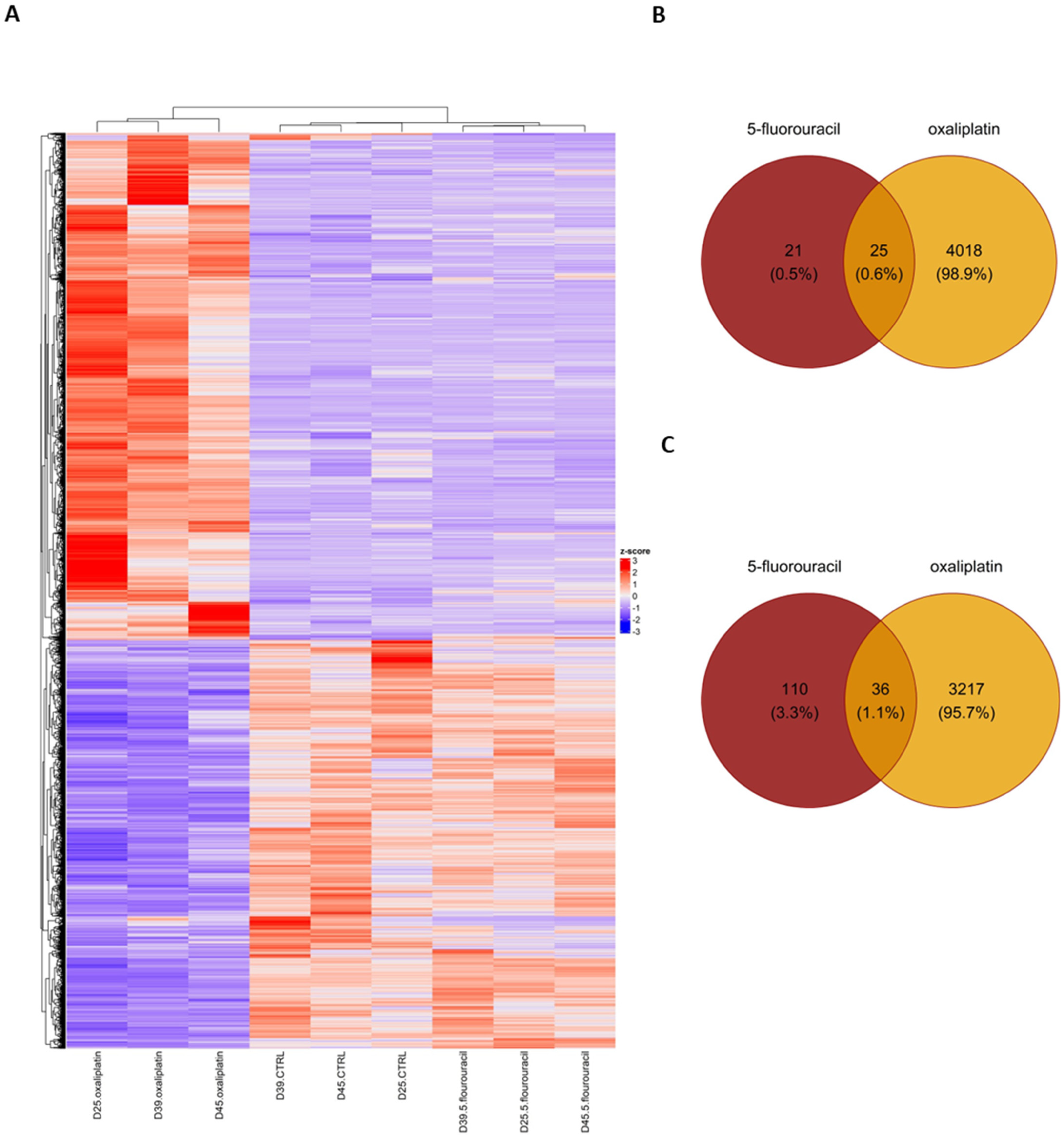
2.2. OXP Treatment-Induced Pronounced Gene Expression Changes Compared with 5-FU Treatment and Untreated Controls in ASCs
2.3. Gene Ontology (GO) Enrichment Analysis of DEGs in ASCs Treated with OXP and 5-FU
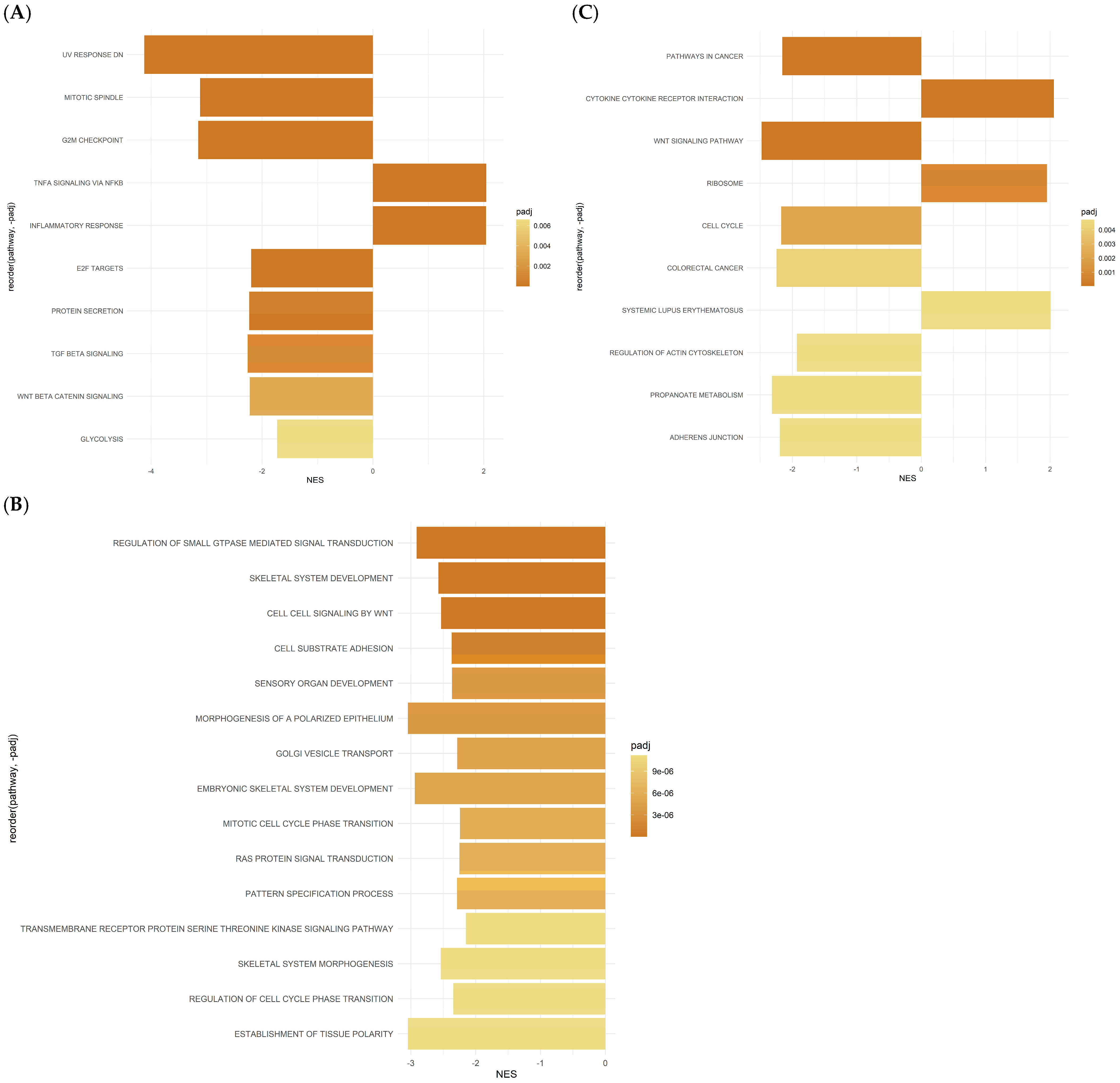
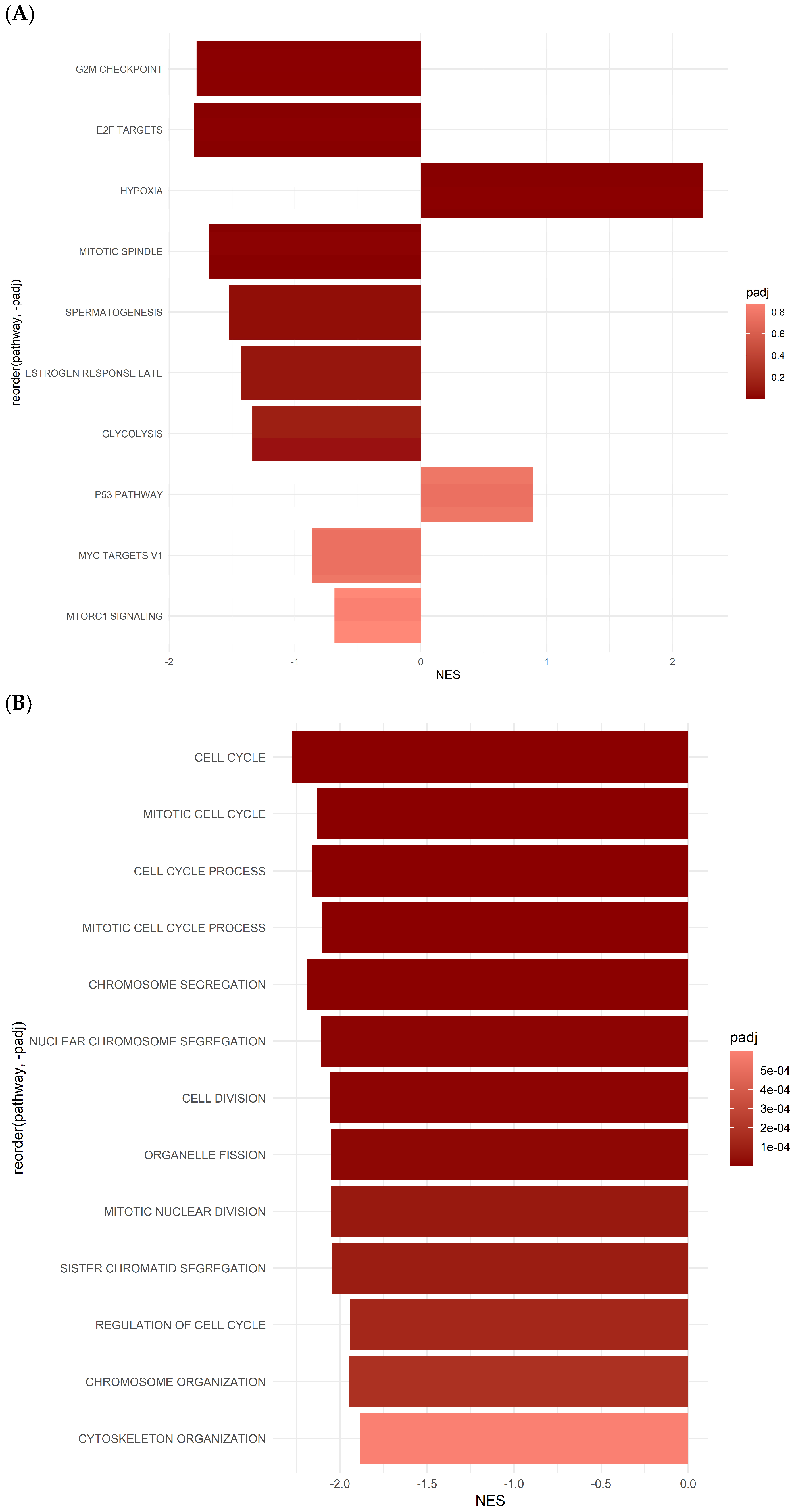
2.4. Differentially Expressed Gene Set Enrichment (GSEA) Analysis Results for ASCs Treated with Oxaliplatin
2.5. Gene Set Enrichment Analysis (GSEA) Reveals Hallmark and GO Pathways in ASCs Treated with 5-FU
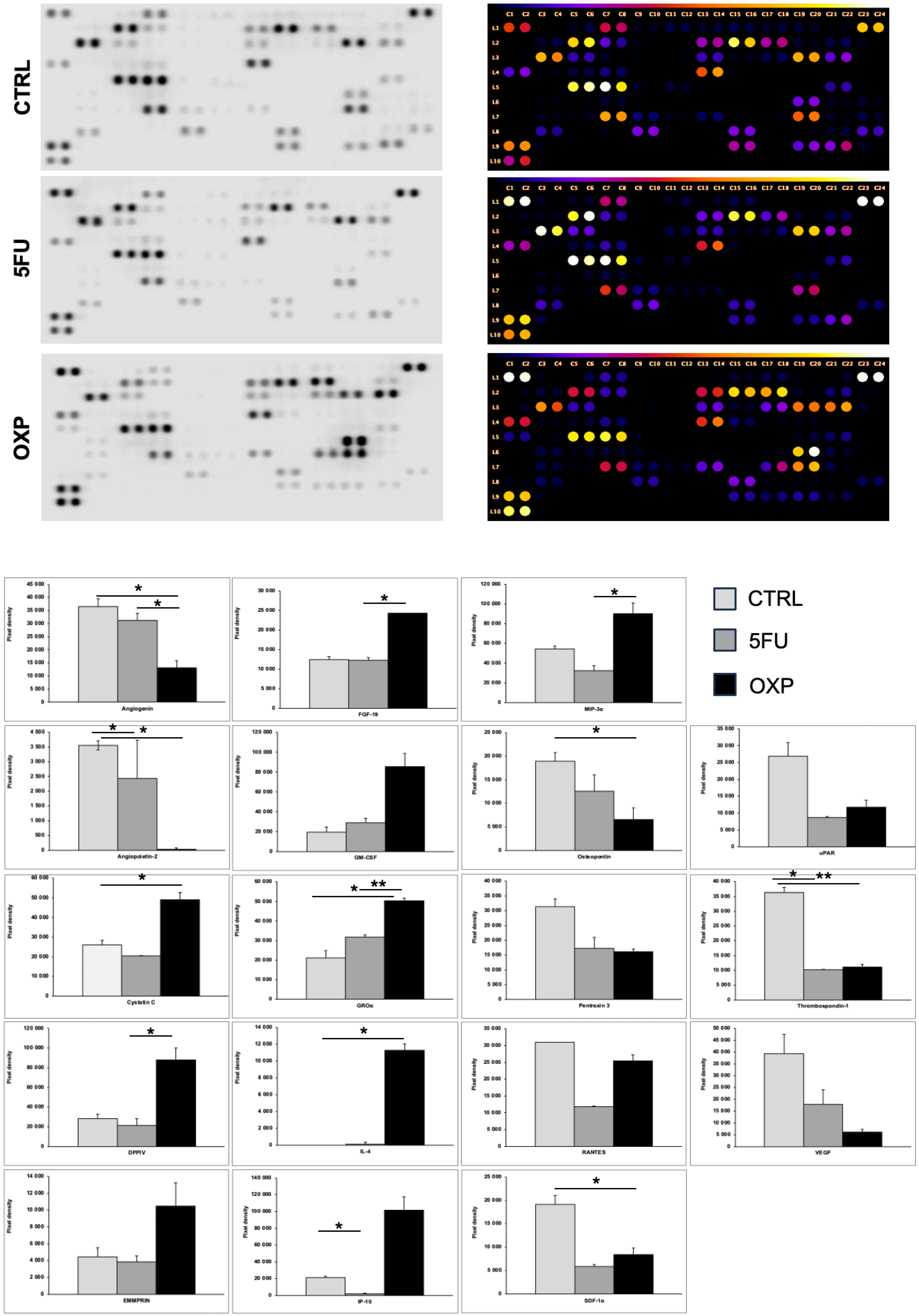
2.6. Protein Array Analysis of ASCs Supernatant Treated with OXP and 5-FU
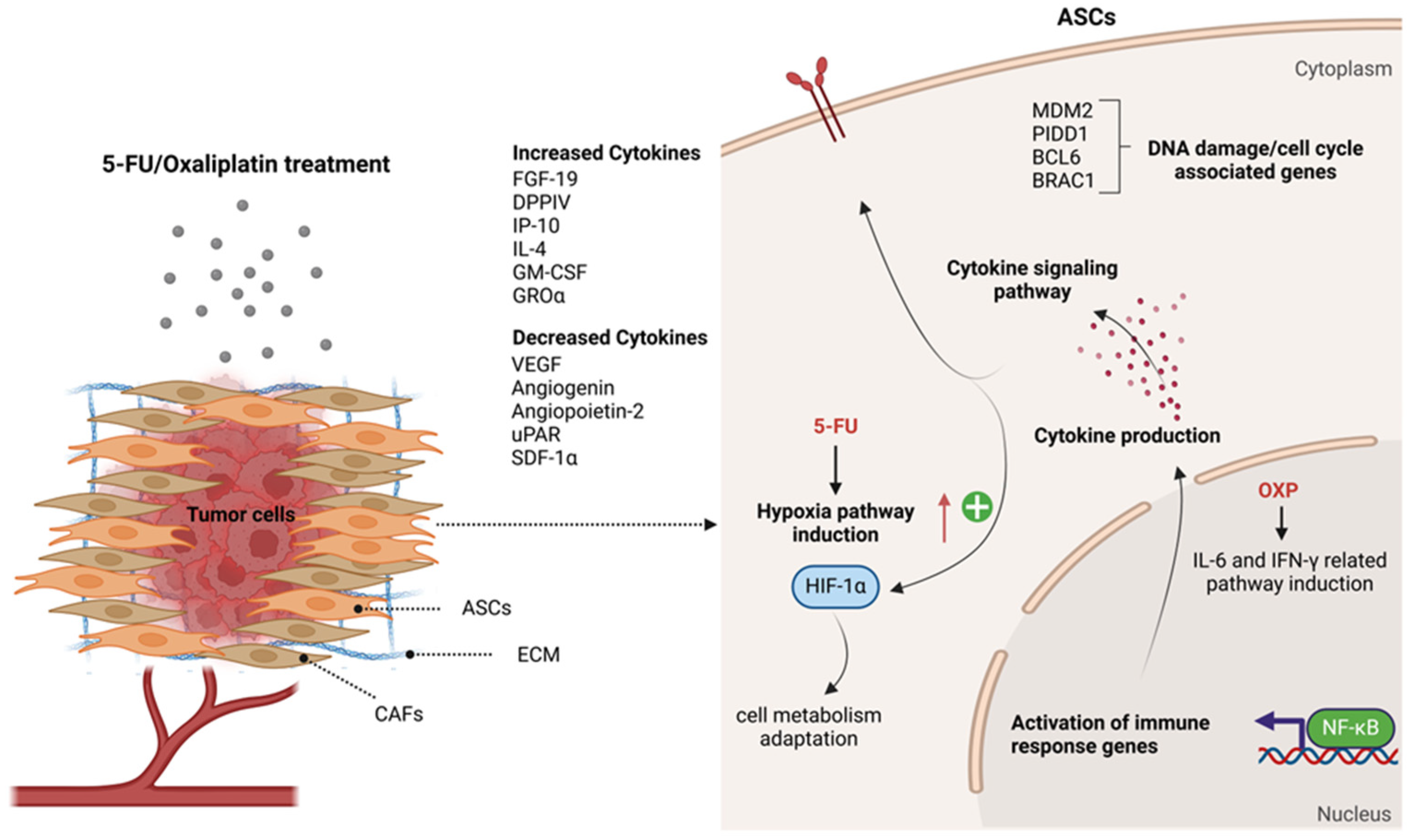
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Tissue Culture
4.1.1. Capan-1 Cell Culture
4.1.2. Adipose-Derived Mesenchymal Stem Cells (ASCs) Isolation and Culture
4.1.3. Viability and Metabolic Assay
4.2. Validation of Adipose Tissue-Derived Mesenchymal Stem Cell Differentiation Potential into Adipocytes, Chondrocytes, and Osteocytes
4.3. Flow Cytometry
4.4. MTT Cell Proliferation Assay
4.5. Treatment of ASCs for RNA-Seq and Protein Array
4.6. RNA Isolation for RNA Sequencing
4.7. RNA Sequencing
4.8. Protein Array
4.9. Data Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanno, A.; Masamune, A.; Hanada, K.; Kikuyama, M.; Kitano, M. Advances in Early Detection of Pancreatic Cancer. Diagnostics 2019, 9, 18. [Google Scholar] [CrossRef]
- Bausch, D.; Keck, T. Minimally Invasive Surgery of Pancreatic Cancer: Feasibility and Rationale. Visc. Med. 2018, 34, 440–443. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Sunami, Y.; Kleeff, J. Immunotherapy of Pancreatic Cancer. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2019; Volume 164, pp. 189–216. ISBN 978-0-12-816575-1. [Google Scholar]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of Tumor Microenvironment in Cancer Progression and Therapeutic Strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Crippa, S.; Belfiori, G.; Bissolati, M.; Partelli, S.; Pagnanelli, M.; Tamburrino, D.; Gasparini, G.; Rubini, C.; Zamboni, G.; Falconi, M. Recurrence after Surgical Resection of Pancreatic Cancer: The Importance of Postoperative Complications beyond Tumor Biology. HPB 2021, 23, 1666–1673. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Zhai, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer Stem Cells: Advances in Knowledge and Implications for Cancer Therapy. Signal Transduct. Target. Ther. 2024, 9, 170. [Google Scholar] [CrossRef]
- Neesse, A.; Algül, H.; Tuveson, D.A.; Gress, T.M. Stromal Biology and Therapy in Pancreatic Cancer: A Changing Paradigm. Gut 2015, 64, 1476–1484. [Google Scholar] [CrossRef]
- El-Haibi, C.P.; Karnoub, A.E. Mesenchymal Stem Cells in the Pathogenesis and Therapy of Breast Cancer. J. Mammary Gland Biol. Neoplasia 2010, 15, 399–409. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. Stromal Cells in the Tumor Microenvironment: Accomplices of Tumor Progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct Evidence of Mesenchymal Stem Cell Tropism for Tumor and Wounding Microenvironments Using In Vivo Bioluminescent Imaging. Stem Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, P.; Bijlsma, M.; Van Laarhoven, H. The Cellular Origins of Cancer-Associated Fibroblasts and Their Opposing Contributions to Pancreatic Cancer Growth. Front. Cell Dev. Biol. 2021, 9, 743907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, P. Cancer-Stromal Interactions: Role in Cell Survival, Metabolism and Drug Sensitivity. Cancer Biol. Ther. 2011, 11, 150–156. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; Yang, J.; Zhang, X.; Zhang, H.; Zhang, T.; Zhao, S.; Zheng, P.; Huo, J.; Wu, H. Gastric Cancer-Derived Mesenchymal Stem Cells Prompt Gastric Cancer Progression through Secretion of Interleukin-8. J. Exp. Clin. Cancer Res. CR 2015, 34, 52. [Google Scholar] [CrossRef]
