Neutrophil and Colorectal Cancer
Abstract
1. Introduction
2. Multifaceted Roles of Neutrophils in Cancer
3. Tumor-Associated Neutrophils (TANs)
4. Antitumor Role of TANs
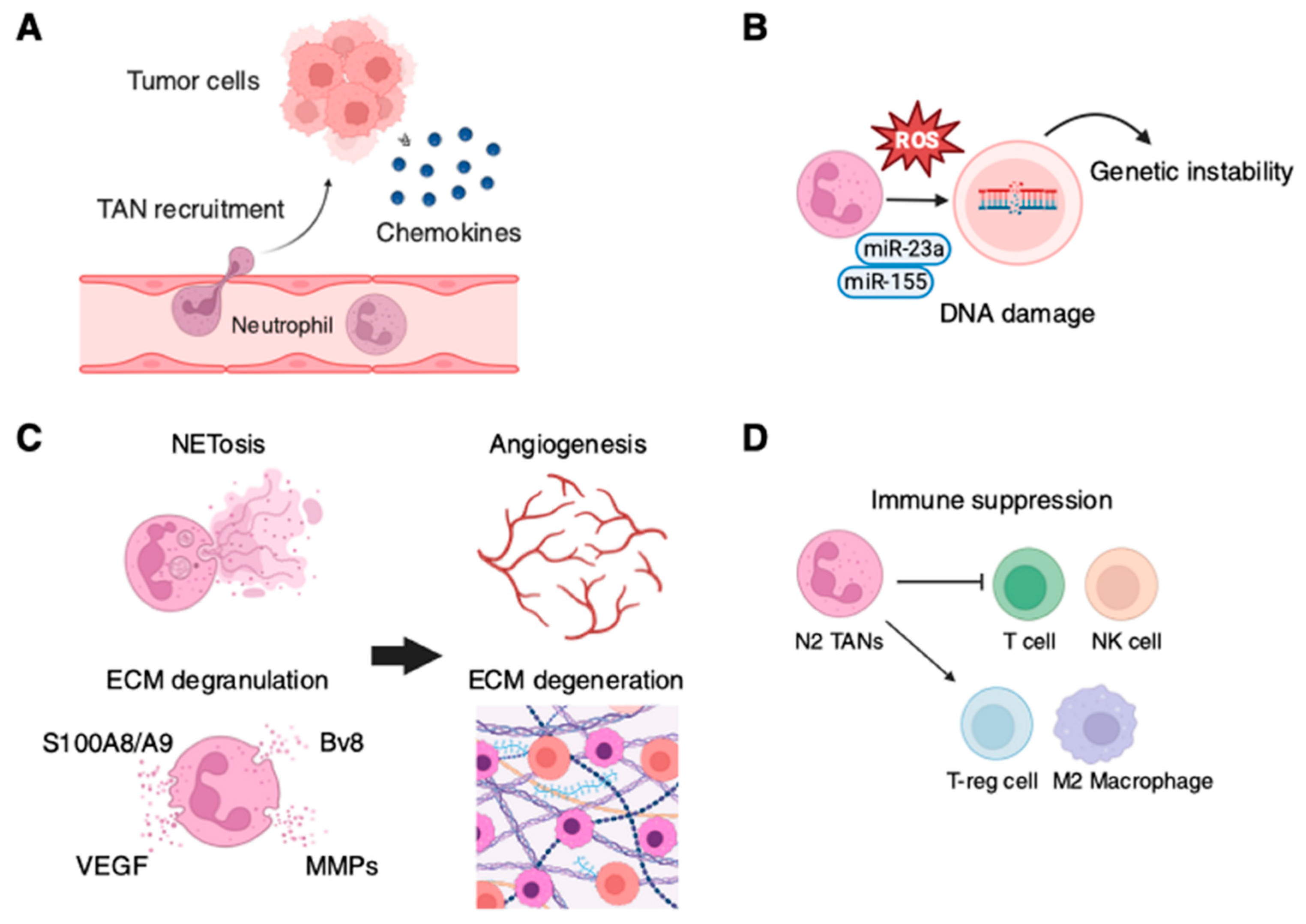
5. Role of Neutrophils in Enhancing Tumor Progression
6. Recruitment of TANs
7. Role of TANs in Angiogenesis and Metastasis
8. Neutrophil Extracellular Traps (NETs) and Tumor Progression
9. Therapeutic Approach and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Sanchis-Gomar, F.; Lippi, G. Concise update on colorectal cancer epidemiology. Ann. Transl. Med. 2019, 7, 609. [Google Scholar] [CrossRef]
- Suwaidan, A.A.; Lau, D.K.; Chau, I. HER2 targeted therapy in colorectal cancer: New horizons. Cancer Treat. Rev. 2022, 105, 102363. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wu, J.; Peng, Y.; Sun, J.; Cheng, P.; Huang, Q. Tumor-associated neutrophils in colorectal cancer development, progression and immunotherapy. Cancers 2022, 14, 4755. [Google Scholar] [CrossRef]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K. Optimizing immunotherapy for colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Antuamwine, B.B.; Bosnjakovic, R.; Hofmann-Vega, F.; Wang, X.; Theodosiou, T.; Iliopoulos, I.; Brandau, S. N1 versus N2 and PMN-MDSC: A critical appraisal of current concepts on tumor-associated neutrophils and new directions for human oncology. Immunol. Rev. 2023, 314, 250–279. [Google Scholar] [CrossRef]
- Awasthi, D.; Sarode, A. Neutrophils at the crossroads: Unraveling the multifaceted role in the tumor microenvironment. Int. J. Mol. Sci. 2024, 25, 2929. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; McDowell, S.A.C.; He, X.Y.; Quail, D.F.; Egeblad, M. NETworking with cancer: The bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell 2023, 41, 505–526. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, H.; Mao, Y.; Jiang, S.; Yi, H.; Zhang, Z.; Wang, M.; Zong, Z. Myeloid-derived suppressor cells: Key immunosuppressive regulators and therapeutic targets in colorectal cancer (Review). Int. J. Oncol. 2024, 65, 85. [Google Scholar] [CrossRef]
- Ghosh, S.; Zanoni, I. The dark knight: Functional reprogramming of neutrophils in the pathogenesis of colitis-associated cancer. Cancer Immunol. Res. 2024, 12, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, C.C.; Malanchi, I. Neutrophils in cancer: Heterogeneous and multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef] [PubMed]
- Grenader, T.; Nash, S.; Adams, R.; Kaplan, R.; Fisher, D.; Maughan, T.; Bridgewater, J. Derived neutrophil lymphocyte ratio is predictive of survival from intermittent therapy in advanced colorectal cancer: A post hoc analysis of the MRC COIN study. Br. J. Cancer 2016, 114, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, S.; Kawada, K.; Okamura, R.; Hida, K.; Sakai, Y. Prognostic impact of the combination of neutrophil-to-lymphocyte ratio and Glasgow prognostic score in colorectal cancer: A retrospective cohort study. Int. J. Colorectal Dis. 2019, 34, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, W.; Du, Y.; Yin, H.; Chen, Q.; Yu, W.; Wang, W.; Yu, J.; Liu, L.; Lou, W.; et al. The evolution and heterogeneity of neutrophils in cancers: Origins, subsets, functions, orchestrations and clinical applications. Mol. Cancer 2023, 22, 148. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Governa, V.; Trella, E.; Mele, V.; Tornillo, L.; Amicarella, F.; Cremonesi, E.; Muraro, M.G.; Xu, H.; Droeser, R.; Däster, S.R.; et al. The interplay between neutrophils and CD8(+) T cells improves survival in human colorectal cancer. Clin. Cancer Res. 2017, 23, 3847–3858. [Google Scholar] [CrossRef]
- Rottmann, B.G.; Patel, N.; Ahmed, M.; Deng, Y.; Ciarleglio, M.; Vyas, M.; Jain, D.; Zhang, X. Clinicopathological significance of neutrophil-rich colorectal carcinoma. J. Clin. Pathol. 2023, 76, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.S.; Xiong, M.J.; Greenbaum, A.; Mortaji, P.; Nofchissey, R.A.; Schultz, F.; Martinez, C.; Luo, L.; Morris, K.T.; Hanson, J.A. High levels of tumor-associated neutrophils are associated with improved overall survival in patients with stage II colorectal cancer. PLoS ONE 2017, 12, e0188799. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S.; Zhao, Y.; Dinh, T.; Jiang, D.; Selfridge, J.E.; Myers, G.; Wang, Y.; Zhao, X.; Tomchuck, S.; et al. Neutrophil extracellular traps induced by chemotherapy inhibit tumor growth in murine models of colorectal cancer. J. Clin. Investig. 2024, 134, e175031. [Google Scholar] [CrossRef]
