Early-Stage Alcoholic Cardiomyopathy Highlighted by Metabolic Remodeling, Oxidative Stress, and Cardiac Myosin Dysfunction in Male Rats
Abstract
1. Introduction
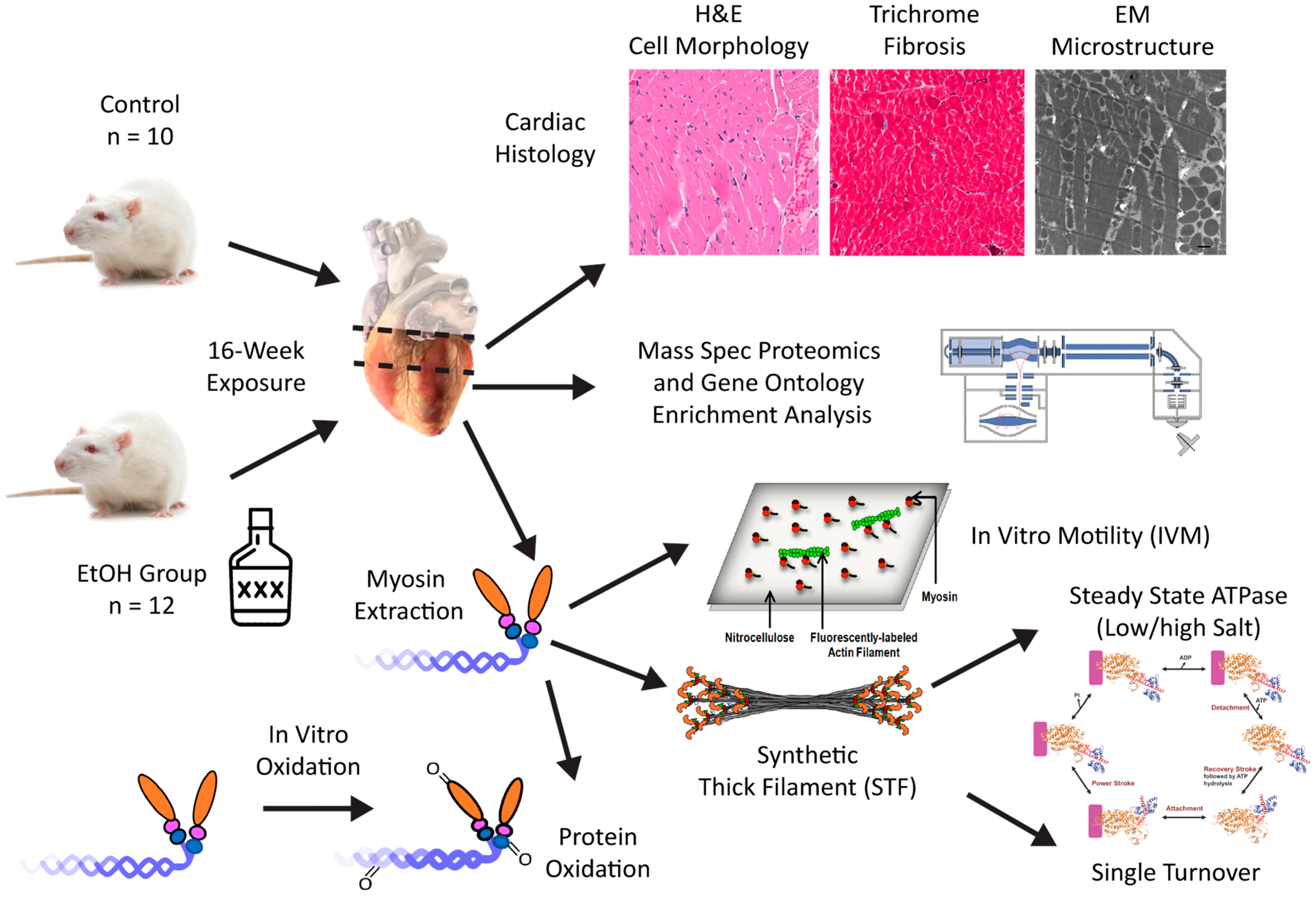
2. Results
2.1. Animal Morphometrics
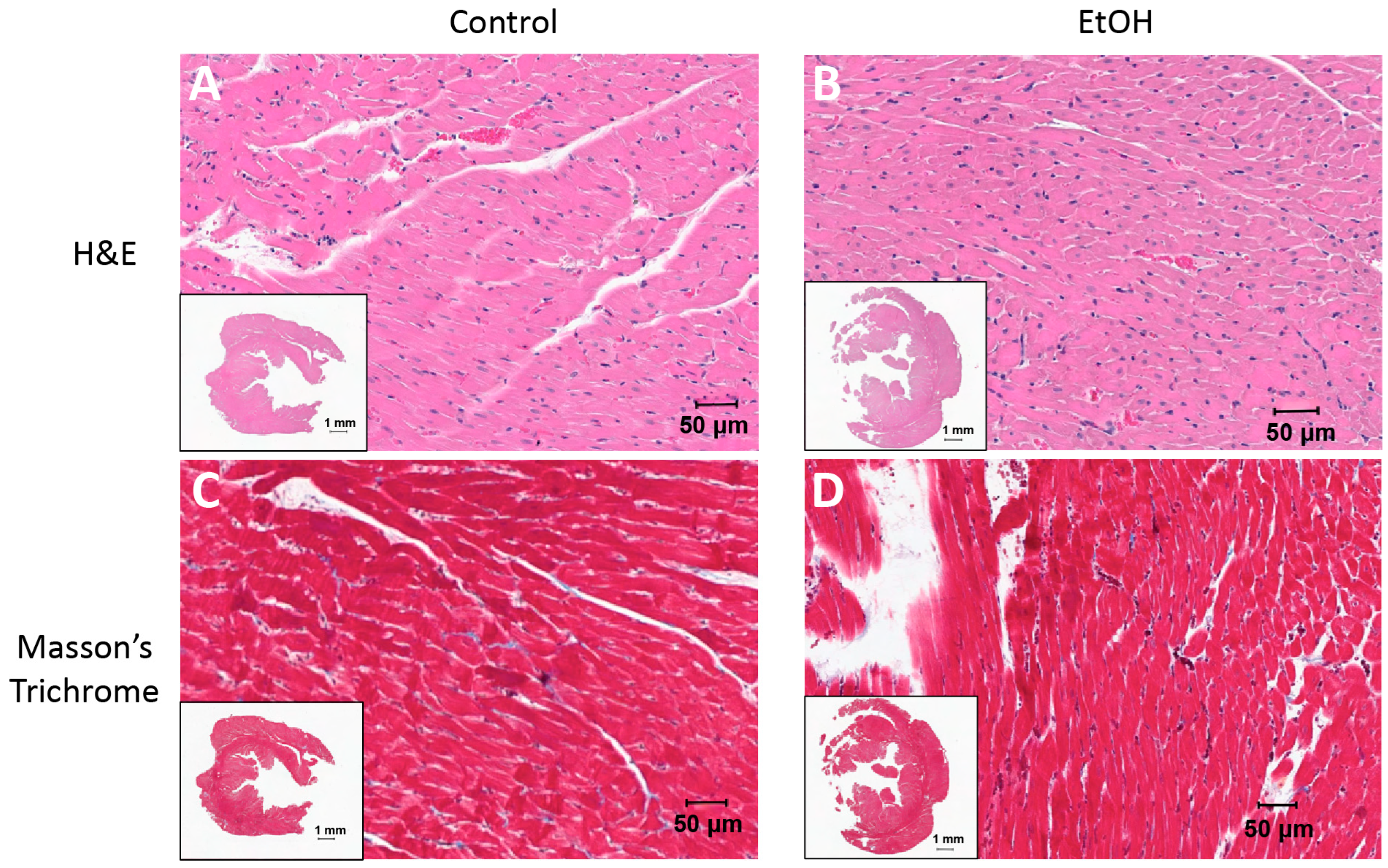
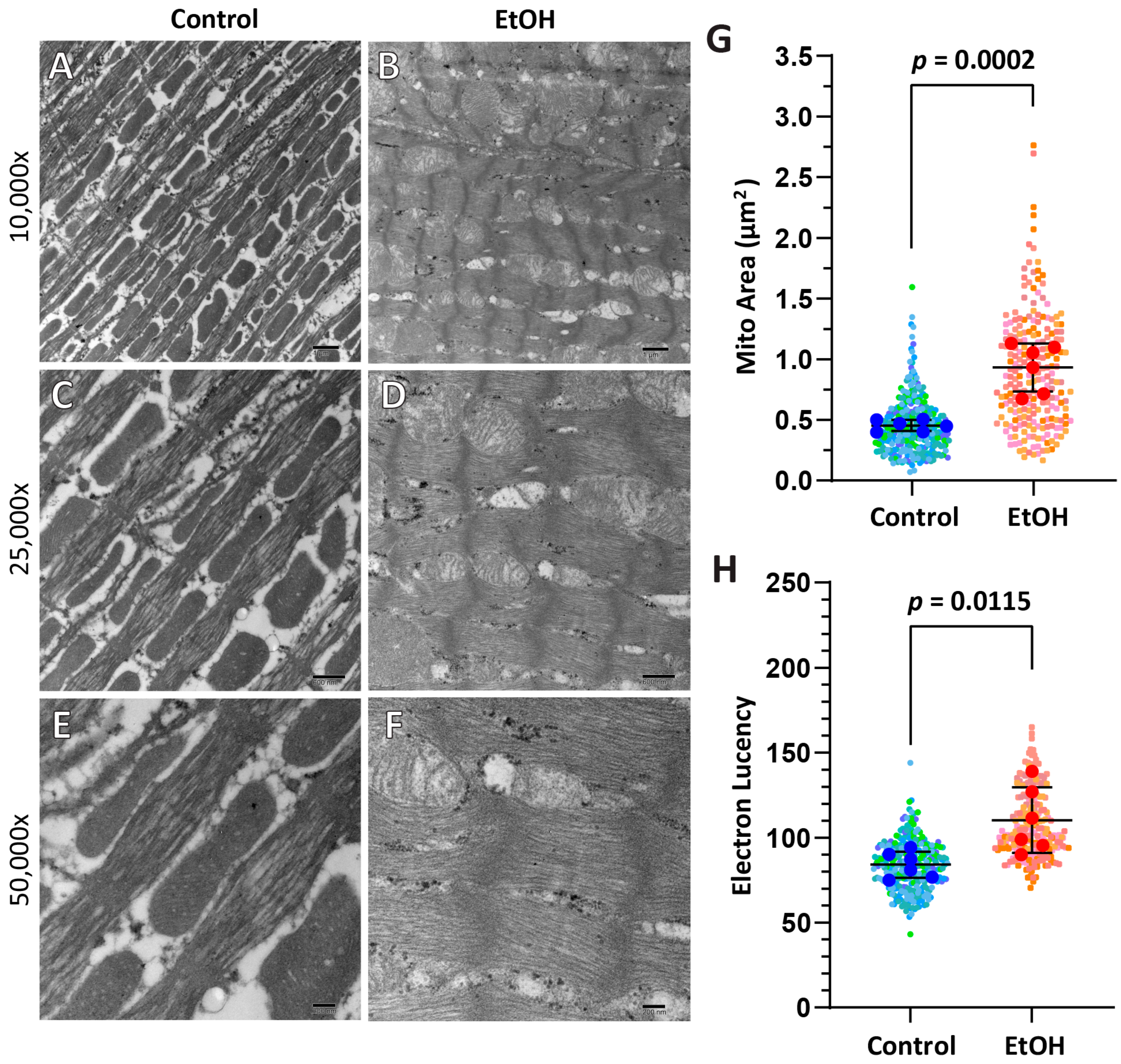
2.2. Routine Histology and Electron Microscopy
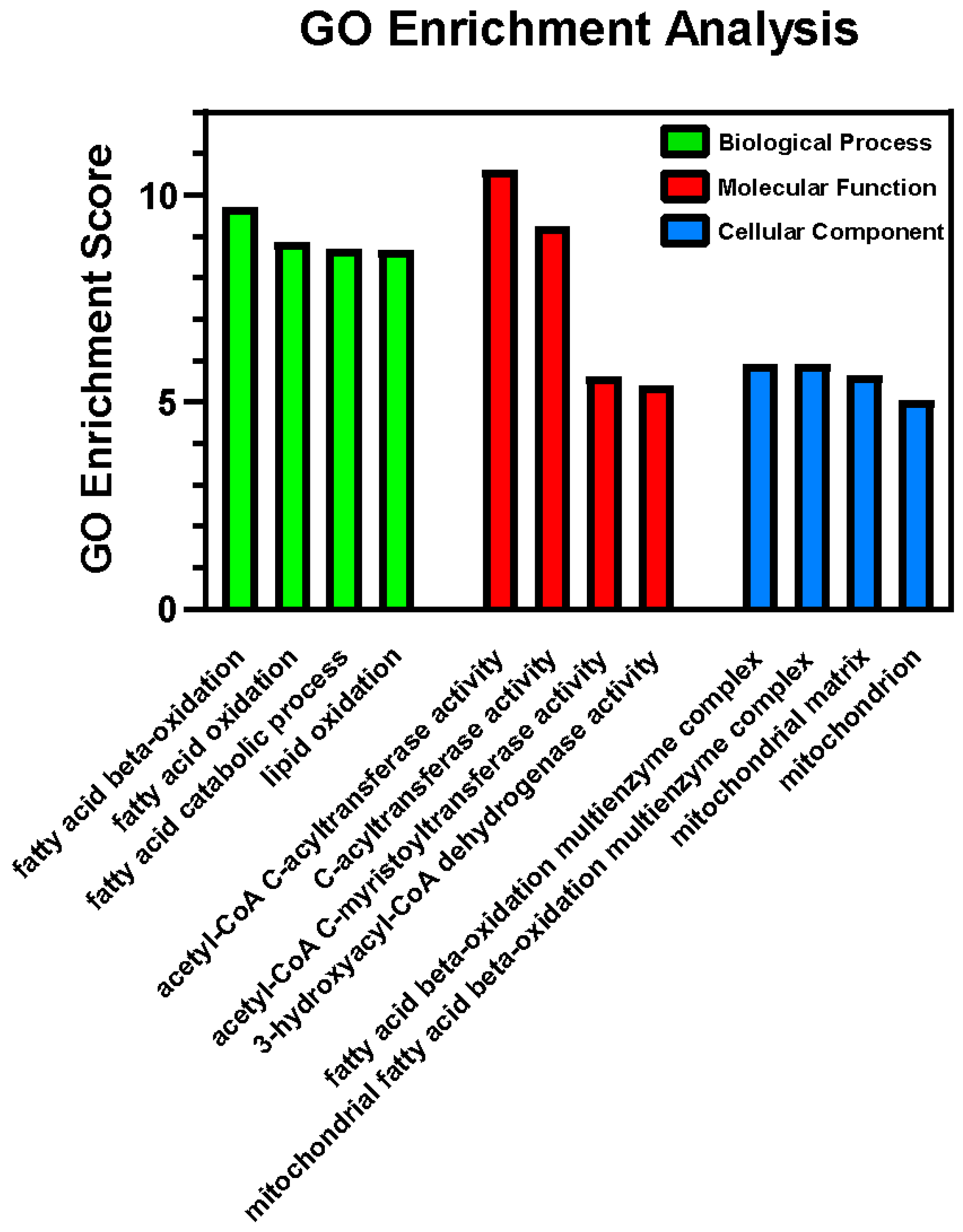
2.3. Proteomics via Mass Spectrometry
2.4. Mechanical Properties—In Vitro Motility
2.5. Synthetic Thick Filament (STF) Formation
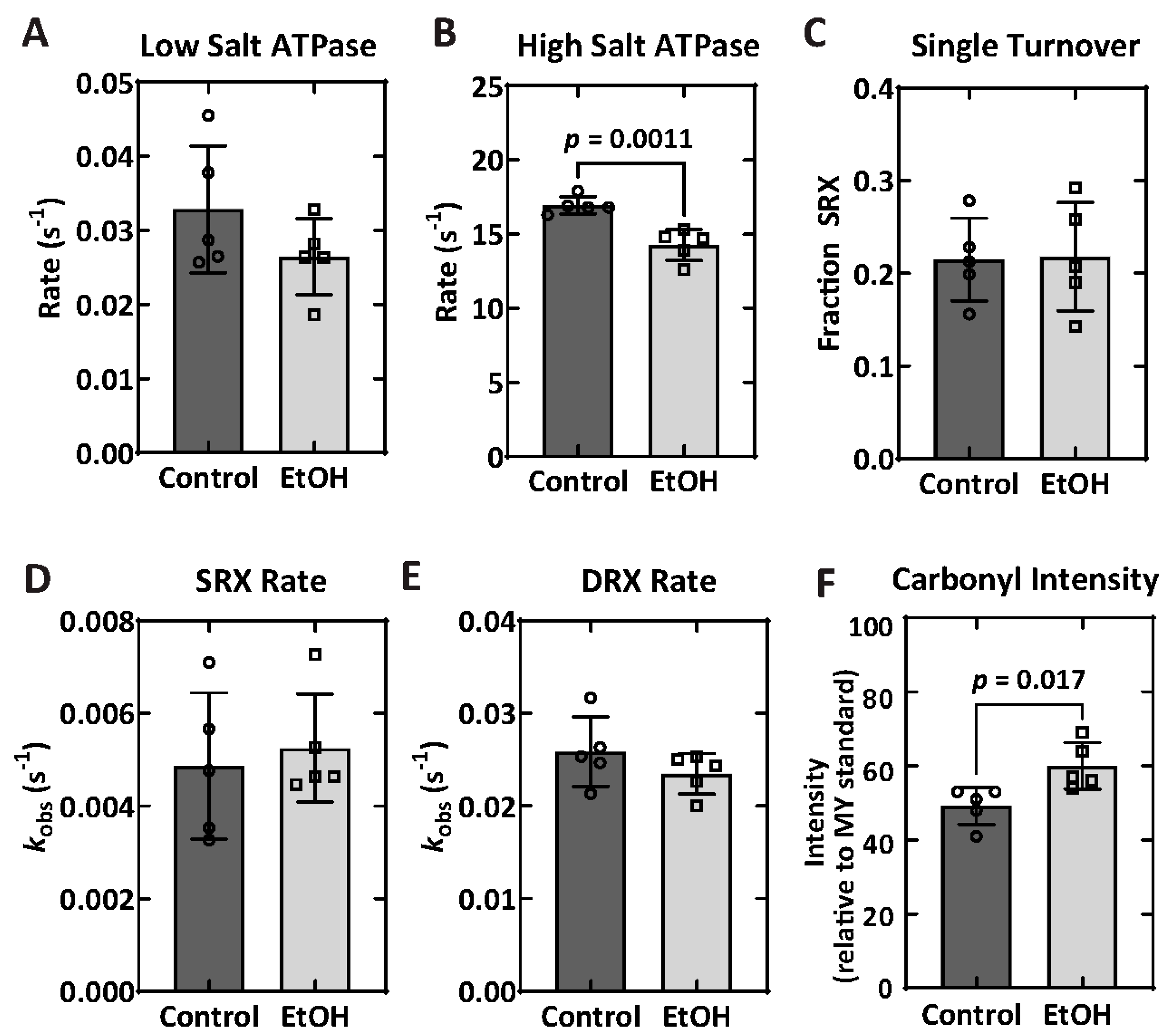
2.6. Biochemical Properties—ATPase and Single Turnover
2.7. Protein Carbonylation
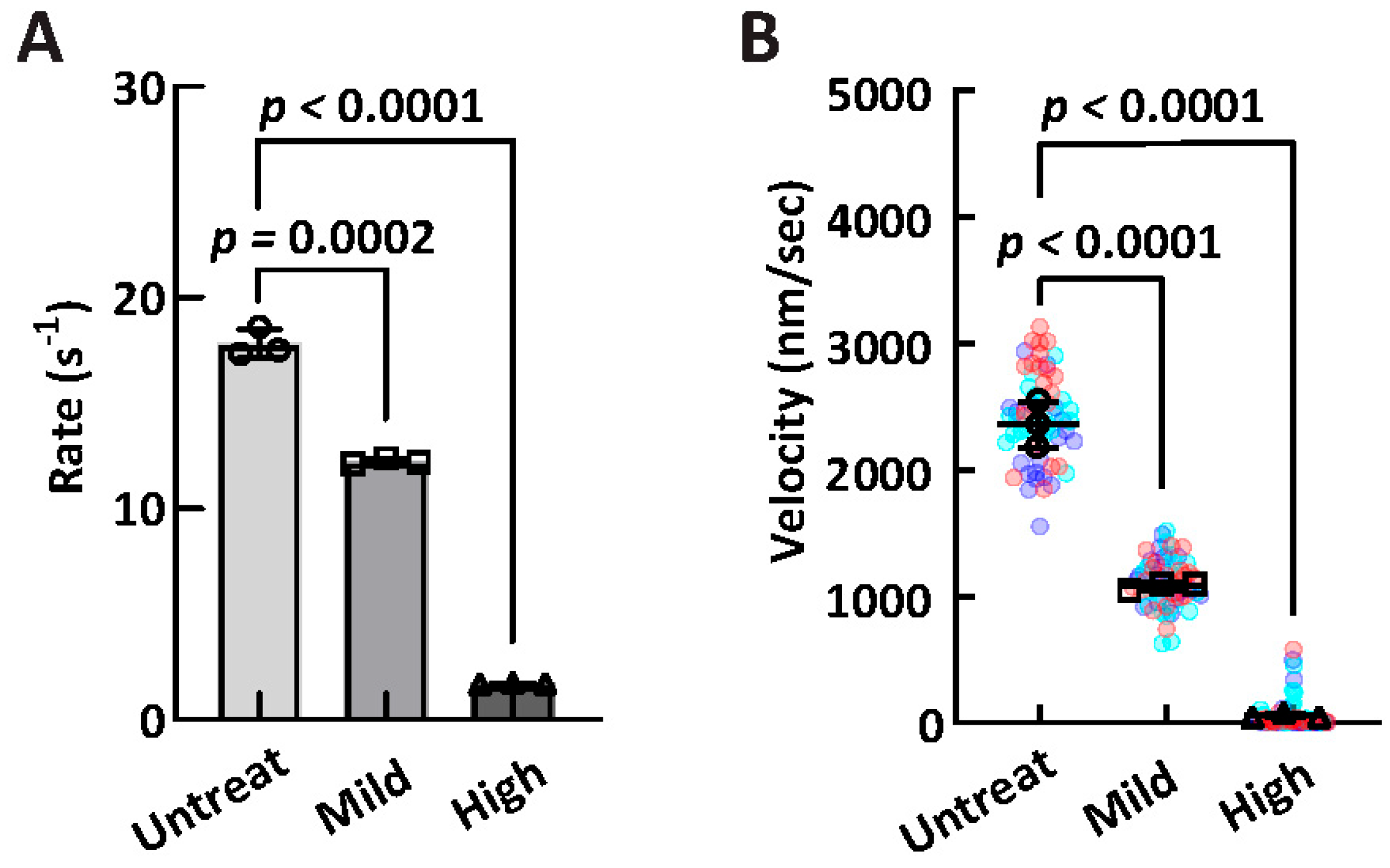
2.8. In Vitro Oxidation Treatment of Myosin
2.9. mRNA Expression
3. Discussion
3.1. Morphometrics and Cardiac Structure
3.2. Proteomics and GO Enrichment Analysis
3.3. Mechanochemical Properties of Myosin
3.4. Ethanol and Oxidation
3.5. Limitations
3.6. Summary
4. Materials and Methods
4.1. Animal Care and Handling
4.2. Sacrifice and Harvesting of Tissue
4.3. Blood Alcohol Content
4.4. Routine Histology, Electron Microscopy, and Analysis
4.5. Mass Spectrometry
4.6. Myosin Extraction
4.7. In Vitro Motility
4.8. Synthetic Thick Filament Formation
4.9. Synthetic Thick Filament ATPase
4.10. Single Turnover Assay
4.11. Actin Preparation
4.12. Protein Carbonylation of Cardiac Myosin
4.13. In Vitro Oxidative Treatment
4.14. mRNA Expression
4.15. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACM | Alcoholic cardiomyopathy |
| DCM | Dilated cardiomyopathy |
| SRX | Super-relaxed state |
| DRX | Disordered-relaxed state |
| IVM | In vitro motility |
| EM | Electron microscopy |
| H&E | Hematoxylin and eosin |
| BAC | Blood alcohol content |
| DEPs | Differentially expressed proteins |
| GO | Gene Ontology |
| CAT | Catalase |
| TNF⍺ | Tumor necrosis factor alpha |
| IFN⍺/IFNβ | Interferon alpha/beta |
| SOD1/SOD2 | Superoxide dismutase 1/2 |
| GPX1 | Glutathione peroxidase 1 |
| NQ01 | NAD(P)H quinone dehydrogenase 1 |
| HO-1 | Heme oxygenase 1 |
| REDD1 | Regulated in Development and DNA Damage Responses 1 |
| CCL2/CCL5 | Chemokine (C-C motif) Ligand 2/5 |
| ICAM-1 | Intercellular adhesion molecule 1 |
| STF | Synthetic thick filament |
| MTP | Mitochondrial trifunctional protein |
| OXPHOS | Oxidative phosphorylation |
| PCR | Polymerase chain reaction |
| TFA | Trifluoroacetic acid |
| SDS-PAGE | Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis |
| DNPH | 2,4-Dinitrophenylhydrazine |
| MHC | Myosin heavy chain |
| BSA | Bovine serum albumin |
| ECL | Enhanced chemiluminescence |
| PBS | Phosphate buffered saline |
| DTT | Dithiothreitol |
| EGTA | Ethylene glycol tetraacetic acid |
