Combination of HSP90 Inhibitors and HSP70 Inducers Prevent Hydrochloric Acid-Induced Pulmonary Fibrosis in Rabbits
Abstract
1. Introduction
2. Results
2.1. TAS-116 and GGA Ameliorate HCl-Induced Inflammation in Bronchoalveolar Lavage Fluid
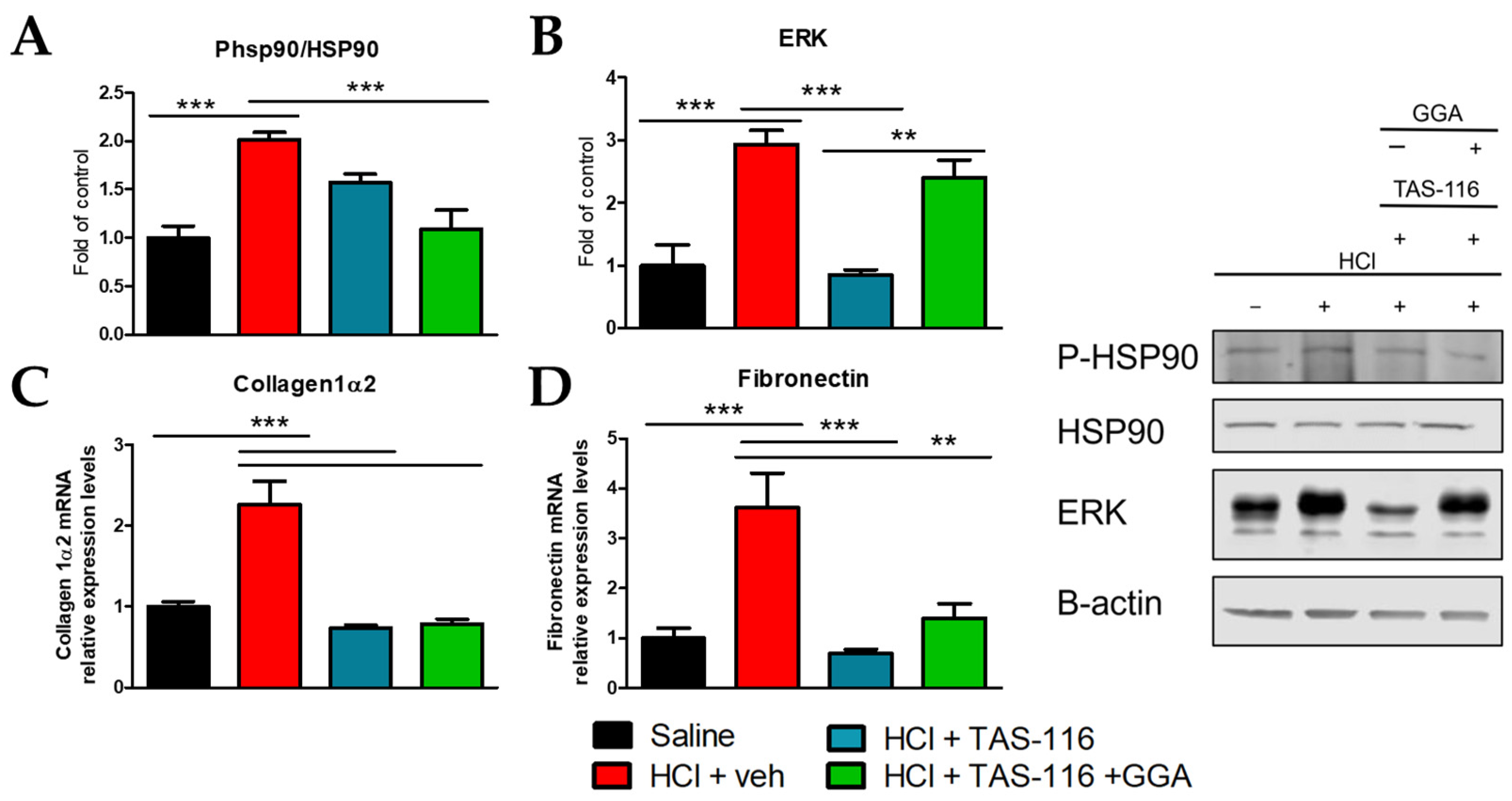