- Mizuno, R.; Kawada, K.; Itatani, Y.; Ogawa, R.; Kiyasu, Y.; Sakai, Y. The role of tumor-associated neutrophils in colorectal cancer. Int. J. Mol. Sci. 2019, 20, 529. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef]
- Zhou, G.; Peng, K.; Song, Y.; Yang, W.; Shu, W.; Yu, T.; Yu, L.; Lin, M.; Wei, Q.; Chen, C.; et al. CD177+ neutrophils suppress epithelial cell tumourigenesis in colitis-associated cancer and predict good prognosis in colorectal cancer. Carcinogenesis 2018, 39, 272–282. [Google Scholar] [CrossRef]
- Qin, F.; Liu, X.; Chen, J.; Huang, S.; Wei, W.; Zou, Y.; Liu, X.; Deng, K.; Mo, S.; Chen, J.; et al. Anti-TGF-β attenuates tumor growth via polarization of tumor associated neutrophils towards an anti-tumor phenotype in colorectal cancer. J. Cancer 2020, 11, 2580–2592. [Google Scholar] [CrossRef]
- Veglia, F.; Hashimoto, A.; Dweep, H.; Sanseviero, E.; De Leo, A.; Tcyganov, E.; Kossenkov, A.; Mulligan, C.; Nam, B.; Masters, G.; et al. Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice. J. Exp. Med. 2021, 218, e20201803. [Google Scholar] [CrossRef] [PubMed]
- Kiyasu, Y.; Kawada, K.; Hirai, H.; Ogawa, R.; Hanada, K.; Masui, H.; Nishikawa, G.; Yamamoto, T.; Mizuno, R.; Itatani, Y.; et al. Disruption of CCR1-mediated myeloid cell accumulation suppresses colorectal cancer progression in mice. Cancer Lett. 2020, 487, 53–62. [Google Scholar] [CrossRef]
- Masui, H.; Kawada, K.; Itatani, Y.; Hirai, H.; Nakanishi, Y.; Kiyasu, Y.; Hanada, K.; Okamoto, M.; Hirata, W.; Nishikawa, Y.; et al. Synergistic antitumor activity by dual blockade of CCR1 and CXCR2 expressed on myeloid cells within the tumor microenvironment. Br. J. Cancer 2024, 131, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Fujishita, T.; Kurimoto, K.; Miyachi, H.; Kitano, S.; Inamoto, S.; Itatani, Y.; Saitou, M.; Maekawa, T.; Taketo, M.M. CCR1-mediated accumulation of myeloid cells in the liver microenvironment promoting mouse colon cancer metastasis. Clin. Exp. Metastasis 2014, 31, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Jackstadt, R.; van Hooff, S.R.; Leach, J.D.; Cortes-Lavaud, X.; Lohuis, J.O.; Ridgway, R.A.; Wouters, V.M.; Roper, J.; Kendall, T.J.; Roxburgh, C.S.; et al. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell 2019, 36, 319–336.e7. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Overman, M.J.; Boutin, A.T.; Shang, X.; Zhao, D.; Dey, P.; Li, J.; Wang, G.; Lan, Z.; Li, J.; et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 2019, 35, 559–572.e7. [Google Scholar] [CrossRef]
- Germann, M.; Zangger, N.; Sauvain, M.O.; Sempoux, C.; Bowler, A.D.; Wirapati, P.; Kandalaft, L.E.; Delorenzi, M.; Tejpar, S.; Coukos, G.; et al. Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFβ. EMBO Mol. Med. 2020, 12, e10681. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef]
- Varney, M.L.; Singh, S.; Li, A.; Mayer-Ezell, R.; Bond, R.; Singh, R.K. Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Lett. 2011, 300, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; von Köckritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef]
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188762. [Google Scholar] [CrossRef]
- Quail, D.F.; Amulic, B.; Aziz, M.; Barnes, B.J.; Eruslanov, E.; Fridlender, Z.G.; Goodridge, H.S.; Granot, Z.; Hidalgo, A.; Huttenlocher, A.; et al. Neutrophil phenotypes and functions in cancer: A consensus statement. J. Exp. Med. 2022, 219, e20220011. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, I.; Rubio-Ponce, A.; Genua, M.; Lusito, E.; Kwok, I.; Fernández-Calvo, G.; Khoyratty, T.E.; van Grinsven, E.; González-Hernández, S.; Nicolás-Ávila, J.; et al. Co-option of neutrophil fates by tissue environments. Cell 2020, 183, 1282–1297.e18. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil diversity in health and disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Shi, Q.; Wu, P.; Zhang, X.; Kambara, H.; Su, J.; Yu, H.; Park, S.Y.; Guo, R.; Ren, Q.; et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat. Immunol. 2020, 21, 1119–1133. [Google Scholar] [CrossRef]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef] [PubMed]
- Gershkovitz, M.; Caspi, Y.; Fainsod-Levi, T.; Katz, B.; Michaeli, J.; Khawaled, S.; Lev, S.; Polyansky, L.; Shaul, M.E.; Sionov, R.V.; et al. TRPM2 mediates neutrophil killing of disseminated tumor cells. Cancer Res. 2018, 78, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Finisguerra, V.; Di Conza, G.; Di Matteo, M.; Serneels, J.; Costa, S.; Thompson, A.A.; Wauters, E.; Walmsley, S.; Prenen, H.; Granot, Z.; et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature 2015, 522, 349–353. [Google Scholar] [CrossRef]