| MOPS | 3-(N-morpholino)propanesulfonic acid |
| UHPLC | Ultra-high performance liquid chromatography |
| LCMS | Liquid chromatography–mass spectrometry |
| NIH | National Institutes of Health |
| IACUC | Institutional Animal Care and Use Committee |
| PVDF | Polyvinylidene difluoride |
| HRP | Horseradish peroxidase |
References
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef] [PubMed]
- Sudhinaraset, M.; Wigglesworth, C.; Takeuchi, D.T. Social and Cultural Contexts of Alcohol Use: Influences in a Social-Ecological Framework. Alcohol Res. 2016, 38, 35–45. [Google Scholar] [PubMed]
- Roerecke, M. Alcohol’s Impact on the Cardiovascular System. Nutrients 2021, 13, 3419. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Solà, J. The Effects of Ethanol on the Heart: Alcoholic Cardiomyopathy. Nutrients 2020, 12, 572. [Google Scholar] [CrossRef]
- Domínguez, F.; Adler, E.; García-Pavía, P. Alcoholic cardiomyopathy: An update. Eur. Heart J. 2024, 45, 2294–2305. [Google Scholar] [CrossRef]
- Steiner, J.L.; Lang, C.H. Alcoholic Cardiomyopathy: Disrupted Protein Balance and Impaired Cardiomyocyte Contractility. Alcohol. Clin. Exp. Res. 2017, 41, 1392–1401. [Google Scholar] [CrossRef]
- Preedy, V.R.; Reilly, M.E.; Patel, V.B.; Richardson, P.J.; Peters, T.J. Protein metabolism in alcoholism: Effects on specific tissues and the whole body. Nutrition 1999, 15, 604–608. [Google Scholar] [CrossRef]
- Hu, C.; Ge, F.; Hyodo, E.; Arai, K.; Iwata, S.; Lobdell, H.; Walewski, J.L.; Zhou, S.; Clugston, R.D.; Jiang, H.; et al. Chronic ethanol consumption increases cardiomyocyte fatty acid uptake and decreases ventricular contractile function in C57BL/6J mice. J. Mol. Cell. Cardiol. 2013, 59, 30–40. [Google Scholar] [CrossRef]
- Matyas, C.; Varga, Z.V.; Mukhopadhyay, P.; Paloczi, J.; Lajtos, T.; Erdelyi, K.; Nemeth, B.T.; Nan, M.; Hasko, G.; Gao, B.; et al. Chronic plus binge ethanol feeding induces myocardial oxidative stress, mitochondrial and cardiovascular dysfunction, and steatosis. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1658–H1670. [Google Scholar] [CrossRef]
- Steiner, J.L.; Lang, C.H. Etiology of alcoholic cardiomyopathy: Mitochondria, oxidative stress and apoptosis. Int. J. Biochem. Cell Biol. 2017, 89, 125–135. [Google Scholar] [CrossRef]
- Urbano-Márquez, A.; Fernández-Solà, J. Alcohol consumption and heart failure. J. Card. Fail. 2005, 11, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Gollapudi, S.K.; Yu, M.; Gan, Q.F.; Nag, S. Synthetic thick filaments: A new avenue for better understanding the myosin super-relaxed state in healthy, diseased, and mavacamten-treated cardiac systems. J. Biol. Chem. 2021, 296, 100114. [Google Scholar] [CrossRef] [PubMed]
