Epigenetic Regulatory Processes Involved in the Establishment and Maintenance of Skin Homeostasis—The Role of Microbiota
Abstract
:1. Introduction
2. Epigenetic Regulation
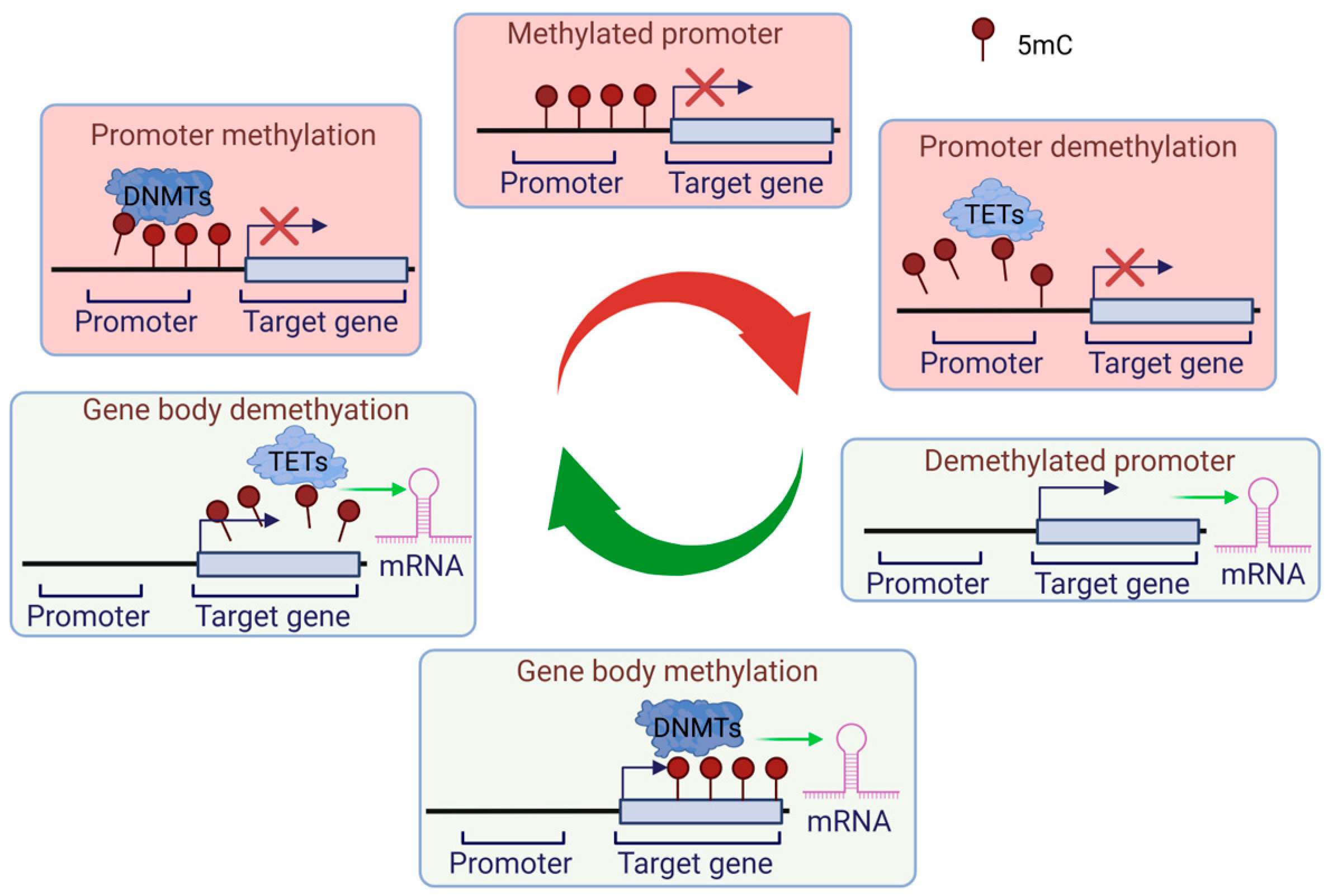
2.1. DNA-Associated Epigenetic Regulation: Methylation and Demethylation
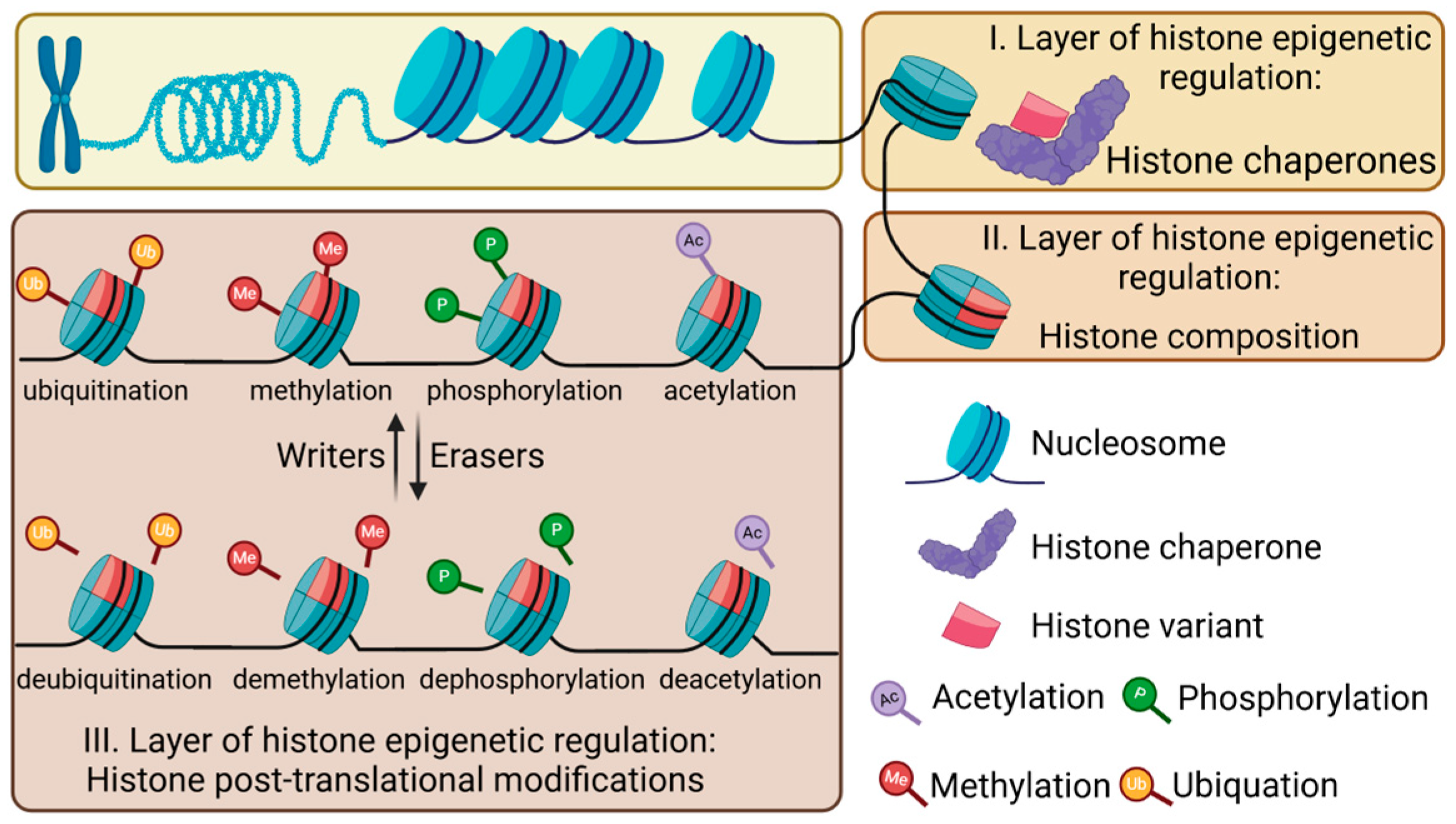
2.2. Epigenetic Regulation of Histones
2.2.1. Histone Chaperones
2.2.2. Histone Variants
2.2.3. Post-Translational Modifications of Histones
2.2.4. Crosstalk Among Different Epigenetic Regulatory Mechanisms
2.3. Non-Coding RNAs
3. Epigenetic Regulation in the Skin Under Healthy Conditions
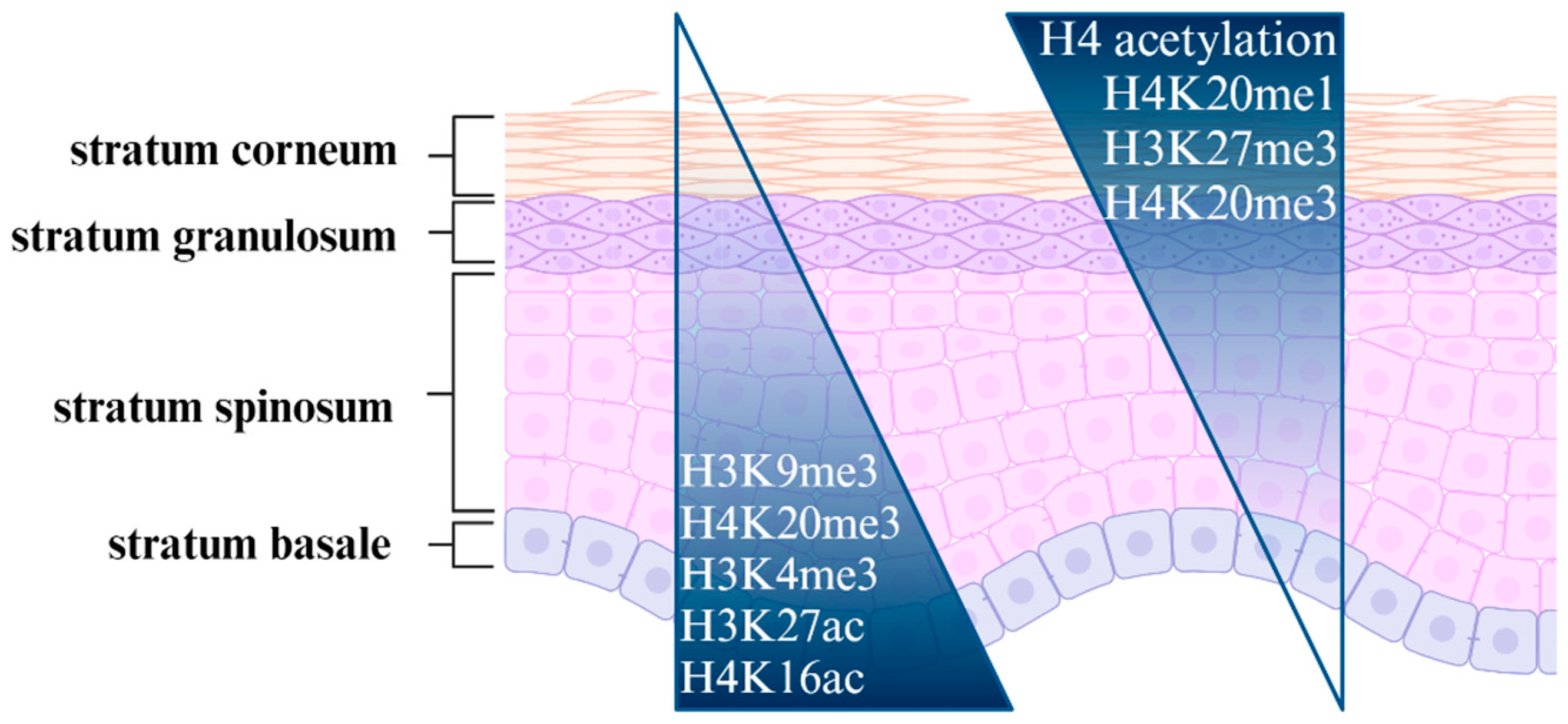
3.1. Skin Development
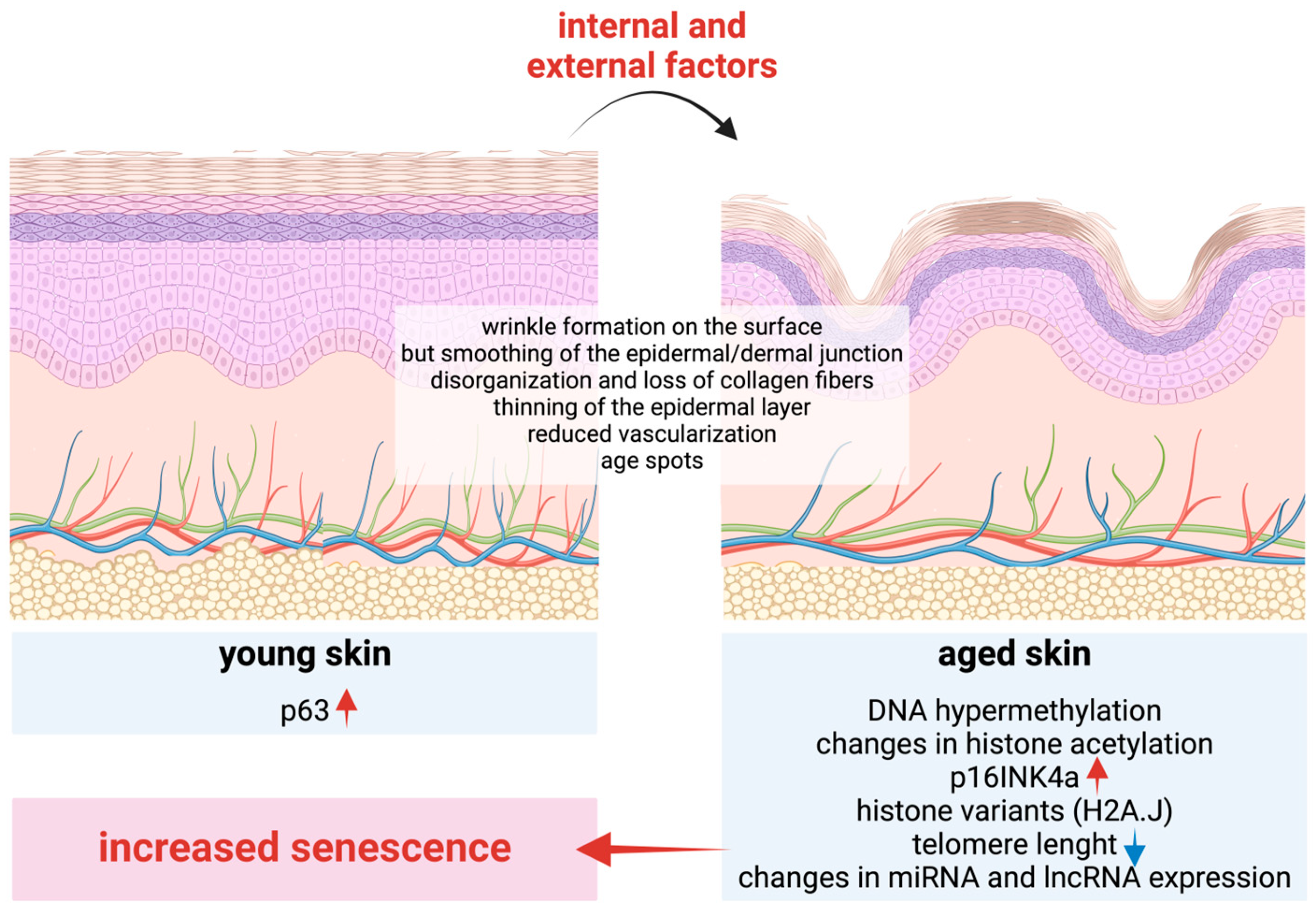
3.2. Skin Aging
| ncRNA Biomarker | Type | Major Function Related to Aging | References |
|---|---|---|---|
| let-7 family | miRNA | longevity-related | [125] |
| miR-34 family | miRNA | regulation of senescence and apoptosis | [126,128] |
| miR-130 family | miRNA | regulation of keratinocyte proliferation and differentiation (senescence) | [126] |
| miR-146a | miRNA | anti-inflammatory and promotes survival under stress conditions | [126,129] |
| miR-181family | miRNA | regulation of immunosenescence and mitochondrial function | [126,130] |
| miR-221/222 | miRNA | regulation of proliferation and survival pathways | [126,131] |
| H19 | lncRNA | regulation of senescence | [132] |
| PANDA | lncRNA | regulation of p21 expression, which is implicated in DNA damage response | [133,134] |
| SPRR2C | lncRNA | modulating calcium signaling pathways, influencing cutaneous differentiation | [132,135] |
| GAS5 | lncRNA | regulation of growth arrest and apoptosis, influencing cellular responses to stress | [136] |
| TERRA | lncRNA | telomere maintenance and DNA damage response | [137] |
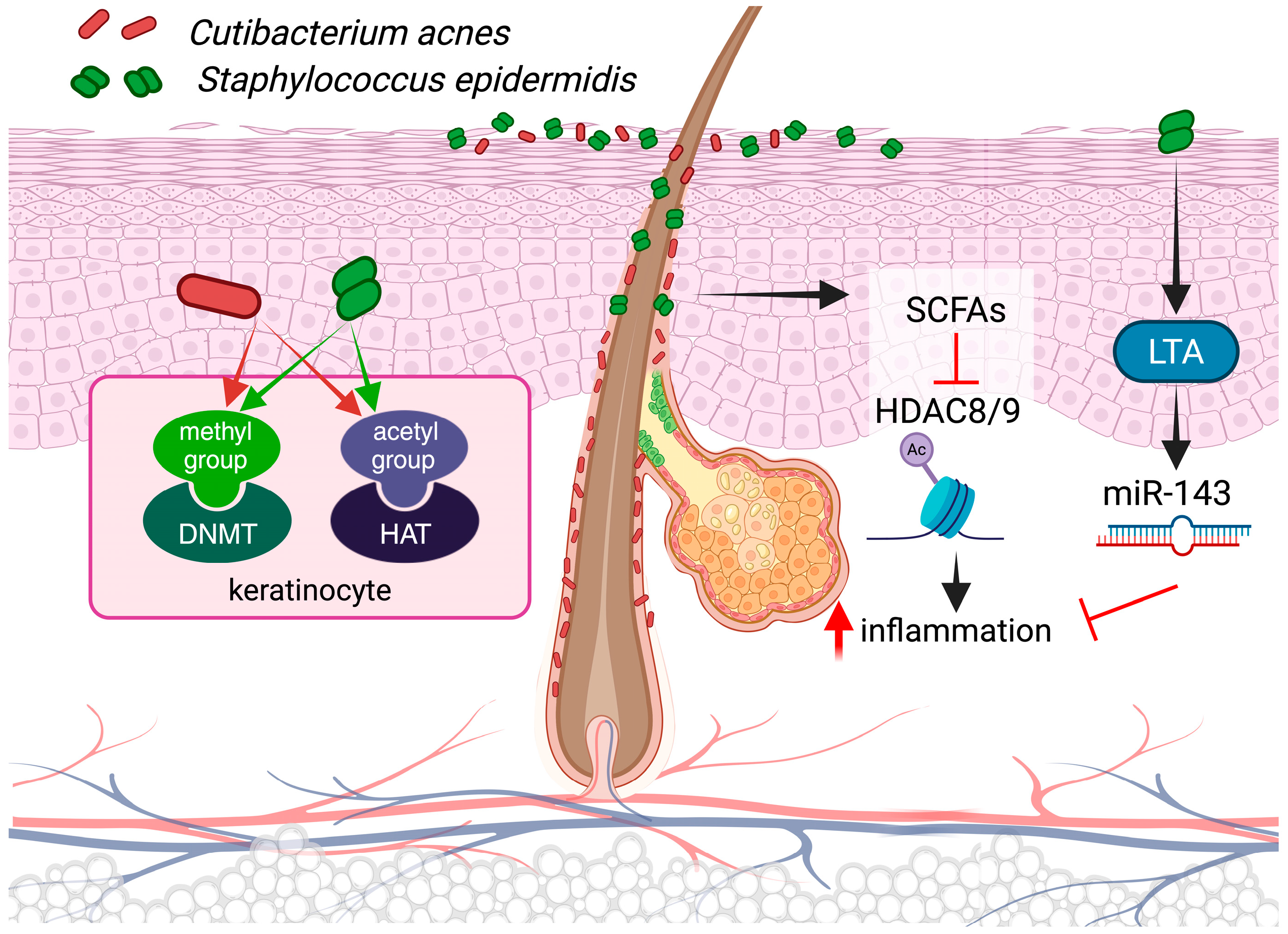
3.3. Epigenetics and Microbiota
4. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Fincham, J.R.S. Epigenetic Mechanisms of Gene Regulation. Edited by V. E. A. Russo, R.A. Martienssen and A. D. Riggs. Cold Spring Harbor Laboratory Press, 1996. 693+xii Pages. Price $125. ISBN 0 87969 490 4. Genet. Res. 1997, 69, 159–162. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and Gene Expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.; Millar, S. Skin Epigenetics. Exp. Dermatol. 2021, 30, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Chovatiya, G.; Tumbar, T. Epigenetic Control in Skin Development, Homeostasis and Injury Repair. Exp. Dermatol. 2019, 28, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, S.; Cui, Y.; Klaassen, C.D. Molecular Targets of Epigenetic Regulation and Effectors of Environmental Influences. Toxicol. Appl. Pharmacol. 2010, 245, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.A.; Zylicz, J.J.; Surani, M.A. Parallel Mechanisms of Epigenetic Reprogramming in the Germline. Trends Genet. 2012, 28, 164–174. [Google Scholar] [CrossRef]
- Prokhortchouk, E.; Defossez, P.-A. The Cell Biology of DNA Methylation in Mammals. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 2008, 1783, 2167–2173. [Google Scholar] [CrossRef]
- Smith, Z.D.; Meissner, A. DNA Methylation: Roles in Mammalian Development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-Methylcytosine to 5-Hydroxymethylcytosine in Mammalian DNA by MLL Partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef]
- Ito, S.; Shen, L.; Dai, Q.; Wu, S.C.; Collins, L.B.; Swenberg, J.A.; He, C.; Zhang, Y. Tet Proteins Can Convert 5-Methylcytosine to 5-Formylcytosine and 5-Carboxylcytosine. Science 2011, 333, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Booth, M.J.; Branco, M.R.; Ficz, G.; Oxley, D.; Krueger, F.; Reik, W.; Balasubramanian, S. Quantitative Sequencing of 5-Methylcytosine and 5-Hydroxymethylcytosine at Single-Base Resolution. Science 2012, 336, 934–937. [Google Scholar] [CrossRef]
- Edwards, J.R.; Yarychkivska, O.; Boulard, M.; Bestor, T.H. DNA Methylation and DNA Methyltransferases. Epigenetics Chromatin 2017, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, A. Beyond Watson and Crick: DNA Methylation and Molecular Enzymology of DNA Methyltransferases. ChemBioChem 2002, 3, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA Methyltransferases Dnmt3a and Dnmt3b Are Essential for De Novo Methylation and Mammalian Development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Law, J.A.; Jacobsen, S.E. Establishing, Maintaining and Modifying DNA Methylation Patterns in Plants and Animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R.M.; Zhang, Y. TET Enzymes, TDG and the Dynamics of DNA Demethylation. Nature 2013, 502, 472–479. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA Methylation: Islands, Start Sites, Gene Bodies and Beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Daban, J.-R.; Cantor, C.R. Role of Histone Pairs H2A, H2B and H3, H4 in the Self-Assembly of Nucleosome Core Particles. J. Mol. Biol. 1982, 156, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Nandy, D.; Rajam, S.M.; Dutta, D. A Three Layered Histone Epigenetics in Breast Cancer Metastasis. Cell Biosci. 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, Y.; Wang, S. Histone Citrullination: A New Target for Tumors. Mol. Cancer 2021, 20, 90. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shangguan, Y.; Tang, D.; Dai, Y. Histone Succinylation and Its Function on the Nucleosome. J. Cell Mol. Med. 2021, 25, 7101–7109. [Google Scholar] [CrossRef] [PubMed]