- Riise, R.E.; Bernson, E.; Aurelius, J.; Martner, A.; Pesce, S.; Della Chiesa, M.; Marcenaro, E.; Bylund, J.; Hellstrand, K.; Moretta, L.; et al. TLR-stimulated neutrophils instruct NK cells to trigger dendritic cell maturation and promote adaptive T cell responses. J. Immunol. 2015, 195, 1121–1128. [Google Scholar] [CrossRef]
- Raftopoulou, S.; Valadez-Cosmes, P.; Mihalic, Z.N.; Schicho, R.; Kargl, J. Tumor-mediated neutrophil polarization and therapeutic implications. Int. J. Mol. Sci. 2022, 23, 3218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Diao, N.; Lee, C.K.; Chu, H.W.; Bai, L.; Li, L. Neutrophils deficient in innate suppressor IRAK-M enhances anti-tumor immune responses. Mol. Ther. 2020, 28, 89–99. [Google Scholar] [CrossRef]
- Singhal, S.; Bhojnagarwala, P.S.; O'Brien, S.; Moon, E.K.; Garfall, A.L.; Rao, A.S.; Quatromoni, J.G.; Stephen, T.L.; Litzky, L.; Deshpande, C.; et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell 2016, 30, 120–135. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef]
- Spiegel, A.; Brooks, M.W.; Houshyar, S.; Reinhardt, F.; Ardolino, M.; Fessler, E.; Chen, M.B.; Krall, J.A.; DeCock, J.; Zervantonakis, I.K.; et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016, 6, 630–649. [Google Scholar] [CrossRef]
- Loffredo, S.; Borriello, F.; Iannone, R.; Ferrara, A.L.; Galdiero, M.R.; Gigantino, V.; Esposito, P.; Varricchi, G.; Lambeau, G.; Cassatella, M.A.; et al. Group V secreted phospholipase A(2) induces the release of proangiogenic and antiangiogenic factors by human neutrophils. Front. Immunol. 2017, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Triner, D.; Devenport, S.N.; Ramakrishnan, S.K.; Ma, X.; Frieler, R.A.; Greenson, J.K.; Inohara, N.; Nunez, G.; Colacino, J.A.; Mortensen, R.M.; et al. Neutrophils restrict tumor-associated microbiota to reduce growth and invasion of colon tumors in mice. Gastroenterology 2019, 156, 1467–1482. [Google Scholar] [CrossRef]
- Dmitrieva-Posocco, O.; Dzutsev, A.; Posocco, D.F.; Hou, V.; Yuan, W.; Thovarai, V.; Mufazalov, I.A.; Gunzer, M.; Shilovskiy, I.P.; Khaitov, M.R.; et al. Cell-type-specific responses to interleukin-1 control microbial invasion and tumor-elicited inflammation in colorectal cancer. Immunity 2019, 50, 166–180.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [PubMed]
- Shah, S.C.; Itzkowitz, S.H. Colorectal cancer in inflammatory bowel disease: Mechanisms and management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014, 345, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and inflammatory cytokine contribute to the initiation and development of ulcerative colitis and its associated cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Canli, Ö.; Nicolas, A.M.; Gupta, J.; Finkelmeier, F.; Goncharova, O.; Pesic, M.; Neumann, T.; Horst, D.; Löwer, M.; Sahin, U.; et al. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell 2017, 32, 869–883.e5. [Google Scholar] [CrossRef]
- Azzouz, D.; Khan, M.A.; Palaniyar, N. ROS induces NETosis by oxidizing DNA and initiating DNA repair. Cell Death Discov. 2021, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Aubé, F.A.; Bidias, A.; Pépin, G. Who and how, DNA sensors in NETs-driven inflammation. Front. Immunol. 2023, 14, 1190177. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yi, M.; Niu, M.; Mei, Q.; Wu, K. Myeloid-derived suppressor cells: An emerging target for anticancer immunotherapy. Mol. Cancer 2022, 21, 184. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Corzo, C.A.; Cotter, M.J.; Cheng, P.; Cheng, F.; Kusmartsev, S.; Sotomayor, E.; Padhya, T.; McCaffrey, T.V.; McCaffrey, J.C.; Gabrilovich, D.I. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 2009, 182, 5693–5701. [Google Scholar] [CrossRef] [PubMed]
- OuYang, L.Y.; Wu, X.J.; Ye, S.B.; Zhang, R.X.; Li, Z.L.; Liao, W.; Pan, Z.Z.; Zheng, L.M.; Zhang, X.S.; Wang, Z.; et al. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J. Transl. Med. 2015, 13, 47. [Google Scholar] [CrossRef]
- Nagaraj, S.; Gupta, K.; Pisarev, V.; Kinarsky, L.; Sherman, S.; Kang, L.; Herber, D.L.; Schneck, J.; Gabrilovich, D.I. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 2007, 13, 828–835. [Google Scholar] [CrossRef]
- Butin-Israeli, V.; Bui, T.M.; Wiesolek, H.L.; Mascarenhas, L.; Lee, J.J.; Mehl, L.C.; Knutson, K.R.; Adam, S.A.; Goldman, R.D.; Beyder, A.; et al. Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. J. Clin. Investig. 2019, 129, 712–726. [Google Scholar] [CrossRef]