- Addolorato, G. Chronic alcohol abuse and nutritional status: Recent acquisitions. Eur. Rev. Med. Pharmacol. Sci. 1998, 2, 165–167. [Google Scholar]
- Javadov, S.; Chapa-Dubocq, X.; Makarov, V. Different approaches to modeling analysis of mitochondrial swelling. Mitochondrion 2018, 38, 58–70. [Google Scholar] [CrossRef]
- Feldmann, G.; Haouzi, D.; Moreau, A.; Durand-Schneider, A.M.; Bringuier, A.; Berson, A.; Mansouri, A.; Fau, D.; Pessayre, D. Opening of the mitochondrial permeability transition pore causes matrix expansion and outer membrane rupture in Fas-mediated hepatic apoptosis in mice. Hepatology 2000, 31, 674–683. [Google Scholar] [CrossRef]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.S.; Soukas, A.A. Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- Diebold, L.P.; Gil, H.J.; Gao, P.; Martinez, C.A.; Weinberg, S.E.; Chandel, N.S. Mitochondrial complex III is necessary for endothelial cell proliferation during angiogenesis. Nat. Metab. 2019, 1, 158–171. [Google Scholar] [CrossRef]
- Miklas, J.W.; Clark, E.; Levy, S.; Detraux, D.; Leonard, A.; Beussman, K.; Showalter, M.R.; Smith, A.T.; Hofsteen, P.; Yang, X.; et al. Author Correction: TFPa/HADHA is required for fatty acid beta-oxidation and cardiolipin re-modeling in human cardiomyocytes. Nat. Commun. 2020, 11, 2387. [Google Scholar] [CrossRef]
- Taylor, W.A.; Mejia, E.M.; Mitchell, R.W.; Choy, P.C.; Sparagna, G.C.; Hatch, G.M. Human trifunctional protein alpha links cardiolipin remodeling to beta-oxidation. PLoS ONE 2012, 7, e48628. [Google Scholar] [CrossRef]
- Wang, Y.; Mohsen, A.W.; Mihalik, S.J.; Goetzman, E.S.; Vockley, J. Evidence for physical association of mitochondrial fatty acid oxidation and oxidative phosphorylation complexes. J. Biol. Chem. 2010, 285, 29834–29841. [Google Scholar] [CrossRef]
- Lang, C.H.; Frost, R.A.; Summer, A.D.; Vary, T.C. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int. J. Biochem. Cell Biol. 2005, 37, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.L.; Lang, C.H. Dysregulation of skeletal muscle protein metabolism by alcohol. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E699–E712. [Google Scholar] [CrossRef] [PubMed]
- Vendemiale, G.; Grattagliano, I.; Altomare, E.; Serviddio, G.; Portincasa, P.; Prigigallo, F.; Palasciano, G. Mitochondrial oxidative damage and myocardial fibrosis in rats chronically intoxicated with moderate doses of ethanol. Toxicol. Lett. 2001, 123, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Fukushima, A.; Lopaschuk, G.D. Cardiac fatty acid oxidation in heart failure associated with obesity and diabetes. Biochim. Biophys. Acta 2016, 1861, 1525–1534. [Google Scholar] [CrossRef]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Griffin, T.M.; Humphries, K.M.; Kinter, M.; Lim, H.Y.; Szweda, L.I. Nutrient sensing and utilization: Getting to the heart of metabolic flexibility. Biochimie 2016, 124, 74–83. [Google Scholar] [CrossRef]