- Kabashima-Niibe, A.; Higuchi, H.; Takaishi, H.; Masugi, Y.; Matsuzaki, Y.; Mabuchi, Y.; Funakoshi, S.; Adachi, M.; Hamamoto, Y.; Kawachi, S.; et al. Mesenchymal Stem Cells Regulate Epithelial-Mesenchymal Transition and Tumor Progression of Pancreatic Cancer Cells. Cancer Sci. 2013, 104, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Zhao, J.; Li, Y.; Wang, Z.; Yan, H.; Liu, Y.; Sun, M.; Zhuang, J.; Wang, J. Rat Bone Marrow-Derived Mesenchymal Stem Cells Promote the Migration and Invasion of Colorectal Cancer Stem Cells. OncoTargets Ther. 2020, 13, 6617–6628. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Kreis, N.-N.; Hoock, S.C.; Solbach, C.; Louwen, F.; Yuan, J. Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells, Obesity and the Tumor Microenvironment of Breast Cancer. Cancers 2022, 14, 3908. [Google Scholar] [CrossRef]
- Del Vecchio, V.; Rehman, A.; Panda, S.K.; Torsiello, M.; Marigliano, M.; Nicoletti, M.M.; Ferraro, G.A.; De Falco, V.; Lappano, R.; Lieto, E.; et al. Mitochondrial Transfer from Adipose Stem Cells to Breast Cancer Cells Drives Multi-Drug Resistance. J. Exp. Clin. Cancer Res. 2024, 43, 166. [Google Scholar] [CrossRef] [PubMed]
- Salaud, C.; Alvarez-Arenas, A.; Geraldo, F.; Belmonte-Beitia, J.; Calvo, G.F.; Gratas, C.; Pecqueur, C.; Garnier, D.; Pérez-Garcià, V.; Vallette, F.M.; et al. Mitochondria Transfer from Tumor-Activated Stromal Cells (TASC) to Primary Glioblastoma Cells. Biochem. Biophys. Res. Commun. 2020, 533, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Sakaguchi, M.; Maruyama, S.; Iioka, H.; Putranto, E.W.; Sumardika, I.W.; Tomonobu, N.; Kawasaki, T.; Homma, K.; Kondo, E. Stromal Mesenchymal Stem Cells Facilitate Pancreatic Cancer Progression by Regulating Specific Secretory Molecules through Mutual Cellular Interaction. J. Cancer 2018, 9, 2916–2929. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, L.; Li, D.; Andl, T.; Zhang, Y. Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironment. Front. Cell Dev. Biol. 2019, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Ohuchida, K.; Kibe, S.; Iwamoto, C.; Ando, Y.; Takesue, S.; Nakayama, H.; Abe, T.; Endo, S.; Koikawa, K.; et al. Adipose Tissue-Derived Stromal Cells Are Sources of Cancer-Associated Fibroblasts and Enhance Tumor Progression by Dense Collagen Matrix. Int. J. Cancer 2019, 144, 1401–1413. [Google Scholar] [CrossRef]
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016, 6, 852–869. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Oda, T.; Inagaki, Y.; Kushige, H.; Saito, Y.; Mori, N.; Takayama, Y.; Kumagai, Y.; Mitsuyama, T.; Kida, Y.S. Adipose-Derived Mesenchymal Stem Cells Differentiate into Heterogeneous Cancer-Associated Fibroblasts in a Stroma-Rich Xenograft Model. Sci. Rep. 2021, 11, 4690. [Google Scholar] [CrossRef]
- Sperb, N.; Tsesmelis, M.; Wirth, T. Crosstalk between Tumor and Stromal Cells in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 5486. [Google Scholar] [CrossRef] [PubMed]
- Antoon, R.; Overdevest, N.; Saleh, A.H.; Keating, A. Mesenchymal Stromal Cells as Cancer Promoters. Oncogene 2024, 43, 3545–3555. [Google Scholar] [CrossRef]
- Duluc, C.; Moatassim-Billah, S.; Chalabi-Dchar, M.; Perraud, A.; Samain, R.; Breibach, F.; Gayral, M.; Cordelier, P.; Delisle, M.-B.; Bousquet-Dubouch, M.-P.; et al. Pharmacological Targeting of the Protein Synthesis mTOR/4E-BP1 Pathway in Cancer-Associated Fibroblasts Abrogates Pancreatic Tumour Chemoresistance. EMBO Mol. Med. 2015, 7, 735–753. [Google Scholar] [CrossRef]
- Garg, B.; Giri, B.; Modi, S.; Sethi, V.; Castro, I.; Umland, O.; Ban, Y.; Lavania, S.; Dawra, R.; Banerjee, S.; et al. NFκB in Pancreatic Stellate Cells Reduces Infiltration of Tumors by Cytotoxic T Cells and Killing of Cancer Cells, via Up-Regulation of CXCL12. Gastroenterology 2018, 155, 880–891.e8. [Google Scholar] [CrossRef] [PubMed]
- Wörmann, S.M.; Song, L.; Ai, J.; Diakopoulos, K.N.; Kurkowski, M.U.; Görgülü, K.; Ruess, D.; Campbell, A.; Doglioni, C.; Jodrell, D.; et al. Loss of P53 Function Activates JAK2–STAT3 Signaling to Promote Pancreatic Tumor Growth, Stroma Modification, and Gemcitabine Resistance in Mice and Is Associated With Patient Survival. Gastroenterology 2016, 151, 180–193.e12. [Google Scholar] [CrossRef]