2.2. TAS-116 and GGA Ameliorate the HCl-Induced Activation of Pro-Fibrotic Markers
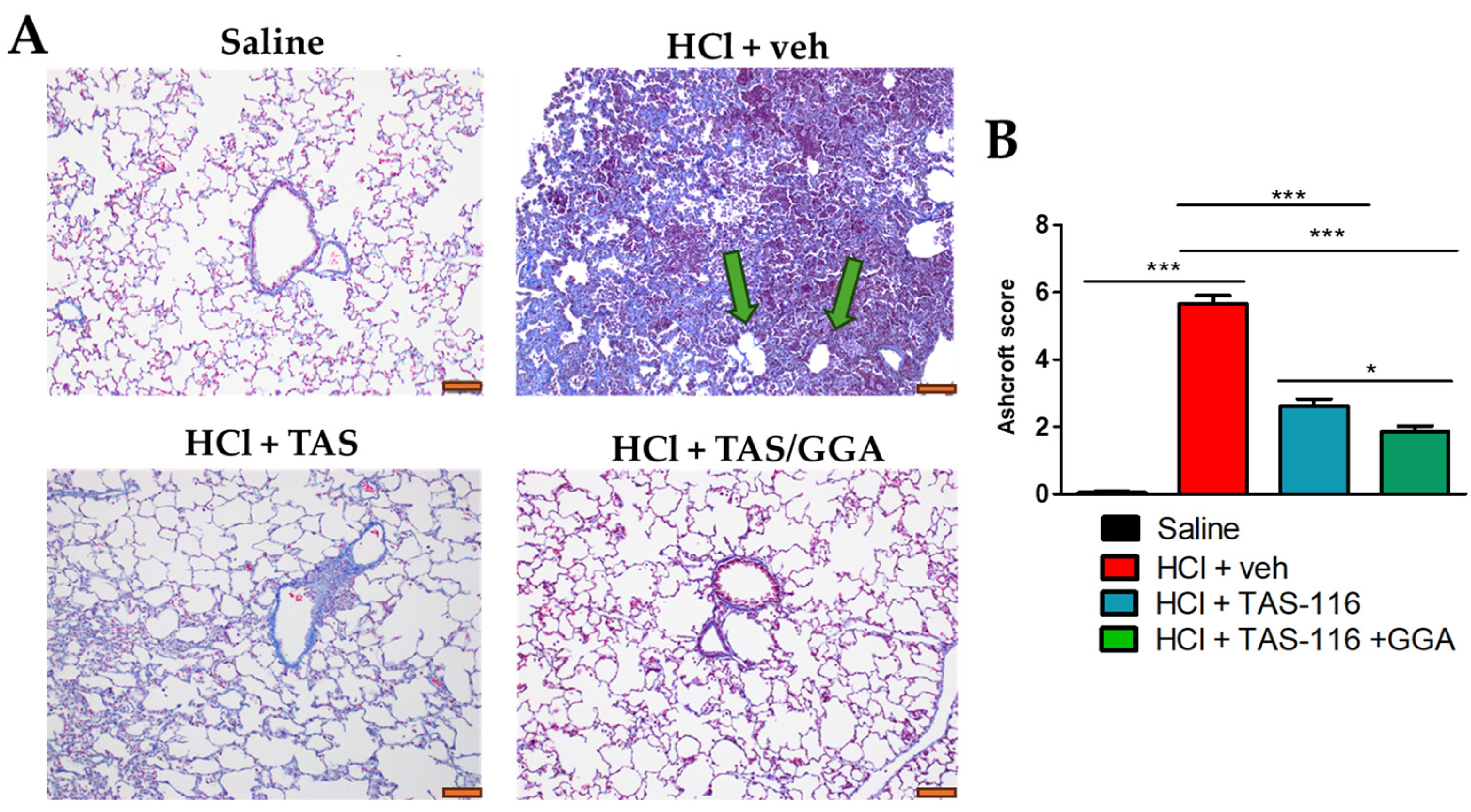
2.3. TAS-116 and GGA Prevent the Development of Pulmonary Fibrosis
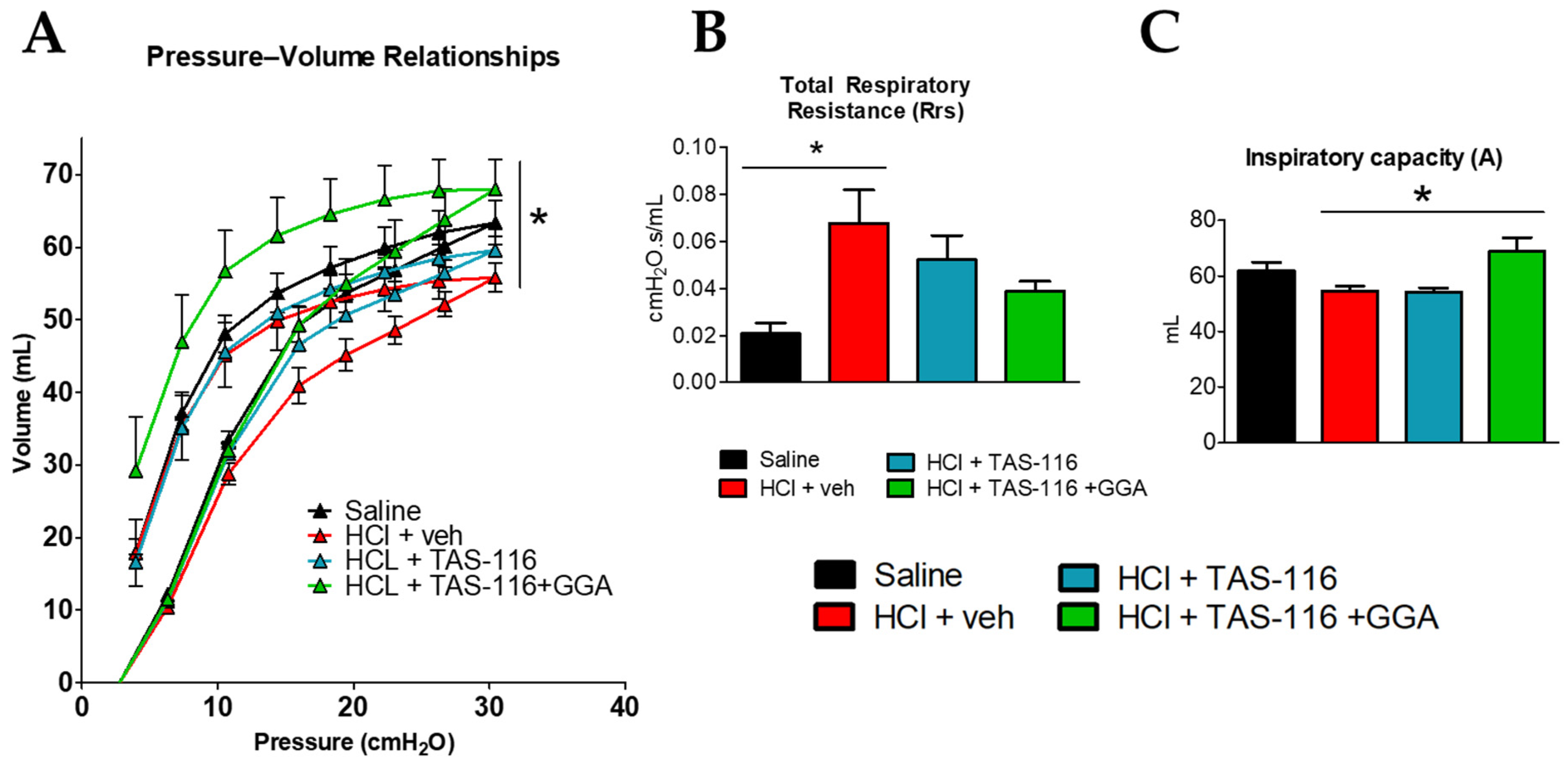
2.4. TAS-116 and GGA Improve HCl-Induced Lung Dysfunction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Ethical Statement
4.3. Animals and Treatment Groups
4.4. TAS-116 and GGA Rabbit Equivalent Dose
- For TAS-116, we calculated the human equivalent dose (HED) using a mouse-to-human conversion factor of 12.3.