- He, W.; Zeng, L.; Zhao, R.; Qiu, K.; He, P.; Sun, Z.; Tan, N. Loss of Lipid Droplet-Mitochondria contacts confers protection against Ethanol-induced cardiotoxicity. Exp. Cell Res. 2025, 447, 114517. [Google Scholar] [CrossRef]
- Vafiadaki, E.; Arvanitis, D.A.; Sanoudou, D. Muscle LIM Protein: Master regulator of cardiac and skeletal muscle functions. Gene 2015, 566, 1–7. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Y.; Jin, J.; Du, R.; Tang, K.; Fan, L.; Xiang, R. CSRP3, p.Arg122*, is responsible for hypertrophic cardiomyopathy in a Chinese family. J. Gene Med. 2022, 24, e3390. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Parks, S.B.; Kushner, J.D.; Li, D.; Ludwigsen, S.; Jakobs, P.; Nauman, D.; Burgess, D.; Partain, J.; Litt, M. Coding sequence mutations identified in MYH7, TNNT2, SCN5A, CSRP3, LBD3, and TCAP from 313 patients with familial or idiopathic dilated cardiomyopathy. Clin. Transl. Sci. 2008, 1, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kostin, S.; Hein, S.; Arnon, E.; Scholz, D.; Schaper, J. The cytoskeleton and related proteins in the human failing heart. Heart Fail. Rev. 2000, 5, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Zahavich, L.; Akilen, R.; George, K.; Mital, S. Heart Failure with Recovered Ejection Fraction in Patients with Vinculin Loss-of-function Variants. J. Cardiovasc. Transl. Res. 2023, 16, 1303–1309. [Google Scholar] [CrossRef]
- Maggiorani, D.; Manzella, N.; Edmondson, D.E.; Mattevi, A.; Parini, A.; Binda, C.; Mialet-Perez, J. Monoamine Oxidases, Oxidative Stress, and Altered Mitochondrial Dynamics in Cardiac Ageing. Oxidative Med. Cell. Longev. 2017, 2017, 3017947. [Google Scholar] [CrossRef]
- Kaludercic, N.; Arusei, R.J.; Di Lisa, F. Recent advances on the role of monoamine oxidases in cardiac pathophysiology. Basic Res. Cardiol. 2023, 118, 41. [Google Scholar] [CrossRef]
- Shih, A.W.; McFarlane, A.; Verhovsek, M. Haptoglobin testing in hemolysis: Measurement and interpretation. Am. J. Hematol. 2014, 89, 443–447. [Google Scholar] [CrossRef]
- Niemelä, O.; Halkola, A.S.; Bloigu, A.; Bloigu, R.; Nivukoski, U.; Pohjasniemi, H.; Kultti, J. Blood Cell Responses Following Heavy Alcohol Consumption Coincide with Changes in Acute Phase Reactants of Inflammation, Indices of Hemolysis and Immune Responses to Ethanol Metabolites. Int. J. Mol. Sci. 2022, 23, 12738. [Google Scholar] [CrossRef]
- Sweeney, H.L.; Hammers, D.W. Muscle Contraction. Cold Spring Harb. Perspect. Biol. 2018, 10, a023200. [Google Scholar] [CrossRef]
- Geeves, M.A. Review: The ATPase mechanism of myosin and actomyosin. Biopolymers 2016, 105, 483–491. [Google Scholar] [CrossRef]