- Li, L.; Tian, H.; Yue, W.; Zhu, F.; Li, S.; Li, W. Human Mesenchymal Stem Cells Play a Dual Role on Tumor Cell Growth in Vitro and in Vivo. J. Cell. Physiol. 2011, 226, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal Elements Act to Restrain, Rather Than Support, Pancreatic Ductal Adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef]
- Ji, N.; Yu, J.-W.; Ni, X.-C.; Wu, J.-G.; Wang, S.-L.; Jiang, B.-J. Bone Marrow-Derived Mesenchymal Stem Cells Increase Drug Resistance in CD133-Expressing Gastric Cancer Cells by Regulating the PI3K/AKT Pathway. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 14637–14651. [Google Scholar] [CrossRef] [PubMed]
- Citterio, C.; Baccini, M.; Orlandi, E.; Di Nunzio, C.; Cavanna, L. Second-Line Chemotherapy for the Treatment of Metastatic Pancreatic Cancer after First-Line Gemcitabine-Based Chemotherapy: A Network Meta-Analysis. Oncotarget 2018, 9, 29801–29809. [Google Scholar] [CrossRef][Green Version]
- Saung, M.T.; Zheng, L. Current Standards of Chemotherapy for Pancreatic Cancer. Clin. Ther. 2017, 39, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Phua, L.C.; Mal, M.; Koh, P.K.; Cheah, P.Y.; Chan, E.C.Y.; Ho, H.K. Investigating the Role of Nucleoside Transporters in the Resistance of Colorectal Cancer to 5-Fluorouracil Therapy. Cancer Chemother. Pharmacol. 2013, 71, 817–823. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Wang, W.-B.; Yang, Y.; Zhao, Y.-P.; Zhang, T.-P.; Liao, Q.; Shu, H. Recent Studies of 5-Fluorouracil Resistance in Pancreatic Cancer. World J. Gastroenterol. 2014, 20, 15682–15690. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Houthuijzen, J.M.; Daenen, L.G.M.; Roodhart, J.M.L.; Voest, E.E. The Role of Mesenchymal Stem Cells in Anti-Cancer Drug Resistance and Tumour Progression. Br. J. Cancer 2012, 106, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, M.S.; Ward, C.M.; Davies, C.C. DNA Repair and Therapeutic Strategies in Cancer Stem Cells. Cancers 2023, 15, 1897. [Google Scholar] [CrossRef]
- Yuan, M.; Eberhart, C.G.; Kai, M. RNA Binding Protein RBM14 Promotes Radio-Resistance in Glioblastoma by Regulating DNA Repair and Cell Differentiation. Oncotarget 2014, 5, 2820–2826. [Google Scholar] [CrossRef]
- Wade, M.; Wang, Y.V.; Wahl, G.M. The P53 Orchestra: Mdm2 and Mdmx Set the Tone. Trends Cell Biol. 2010, 20, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Pourebrahim, R.; Heinz Montoya, R.; Alaniz, Z.; Ostermann, L.; Lin, P.P.; Liu, B.; Ayoub, E.; Burks, J.K.; Andreeff, M. Mdm2/P53 Levels in Bone Marrow Mesenchymal Stromal Cells Are Essential for Maintaining the Hematopoietic Niche in Response to DNA Damage. Cell Death Dis. 2023, 14, 371. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, T.; Matthews, W.C.; Jackson, E.R.; Staudt, D.E.; Douglas, A.M.; Findlay, I.J.; Persson, M.L.; Duchatel, R.J.; Mannan, A.; Germon, Z.P.; et al. B-Cell Lymphoma 6 (BCL6): From Master Regulator of Humoral Immunity to Oncogenic Driver in Pediatric Cancers. Mol. Cancer Res. MCR 2022, 20, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, J.; Yuan, K.; Wu, Z.; Hu, L.; Lu, Y.; Li, K.; Guo, J.; Chen, J.; Ma, C.; et al. The Oncoprotein BCL6 Enables Solid Tumor Cells to Evade Genotoxic Stress. eLife 2022, 11, e69255. [Google Scholar] [CrossRef]
- Hatzi, K.; Nance, J.P.; Kroenke, M.A.; Bothwell, M.; Haddad, E.K.; Melnick, A.; Crotty, S. BCL6 Orchestrates Tfh Cell Differentiation via Multiple Distinct Mechanisms. J. Exp. Med. 2015, 212, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.R.; Liu, S.; Xiang, M.; Nicolais, M.; Hatzi, K.; Giannopoulou, E.; Elemento, O.; Cerchietti, L.; Melnick, A.; Frank, D.A. The Transcriptional Modulator BCL6 as a Molecular Target for Breast Cancer Therapy. Oncogene 2015, 34, 1073–1082. [Google Scholar] [CrossRef]