- 2.
- Next, we computed the rabbit animal equivalent dose (AED) from the HED using a human-to-rabbit conversion factor of 3.1.
4.5. Bronchoalveolar Lavage Fluid (BALF)
4.6. Histopathology, Immunohistochemistry, and Lung Fibrosis Scoring
4.7. Lung Tissue Collection
4.8. Western Blot Analysis
4.9. Lung Mechanics Measurements
4.10. RNA Isolation and Quantitative Real-Time PCR (qPCR)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Genest, O.; Wickner, S.; Doyle, S.M. Hsp90 and Hsp70 chaperones: Collaborators in protein remodeling. J. Biol. Chem. 2019, 294, 2109–2120. [Google Scholar] [CrossRef]
- Evans, C.G.; Chang, L.; Gestwicki, J.E. Heat shock protein 70 (hsp70) as an emerging drug target. J. Med. Chem. 2010, 53, 4585–4602. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.S.; Hasday, J.D. Fever, hyperthermia and the heat shock response. Int. J. Hyperth. 2013, 29, 423–435. [Google Scholar] [CrossRef]
- Wheeler, D.S.; Wong, H.R. Heat shock response and acute lung injury. Free Radic. Biol. Med. 2007, 42, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Edkins, A.L.; Price, J.T.; Pockley, A.G.; Blatch, G.L. Heat shock proteins as modulators and therapeutic targets of chronic disease: An integrated perspective. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160521. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cox, I.A.; Campbell, J.A.; Xia, Q.; Otahal, P.; de Graaff, B.; Corte, T.J.; Teoh, A.K.Y.; Walters, E.H.; Palmer, A.J. Mortality and survival in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. ERJ Open Res. 2022, 8, 00591-2021. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Tonelli, R.; Murray, M.; Samarelli, A.V.; Spagnolo, P. Environmental Causes of Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2023, 24, 16481. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Ahn, C.; Kim, T.-H. Occupational and environmental risk factors of idiopathic pulmonary fibrosis: A systematic review and meta-analyses. Sci. Rep. 2021, 11, 4318. [Google Scholar] [CrossRef] [PubMed]
- Nett, R.J.; Wood, J.M.; Blackley, D.J. Collecting Occupational Exposure Data Would Strengthen Idiopathic Pulmonary Fibrosis Registries. Am. J. Respir. Crit. Care Med. 2020, 201, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Ebina, M.; Kondoh, Y.; Ogura, T.; Azuma, A.; Suga, M.; Taguchi, Y.; Takahashi, H.; Nakata, K.; Sato, A.; et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010, 35, 821. [Google Scholar] [CrossRef]
- Štefániková, M.; Doubková, M.; Ovesná, P.; Šterclová, M.; Lacina, L.; Žurková, M.; Plačková, M.; Bartoš, V.; Janíčková, I.; Bittenglová, R.; et al. The effect of nintedanib on lung functions and survival in idiopathic pulmonary fibrosis: Real-life analysis of the Czech EMPIRE registry. BMC Pulm. Med. 2023, 23, 154. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; De Los Santos, F.G.; Phan, S.H. The Bleomycin Model of Pulmonary Fibrosis. Methods Mol. Biol. 2017, 1627, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Gorguner, M.; Aslan, S.; Inandi, T.; Cakir, Z. Reactive airways dysfunction syndrome in housewives due to a bleach-hydrochloric acid mixture. Inhal. Toxicol. 2004, 16, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kamrin, M.A. Workshop on the health effects of HCl in ambient air. Regul. Toxicol. Pharmacol. 1992, 15, 73–82. [Google Scholar] [CrossRef]