- Reddy, Y.S.; Beesley, R.C. Effects of acute and chronic ethanol on cardiac contractile protein ATPase activity of Syrian hamsters. Biochem. Med. Metab. Biol. 1990, 44, 259–265. [Google Scholar] [CrossRef]
- Meehan, J.; Piano, M.R.; Solaro, R.J.; Kennedy, J.M. Heavy long-term ethanol consumption induces an alpha- to beta-myosin heavy chain isoform transition in rat. Basic Res. Cardiol. 1999, 94, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Deacon, J.C.; Bloemink, M.J.; Rezavandi, H.; Geeves, M.A.; Leinwand, L.A. Identification of functional differences between recombinant human α and β cardiac myosin motors. Cell. Mol. Life Sci. 2012, 69, 2261–2277. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lekarski 2020, 48, 124–127. [Google Scholar] [PubMed]
- Cai, Z.; Yan, L.J. Protein Oxidative Modifications: Beneficial Roles in Disease and Health. J. Biochem. Pharmacol. Res. 2013, 1, 15–26. [Google Scholar]
- Tiago, T.; Ramos, S.; Aureliano, M.; Gutiérrez-Merino, C. Peroxynitrite induces F-actin depolymerization and blockade of myosin ATPase stimulation. Biochem. Biophys. Res. Commun. 2006, 342, 44–49. [Google Scholar] [CrossRef]
- Klein, J.C.; Moen, R.J.; Smith, E.A.; Titus, M.A.; Thomas, D.D. Structural and functional impact of site-directed methionine oxidation in myosin. Biochemistry 2011, 50, 10318–10327. [Google Scholar] [CrossRef]
- Moen, R.J.; Cornea, S.; Oseid, D.E.; Binder, B.P.; Klein, J.C.; Thomas, D.D. Redox-sensitive residue in the actin-binding interface of myosin. Biochem. Biophys. Res. Commun. 2014, 453, 345–349. [Google Scholar] [CrossRef]
- Snook, J.H.; Li, J.; Helmke, B.P.; Guilford, W.H. Peroxynitrite inhibits myofibrillar protein function in an in vitro assay of motility. Free Radic. Biol. Med. 2008, 44, 14–23. [Google Scholar] [CrossRef]
- Elkrief, D.; Cheng, Y.S.; Matusovsky, O.S.; Rassier, D.E. Oxidation alters myosin-actin interaction and force generation in skeletal muscle filaments. Am. J. Physiol. Cell Physiol. 2022, 323, C1206–C1214. [Google Scholar] [CrossRef]
- Elkrief, D.; Matusovsky, O.; Cheng, Y.S.; Rassier, D.E. From amino-acid to disease: The effects of oxidation on actin-myosin interactions in muscle. J. Muscle Res. Cell Motil. 2023, 44, 225–254. [Google Scholar] [CrossRef]
- Fogle, R.L.; Hollenbeak, C.S.; Stanley, B.A.; Vary, T.C.; Kimball, S.R.; Lynch, C.J. Functional proteomic analysis reveals sex-dependent differences in structural and energy-producing myocardial proteins in rat model of alcoholic cardiomyopathy. Physiol. Genom. 