- Cardenas, M.G.; Oswald, E.; Yu, W.; Xue, F.; MacKerell, A.D.; Melnick, A.M. The Expanding Role of the BCL6 Oncoprotein as a Cancer Therapeutic Target. Clin. Cancer Res. 2017, 23, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Ray Chaudhuri, A.; Callen, E.; Ding, X.; Gogola, E.; Duarte, A.A.; Lee, J.-E.; Wong, N.; Lafarga, V.; Calvo, J.A.; Panzarino, N.J.; et al. Replication Fork Stability Confers Chemoresistance in BRCA-Deficient Cells. Nature 2016, 535, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Pei, Q.; Li, J.; Wan, X.; Ye, T. Emerging Role of E2F Family in Cancer Stem Cells. Front. Oncol. 2021, 11, 723137. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in Tumor Progression and Regression: A Review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Dong, S.; Liang, S.; Cheng, Z.; Zhang, X.; Luo, L.; Li, L.; Zhang, W.; Li, S.; Xu, Q.; Zhong, M.; et al. ROS/PI3K/Akt and Wnt/β-Catenin Signalings Activate HIF-1α-Induced Metabolic Reprogramming to Impart 5-Fluorouracil Resistance in Colorectal Cancer. J. Exp. Clin. Cancer Res. CR 2022, 41, 15. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, B.M. Hypoxia-Inducible Factor in Cancer: From Pathway Regulation to Therapeutic Opportunity. BMJ Oncol. 2024, 3, e000154. [Google Scholar] [CrossRef]
- Akakura, N.; Kobayashi, M.; Horiuchi, I.; Suzuki, A.; Wang, J.; Chen, J.; Niizeki, H.; Kawamura, K.I.; Hosokawa, M.; Asaka, M. Constitutive Expression of Hypoxia-Inducible Factor-1alpha Renders Pancreatic Cancer Cells Resistant to Apoptosis Induced by Hypoxia and Nutrient Deprivation. Cancer Res. 2001, 61, 6548–6554. [Google Scholar]
- Kumar, H.; Choi, D.-K. Hypoxia Inducible Factor Pathway and Physiological Adaptation: A Cell Survival Pathway? Mediators Inflamm. 2015, 2015, 584758. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Monaci, S.; Coppola, F.; Filippi, I.; Falsini, A.; Carraro, F.; Naldini, A. Targeting Hypoxia Signaling Pathways in Angiogenesis. Front. Physiol. 2024, 15, 1408750. [Google Scholar] [CrossRef]
- Szűcs, D.; Monostori, T.; Miklós, V.; Páhi, Z.G.; Póliska, S.; Kemény, L.; Veréb, Z. Licensing Effects of Inflammatory Factors and TLR Ligands on the Regenerative Capacity of Adipose-Derived Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2024, 12, 1367242. [Google Scholar] [CrossRef]
- Czajka-Francuz, P.; Prendes, M.J.; Mankan, A.; Quintana, Á.; Pabla, S.; Ramkissoon, S.; Jensen, T.J.; Peiró, S.; Severson, E.A.; Achyut, B.R.; et al. Mechanisms of Immune Modulation in the Tumor Microenvironment and Implications for Targeted Therapy. Front. Oncol. 2023, 13, 1200646. [Google Scholar] [CrossRef]
- Popova, N.V.; Jücker, M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Dubrovska, A. CD98 Heavy Chain as a Prognostic Biomarker and Target for Cancer Treatment. Front. Oncol. 2023, 13, 1251100. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, H.; Jihu, R.; Zhou, J.; Zeng, R.; Yan, H. Novel Characterization of Myeloid-Derived Suppressor Cells in Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 698532. [Google Scholar] [CrossRef]
- Seetharam, R.N. Oxaliplatin: Preclinical Perspectives on the Mechanisms of Action, Response and Resistance. ecancermedicalscience 2010. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Buckley, B.J.; Ranson, M. The Urokinase Plasminogen Activation System in Pancreatic Cancer: Prospective Diagnostic and Therapeutic Targets. Biomolecules 2022, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Y.; Yao, S.; Gaedcke, J.; Baart, V.M.; Sier, C.F.M.; Neesse, A.; Ellenrieder, V.; Bohnenberger, H.; Fuchs, F.; et al. Urokinase-Type Plasminogen Activator Receptor (uPAR) Cooperates with Mutated KRAS in Regulating Cellular Plasticity and Gemcitabine Response in Pancreatic Adenocarcinomas. Cancers 2023, 15, 1587. [Google Scholar] [CrossRef]
- Li, X.; Ma, Q.; Xu, Q.; Liu, H.; Lei, J.; Duan, W.; Bhat, K.; Wang, F.; Wu, E.; Wang, Z. SDF-1/CXCR4 Signaling Induces Pancreatic Cancer Cell Invasion and Epithelial–Mesenchymal Transition in Vitro through Non-Canonical Activation of Hedgehog Pathway. Cancer Lett. 2012, 322, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Lu, Z.; Xu, Q.; Wu, P.; Tian, L.; Zhao, L.; Cai, B.; Yin, J.; Wu, Y.; Staveley-O’Carroll, K.F.; et al. Galectin-1-Driven Upregulation of SDF-1 in Pancreatic Stellate Cells Promotes Pancreatic Cancer Metastasis. Cancer Lett. 2017, 397, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Szűcs, D.; Miklós, V.; Monostori, T.; Guba, M.; Kun-Varga, A.; Póliska, S.; Kis, E.; Bende, B.; Kemény, L.; Veréb, Z. Effect of Inflammatory Microenvironment on the Regenerative Capacity of Adipose-Derived Mesenchymal Stem Cells. Cells 2023, 12, 1966. [Google Scholar] [CrossRef] [PubMed]