- Bui, T.M.; Butin-Israeli, V.; Wiesolek, H.L.; Zhou, M.; Rehring, J.F.; Wiesmüller, L.; Wu, J.D.; Yang, G.Y.; Hanauer, S.B.; Sebag, J.A.; et al. Neutrophils alter DNA repair landscape to impact survival and shape distinct therapeutic phenotypes of colorectal cancer. Gastroenterology 2021, 161, 225–238.e15. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M. Neutrophils deregulate tumoral DNA damage response and contribute to non-homologous end-joining (NHEJ)-dependent survival of colorectal cancer. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Lu, Y.; Sanchez, D.J.; Li, J.; Wang, L.; Meng, X.; Chen, J.; Kien, T.T.; Zhong, M.; et al. Region-specific CD16(+) neutrophils promote colorectal cancer progression by inhibiting natural killer cells. Adv. Sci. 2024, 11, e2403414. [Google Scholar] [CrossRef]
- Carnevale, S.; Di Ceglie, I.; Grieco, G.; Rigatelli, A.; Bonavita, E.; Jaillon, S. Neutrophil diversity in inflammation and cancer. Front. Immunol. 2023, 14, 1180810. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, K.; Tian, J.; Xia, X.; Ma, J.; Tang, X.; Xu, H.; Wang, S. Granulocytic myeloid-derived suppressor cells promote the stemness of colorectal cancer cells through exosomal S100A9. Adv. Sci. 2019, 6, 1901278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.Y.; Zhang, P.; He, T.C.; Han, J.H.; Zhang, R.; Lin, J.; Fan, J.; Lu, L.; Zhu, W.W.; et al. Cancer-derived exosomal HSPC111 promotes colorectal cancer liver metastasis by reprogramming lipid metabolism in cancer-associated fibroblasts. Cell Death Dis. 2022, 13, 57. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Z.; Fu, W.; Lu, K.; Gu, X.; Xu, F.; Dai, J.; Yang, Y.; Jiang, J. Exosome-derived ADAM17 promotes liver metastasis in colorectal cancer. Front. Pharmacol. 2021, 12, 734351. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Zhang, Z.; Bian, D.; Shao, K.; Wang, S.; Ding, Y. G-MDSC-derived exosomes mediate the differentiation of M-MDSC into M2 macrophages promoting colitis-to-cancer transition. J. Immunother. Cancer 2023, 11, e006166. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.L.; Lan, H.Y.; Cheng, W.C.; Huang, S.C.; Yang, M.H. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. J. Hematol. Oncol. 2019, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Lv, X.; Li, W.; Li, G.; He, X.; Zhang, Y.; Shi, L.; Zhang, X. Deciphering the mechanism of Peptostreptococcus anaerobius-induced chemoresistance in colorectal cancer: The important roles of MDSC recruitment and EMT activation. Front. Immunol. 2023, 14, 1230681. [Google Scholar] [CrossRef]
- Abed, J.; Emgård, J.E.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, B.; Zhang, Y.; Yin, T.; Cui, Y.; Liu, J.; Yang, Y.; Song, H.; Shang, D. Gut microbiome: Decision-makers in the microenvironment of colorectal cancer. Front. Cell Infect. Microbiol. 2023, 13, 1299977. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Heidarzadeh, S.; Jahangiri, S.; Farhood, B.; Mortezaee, K.; Khanlarkhani, N.; Negahdari, B. Fusobacterium nucleatum and colorectal cancer: A mechanistic overview. J. Cell Physiol. 2019, 234, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Li, B.H.; Garstka, M.A.; Li, Z.F. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol. Immunol. 2020, 117, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.P.; Belperio, J.A.; Xue, Y.Y.; Burdick, M.D.; Strieter, R.M. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J. Immunol. 2004, 172, 2853–2860. [Google Scholar] [CrossRef]
- Jamieson, T.; Clarke, M.; Steele, C.W.; Samuel, M.S.; Neumann, J.; Jung, A.; Huels, D.; Olson, M.F.; Das, S.; Nibbs, R.J.; et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J. Clin. Investig. 2012, 122, 3127–3144. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Wang, D.; Daikoku, T.; Sun, H.; Dey, S.K.; Dubois, R.N. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 2013, 24, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Triner, D.; Xue, X.; Schwartz, A.J.; Jung, I.; Colacino, J.A.; Shah, Y.M. Epithelial hypoxia-inducible factor 2α facilitates the progression of colon tumors through recruiting neutrophils. Mol. Cell Biol. 2017, 37, e00481-16. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Itatani, Y.; Kawada, K.; Fujishita, T.; Kakizaki, F.; Hirai, H.; Matsumoto, T.; Iwamoto, M.; Inamoto, S.; Hatano, E.; Hasegawa, S.; et al. Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis. Gastroenterology 2013, 145, 1064–1075.e11. [Google Scholar] [CrossRef]