- De Koning, L.; Corpet, A.; Haber, J.E.; Almouzni, G. Histone Chaperones: An Escort Network Regulating Histone Traffic. Nat. Struct. Mol. Biol. 2007, 14, 997–1007. [Google Scholar] [CrossRef]
- Warren, C.; Shechter, D. Fly Fishing for Histones: Catch and Release by Histone Chaperone Intrinsically Disordered Regions and Acidic Stretches. J. Mol. Biol. 2017, 429, 2401–2426. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Feng, H.; Zhou, B.-R.; Ghirlando, R.; Hu, K.; Zwolak, A.; Miller Jenkins, L.M.; Xiao, H.; Tjandra, N.; Wu, C.; et al. Structural Basis for Recognition of Centromere Histone Variant CenH3 by the Chaperone Scm3. Nature 2011, 472, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Stillman, B. Purification and Characterization of CAF-I, a Human Cell Factor Required for Chromatin Assembly during DNA Replication in Vitro. Cell 1989, 58, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ray-Gallet, D.; Quivy, J.-P.; Scamps, C.; Martini, E.M.-D.; Lipinski, M.; Almouzni, G. HIRA Is Critical for a Nucleosome Assembly Pathway Independent of DNA Synthesis. Mol. Cell 2002, 9, 1091–1100. [Google Scholar] [CrossRef]
- English, C.M.; Maluf, N.K.; Tripet, B.; Churchill, M.E.A.; Tyler, J.K. ASF1 Binds to a Heterodimer of Histones H3 and H4: A Two-Step Mechanism for the Assembly of the H3−H4 Heterotetramer on DNA. Biochemistry 2005, 44, 13673–13682. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Chodaparambil, J.V.; Bao, Y.; McBryant, S.J.; Luger, K. Nucleosome Assembly Protein 1 Exchanges Histone H2A-H2B Dimers and Assists Nucleosome Sliding. J. Biol. Chem. 2005, 280, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Sarkar, S.; Tan, D. Histone Variants and Chromatin Structure, Update of Advances. Comput. Struct. Biotechnol. J. 2023, 21, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bassett, E.; Chakravarti, A.; Parthun, M.R. Replication-Dependent Histone Isoforms: A New Source of Complexity in Chromatin Structure and Function. Nucleic Acids Res. 2018, 46, 8665–8678. [Google Scholar] [CrossRef]
- Albig, W.; Doenecke, D. The Human Histone Gene Cluster at the D6S105 Locus. Hum. Genet. 1997, 101, 284–294. [Google Scholar] [CrossRef]
- Marzluff, W.F.; Gongidi, P.; Woods, K.R.; Jin, J.; Maltais, L.J. The Human and Mouse Replication-Dependent Histone Genes. Genomics 2002, 80, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Susano Pinto, D.M.; Flaus, A. The Human Canonical Core Histone Catalogue. bioRxiv 2019, 720235. [Google Scholar] [CrossRef]
- Marzluff, W.F.; Wagner, E.J.; Duronio, R.J. Metabolism and Regulation of Canonical Histone MRNAs: Life without a Poly(A) Tail. Nat. Rev. Genet. 2008, 9, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, G.; Manchanda, P.; Krishna Rapalli, V.; Kumar Dubey, S.; Gupta, G.; Dua, K. MicroRNAs as Biological Regulators in Skin Disorders. Biomed. Pharmacother. 2018, 108, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Buschbeck, M.; Hake, S.B. Variants of Core Histones and Their Roles in Cell Fate Decisions, Development and Cancer. Nat. Rev. Mol. Cell Biol. 2017, 18, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Maze, I.; Noh, K.-M.; Soshnev, A.A.; Allis, C.D. Every Amino Acid Matters: Essential Contributions of Histone Variants to Mammalian Development and Disease. Nat. Rev. Genet. 2014, 15, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.M. Histone Acetylation and Control of Gene Expression. J. Cell Sci. 1991, 99, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, D.; Avvakumov, N.; Côté, J. Histone Phosphorylation. Epigenetics 2012, 7, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Halmer, L. Effects of Cell Cycle Dependent Histone H1 Phosphorylation on Chromatin Structure and Chromatin Replication. Nucleic Acids Res. 1996, 24, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Cho, K.-O. The Role of Epigenetics in the Pathophysiology of Epilepsy. In Neuropsychiatric Disorders and Epigenetics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 233–260. [Google Scholar] [CrossRef]
- Husmann, D.; Gozani, O. Histone Lysine Methyltransferases in Biology and Disease. Nat. Struct. Mol. Biol. 2019, 26, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Shi, Y. Histone Methylation: A Dynamic Mark in Health, Disease and Inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Grice, G.L.; Nathan, J.A. The Recognition of Ubiquitinated Proteins by the Proteasome. Cell. Mol. Life Sci. 2016, 73, 3497–3506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhu, P.; Wang, J.; Pascual, G.; Ohgi, K.A.; Lozach, J.; Glass, C.K.; Rosenfeld, M.G. Histone H2A Monoubiquitination Represses Transcription by Inhibiting RNA Polymerase II Transcriptional Elongation. Mol. Cell 2008, 29, 69–80. [Google Scholar] [CrossRef]
- Zhu, Q.; Pao, G.M.; Huynh, A.M.; Suh, H.; Tonnu, N.; Nederlof, P.M.; Gage, F.H.; Verma, I.M. BRCA1 Tumour Suppression Occurs via Heterochromatin-Mediated Silencing. Nature 2011, 477, 179–184. [Google Scholar] [CrossRef]