- Kun-Varga, A.; Gubán, B.; Miklós, V.; Parvaneh, S.; Guba, M.; Szűcs, D.; Monostori, T.; Varga, J.; Varga, Á.; Rázga, Z.; et al. Herpes Simplex Virus Infection Alters the Immunological Properties of Adipose-Tissue-Derived Mesenchymal-Stem Cells. Int. J. Mol. Sci. 2023, 24, 11989. [Google Scholar] [CrossRef] [PubMed]
| Positively Enriched GO Terms | |||||||
|---|---|---|---|---|---|---|---|
| GO ID | Description | Bg Ratio | p-Value | p. Adjust | q-Value | Gene ID | Count |
| 0006977 | DNA damage response, signal transduction by p53 class mediator resulting in cell cycle arrest | 18/18,903 | 0.000101960656047455 | 0.0253341548649552 | 0.0162517934743993 | MDM2/PIDD1 | 2 |
| 0010948 | Negative regulation of cell cycle process | 318/18,903 | 0.000121909527970945 | 0.0253341548649552 | 0.0162517934743993 | BCL6/MDM2/PIDD1/RBM14 | 4 |
| 0046599 | Regulation of centriole replication | 23/18,903 | 0.000168185906455739 | 0.0253341548649552 | 0.0162517934743993 | CEP295NL/RBM14 | 2 |
| 0071456 | Cellular response to hypoxia | 151/18,903 | 0.000259259235342849 | 0.0253341548649552 | 0.0162517934743993 | MDM2/STC1/TIGAR | 3 |
| 0031571 | Mitotic G1 DNA damage checkpoint signaling | 29/18,903 | 0.000269096926508324 | 0.0253341548649552 | 0.0162517934743993 | MDM2/PIDD1 | 2 |
| 0044819 | Mitotic G1/S transition checkpoint signaling | 30/18,903 | 0.000288175849364675 | 0.0253341548649552 | 0.0162517934743993 | MDM2/PIDD1 | 2 |
| 0036294 | Cellular response to decreased oxygen levels | 159/18,903 | 0.000301744137941842 | 0.0253341548649552 | 0.0162517934743993 | MDM2/STC1/TIGAR | 3 |
| 0045786 | Negative regulation of cell cycle | 405/18,903 | 0.000308014040911309 | 0.0253341548649552 | 0.0162517934743993 | BCL6/MDM2/PIDD1/RBM14 | 4 |
| 0072331 | Signal transduction by p53 class mediator | 173/18,903 | 0.000386468887239921 | 0.0262999193876633 | 0.0168713288665062 | EDA2R/MDM2/PIDD1 | 3 |
| 0071453 | Cellular response to oxygen levels | 175/18,903 | 0.00039969482352072 | 0.0262999193876633 | 0.0168713288665062 | MDM2/STC1/TIGAR | 3 |
| 0007099 | Centriole replication | 42/18,903 | 0.000567021211169682 | 0.0339181779045137 | 0.0217584215960806 | CEP295NL/RBM14 | 2 |
| 0098534 | Centriole assembly | 46/18,903 | 0.000680266788329752 | 0.0359445339843582 | 0.0230583236725766 | CEP295NL/RBM14 | 2 |
| 0010824 | Regulation of centrosome duplication | 47/18,903 | 0.000710150367472122 | 0.0359445339843582 | 0.0230583236725766 | CEP295NL/RBM14 | 2 |
| 0046605 | Regulation of centrosome cycle | 54/18,903 | 0.000936837607682431 | 0.0440313675610742 | 0.0282460060662146 | CEP295NL/RBM14 | 2 |
| 0010332 | Response to gamma radiation | 57/18,903 | 0.00104331346356804 | 0.0442341420950184 | 0.0283760853945007 | MDM2/TIGAR | 2 |
| 0045930 | Negative regulation of mitotic cell cycle | 246/18,903 | 0.00107560223939255 | 0.0442341420950184 | 0.0283760853945007 | BCL6/MDM2/PIDD1 | 3 |
| 0043122 | Regulation of I-kappaB kinase/NF-kappaB signaling | 255/18,903 | 0.00119298433051044 | 0.0461755111456394 | 0.0296214685160797 | EDA2R/PIDD1/TNFRSF10B | 3 |
| Negatively Enriched GO Terms | |||||||
| GO ID | Description | Bg Ratio | p-Value | p. Adjust | q-Value | Gene ID | Count |
| 0000280 | Nuclear division | 481/18,903 | 1.76881066719348 × 10−18 | 1.47872571777375 × 10−15 | 1.03894352873049 × 10−15 | AURKA/BUB1/BUB1B/CCNB1/CCNB2/CENPE/CENPF/DLGAP5/KIF11/KIF20B/KIF23/NDC80/NUF2/NUSAP1/RAD51/TOP2A/TTK | 17 |
| 0007088 | Regulation of mitotic nuclear division | 118/18,903 | 1.28092889781954 × 10−16 | 4.8902786348109 × 10−14 | 3.43587947014396686 × 10−14 | AURKA/BUB1/BUB1B/CCNB1/CENPF/DLGAP5/KIF20B/NDC80/NUF2/NUSAP1/TTK | 11 |