- Inamoto, S.; Itatani, Y.; Yamamoto, T.; Minamiguchi, S.; Hirai, H.; Iwamoto, M.; Hasegawa, S.; Taketo, M.M.; Sakai, Y.; Kawada, K. Loss of SMAD4 promotes colorectal cancer progression by accumulation of myeloid-derived suppressor cells through the CCL15-CCR1 chemokine axis. Clin. Cancer Res. 2016, 22, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kawada, K.; Itatani, Y.; Inamoto, S.; Okamura, R.; Iwamoto, M.; Miyamoto, E.; Chen-Yoshikawa, T.F.; Hirai, H.; Hasegawa, S.; et al. Loss of SMAD4 promotes lung metastasis of colorectal cancer by accumulation of CCR1+ tumor-associated neutrophils through CCL15-CCR1 axis. Clin. Cancer Res. 2017, 23, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Nywening, T.M.; Belt, B.A.; Cullinan, D.R.; Panni, R.Z.; Han, B.J.; Sanford, D.E.; Jacobs, R.C.; Ye, J.; Patel, A.A.; Gillanders, W.E.; et al. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 2018, 67, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Guo, S.; Liu, M.; Burow, M.E.; Wang, G. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr. Med. Chem. 2019, 26, 3026–3041. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Biasci, D.; Smoragiewicz, M.; Connell, C.M.; Wang, Z.; Gao, Y.; Thaventhiran, J.E.D.; Basu, B.; Magiera, L.; Johnson, T.I.; Bax, L.; et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc. Natl. Acad. Sci. USA 2020, 117, 28960–28970. [Google Scholar] [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- Zou, Q.; Lei, X.; Xu, A.; Li, Z.; He, Q.; Huang, X.; Xu, G.; Tian, F.; Ding, Y.; Zhu, W. Chemokines in progression, chemoresistance, diagnosis, and prognosis of colorectal cancer. Front. Immunol. 2022, 13, 724139. [Google Scholar] [CrossRef]
- Ogawa, R.; Yamamoto, T.; Hirai, H.; Hanada, K.; Kiyasu, Y.; Nishikawa, G.; Mizuno, R.; Inamoto, S.; Itatani, Y.; Sakai, Y.; et al. Loss of SMAD4 promotes colorectal cancer progression by recruiting tumor-associated neutrophils via the CXCL1/8-CXCR2 axis. Clin. Cancer Res. 2019, 25, 2887–2899. [Google Scholar] [CrossRef]
- Huang, K.; Luo, W.; Fang, J.; Yu, C.; Liu, G.; Yuan, X.; Liu, Y.; Wu, W. Notch3 signaling promotes colorectal tumor growth by enhancing immunosuppressive cells infiltration in the microenvironment. BMC Cancer 2023, 23, 55. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Li, R.; Chen, J.; Xu, G.; Zhu, Y.; Li, J.; Liang, Q.; Hua, Q.; Wang, L.; et al. KIAA1199 drives immune suppression to promote colorectal cancer liver metastasis by modulating neutrophil infiltration. Hepatology 2022, 76, 967–981. [Google Scholar] [CrossRef]
- Zhou, Q.; Peng, Y.; Ji, F.; Chen, H.; Kang, W.; Chan, L.S.; Gou, H.; Lin, Y.; Huang, P.; Chen, D.; et al. Targeting of SLC25A22 boosts the immunotherapeutic response in KRAS-mutant colorectal cancer. Nat. Commun. 2023, 14, 4677. [Google Scholar] [CrossRef]
- Yan, R.; Li, J.; Xiao, Z.; Fan, X.; Liu, H.; Xu, Y.; Sun, R.; Liu, J.; Yao, J.; An, G.; et al. DCLK1 suppresses tumor-specific cytotoxic T lymphocyte function through recruitment of MDSCs via the CXCL1-CXCR2 axis. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 463–485. [Google Scholar] [CrossRef]
- Chen, H.; Pan, Y.; Zhou, Q.; Liang, C.; Wong, C.C.; Zhou, Y.; Huang, D.; Liu, W.; Zhai, J.; Gou, H.; et al. METTL3 inhibits antitumor immunity by targeting m(6)A-BHLHE41-CXCL1/CXCR2 axis to promote colorectal cancer. Gastroenterology 2022, 163, 891–907. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, M.; Wu, X.; Lee, J.; Tan, M.; Hagenbeek, T.; Qu, X.; Yu, L.; Ross, J.; Korsisaari, N.; Cao, T.; et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 21248–21255. [Google Scholar] [CrossRef]
- Coussens, L.M.; Tinkle, C.L.; Hanahan, D.; Werb, Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000, 103, 481–490. [Google Scholar] [CrossRef]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Yalom, L.K.; Ning, E.; Urbanczyk, J.M.; Ren, X.; Herrnreiter, C.J.; Disario, J.A.; Wray, B.; Schipma, M.J.; Velichko, Y.S.; et al. Tissue-specific reprogramming leads to angiogenic neutrophil specialization and tumor vascularization in colorectal cancer. J. Clin. Investig. 2024, 134, e174545. [Google Scholar] [CrossRef]
- Korbecki, J.; Bosiacki, M.; Chlubek, D.; Baranowska-Bosiacka, I. Bioinformatic analysis of the CXCR2 ligands in cancer processes. Int. J. Mol. Sci. 2023, 24, 13287. [Google Scholar] [CrossRef] [PubMed]
- Najdaghi, S.; Razi, S.; Rezaei, N. An overview of the role of interleukin-8 in colorectal cancer. Cytokine 2020, 135, 155205. [Google Scholar] [CrossRef] [PubMed]
- Teijeira, A.; Garasa, S.; Ochoa, M.C.; Villalba, M.; Olivera, I.; Cirella, A.; Eguren-Santamaria, I.; Berraondo, P.; Schalper, K.A.; de Andrea, C.E.; et al. IL8, neutrophils, and NETs in a collusion against cancer immunity and immunotherapy. Clin. Cancer Res. 2021, 27, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Liao, X.; Qiu, S.; Xu, H.; Zhang, S.; Wang, S.; Ai, J.; Yang, L. CXCL8 in tumor biology and its implications for clinical translation. Front. Mol. Biosci. 2022, 9, 723846. [Google Scholar] [CrossRef]