- Stevens, B.; Koenig, J.Q.; Rebolledo, V.; Hanley, Q.S.; Covert, D.S. Respiratory effects from the inhalation of hydrogen chloride in young adult asthmatics. J. Occup. Med. 1992, 34, 923–929. [Google Scholar]
- Agabiti, N.; Ancona, C.; Forastiere, F.; Di Napoli, A.; Lo Presti, E.; Corbo, G.M.; D’Orsi, F.; Perucci, C.A. Short term respiratory effects of acute exposure to chlorine due to a swimming pool accident. Occup. Environ. Med. 2001, 58, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Heidari, H.; Mohammadbeigi, A.; Soltanzadeh, A.; Darabi, M.; Asadi-Ghalhari, M. Respiratory effects of occupational exposure to low concentration of hydrochloric acid among exposed workers: A case study in steel industry. Med. Gas Res. 2019, 9, 208–212. [Google Scholar] [CrossRef]
- Wiergowski, M.; Sołtyszewski, I.; Sein Anand, J.; Kaliszan, M.; Wilmanowska, J.A.; Jankowski, Z.; Łukasik, M. Difficulties in interpretation when assessing prolonged and subacute exposure to the toxic effects of chlorine. J. Forensic Leg. Med. 2018, 58, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Marinova, M.; Solopov, P.; Dimitropoulou, C.; Colunga Biancatelli, R.M.L.; Catravas, J.D. Acute exposure of mice to hydrochloric acid leads to the development of chronic lung injury and pulmonary fibrosis. Inhal. Toxicol. 2019, 31, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Solopov, P.; Colunga Biancatelli, R.M.L.; Dimitropoulou, C.; Catravas, J.D. Sex-Related Differences in Murine Models of Chemically Induced Pulmonary Fibrosis. Int. J. Mol. Sci. 2021, 22, 5909. [Google Scholar] [CrossRef] [PubMed]
- Solopov, P.; Marinova, M.; Dimitropoulou, C.; Colunga Biancatelli, R.M.L.; Catravas, J.D. Development of chronic lung injury and pulmonary fibrosis in mice following acute exposure to nitrogen mustard. Inhal. Toxicol. 2020, 32, 141–154. [Google Scholar] [CrossRef]
- Westphalen, K.; Monma, E.; Islam, M.N.; Bhattacharya, J. Acid contact in the rodent pulmonary alveolus causes proinflammatory signaling by membrane pore formation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L107–L116. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Solopov, P.; Dimitropoulou, C.; Gregory, B.; Day, T.; Catravas, J.D. The Heat Shock Protein 90 Inhibitor, AT13387, Protects the Alveolo-Capillary Barrier and Prevents HCl-Induced Chronic Lung Injury and Pulmonary Fibrosis. Cells 2022, 11, 1046. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Solopov, P.; Dimitropoulou, C.; Catravas, J.D. Age-Dependent Chronic Lung Injury and Pulmonary Fibrosis following Single Exposure to Hydrochloric Acid. Int. J. Mol. Sci. 2021, 22, 8833. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Solopov, P.; Gregory, B.; Catravas, J.D. The HSP90 Inhibitor, AUY-922, Protects and Repairs Human Lung Microvascular Endothelial Cells from Hydrochloric Acid-Induced Endothelial Barrier Dysfunction. Cells 2021, 10, 1489. [Google Scholar] [CrossRef]
- Solopov, P.A.; Colunga Biancatelli, R.M.L.; Dimitropolou, C.; Day, T.; Catravas, J.D. Optimizing antidotal treatment with the oral HSP90 inhibitor TAS-116 against hydrochloric acid-induced pulmonary fibrosis in mice. Front. Pharmacol. 2022, 13, 1034464. [Google Scholar] [CrossRef] [PubMed]