| 0000819 | Sister chromatid segregation | 239/18,903 | 1.75488467756372 × 10−16 | 4.8902786348109 × 10−14 | 3.43587947396686 × 10−14 | BUB1/BUB1B/CCNB1/CENPE/CENPF/DLGAP5/KIF11/KIF23/NDC80/NUF2/NUSAP1/TOP2A/TTK | 13 |
| 0140014 | Mitotic nuclear division | 325/18,903 | 2.50022223332157 × 10−16 | 5.22546446764208 × 10−14 | 3.67137896366693 × 10−14 | AURKA/BUB1/BUB1B/CCNB1/CENPE/CENPF/DLGAP5/KIF11/KIF20B/KIF23/NDC80/NUF2/NUSAP1/TTK | 14 |
| 0000070 | Mitotic sister chromatid segregation | 204/18,903 | 1.20987483051498 × 10−15 | 1.82830384900337 × 10−13 | 1.28455495812627 × 10−13 | BUB1/BUB1B/CCNB1/CENPE/CENPF/DLGAP5/KIF11/KIF23/NDC80/NUF2/NUSAP1/TTK | 12 |
| 0051783 | Regulation of nuclear division | 145/18,903 | 1.31217979593543 × 10−15 | 1.82830384900337 × 10−13 | 1.28455495812627 × 10−13 | AURKA/BUB1/BUB1B/CCNB1/CENPF/DLGAP5/KIF20B/NDC80/NUF2/NUSAP1/TTK | 11 |
| 0044772 | Mitotic cell cycle phase transition | 473/18,903 | 1.55567542073125 × 10−15 | 1.85792093104475 × 10−13 | 1.30536373649329 × 10−13 | AURKA/BRCA1/BUB1/BUB1B/CCNA2/CCNB1/CCNB2/CENPE/CENPF/CIT/DLGAP5/MELK/NDC80/NUF2/TTK | 15 |
| 0007059 | Chromosome segregation | 382/18,903 | 2.36061743337282 × 10−15 | 2.4668452178746 × 10−13 | 1.73319016818689 × 10−13 | BRCA1/BUB1/BUB1B/CCNB1/CENPE/CENPF/DLGAP5/KIF11/KIF23/NDC80/NUF2/NUSAP1/TOP2A/TTK | 14 |
| 0098813 | Nuclear chromosome segregation | 321/18,903 | 8.09097373728085 × 10−15 | 7.51561560485199 × 10−13 | 5.28042496538329 × 10−13 | BUB1/BUB1B/CCNB1/CENPE/CENPF/DLGAP5/KIF11/KIF23/NDC80/NUF2/NUSAP1/TOP2A/TTK | 13 |
| 0051304 | Chromosome separation | 135/18,903 | 3.88243219597149 × 10−14 | 3.24571331583216 × 10−12 | 2.28041806879167 × 10−12 | BUB1/BUB1B/CCNB1/CENPE/CENPF/DLGAP5/NDC80/NUF2/TOP2A/TTK | 10 |
| Hallmark Gene Sets of ASDMSCs Treated by OXP | |||||||
|---|---|---|---|---|---|---|---|
| Pathway | p-Value | p-Adj | log2err | ES | NES | Size | |
| 1 | HALLMARK_E2F_TARGETS | 2.64139101652653 × 10−5 | 0.000220115918043877 | 0.575610261071129 | −0.340157293053717 | −2.19499534337419 | 66 |
| 2 | HALLMARK_PROTEIN_SECRETION | 5.91563187836908 × 10−5 | 0.00042254513416922 | 0.557332238758646 | −0.37601381727437 | −2.23266725677663 | 48 |
| 3 | HALLMARK_TGF_BETA_SIGNALING | 0.000218576664884453 | 0.00136610415552783 | 0.518848077743792 | −0.495322402827272 | −2.25846306096561 | 22 |
| 4 | HALLMARK_WNT_BETA_CATENIN_SIGNALING | 0.000590324063336932 | 0.00327957812964962 | 0.477270815362862 | −0.52540555272117 | −2.22228354009291 | 18 |
| 5 | HALLMARK_GLYCOLYSIS | 0.0013175339500311 | 0.00658766975015548 | 0.45505986738723 | −0.263389231583071 | −1.72899685956862 | 71 |
| 6 | HALLMARK_APICAL_SURFACE | 0.0017804176406084 | 0.00809280745731091 | 0.45505986738723 | −0.491393827829415 | −2.04389947310002 | 17 |
| 7 | HALLMARK_ANDROGEN_RESPONSE | 0.0026784748393661 | 0.0111603118306921 | 0.431707695803346 | −0.310678503446223 | −1.77233833738595 | 43 |
| 8 | HALLMARK_IL6_JAK_STAT3_SIGNALING | 0.00834837545126354 | 0.0321091363510136 | 0.167658528065765 | 0.503377946262252 | 1.68855652293838 | 31 |
| 9 | HALLMARK_INTERFERON_GAMMA_RESPONSE | 0.0132659507264687 | 0.047378395451674 | 0.12797030576243 | 0.423017595767414 | 1.56993531942059 | 57 |
| 10 | HALLMARK_OXIDATIVE_PHOSPHORYLATION | 0.0169363189037552 | 0.0564543963458505 | 0.352487857583619 | 0.190907632331622 | 1.40892610341346 | 116 |
| KEGG Pathway Gene Sets of ASCs Treated by OXP | |||||||
| Pathway | p-Value | p-Adj | log2err | ES | NES | Size | |
| 1 | KEGG_CYTOKINE_CYTOKINE_RECEPTOR_INTERACTION | 3.93732090514141 × 10−7 | 0.0000346492769444532 | 0.674962860011025 | 0.532331938126544 | 2.05892651987083 | 80 |
| 2 | KEGG_PATHWAYS_IN_CANCER | 4.22552157859185 × 10−7 | 0.0000346492769444532 | 0.674962860011025 | −0.294701843495029 | −2.15756612113785 | 130 |
| 3 | KEGG_WNT_SIGNALING_PATHWAY | 7.93012559407585 × 10−7 | 0.0000433513532476146 | 0.659444398037935 | −0.393751027771448 | −2.47883362699721 | 63 |
| 4 | KEGG_RIBOSOME | 1.8006005614366 × 10−5 | 0.000738246230189004 | 0.575610261071129 | 0.508195995347252 | 1.95272043832977 | 74 |
| 5 | KEGG_CELL_CYCLE | 6.56683118791417 × 10−5 | 0.00215392062963585 | 0.538434096309916 | −0.368250635338693 | −2.17428371995909 | 49 |