- Schalper, K.A.; Carleton, M.; Zhou, M.; Chen, T.; Feng, Y.; Huang, S.P.; Walsh, A.M.; Baxi, V.; Pandya, D.; Baradet, T.; et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 2020, 26, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.C.; Liu, L.F.; Gupta, V.; Madireddi, S.; Keerthivasan, S.; Li, C.; Rishipathak, D.; Williams, P.; Kadel, E.E., 3rd; Koeppen, H.; et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 2020, 26, 693–698. [Google Scholar] [CrossRef]
- Hirai, H.; Zhang, P.; Dayaram, T.; Hetherington, C.J.; Mizuno, S.; Imanishi, J.; Akashi, K.; Tenen, D.G. C/EBPbeta is required for ‘emergency’ granulopoiesis. Nat. Immunol. 2006, 7, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Welte, T.; Kim, I.S.; Tian, L.; Gao, X.; Wang, H.; Li, J.; Holdman, X.B.; Herschkowitz, J.I.; Pond, A.; Xie, G.; et al. Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nat. Cell Biol. 2016, 18, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Benedicto, A.; Marquez, J.; Herrero, A.; Olaso, E.; Kolaczkowska, E.; Arteta, B. Decreased expression of the β(2) integrin on tumor cells is associated with a reduction in liver metastasis of colorectal cancer in mice. BMC Cancer 2017, 17, 827. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, F.; Wu, X.; Zhong, C.; Yu, L.; Liang, X.H.; Yao, J.; Blanchard, D.; Bais, C.; Peale, F.V.; van Bruggen, N.; et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 2007, 450, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Itatani, Y.; Yamamoto, T.; Zhong, C.; Molinolo, A.A.; Ruppel, J.; Hegde, P.; Taketo, M.M.; Ferrara, N. Suppressing neutrophil-dependent angiogenesis abrogates resistance to anti-VEGF antibody in a genetic model of colorectal cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 21598–21608. [Google Scholar] [CrossRef]
- Palmieri, V.; Lazaris, A.; Mayer, T.Z.; Petrillo, S.K.; Alamri, H.; Rada, M.; Jarrouj, G.; Park, W.Y.; Gao, Z.H.; McDonald, P.P.; et al. Neutrophils expressing lysyl oxidase-like 4 protein are present in colorectal cancer liver metastases resistant to anti-angiogenic therapy. J. Pathol. 2020, 251, 213–223. [Google Scholar] [CrossRef]
- Veglia, F.; Tyurin, V.A.; Blasi, M.; De Leo, A.; Kossenkov, A.V.; Donthireddy, L.; To, T.K.J.; Schug, Z.; Basu, S.; Wang, F.; et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019, 569, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Mouchemore, K.A.; Anderson, R.L. Immunomodulatory effects of G-CSF in cancer: Therapeutic implications. Semin. Immunol. 2021, 54, 101512. [Google Scholar] [CrossRef] [PubMed]
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Küttner, V.; et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361, eaao4227. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, H.O.; Roy, E.; Comerci, A.J.; van der Windt, D.J.; Zhang, H.; Huang, H.; Loughran, P.; Shiva, S.; Geller, D.A.; Bartlett, D.L.; et al. Neutrophil extracellular traps drive mitochondrial homeostasis in tumors to augment growth. Cancer Res. 2019, 79, 5626–5639. [Google Scholar] [CrossRef]
- Okamoto, M.; Mizuno, R.; Kawada, K.; Itatani, Y.; Kiyasu, Y.; Hanada, K.; Hirata, W.; Nishikawa, Y.; Masui, H.; Sugimoto, N.; et al. Neutrophil extracellular traps promote metastases of colorectal cancers through activation of ERK signaling by releasing neutrophil elastase. Int. J. Mol. Sci. 2023, 24, 1118. [Google Scholar] [CrossRef] [PubMed]
- Rayes, R.F.; Vourtzoumis, P.; Bou Rjeily, M.; Seth, R.; Bourdeau, F.; Giannias, B.; Berube, J.; Huang, Y.H.; Rousseau, S.; Camilleri-Broet, S.; et al. Neutrophil extracellular trap-associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. J. Immunol. 2020, 204, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Yu, M.; Zhao, X.; Du, J.; Li, Y.; Jing, H.; Dong, Z.; Kou, J.; Bi, Y.; et al. Neutrophil extracellular traps induced by activated platelets contribute to procoagulant activity in patients with colorectal cancer. Thromb. Res. 2019, 180, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Poto, R.; Cristinziano, L.; Modestino, L.; de Paulis, A.; Marone, G.; Loffredo, S.; Galdiero, M.R.; Varricchi, G. Neutrophil extracellular traps, angiogenesis and cancer. Biomedicines 2022, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef]