- Minsky, N.; Shema, E.; Field, Y.; Schuster, M.; Segal, E.; Oren, M. Monoubiquitinated H2B Is Associated with the Transcribed Region of Highly Expressed Genes in Human Cells. Nat. Cell Biol. 2008, 10, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Miroshnikova, Y.A.; Cohen, I.; Ezhkova, E.; Wickström, S.A. Epigenetic Gene Regulation, Chromatin Structure, and Force-Induced Chromatin Remodelling in Epidermal Development and Homeostasis. Curr. Opin. Genet. Dev. 2019, 55, 46–51. [Google Scholar] [CrossRef]
- Frye, M.; Fisher, A.G.; Watt, F.M. Epidermal Stem Cells Are Defined by Global Histone Modifications That Are Altered by Myc-Induced Differentiation. PLoS ONE 2007, 2, e763. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Li, L.; Fuchs, E. Transit-Amplifying Cells Orchestrate Stem Cell Activity and Tissue Regeneration. Cell 2014, 157, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Shue, Y.T.; Lee, K.T.; Walters, B.W.; Ong, H.B.; Silvaraju, S.; Lam, W.J.; Lim, C.Y. Dynamic Shifts in Chromatin States Differentially Mark the Proliferative Basal Cells and Terminally Differentiated Cells of the Developing Epidermis. Epigenetics 2020, 15, 932–948. [Google Scholar] [CrossRef] [PubMed]
- Hake, S.B.; Garcia, B.A.; Kauer, M.; Baker, S.P.; Shabanowitz, J.; Hunt, D.F.; Allis, C.D. Serine 31 Phosphorylation of Histone Variant H3.3 Is Specific to Regions Bordering Centromeres in Metaphase Chromosomes. Proc. Natl. Acad. Sci. USA 2005, 102, 6344–6349. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Henikoff, S. The Histone Variant H3.3 Marks Active Chromatin by Replication-Independent Nucleosome Assembly. Mol. Cell 2002, 9, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Liu, Y.; Geng, F.; Daman, A.W.; Liu, X.; Zhong, L.; Ravishankar, A.; Lis, R.; Barcia Durán, J.G.; Itkin, T.; et al. Histone Variant H3.3 Maintains Adult Haematopoietic Stem Cell Homeostasis by Enforcing Chromatin Adaptability. Nat. Cell Biol. 2022, 24, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Rando, O.J. Combinatorial Complexity in Chromatin Structure and Function: Revisiting the Histone Code. Curr. Opin. Genet. Dev. 2012, 22, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Rao, C.M. Epigenetic Tools (The Writers, The Readers and The Erasers) and Their Implications in Cancer Therapy. Eur. J. Pharmacol. 2018, 837, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.M. Epigenetic Responses to Environmental Change and Their Evolutionary Implications. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3403–3418. [Google Scholar] [CrossRef] [PubMed]
- Tagami, H.; Ray-Gallet, D.; Almouzni, G.; Nakatani, Y. Histone H3.1 and H3.3 Complexes Mediate Nucleosome Assembly Pathways Dependent or Independent of DNA Synthesis. Cell 2004, 116, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Lucia, M.S.; Hansen, K.C.; Tyler, J.K. CBP/P300-Mediated Acetylation of Histone H3 on Lysine 56. Nature 2009, 459, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Long, H.; Yu, J.; Dong, L.; Wassef, M.; Zhuo, B.; Li, X.; Zhao, J.; Wang, M.; Liu, C.; et al. Histone Variants H2A.Z and H3.3 Coordinately Regulate PRC2-Dependent H3K27me3 Deposition and Gene Expression Regulation in MES Cells. BMC Biol. 2018, 16, 107. [Google Scholar] [CrossRef]
- D’Alessio, A.C.; Weaver, I.C.G.; Szyf, M. Acetylation-Induced Transcription Is Required for Active DNA Demethylation in Methylation-Silenced Genes. Mol. Cell Biol. 2007, 27, 7462–7474. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Bonora, G.; Pellegrini, M. Interactions between Core Histone Marks and DNA Methyltransferases Predict DNA Methylation Patterns Observed in Human Cells and Tissues. Epigenetics 2020, 15, 272–282. [Google Scholar] [CrossRef]
- Mangiavacchi, A.; Morelli, G.; Orlando, V. Behind the Scenes: How RNA Orchestrates the Epigenetic Regulation of Gene Expression. Front. Cell Dev. Biol. 2023, 11, 1123975. [Google Scholar] [CrossRef] [PubMed]
- Guttman, M.; Rinn, J.L. Modular Regulatory Principles of Large Non-Coding RNAs. Nature 2012, 482, 339–346. [Google Scholar] [CrossRef]
- Marchese, F.P.; Huarte, M. Long Non-Coding RNAs and Chromatin Modifiers. Epigenetics 2014, 9, 21–26. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Epidermal Homeostasis: A Balancing Act of Stem Cells in the Skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217. [Google Scholar] [CrossRef]
- Wagner, R.N.; Piñón Hofbauer, J.; Wally, V.; Kofler, B.; Schmuth, M.; De Rosa, L.; De Luca, M.; Bauer, J.W. Epigenetic and Metabolic Regulation of Epidermal Homeostasis. Exp. Dermatol. 2021, 30, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S. THE EPIDERMAL PROLIFERATIVE UNIT: THE POSSIBLE ROLE OF THE CENTRAL BASAL CELL. Cell Prolif. 1974, 7, 77–88. [Google Scholar] [CrossRef]
- Potten, C.S.; Loeffler, M. Stem Cells: Attributes, Cycles, Spirals, Pitfalls and Uncertainties. Lessons for and from the Crypt. Development 1990, 110, 1001–1020. [Google Scholar] [CrossRef] [PubMed]