| 6 | KEGG_COLORECTAL_CANCER | 0.000153127064422625 | 0.00418547309421842 | 0.518848077743792 | −0.424324314956772 | −2.2464797217589 | 34 |
| 7 | KEGG_ADHERENS_JUNCTION | 0.000344717035697592 | 0.00471113282120042 | 0.49849310876659 | −0.417114186960923 | −2.19630436832298 | 33 |
| 8 | KEGG_PROPANOATE_METABOLISM | 0.000254144405157146 | 0.00471113282120042 | 0.49849310876659 | −0.587093416395374 | −2.31891722502165 | 15 |
| 9 | KEGG_REGULATION_OF_ACTIN_CYTOSKELETON | 0.000223272560199641 | 0.00471113282120042 | 0.518848077743792 | −0.289865264978343 | −1.93135681744662 | 75 |
| 10 | KEGG_SYSTEMIC_LUPUS_ERYTHEMATOSUS | 0.000325945507623177 | 0.00471113282120042 | 0.49849310876659 | 0.624911870842555 | 2.01016409806685 | 25 |
| GO Pathway Gene Sets of ASCs Treated by OXP | |||||||
| Pathway | p-Value | p-Adj | log2err | ES | NES | Size | |
| 1 | GOCC_CILIARY_BASAL_BODY | 7.82720516782125 × 10−7 | 0.0000919926819135698 | 0.659444398037935 | −0.39610119737703 | −2.44040401476571 | 60 |
| 2 | GOMF_CYTOKINE_RECEPTOR_BINDING | 1.51281379650273 × 10−9 | 0.000000906780589623734 | 0.788186810800237 | 0.582963622296549 | 2.24926964536283 | 80 |
| 3 | GOMF_PHOSPHORIC_ESTER_HYDROLASE_ACTIVITY | 1.3092422431839 × 10−9 | 0.000000871955333960478 | 0.788186810800237 | −0.333513038562531 | −2.41942991352463 | 126 |
| 4 | GOBP_REGULATION_OF_PROTEIN_SERINE_THREONINE_KINASE_ACTIVITY | 7.01033059960302 × 10−7 | 0.000084039843228041 | 0.659444398037935 | −0.257783461836265 | −1.98310722278176 | 152 |
| 5 | GOBP_CELLULAR_RESPONSE_TO_ORGANIC_CYCLIC_COMPOUND | 6.54759701956862 × 10−7 | 0.0000800944827250904 | 0.659444398037935 | −0.237847374889051 | −2.05107083041299 | 201 |
| 6 | GOMF_PHOSPHATASE_ACTIVITY | 2.66353767958835 × 10−8 | 0.0000079826224257263 | 0.73376198835648 | −0.352246801743327 | −2.49094501452385 | 101 |
| 7 | GOBP_CILIUM_ORGANIZATION | 6.36062716975486 × 10−7 | 0.0000794283317823138 | 0.659444398037935 | −0.275699356833308 | −2.05428254765 | 141 |
| 8 | GOBP_HEART_MORPHOGENESIS | 6.08811861023075 × 10−7 | 0.0000776429424462194 | 0.659444398037935 | −0.358748271702841 | −2.38680754796361 | 82 |
| 9 | GOBP_REGIONALIZATION | 6.07629933943173 × 10−7 | 0.0000776429424462194 | 0.659444398037935 | −0.319006359814004 | −2.2465575880912 | 103 |
| 10 | GOBP_PATTERN_SPECIFICATION_PROCESS | 1.97618354957955 × 10−8 | 0.00000633035626394535 | 0.73376198835648 | −0.307348031514522 | −2.28721000698764 | 135 |
| Hallmark Pathway Gene Sets of ASCs Treated by 5-FU | |||||||
|---|---|---|---|---|---|---|---|
| Pathway | p-Value | p-Adj | log2err | ES | NES | Size | |
| 1 | HALLMARK_G2M_CHECKPOINT | 0.0000733471109727229 | 0.000682362573410719 | 0.538434096309916 | −0.558868261837081 | −1.7830668390697 | 45 |
| 2 | HALLMARK_E2F_TARGETS | 0.000136472514682144 | 0.000682362573410719 | 0.518848077743792 | −0.574875140341789 | −1.80515308646078 | 38 |
| 3 | HALLMARK_HYPOXIA | 0.000934151233258401 | 0.00311383744419467 | 0.477270815362862 | 0.769430870017944 | 2.24004089128129 | 7 |
| 4 | HALLMARK_MITOTIC_SPINDLE | 0.00196565280364163 | 0.00491413200910408 | 0.335068558717014 | −0.567565074528727 | −1.68661253301052 | 25 |
| 5 | HALLMARK_SPERMATOGENESIS | 0.0238582140422631 | 0.0477164280845262 | 0.0986244904449457 | −0.61558780552373 | −1.52724795175338 | 10 |
| 6 | HALLMARK_ESTROGEN_RESPONSE_LATE | 0.0531142529614062 | 0.088523754935677 | 0.0692037251597512 | −0.691973854147228 | −1.42865347619181 | 5 |
| 7 | HALLMARK_GLYCOLYSIS | 0.100535460605398 | 0.14362208657914 | 0.0453371931652217 | −0.507474988249809 | −1.3390527344082 | 13 |
| 8 | HALLMARK_P53_PATHWAY | 0.605299860529986 | 0.751921198998004 | 0.0372972016676791 | 0.363636363636364 | 0.891284030826118 | 5 |
| 9 | HALLMARK_MYC_TARGETS_V1 | 0.676729079098204 | 0.751921198998004 | 0.0135521440941112 | −0.420312088040893 | −0.867778922666198 | 5 |
| 10 | HALLMARK_MTORC1_SIGNALING | 0.874798619102417 | 0.874798619102417 | 0.00810455465169815 | −0.283125336361726 | −0.685983995043019 | 9 |
| GO Pathway Gene Sets of ASCs Treated by 5-FU | |||||||