- Xia, Y.; He, J.; Zhang, H.; Wang, H.; Tetz, G.; Maguire, C.A.; Wang, Y.; Onuma, A.; Genkin, D.; Tetz, V.; et al. AAV-mediated gene transfer of DNase I in the liver of mice with colorectal cancer reduces liver metastasis and restores local innate and adaptive immune response. Mol. Oncol. 2020, 14, 2920–2935. [Google Scholar] [CrossRef] [PubMed]
- Garten, A.; Schuster, S.; Penke, M.; Gorski, T.; de Giorgis, T.; Kiess, W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 2015, 11, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Pylaeva, E.; Harati, M.D.; Spyra, I.; Bordbari, S.; Strachan, S.; Thakur, B.K.; Höing, B.; Franklin, C.; Skokowa, J.; Welte, K.; et al. NAMPT signaling is critical for the proangiogenic activity of tumor-associated neutrophils. Int. J. Cancer 2019, 144, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, T.; Liu, J.; Wang, Y.; Zhang, C.; Guo, L.; Shi, D.; Zhang, T.; Wang, X.; Li, J. FGF19-induced inflammatory CAF promoted neutrophil extracellular trap formation in the liver metastasis of colorectal cancer. Adv. Sci. 2023, 10, e2302613. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, W.; Pan, Y.; Wang, J.; Wu, M.; Yu, Y. Crosstalk between gut microbiota and metastasis in colorectal cancer: Implication of neutrophil extracellular traps. Front. Immunol. 2023, 14, 1296783. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, Y.; Xiang, L.; You, Y.; Duan, Y.; Zhao, Y.; Li, S.; Wu, R.; Zhang, J.; Zhou, L.; et al. Fusobacterium nucleatum-triggered neutrophil extracellular traps facilitate colorectal carcinoma progression. J. Exp. Clin. Cancer Res. 2023, 42, 236. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wu, J.; Zong, G.; Wang, F.; Deng, R.; Tao, R.; Qian, C.; Shan, Y.; Wang, A.; Zhao, Y.; et al. Capsaicin shapes gut microbiota and pre-metastatic niche to facilitate cancer metastasis to liver. Pharmacol. Res. 2023, 188, 106643. [Google Scholar] [CrossRef]
- Ansari, M.A.; Nadeem, A.; Attia, S.M.; Bakheet, S.A.; Shahid, M.; Rehman, M.U.; Alanazi, M.M.; Alhamed, A.S.; Ibrahim, K.E.; Albekairi, N.A.; et al. CCR1 antagonist J-113863 corrects the imbalance of pro- and anti-inflammatory cytokines in a SJL/J mouse model of relapsing-remitting multiple sclerosis. Immunobiology 2022, 227, 152245. [Google Scholar] [CrossRef] [PubMed]
- Tapmeier, T.T.; Howell, J.H.; Zhao, L.; Papiez, B.W.; Schnabel, J.A.; Muschel, R.J.; Gal, A. Evolving polarisation of infiltrating and alveolar macrophages in the lung during metastatic progression of melanoma suggests CCR1 as a therapeutic target. Oncogene 2022, 41, 5032–5045. [Google Scholar] [CrossRef]
- Al-Mazroua, H.A.; Nadeem, A.; Ansari, M.A.; Attia, S.M.; Bakheet, S.A.; Albekairi, T.H.; Ali, N.; Alasmari, F.; Algahtani, M.; Alsaad, A.M.S.; et al. CCR1 antagonist ameliorates experimental autoimmune encephalomyelitis by inhibition of Th9/Th22-related markers in the brain and periphery. Mol. Immunol. 2022, 144, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, F.; Zhang, C.; Li, N.; Huang, H.; Shao, Z.; Zhang, M.; Zhan, X.; He, Y.; Ju, Z.; et al. Eosinophil-derived chemokine (hCCL15/23, mCCL6) interacts with CCR1 to promote eosinophilic airway inflammation. Signal Transduct. Target. Ther. 2021, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G.; Rajarathnam, K.; Richmond, A. CXCR2 chemokine receptor—A master regulator in cancer and physiology. Trends Mol. Med. 2024, 30, 37–55. [Google Scholar] [CrossRef]
- Lin, H.; Wu, Y.; Chen, J.; Huang, S.; Wang, Y. (-)-4-O-(4-O-β-D-glucopyranosylcaffeoyl) quinic acid inhibits the function of myeloid-derived suppressor cells to enhance the efficacy of anti-PD1 against colon cancer. Pharm. Res. 2018, 35, 183. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, X.; Zhang, J.; Jiang, X.; Wang, J.; Li, Y.; Li, X.; Shen, G.; Peng, J.; Zheng, P.; et al. CD300ld on neutrophils is required for tumour-driven immune suppression. Nature 2023, 621, 830–839. [Google Scholar] [CrossRef]
- Lote, H.; Starling, N.; Pihlak, R.; Gerlinger, M. Advances in immunotherapy for MMR proficient colorectal cancer. Cancer Treat. Rev. 2022, 111, 102480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tang, L.; Tian, Y.; Ji, X.; Hu, Q.; Zhou, B.; Zhenyu, D.; Heng, X.; Yang, L. Cholesterol-modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials 2020, 241, 119852. [Google Scholar] [CrossRef]
- Chauhan, S.; Dhawan, D.K.; Saini, A.; Preet, S. Antimicrobial peptides against colorectal cancer-a focused review. Pharmacol. Res. 2021, 167, 105529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, X.; Dai, Y.; Xiong, S.; Wei, C.; Yu, P.; Tang, Y.; Wu, L.; Li, J.; Liu, D.; et al. Chemical cocktail induces hematopoietic reprogramming and expands hematopoietic stem/progenitor cells. Adv. Sci. 2020, 7, 1901785. [Google Scholar] [CrossRef] [PubMed]