- Mascré, G.; Dekoninck, S.; Drogat, B.; Youssef, K.K.; Brohée, S.; Sotiropoulou, P.A.; Simons, B.D.; Blanpain, C. Distinct Contribution of Stem and Progenitor Cells to Epidermal Maintenance. Nature 2012, 489, 257–262. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, E.A.; Till, J.E. Perspectives on the Properties of Stem Cells. Nat. Med. 2005, 11, 1026–1028. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Mastrogiacomo, M. Transit Amplifying Cells (TACs): A Still Not Fully Understood Cell Population. Front. Bioeng. Biotechnol. 2023, 11, 1189225. [Google Scholar] [CrossRef] [PubMed]
- Moltrasio, C.; Romagnuolo, M.; Marzano, A.V. Epigenetic Mechanisms of Epidermal Differentiation. Int. J. Mol. Sci. 2022, 23, 4874. [Google Scholar] [CrossRef] [PubMed]
- Sen, G.L.; Reuter, J.A.; Webster, D.E.; Zhu, L.; Khavari, P.A. DNMT1 Maintains Progenitor Function in Self-Renewing Somatic Tissue. Nature 2010, 463, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.; Beerman, I.; Lien, W.-H.; Smith, Z.D.; Gu, H.; Boyle, P.; Gnirke, A.; Fuchs, E.; Rossi, D.J.; Meissner, A. DNA Methylation Dynamics during In Vivo Differentiation of Blood and Skin Stem Cells. Mol. Cell 2012, 47, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Perdigoto, C.N.; Valdes, V.J.; Bardot, E.S.; Ezhkova, E. Epigenetic Regulation of Epidermal Differentiation. Cold Spring Harb. Perspect. Med. 2014, 4, a015263. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Datta, D.; Serrat, J.; Morey, L.; Solanas, G.; Avgustinova, A.; Blanco, E.; Pons, J.I.; Matallanas, D.; Von Kriegsheim, A.; et al. Dnmt3a and Dnmt3b Associate with Enhancers to Regulate Human Epidermal Stem Cell Homeostasis. Cell Stem. Cell 2016, 19, 491–501. [Google Scholar] [CrossRef]
- Watt, F.M. The Stem Cell Compartment in Human Interfollicular Epidermis. J. Dermatol. Sci. 2002, 28, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.L.; Patel, D.M.; Green, K.J. Deconstructing the Skin: Cytoarchitectural Determinants of Epidermal Morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Divo, M.; Langbein, L. The Human Keratins: Biology and Pathology. Histochem. Cell Biol. 2008, 129, 705. [Google Scholar] [CrossRef]
- Oh, I.Y.; de Guzman Strong, C. The Molecular Revolution in Cutaneous Biology: EDC and Locus Control. J. Investig. Dermatol. 2017, 137, e101–e104. [Google Scholar] [CrossRef] [PubMed]
- Kypriotou, M.; Huber, M.; Hohl, D. The Human Epidermal Differentiation Complex: Cornified Envelope Precursors, S100 Proteins and the ‘Fused Genes’ Family. Exp. Dermatol. 2012, 21, 643–649. [Google Scholar] [CrossRef]
- Gdula, M.R.; Poterlowicz, K.; Mardaryev, A.N.; Sharov, A.A.; Peng, Y.; Fessing, M.Y.; Botchkarev, V.A. Remodeling of Three-Dimensional Organization of the Nucleus during Terminal Keratinocyte Differentiation in the Epidermis. J. Investig. Dermatol. 2013, 133, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Jiang, K.; Hope, E.; Cross, M.; Overmiller, A.; Naz, F.; Worrell, S.; Bajpai, D.; Hasneen, K.; Brooks, S.R.; et al. Chromatin Landscape Governing Murine Epidermal Differentiation. J. Investig. Dermatol. 2023, 143, 1220–1232.e9. [Google Scholar] [CrossRef] [PubMed]
- Andl, T.; Murchison, E.P.; Liu, F.; Zhang, Y.; Yunta-Gonzalez, M.; Tobias, J.W.; Andl, C.D.; Seykora, J.T.; Hannon, G.J.; Millar, S.E. The MiRNA-Processing Enzyme Dicer Is Essential for the Morphogenesis and Maintenance of Hair Follicles. Curr. Biol. 2006, 16, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; O’Carroll, D.; Pasolli, H.A.; Zhang, Z.; Dietrich, F.S.; Tarakhovsky, A.; Fuchs, E. Morphogenesis in Skin Is Governed by Discrete Sets of Differentially Expressed MicroRNAs. Nat. Genet. 2006, 38, 356–362. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef]
- Zhang, Z.; Shu, L.; Hu, M.; Yang, F.; Zhou, X.-H. Emerging Role of LncRNA DANCR in Progenitor Cells: Beyond Cancer. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1399–1409. [Google Scholar]
- Kretz, M.; Siprashvili, Z.; Chu, C.; Webster, D.E.; Zehnder, A.; Qu, K.; Lee, C.S.; Flockhart, R.J.; Groff, A.F.; Chow, J.; et al. Control of Somatic Tissue Differentiation by the Long Non-Coding RNA TINCR. Nature 2013, 493, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Tanis, S.E.J.; Köksal, E.S.; van Buggenum, J.A.G.L.; Mulder, K.W. BLNCR Is a Long Non-Coding RNA Adjacent to Integrin Beta-1 That Is Rapidly Lost during Epidermal Progenitor Cell Differentiation. Sci. Rep. 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.; Graf, J.; Faderl, S.; Schedlbauer, J.; Strieder, N.; Förstl, B.; Spang, R.; Bruckmann, A.; Merkl, R.; Hombach, S.; et al. The Long Non-coding RNA LINC00941 and SPRR5 Are Novel Regulators of Human Epidermal Homeostasis. EMBO Rep. 2019, 20, e46612. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Tárraga, C.; Jiménez-Conde, J.; Roquer, J. Epigenetics and Aging. In Handbook of Nutrition, Diet, and Epigenetics; Springer International Publishing: Cham, Switzerland, 2019; pp. 1413–1433. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular Senescence: From Physiology to Pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Clatici, V.G.; Racoceanu, D.; Dalle, C.; Voicu, C.; Tomas-Aragones, L.; Marron, S.E.; Wollina, U.; Fica, S. Perceived Age and Life Style. The Specific Contributions of Seven Factors Involved in Health and Beauty. Maedica 2017, 12, 191–201. [Google Scholar] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin Anti-Aging Strategies. Dermatoendocrinol 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and Extrinsic Factors in Skin Ageing: A Review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Michalak, E.M.; Burr, M.L.; Bannister, A.J.; Dawson, M.A. The Roles of DNA, RNA and Histone Methylation in Ageing and Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 573–589. [Google Scholar] [CrossRef]