| Pathway | p-Value | p-Adj | log2err | ES | NES | Size | |
| 1 | GOBP_CELL_CYCLE | 2.79067384940996 × 10−11 | 1.58231207261545 × 10−8 | 0.863415391606693 | −0.685309035511196 | −2.27333326581027 | 97 |
| 2 | GOBP_CELL_CYCLE_PROCESS | 3.82674947408738 × 10−9 | 7.23255650602515 × 10−7 | 0.761460801445585 | −0.651897388248435 | −2.16371155173349 | 88 |
| 3 | GOBP_CELL_DIVISION | 1.40683280616485 × 10−7 | 1.13953457299353 × 10−5 | 0.690132458796796 | −0.632482709801711 | −2.055589722267 | 56 |
| 4 | GOBP_CHROMOSOME_ORGANIZATION | 4.06803322002345 × 10−6 | 0.000177428833519484 | 0.610526878385931 | −0.603365321057273 | −1.94936769929251 | 52 |
| 5 | GOBP_CHROMOSOME_SEGREGATION | 1.29447171412157 × 10−8 | 1.46793092381386 × 10−6 | 0.747739663149885 | −0.68303550124922 | −2.18639772376246 | 46 |
| 6 | GOBP_CYTOSKELETON_ORGANIZATION | 1.58714316917903 × 10−5 | 0.000599940117949674 | 0.575610261071129 | −0.58390599218358 | −1.88649801515187 | 52 |
| 7 | GOBP_MITOTIC_CELL_CYCLE | 2.96260527345182 × 10−9 | 7.23255650602515 × 10−7 | 0.774939030136436 | −0.644228764699433 | −2.13171387737599 | 79 |
| 8 | GOBP_MITOTIC_CELL_CYCLE_PROCESS | 8.47450055550495 × 10−9 | 1.20126045374283 × 10−6 | 0.747739663149885 | −0.635828913368128 | −2.10045721627125 | 76 |
| 9 | GOBP_MITOTIC_NUCLEAR_DIVISION | 1.12901172508325 × 10−06 | 7.11277386802448 × 10−5 | 0.643551836150722 | −0.640638667096561 | −2.05068539033824 | 46 |
| 10 | GOBP_NUCLEAR_CHROMOSOME_SEGREGATION | 1.26791003202673 × 10−7 | 1.13953457299353 × 10−5 | 0.690132458796796 | −0.668286487412083 | −2.11091785107083 | 40 |
| 11 | GOBP_ORGANELLE_FISSION | 2.5763252011723 × 10−7 | 1.82597048633087. × 10−5 | 0.674962860011025 | −0.632903138174849 | −2.05103119716214 | 54 |
| 12 | GOBP_REGULATION_OF_CELL_CYCLE | 2.5659740132735 x. 10−6 | 0.000132264296866007 | 0.627256739718528 | −0.590061640981375 | −1.94396251426521 | 70 |
| 13 | GOBP_SISTER_CHROMATID_SEGREGATION | 1.53348302367881 × 10−6 | 8.69484874425883 × 10−5 | 0.643551836150722 | −0.650394050059615 | −2.04361563736296 | 38 |
| 14 | GOCC_MICROTUBULE_CYTOSKELETON | 4.61134563187446 × 10−6 | 0.000186759498090915 | 0.610526878385931 | −0.592507368359088 | −1.92870493250568 | 57 |
| 15 | GOCC_SPINDLE | 3.52663387478052 × 10−6 | 0.000166633450583379 | 0.627256739718528 | −0.617148091472953 | −1.95344908812434 | 41 |
| CD Markers | Mean | SD |
|---|---|---|
| HLA-DR | 0.9 | 0.7 |
| CD29 | 99.0 | 0.4 |
| CD34 | 1.9 | 1.5 |
| CD47 | 96.1 | 1.6 |
| CD49A | 92.5 | 5.6 |
| CD51 | 82.9 | 5.8 |
| CD73 | 99.0 | 0.1 |
| CD90 | 90.9 | 1.0 |
| CD105 | 88.1 | 6.0 |
| CD166 | 95.9 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvaneh, S.; Miklós, V.; Páhi, Z.G.; Szűcs, D.; Monostori, T.; Póliska, S.; Venglovecz, V.; Pankotai, T.; Kemény, L.; Veréb, Z. Chemoresistance in Pancreatic Cancer: The Role of Adipose-Derived Mesenchymal Stem Cells and Key Resistance Genes. Int. J. Mol. Sci. 2025, 26, 390. https://doi.org/10.3390/ijms26010390
Parvaneh S, Miklós V, Páhi ZG, Szűcs D, Monostori T, Póliska S, Venglovecz V, Pankotai T, Kemény L, Veréb Z. Chemoresistance in Pancreatic Cancer: The Role of Adipose-Derived Mesenchymal Stem Cells and Key Resistance Genes. International Journal of Molecular Sciences. 2025; 26(1):390. https://doi.org/10.3390/ijms26010390
Chicago/Turabian StyleParvaneh, Shahram, Vanda Miklós, Zoltán Gábor Páhi, Diána Szűcs, Tamás Monostori, Szilárd Póliska, Viktória Venglovecz, Tibor Pankotai, Lajos Kemény, and Zoltán Veréb. 2025. "Chemoresistance in Pancreatic Cancer: The Role of Adipose-Derived Mesenchymal Stem Cells and Key Resistance Genes" International Journal of Molecular Sciences 26, no. 1: 390. https://doi.org/10.3390/ijms26010390
APA StyleParvaneh, S., Miklós, V., Páhi, Z. G., Szűcs, D., Monostori, T., Póliska, S., Venglovecz, V., Pankotai, T., Kemény, L., & Veréb, Z. (2025). Chemoresistance in Pancreatic Cancer: The Role of Adipose-Derived Mesenchymal Stem Cells and Key Resistance Genes. International Journal of Molecular Sciences, 26(1), 390. https://doi.org/10.3390/ijms26010390