- Mysore, V.; Cullere, X.; Mears, J.; Rosetti, F.; Okubo, K.; Liew, P.X.; Zhang, F.; Madera-Salcedo, I.; Rosenbauer, F.; Stone, R.M.; et al. FcγR engagement reprograms neutrophils into antigen cross-presenting cells that elicit acquired anti-tumor immunity. Nat. Commun. 2021, 12, 4791. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cai, X.; Syahirah, R.; Yao, Y.; Xu, Y.; Jin, G.; Bhute, V.J.; Torregrosa-Allen, S.; Elzey, B.D.; Won, Y.Y.; et al. CAR-neutrophil mediated delivery of tumor-microenvironment responsive nanodrugs for glioblastoma chemo-immunotherapy. Nat. Commun. 2023, 14, 2266. [Google Scholar] [CrossRef]
| Role | Year | Model | Mechanism | Reference |
|---|---|---|---|---|
| Anti- tumor | 2017 | Human clinical samples | Neutrophils enhance the responsiveness of CD8+ T cells and CD66+ cell infiltration in CRC is associated with increased OS | [20] |
| 2017 | AOM/DSS-induced CAC model | CD177+ neutrophils suppress epithelial cell tumorigenesis in colitis-associated cancer | [26] | |
| 2020 | Human clinical samples and PDX | Anti-TGFβ attenuates tumor growth via polarization of TANs towards an anti-tumor phenotype | [27] | |
| Pro- tumor | 2021 | Mouse: CT26 | Neutrophils acquire immunosuppressive activity mediated by FATP2 | [28] |
| 2020 2024 | Mouse: MC38 and CMT93 | TANs induce T cell suppression/Angiogenesis in the TME | [29,30] | |
| 2014 | Mouse: CMT93 | Ccl9 in CRC cells recruit CCR1+ neutrophils which produce MMP9 | [31] | |
| 2019 | Mouse: KPN model Human clinical samples | NOTCH1 signaling promotes metastasis via TGFβ-dependent neutrophil recruitment. | [32] | |
| 2019 | Mouse: iKAP, iAP models, and MC38 Human clinical samples | Oncogenic KRAS leads to high expression of CXCL3, binding CXCR2 on TANs to promote their migration | [33] | |
| 2020 | Mouse: Apc/Cdx2CreERT2 model Human clinical samples | Neutrophils suppress tumor-infiltrating T cells viamatrix metalloproteinase mediated activation of TGFβ | [34] | |
| 2020 | Human clinical samples and HCT116 | NETs promote metastasis via binding CCDC25 on cancer cells | [35] | |
| 2011 | KM12L4 human metastatic CRC cells | Systemic inhibition of CXCR1/CXCR2 induced apoptosis and inhibited angiogenesis in the liver metastasis | [36] |
| Target | Agents | Mechanism | Other Interventions | Cancer Type | Phase | Identifier |
|---|---|---|---|---|---|---|
| CCR5 | Maraviroc | Inhibitor | No (Alone) | CRC | I | NCT01736813 |
| Maraviroc | Inhibitor | Pembrolizumab | CRC | I | NCT03274804 | |
| Vicriviroc (MK-7690) | Inhibitor | Pembrolizumab | CRC | II | NCT03631407 | |
| CXCR1/2 | Navarixin | Inhibitor | Pembrolizumab | Various (including CRC) | II | NCT03473925 |
| SX-682 | Inhibitor | Alone or Nivolumab | CRC | I/II | NCT04599140 | |
| CXCR4 | Plerixafor | Inhibitor | No (Alone) | Various (including CRC) | I | NCT02179970 |
| IL-1R | Anakinra | Inhibitor | LV5FU2 and Bevacizumab | CRC | II | NCT02090101 |
| STAT3 | Napabucasin (BBI-608) | Inhibitor | Chemotherapy (FOLFIRI) | CRC | III | NCT02753127 |
| Danvatirsen (AZD9150) | Inhibitor | No (Alone) | Various (including CRC) | I/II | NCT01563302 | |
| Danvatirsen | Inhibitor | Alone or Durvalumab | Various (including CRC) | I | NCT03394144 | |
| IDO1 | Epacadostat | Inhibitor | Pembrolizumab and chemotherapy | Various (including CRC) | I/II | NCT03085914 |
| ARG1 | INCB001158 | Inhibitor | Pembrolizumab | Various (including CRC) | I | NCT02903914 |
| ARG1 vaccine | Peptide vaccine | Adjuvant Montanide ISA-51 | Various (including CRC) | I | NCT03689192 | |
| TRAIL receptor 2 | DS-8273a | Agonist | No (Alone) | Various (including CRC) | I | NCT02076451 |
| (DR5) | DS-8273a | Agonist | Nivolmab | CRC | I | NCT02991196 |
| CS-1008 | Agonist | No (Alone) | CRC | I | NCT01220999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masui, H.; Kawada, K.; Obama, K. Neutrophil and Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 6. https://doi.org/10.3390/ijms26010006
Masui H, Kawada K, Obama K. Neutrophil and Colorectal Cancer. International Journal of Molecular Sciences. 2025; 26(1):6. https://doi.org/10.3390/ijms26010006
Chicago/Turabian StyleMasui, Hideyuki, Kenji Kawada, and Kazutaka Obama. 2025. "Neutrophil and Colorectal Cancer" International Journal of Molecular Sciences 26, no. 1: 6. https://doi.org/10.3390/ijms26010006
APA StyleMasui, H., Kawada, K., & Obama, K. (2025). Neutrophil and Colorectal Cancer. International Journal of Molecular Sciences, 26(1), 6. https://doi.org/10.3390/ijms26010006