- Field, A.E.; Robertson, N.A.; Wang, T.; Havas, A.; Ideker, T.; Adams, P.D. DNA Methylation Clocks in Aging: Categories, Causes, and Consequences. Mol. Cell 2018, 71, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Peng, Q.; Jiang, X.; Tan, S.; Yang, Y.; Yang, W.; Han, Y.; Chen, Y.; Oyang, L.; Lin, J.; et al. Metabolic Reprogramming and Epigenetic Modifications in Cancer: From the Impacts and Mechanisms to the Treatment Potential. Exp. Mol. Med. 2023, 55, 1357–1370. [Google Scholar] [CrossRef]
- Johnson, A.A.; Akman, K.; Calimport, S.R.G.; Wuttke, D.; Stolzing, A.; de Magalhães, J.P. The Role of DNA Methylation in Aging, Rejuvenation, and Age-Related Disease. Rejuvenation Res. 2012, 15, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Köhler, F.; Rodríguez-Paredes, M. DNA Methylation in Epidermal Differentiation, Aging, and Cancer. J. Investig. Dermatol. 2020, 140, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kapetanaki, M.G.; Mora, A.L.; Rojas, M. Influence of Age on Wound Healing and Fibrosis. J. Pathol. 2013, 229, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, N.E.; DePinho, R.A. How Stem Cells Age and Why This Makes Us Grow Old. Nat. Rev. Mol. Cell Biol. 2007, 8, 703–713. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, D.; Tinaburri, L.; Dellambra, E. The Role of P16INK4a Pathway in Human Epidermal Stem Cell Self-Renewal, Aging and Cancer. Int. J. Mol. Sci. 2017, 18, 1591. [Google Scholar] [CrossRef]
- Ressler, S.; Bartkova, J.; Niederegger, H.; Bartek, J.; Scharffetter-Kochanek, K.; Jansen-Dürr, P.; Wlaschek, M. P16 INK4A Is a Robust In Vivo Biomarker of Cellular Aging in Human Skin. Aging Cell 2006, 5, 379–389. [Google Scholar] [CrossRef]
- Safwan-Zaiter, H.; Wagner, N.; Wagner, K.-D. P16INK4A—More Than a Senescence Marker. Life 2022, 12, 1332. [Google Scholar] [CrossRef]
- Shibata, K.R.; Aoyama, T.; Shima, Y.; Fukiage, K.; Otsuka, S.; Furu, M.; Kohno, Y.; Ito, K.; Fujibayashi, S.; Neo, M.; et al. Expression of the P16INK4A Gene Is Associated Closely with Senescence of Human Mesenchymal Stem Cells and Is Potentially Silenced by DNA Methylation During In Vitro Expansion. Stem. Cells 2007, 25, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Giovannini, S.; Wang, T.; Fang, J.; Li, P.; Shao, C.; Wang, Y.; Agostini, M.; Bove, P.; Mauriello, A.; et al. P63: A Crucial Player in Epithelial Stemness Regulation. Oncogene 2023, 42, 3371–3384. [Google Scholar] [CrossRef]
- Ishimi, Y.; Kojima, M.; Takeuchi, F.; Miyamoto, T.; Yamada, M.-A.; Hanaoka, F. Changes in Chromatin Structure during Aging of Human Skin Fibroblasts. Exp. Cell Res. 1987, 169, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Liu, Y.; Du, R.; Wang, B.; Chen, M.; Zhang, Y.; Deng, Z.; Li, J. MiR-377 Induces Senescence in Human Skin Fibroblasts by Targeting DNA Methyltransferase 1. Cell Death Dis. 2017, 8, e2663. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, L.D.; Xu, Z.; Lin, S.; Wang, J.; Wang, Y.; Enechojo, O.S.; Abankwah, J.K.; Peng, Y.; Chu, X.; Zhou, H.; et al. Role of Epigenetics in the Regulation of Skin Aging and Geroprotective Intervention: A New Sight. Biomed. Pharmacother. 2024, 174, 116592. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Orioli, D.; Dellambra, E. Epigenetic Regulation of Skin Cells in Natural Aging and Premature Aging Diseases. Cells 2018, 7, 268. [Google Scholar] [CrossRef]
- Lago, J.C.; Puzzi, M.B. The Effect of Aging in Primary Human Dermal Fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking Older. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Raj, K. DNA Methylation-Based Biomarkers and the Epigenetic Clock Theory of Ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Boroni, M.; Zonari, A.; Reis de Oliveira, C.; Alkatib, K.; Ochoa Cruz, E.A.; Brace, L.E.; Lott de Carvalho, J. Highly Accurate Skin-Specific Methylome Analysis Algorithm as a Platform to Screen and Validate Therapeutics for Healthy Aging. Clin. Epigenetics 2020, 12, 105. [Google Scholar] [CrossRef]
- Talbert, P.B.; Henikoff, S. Transcribing Centromeres: Noncoding RNAs and Kinetochore Assembly. Trends Genet. 2018, 34, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Contrepois, K.; Coudereau, C.; Benayoun, B.A.; Schuler, N.; Roux, P.-F.; Bischof, O.; Courbeyrette, R.; Carvalho, C.; Thuret, J.-Y.; Ma, Z.; et al. Histone Variant H2A.J Accumulates in Senescent Cells and Promotes Inflammatory Gene Expression. Nat. Commun. 2017, 8, 14995. [Google Scholar] [CrossRef] [PubMed]