2011, 43, 346–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garvin, A.M.; Miller-Lee, J.L.; Sharda, D.R.; Kanski, G.M.; Hunter, J.C.; Korzick, D.H. Evidence of Altered Mitochondrial Protein Expression After Chronic Ethanol Consumption in the Aged Estrogen-Deficient Female Rat Heart. Alcohol. Clin. Exp. Res. 2017, 41, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F.W. Randomized block experimental designs can increase the power and reproducibility of laboratory animal experiments. ILAR J. 2014, 55, 472–476. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Mnatsakanyan, N.; Park, H.A.; Wu, J.; He, X.; Llaguno, M.C.; Latta, M.; Miranda, P.; Murtishi, B.; Graham, M.; Weber, J.; et al. Mitochondrial ATP synthase c-subunit leak channel triggers cell death upon loss of its F. Cell Death Differ. 2022, 29, 1874–1887. [Google Scholar] [CrossRef]
- O’Leary, T.S.; Snyder, J.; Sadayappan, S.; Day, S.M.; Previs, M.J. MYBPC3 truncation mutations enhance actomyosin contractile mechanics in human hypertrophic cardiomyopathy. J. Mol. Cell. Cardiol. 2019, 127, 165–173. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.P.; Mi, H. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci. 2022, 31, 8–22. [Google Scholar] [CrossRef]
- Rasicci, D.V.; Ge, J.; Milburn, G.N.; Wood, N.B.; Pruznak, A.M.; Lang, C.H.; Previs, M.J.; Campbell, K.S.; Yengo, C.M. Cardiac myosin motor deficits are associated with left ventricular dysfunction in human ischemic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H198–H209. [Google Scholar] [CrossRef]
- Woodward, M.; Previs, M.J.; Mader, T.J.; Debold, E.P. Modifications of myofilament protein phosphorylation and function in response to cardiac arrest induced in a swine model. Front. Physiol. 2015, 6, 199. [Google Scholar] [CrossRef]
- Kron, S.J.; Toyoshima, Y.Y.; Uyeda, T.Q.; Spudich, J.A. Assays for actin sliding movement over myosin-coated surfaces. Methods Enzymol. 1991, 196, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Aksel, T.; Choe Yu, E.; Sutton, S.; Ruppel, K.M.; Spudich, J.A. Ensemble force changes that result from human cardiac myosin mutations and a small-molecule effector. Cell Rep. 2015, 11, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Rasicci, D.V.; Kirkland, O.; Moonschi, F.H.; Wood, N.B.; Szczesna-Cordary, D.; Previs, M.J.; Wenk, J.F.; Campbell, K.S.; Yengo, C.M. Impact of regulatory light chain mutation K104E on the ATPase and motor properties of cardiac myosin. J. Gen. Physiol. 2021, 153, e202012811. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.J.; Velle, K.B.; Mullins, R.D.; Fritz-Laylin, L.K. SuperPlots: Communicating reproducibility and variability in cell biology. J. Cell Biol. 2020, 219, e202001064. [Google Scholar] [CrossRef]
- Trybus, K.M. Biochemical studies of myosin. Methods 2000, 22, 327–335. [Google Scholar] [CrossRef]