- Rübe, C.E.; Bäumert, C.; Schuler, N.; Isermann, A.; Schmal, Z.; Glanemann, M.; Mann, C.; Scherthan, H. Human Skin Aging Is Associated with Increased Expression of the Histone Variant H2A.J in the Epidermis. NPJ Aging Mech. Dis. 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.N.; Park, Y.S.; Kim, N.H.; Han, J.W.; Lee, H.Y.; Kim, Y.K. HDAC Inhibitors Downregulate MRP2 Expression in Multidrug Resistant Cancer Cells: Implication for Chemosensitization. Int. J. Oncol. 2011, 38, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Cabanel, M.; da Costa, T.P.; EL-Cheikh, M.C.; Carneiro, K. The Epigenome as a Putative Target for Skin Repair: The HDAC Inhibitor Trichostatin A Modulates Myeloid Progenitor Plasticity and Behavior and Improves Wound Healing. J. Transl. Med. 2019, 17, 247. [Google Scholar] [CrossRef] [PubMed]
- Spallotta, F.; Cencioni, C.; Straino, S.; Sbardella, G.; Castellano, S.; Capogrossi, M.C.; Martelli, F.; Gaetano, C. Enhancement of Lysine Acetylation Accelerates Wound Repair. Commun. Integr. Biol. 2013, 6, e25466. [Google Scholar] [CrossRef] [PubMed]
- Gerasymchuk, M.; Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. The Role of MicroRNAs in Organismal and Skin Aging. Int. J. Mol. Sci. 2020, 21, 5281. [Google Scholar] [CrossRef]
- Shin, J.-S.; Hong, A.; Solomon, M.J.; Soon Lee, C. The Role of Telomeres and Telomerase in the Pathology of Human Cancer and Aging. Pathology 2006, 38, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Antonini, D.; Russo, M.T.; De Rosa, L.; Gorrese, M.; Del Vecchio, L.; Missero, C. Transcriptional Repression of MiR-34 Family Contributes to P63-Mediated Cell Cycle Progression in Epidermal Cells. J. Investig. Dermatol. 2010, 130, 1249–1257. [Google Scholar] [CrossRef]
- Bhaumik, D.; Scott, G.K.; Schokrpur, S.; Patil, C.K.; Orjalo, A.V.; Rodier, F.; Lithgow, G.J.; Campisi, J. MicroRNAs MiR-146a/b Negatively Modulate the Senescence-Associated Inflammatory Mediators IL-6 and IL-8. Aging 2009, 1, 402–411. [Google Scholar] [CrossRef]
- Indrieri, A.; Carrella, S.; Romano, A.; Spaziano, A.; Marrocco, E.; Fernandez-Vizarra, E.; Barbato, S.; Pizzo, M.; Ezhova, Y.; Golia, F.M.; et al. MiR-181a/b Downregulation Exerts a Protective Action on Mitochondrial Disease Models. EMBO Mol. Med. 2019, 11, e8734. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, D.; Xiao, Z.; Zhang, M. MiRNA Expression Profiles in Keloid Tissue and Corresponding Normal Skin Tissue. Aesthetic Plast. Surg. 2012, 36, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. LncRNA-MiRNA-MRNA Regulatory Networks in Skin Aging and Therapeutic Potentials. Front. Physiol. 2023, 14, 1303151. [Google Scholar] [CrossRef] [PubMed]
- Puvvula, P.K. LncRNAs Regulatory Networks in Cellular Senescence. Int. J. Mol. Sci. 2019, 20, 2615. [Google Scholar] [CrossRef]
- Zhang, A.; Xu, M.; Mo, Y.-Y. Role of the LncRNA-P53 Regulatory Network in Cancer. J. Mol. Cell Biol. 2014, 6, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Breunig, S.; Wallner, V.; Kobler, K.; Wimmer, H.; Steinbacher, P.; Streubel, M.K.; Bischof, J.; Duschl, J.; Neuhofer, C.; Gruber, W.; et al. The Life in a Gradient: Calcium, the LncRNA SPRR2C and Mir542/Mir196a Meet in the Epidermis to Regulate the Aging Process. Aging 2021, 13, 19127–19144. [Google Scholar] [CrossRef]
- Chen, L.; Yang, H.; Yi, Z.; Jiang, L.; Li, Y.; Han, Q.; Yang, Y.; Zhang, Q.; Yang, Z.; Kuang, Y.; et al. LncRNA GAS5 Regulates Redox Balance and Dysregulates the Cell Cycle and Apoptosis in Malignant Melanoma Cells. J. Cancer Res. Clin. Oncol. 2019, 145, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Chebly, A.; Ropio, J.; Baldasseroni, L.; Prochazkova-Carlotti, M.; Idrissi, Y.; Ferrer, J.; Farra, C.; Beylot-Barry, M.; Merlio, J.-P.; Chevret, E. Telomeric Repeat-Containing RNA (TERRA): A Review of the Literature and First Assessment in Cutaneous T-Cell Lymphomas. Genes 2022, 13, 539. [Google Scholar] [CrossRef]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The Impact of Ultraviolet Radiation on Skin Photoaging—Review of in Vitro Studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef] [PubMed]
- García-Guede, Á.; Vera, O.; Ibáñez-de-Caceres, I. When Oxidative Stress Meets Epigenetics: Implications in Cancer Development. Antioxidants 2020, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The Role of Cellular Senescence in Skin Aging and Age-Related Skin Pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, Q.; Javed, R.; Zhong, J.; Gao, H.; Liang, H. Effect of Tobacco Smoking on the Epigenetic Age of Human Respiratory Organs. Clin. Epigenetics 2019, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Martic, I.; Jansen-Dürr, P.; Cavinato, M. Effects of Air Pollution on Cellular Senescence and Skin Aging. Cells 2022, 11, 2220. [Google Scholar] [CrossRef]
- Prunicki, M.; Cauwenberghs, N.; Lee, J.; Zhou, X.; Movassagh, H.; Noth, E.; Lurmann, F.; Hammond, S.K.; Balmes, J.R.; Desai, M.; et al. Air Pollution Exposure Is Linked with Methylation of Immunoregulatory Genes, Altered Immune Cell Profiles, and Increased Blood Pressure in Children. Sci. Rep. 2021, 11, 4067. [Google Scholar] [CrossRef] [PubMed]