- Rasicci, D.V.; Tiwari, P.; Bodt, S.M.L.; Desetty, R.; Sadler, F.R.; Sivaramakrishnan, S.; Craig, R.; Yengo, C.M. Dilated cardiomyopathy mutation E525K in human beta-cardiac myosin stabilizes the interacting-heads motif and super-relaxed state of myosin. Elife 2022, 11, e77415. [Google Scholar] [CrossRef]
- Duno-Miranda, S.; Nelson, S.R.; Rasicci, D.V.; Bodt, S.M.L.; Cirilo, J.A.; Vang, D.; Sivaramakrishnan, S.; Yengo, C.M.; Warshaw, D.M. Tail length and E525K dilated cardiomyopathy mutant alter human β-cardiac myosin super-relaxed state. J. Gen. Physiol. 2024, 156, e202313522. [Google Scholar] [CrossRef]
- Pardee, J.D.; Spudich, J.A. Purification of muscle actin. Methods Enzymol. 1982, 85 Pt B, 164–181. [Google Scholar] [CrossRef]

| Body Weight (g) | Heart Weight (mg) | HW–BW (mg/g) | Serum (BAC) (mg/dL) | |
|---|---|---|---|---|
| Control (n = 10) | 325 ± 19.5 | 883 ± 54 | 2.72 ± 0.09 | 1.93 ± 0.99 |
| EtOH (n = 12) | 300 ± 23.1 | 834 ± 76 | 2.77 ± 0.11 | 35.3 ± 25.5 |
| p-value | 0.0163 | 0.1031 | 0.2635 | 0.0005 |
| Cell Size (Cross-Section) (µm) | Sarcomere Length * (nm) | Mitochondrial Area * (µm2) | Mitochondrial Lucency * | |
|---|---|---|---|---|
| Control (n = 10) | 15.42 ± 2.44 | 1918 ± 222 | 0.45 ± 0.05 | 84.18 ± 7.68 |
| EtOH (n = 12) | 15.67 ± 1.53 | 1649 ± 377 | 0.93 ± 0.20 | 110.40 ± 19.32 |
| p-value | 0.7761 | 0.164 | 0.0002 | 0.0115 |
| Gene | Accession | Description | Fold Change | p-Value |
|---|---|---|---|---|
| Myl4 | P17209 | Myosin essential (alkali) light chain, atrial/fetal | 0.0711 | 5.98 × 10−4 |
| Nppa | P01161 | Naturietic peptide A | 0.3614 | 2.87 × 10−4 |
| Hp | P06866 | Haptoglobin | 0.5042 | 2.43 × 10−5 |
| Maoa | G3V9Z3 | Monoamine oxidase A | 0.5191 | 1.51 × 10−6 |
| Mug1; A1i3 | Q03626; P14046 | Murinoglobulin 1 + alpha 1-inhibitor 3 | 0.6320 | 3.56 × 10−4 |
| Eno3 | P15429 | Enolase 3 | 0.6765 | 4.64 × 10−3 |
| Ckb | P07335 | Creatine kinase B | 0.6767 | 3.18 × 10−4 |
| Csrp3 | G3V7U0 | Cysteine- and glycine-rich protein | 0.7421 | 6.70 × 10−3 |
| Mug1 | Q03626 | Murinoglobulin 1 | 0.7824 | 8.73 × 10−3 |
| Cs | G3V936 | Citrate synthase | 1.2097 | 6.45 × 10−3 |
| Suclg2 | B1H270 | Succinate-CoA ligase subunit B | 1.2122 | 1.66 × 10−6 |
| Pygb | B2GV03 | Glycogen phosphorylase | 1.2468 | 3.91 × 10−3 |
| Hadhb | Q60587 | Hydroxylacyl-coenzyme A dehydrogenase trifunctional complex subunit B | 1.2563 | 1.86 × 10−7 |
| Vcl | P85972 | Vinculin | 1.2609 | 1.71 × 10−3 |
| Hadha | Q64428 | Hydroxylacyl-coenzyme A dehydrogenase trifunctional complex subunit A | 1.2679 | 9.55 × 10−7 |
| Hadh | Q9WVK7 | Hydroxylacyl-coenzyme A dehydrogenase | 1.2762 | 6.20 × 10−4 |
| ATP8 | Q5UAJ5 | ATP synthase protein 8, mitochondrial | 1.3721 | 2.81 × 10−3 |
| Decr1 | G3V734 | 2,4-dienoyl-CoA reductase | 1.3817 | 6.78 × 10−3 |
| Scp2 | P11915 | Non-specific lipid-transfer protein | 1.3861 | 2.49 × 10−6 |
| Acaa2 | P13437 | Acetyl-CoA acyltransferase 2 | 1.4164 | 1.23 × 10−5 |
| Acot2 | O55171 | Acylcoenzyme A thioesterase 2 | 1.4683 | 6.47 × 10−10 |
| Cat | P04762 | Catalase | 1.5054 | 2.18 × 10−6 |
| Tpm1 | F7FK40 | Tropomyosin alpha-1 chain | 1.8349 | 3.22 × 10−3 |
| Parameter | Control (n = 5) | EtOH (n = 5) | p-Value |
|---|---|---|---|
| Low-salt ATPase (s−1) | 0.033 ± 0.009 | 0.027 ± 0.005 | 0.1906 |
| High-salt ATPase (s−1) | 16.9 ± 0.6 | 14.3 ± 1.1 | 0.0011 |
| SRX fraction | 0.22 ± 0.04 | 0.22 ± 0.06 | 1.000 |
| SRX rate (s−1) | 0.0049 ± 0.0016 | 0.0052 ± 0.0012 | 0.7549 |
| DRX rate (s−1) | 0.026 ± 0.004 | 0.023 ± 0.002 | 0.1720 |
| IVM velocity (nm/s) | 1732 ± 235 | 1732 ± 203 | 1.000 |
| IVM stuck% | 13.3 ± 6.7% | 13.4 ± 4.3% | 0.9902 |
| Protein carbonylation | 49.2 ± 5.0% | 60.0 ± 6.3% | 0.017 |
| High-Salt ATPase (s−1) | IVM Velocity (nm/s) | |
|---|---|---|
| Untreated (n = 3) | 17.83 ± 0.69 | 2414 ± 185 |
| Mild treatment (n = 3) | 12.24 ± 0.12 | 1106 ± 31 |
| High treatment (n = 3) | 1.71 ± 0.03 | 60 ± 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasicci, D.V.; Ge, J.; Chen, A.P.; Wood, N.B.; Bodt, S.M.L.; Toro, A.L.; Evans, A.; Golestanian, O.; Amin, M.S.; Pruznak, A.; et al. Early-Stage Alcoholic Cardiomyopathy Highlighted by Metabolic Remodeling, Oxidative Stress, and Cardiac Myosin Dysfunction in Male Rats. Int. J. Mol. Sci. 2025, 26, 6766. https://doi.org/10.3390/ijms26146766
Rasicci DV, Ge J, Chen AP, Wood NB, Bodt SML, Toro AL, Evans A, Golestanian O, Amin MS, Pruznak A, et al. Early-Stage Alcoholic Cardiomyopathy Highlighted by Metabolic Remodeling, Oxidative Stress, and Cardiac Myosin Dysfunction in Male Rats. International Journal of Molecular Sciences. 2025; 26(14):6766. https://doi.org/10.3390/ijms26146766
Chicago/Turabian StyleRasicci, David V., Jinghua Ge, Adrien P. Chen, Neil B. Wood, Skylar M. L. Bodt, Allyson L. Toro, Alexandra Evans, Omid Golestanian, Md Shahrier Amin, Anne Pruznak, and et al. 2025. "Early-Stage Alcoholic Cardiomyopathy Highlighted by Metabolic Remodeling, Oxidative Stress, and Cardiac Myosin Dysfunction in Male Rats" International Journal of Molecular Sciences 26, no. 14: 6766. https://doi.org/10.3390/ijms26146766
APA StyleRasicci, D. V., Ge, J., Chen, A. P., Wood, N. B., Bodt, S. M. L., Toro, A. L., Evans, A., Golestanian, O., Amin, M. S., Pruznak, A., Mnatsakanyan, N., Silberman, Y., Dennis, M. D., Previs, M. J., Lang, C. H., & Yengo, C. M. (2025). Early-Stage Alcoholic Cardiomyopathy Highlighted by Metabolic Remodeling, Oxidative Stress, and Cardiac Myosin Dysfunction in Male Rats. International Journal of Molecular Sciences, 26(14), 6766. https://doi.org/10.3390/ijms26146766