- Fussell, J.C.; Kelly, F.J. Oxidative Contribution of Air Pollution to Extrinsic Skin Ageing. Free Radic. Biol. Med. 2020, 151, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Li, Z.; Su, J. Air Pollution and Skin Diseases: A Comprehensive Evaluation of the Associated Mechanism. Ecotoxicol. Environ. Saf. 2024, 278, 116429. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Szabó, K.; Bolla, B.S.; Erdei, L.; Balogh, F.; Kemény, L. Are the Cutaneous Microbiota a Guardian of the Skin’s Physical Barrier? The Intricate Relationship between Skin Microbes and Barrier Integrity. Int. J. Mol. Sci. 2023, 24, 15962. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.A.; Zhang, L.-J.; Williams, M.R.; Gangoiti, J.A.; Huang, C.-M.; Gallo, R.L. Inhibition of HDAC8 and HDAC9 by Microbial Short-Chain Fatty Acids Breaks Immune Tolerance of the Epidermis to TLR Ligands. Sci. Immunol. 2016, 1, eaah4609. [Google Scholar] [CrossRef]
- Lin, X.; Han, H.; Wang, N.; Wang, C.; Qi, M.; Wang, J.; Liu, G. The Gut Microbial Regulation of Epigenetic Modification from a Metabolic Perspective. Int. J. Mol. Sci. 2024, 25, 7175. [Google Scholar] [CrossRef] [PubMed]
- Woo, V.; Alenghat, T. Epigenetic Regulation by Gut Microbiota. Gut Microbes 2022, 14, 2022407. [Google Scholar] [CrossRef]
- Pepke, M.L.; Hansen, S.B.; Limborg, M.T. Unraveling Host Regulation of Gut Microbiota through the Epigenome–Microbiome Axis. Trends Microbiol. 2024, 32, 1229–1240. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; González-Fernández, C.; Greses, S.; Kennes, C.; Otero-Logilde, N.; Veiga, M.C.; Bolzonella, D.; Müller, B.; Passoth, V. Production of Short-Chain Fatty Acids (SCFAs) as Chemicals or Substrates for Microbes to Obtain Biochemicals. Biotechnol. Biofuels Bioprod. 2023, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.I.; Kapila, R. Dietary Metabolites Derived from Gut Microbiota: Critical Modulators of Epigenetic Changes in Mammals. Nutr. Rev. 2017, 75, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.P.; Denu, J.M. Short-Chain Fatty Acids Activate Acetyltransferase P300. Elife 2021, 10, e72171. [Google Scholar] [CrossRef]
- Sealy, L. The Effect of Sodium Butyrate on Histone Modification. Cell 1978, 14, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Waldecker, M.; Kautenburger, T.; Daumann, H.; Busch, C.; Schrenk, D. Inhibition of Histone-Deacetylase Activity by Short-Chain Fatty Acids and Some Polyphenol Metabolites Formed in the Colon. J. Nutr. Biochem. 2008, 19, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Tax, G.; Urbán, E.; Palotás, Z.; Puskás, R.; Kónya, Z.; Bíró, T.; Kemény, L.; Szabó, K. Propionic Acid Produced by Propionibacterium Acnes Strains Contributes to Their Pathogenicity. Acta Derm. Venereol. 2016, 96, 43–49. [Google Scholar] [CrossRef]
- Chang, C.-S.; Kao, C.-Y. Current Understanding of the Gut Microbiota Shaping Mechanisms. J. Biomed. Sci. 2019, 26, 59. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.A.; O’Neill, A.M.; Zouboulis, C.C.; Gallo, R.L. Short-Chain Fatty Acids from Cutibacterium acnes Activate Both a Canonical and Epigenetic Inflammatory Response in Human Sebocytes. J. Immunol. 2019, 202, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- McCarville, J.L.; Chen, G.Y.; Cuevas, V.D.; Troha, K.; Ayres, J.S. Microbiota Metabolites in Health and Disease. Annu. Rev. Immunol. 2020, 38, 147–170. [Google Scholar] [CrossRef]
- Miro-Blanch, J.; Yanes, O. Epigenetic Regulation at the Interplay Between Gut Microbiota and Host Metabolism. Front. Genet. 2019, 10, 638. [Google Scholar] [CrossRef] [PubMed]
- Erdei, L.; Bolla, B.S.; Bozó, R.; Tax, G.; Urbán, E.; Kemény, L.; Szabó, K. TNIP1 Regulates Cutibacterium Acnes-Induced Innate Immune Functions in Epidermal Keratinocytes. Front. Immunol. 2018, 9, 2155. [Google Scholar] [CrossRef]
- Erdei, L.; Bolla, B.S.; Bozó, R.; Tax, G.; Urbán, E.; Burián, K.; Kemény, L.; Szabó, K. Tumour Necrosis Factor Alpha-Induced Protein 3 Negatively Regulates Cutibacterium Acnes-Induced Innate Immune Events in Epidermal KeratinocyteseEr. Acta Derm. Venereol. 2021, 13, 1470. [Google Scholar]
- Bolla, B.S.; Erdei, L.; Urbán, E.; Burián, K.; Kemény, L.; Szabó, K. Cutibacterium Acnes Regulates the Epidermal Barrier Properties of HPV-KER Human Immortalized Keratinocyte Cultures. Sci. Rep. 2020, 10, 12815. [Google Scholar] [CrossRef]
- Megyeri, K.; Orosz, L.; Bolla, S.; Erdei, L.; Rázga, Z.; Seprényi, G.; Urbán, E.; Szabó, K.; Kemény, L. Propionibacterium Acnes Induces Autophagy in Keratinocytes: Involvement of Multiple Mechanisms. J. Investig. Dermatol. 2018, 138, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Xu, H.; Liu, Y.; Du, L.; Duan, Z.; Tong, J.; He, Y.; Chen, Q.; Chen, X.; Li, M. MiR-146a Inhibits Biofilm-Derived Cutibacterium Acnes–Induced Inflammatory Reactions in Human Keratinocytes. J. Investig. Dermatol. 2019, 139, 2488–2496.e4. [Google Scholar] [CrossRef]
- Dull, K.; Fazekas, F.; Deák, D.; Kovács, D.; Póliska, S.; Szegedi, A.; Zouboulis, C.C.; Törőcsik, D. MiR-146a Modulates TLR1/2 and 4 Induced Inflammation and Links It with Proliferation and Lipid Production via the Indirect Regulation of GNG7 in Human SZ95 Sebocytes. Sci. Rep. 2021, 11, 21510. [Google Scholar] [CrossRef]
- Xia, X.; Li, Z.; Liu, K.; Wu, Y.; Jiang, D.; Lai, Y. Staphylococcal LTA-Induced MiR-143 Inhibits Propionibacterium Acnes-Mediated Inflammatory Response in Skin. J. Investig. Dermatol. 2016, 136, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial Dysbiosis in the Gut Drives Systemic Autoimmune Diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Microbial Dysbiosis in the Skin Microbiome and Its Psychological Consequences. Microorganisms 2024, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, A.; Dajnoki, Z.; Bíró, T.; Kemény, L.; Törőcsik, D. Acne: Transient Arrest in the Homeostatic Host–Microbiota Dialog? Trends Immunol. 2019, 40, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Ghumra, W.; Lee, N.; Whitehouse, H.; Bhutani, R.; Lagos, D.; Layton, A.M. MicroRNAs as Biomarkers of Atrophic Scarring in Acne: A Cross-sectional Analysis of 41 Patients. Clin. Exp. Dermatol. 2021, 46, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Larsen, S.B.; Gomez, N.C.; Alaverdyan, K.; Sendoel, A.; Yuan, S.; Polak, L.; Kulukian, A.; Chai, S.; Fuchs, E. Inflammatory Memory Sensitizes Skin Epithelial Stem Cells to Tissue Damage. Nature 2017, 550, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Blok, B.A.; Joosten, L.A.B.; Riksen, N.P.; van Crevel, R.; Netea, M.G. In Vitro Experimental Model of Trained Innate Immunity in Human Primary Monocytes. Clin. Vaccine Immunol. 2016, 23, 926–933. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, C.D.C.C.; Noz, M.P.; Joosten, L.A.B.; Netea, M.G.; Riksen, N.P.; Keating, S.T. Epigenetics and Trained Immunity. Antioxid. Redox Signal 2018, 29, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Fanucchi, S.; Domínguez-Andrés, J.; Joosten, L.A.B.; Netea, M.G.; Mhlanga, M.M. The Intersection of Epigenetics and Metabolism in Trained Immunity. Immunity 2021, 54, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarinia, A.; Ayaydin, F.; Póliska, S.; Manczinger, M.; Bolla, B.S.; Flink, L.B.; Balogh, F.; Veréb, Z.; Bozó, R.; Szabó, K.; et al. Psoriatic Resolved Skin Epidermal Keratinocytes Retain Disease-Residual Transcriptomic and Epigenomic Profiles. Int. J. Mol. Sci. 2023, 24, 4556. [Google Scholar] [CrossRef]
- Shibagaki, N.; Suda, W.; Clavaud, C.; Bastien, P.; Takayasu, L.; Iioka, E.; Kurokawa, R.; Yamashita, N.; Hattori, Y.; Shindo, C.; et al. Aging-Related Changes in the Diversity of Women’s Skin Microbiomes Associated with Oral Bacteria. Sci. Rep. 2017, 7, 10567. [Google Scholar] [CrossRef] [PubMed]
- Jugé, R.; Rouaud-Tinguely, P.; Breugnot, J.; Servaes, K.; Grimaldi, C.; Roth, M.-P.; Coppin, H.; Closs, B. Shift in Skin Microbiota of Western European Women across Aging. J. Appl. Microbiol. 2018, 125, 907–916. [Google Scholar] [CrossRef]
- Howard, E.J.; Lam, T.K.T.; Duca, F.A. The Gut Microbiome: Connecting Diet, Glucose Homeostasis, and Disease. Annu. Rev. Med. 2022, 73, 469–481. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary Supplementation with Inulin-Propionate Ester or Inulin Improves Insulin Sensitivity in Adults with Overweight and Obesity with Distinct Effects on the Gut Microbiota, Plasma Metabolome and Systemic Inflammatory Responses: A Randomised Cross-over Trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Jones, E.A.; Popova, L.; Kwinn, L.; Haynes, L.M.; Jones, L.P.; Tripp, R.A.; Walsh, E.E.; Freeman, M.W.; Golenbock, D.T.; Anderson, L.J.; et al. Pattern Recognition Receptors TLR4 and CD14 Mediate Response to Respiratory Syncytial Virus. Nat. Immunol. 2000, 1, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, U.T.; Kalan, L.R. Forgotten Fungi: The Importance of the Skin Mycobiome. Curr. Opin. Microbiol. 2022, 70, 102235. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, G.D.; Meisel, J.S.; Tyldsley, A.S.; Zheng, Q.; Hodkinson, B.P.; SanMiguel, A.J.; Minot, S.; Bushman, F.D.; Grice, E.A. The Human Skin Double-Stranded DNA Virome: Topographical and Temporal Diversity, Genetic Enrichment, and Dynamic Associations with the Host Microbiome. mBio 2015, 6, 10–1128. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Bacteriophages and the Microbiome in Dermatology: The Role of the Phageome and a Potential Therapeutic Strategy. Int. J. Mol. Sci. 2023, 24, 2695. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, K.; Balogh, F.; Romhányi, D.; Erdei, L.; Toldi, B.; Gyulai, R.; Kemény, L.; Groma, G. Epigenetic Regulatory Processes Involved in the Establishment and Maintenance of Skin Homeostasis—The Role of Microbiota. Int. J. Mol. Sci. 2025, 26, 438. https://doi.org/10.3390/ijms26020438
Szabó K, Balogh F, Romhányi D, Erdei L, Toldi B, Gyulai R, Kemény L, Groma G. Epigenetic Regulatory Processes Involved in the Establishment and Maintenance of Skin Homeostasis—The Role of Microbiota. International Journal of Molecular Sciences. 2025; 26(2):438. https://doi.org/10.3390/ijms26020438
Chicago/Turabian StyleSzabó, Kornélia, Fanni Balogh, Dóra Romhányi, Lilla Erdei, Blanka Toldi, Rolland Gyulai, Lajos Kemény, and Gergely Groma. 2025. "Epigenetic Regulatory Processes Involved in the Establishment and Maintenance of Skin Homeostasis—The Role of Microbiota" International Journal of Molecular Sciences 26, no. 2: 438. https://doi.org/10.3390/ijms26020438
APA StyleSzabó, K., Balogh, F., Romhányi, D., Erdei, L., Toldi, B., Gyulai, R., Kemény, L., & Groma, G. (2025). Epigenetic Regulatory Processes Involved in the Establishment and Maintenance of Skin Homeostasis—The Role of Microbiota. International Journal of Molecular Sciences, 26(2), 438. https://doi.org/10.3390/ijms26020438