- Solopov, P.; Biancatelli, R.; Marinova, M.; Dimitropoulou, C.; Catravas, J.D. The HSP90 Inhibitor, AUY-922, Ameliorates the Development of Nitrogen Mustard-Induced Pulmonary Fibrosis and Lung Dysfunction in Mice. Int. J. Mol. Sci. 2020, 21, 4740. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Solopov, P.A.; Day, T.; Gregory, B.; Osei-Nkansah, M.; Dimitropoulou, C.; Catravas, J.D. HSP70 Is a Critical Regulator of HSP90 Inhibitor’s Effectiveness in Preventing HCl-Induced Chronic Lung Injury and Pulmonary Fibrosis. Int. J. Mol. Sci. 2024, 25, 1920. [Google Scholar] [CrossRef] [PubMed]
- Solopov, P.A.; Biancatelli, R.; Day, T.; Dimitropoulou, C.; Catravas, J.D. A novel Non-rodent animal model of hydrochloric acid-induced acute and chronic lung injury. Respir. Res. 2024, 25, 390. [Google Scholar] [CrossRef] [PubMed]
- Mapara, M.; Thomas, B.S.; Bhat, K.M. Rabbit as an animal model for experimental research. Dent. Res. J. (Isfahan) 2012, 9, 111–118. [Google Scholar] [CrossRef]
- Kamaruzaman, N.A.; Kardia, E.; Kamaldin, N.A.; Latahir, A.Z.; Yahaya, B.H. The Rabbit as a Model for Studying Lung Disease and Stem Cell Therapy. BioMed Res. Int. 2013, 2013, 691830. [Google Scholar] [CrossRef]
- Smith, M.L. The histologic diagnosis of usual interstitial pneumonia of idiopathic pulmonary fibrosis. Where we are and where we need to go. Mod. Pathol. 2022, 35, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Amigoni, M.M.D.; Bellani, G.M.D.; Scanziani, M.M.D.; Masson, S.P.D.; Bertoli, E.M.D.; Radaelli, E.D.V.M.; Patroniti, N.M.D.; Di Lelio, A.M.D.; Pesenti, A.M.D.; Latini, R.M.D. Lung Injury and Recovery in a Murine Model of Unilateral Acid Aspiration: Functional, Biochemical, and Morphologic Characterization. Anesthesiol. J. Am. Soc. Anesthesiol. 2008, 108, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.R.; Pechet, T.T.V.; Williams, J.P.; Ma, M.; Frenette, P.S.; Moore, F.D.; Kobzik, L.; Hines, R.O.; Wagner, D.D.; Carroll, M.C.; et al. Experimental murine acid aspiration injury is mediated by neutrophils and the alternative complement pathway. J. Appl. Physiol. 1997, 83, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.; Rubin, S.; Eyal, Z.; Polliack, A. A comparison of the pulmonary response to the endotracheal instillation of 0.1 N hydrochloric acid and Hartmann’s solution in the rabbit. Br. J. Anaesth. 1974, 46, 127–132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, Z.; Lis, R.; Ginsberg, M.; Chavez, D.; Shido, K.; Rabbany, S.Y.; Fong, G.-H.; Sakmar, T.P.; Rafii, S.; Ding, B.-S. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat. Med. 2016, 22, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Luo, L.; Zou, M.; Huang, C.; Wan, X.; Hu, Y.; Le, Y.; Zhao, H.; Li, W.; Zou, F.; et al. Blockade of extracellular heat shock protein 90α by 1G6-D7 attenuates pulmonary fibrosis through inhibiting ERK signaling. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 313, L1006–L1015. [Google Scholar] [CrossRef] [PubMed]
- Decaris, M.L.; Gatmaitan, M.; FlorCruz, S.; Luo, F.; Li, K.; Holmes, W.E.; Hellerstein, M.K.; Turner, S.M.; Emson, C.L. Proteomic analysis of altered extracellular matrix turnover in bleomycin-induced pulmonary fibrosis. Mol. Cell. Proteom. 2014, 13, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Solopov, P.; Colunga Biancatelli, R.M.L.; Dimitropoulou, C.; Catravas, J.D. Dietary Phytoestrogens Ameliorate Hydrochloric Acid-Induced Chronic Lung Injury and Pulmonary Fibrosis in Mice. Nutrients 2021, 13, 3599. [Google Scholar] [CrossRef] [PubMed]
- Marinova, M.; Solopov, P.; Dimitropoulou, C.; Colunga Biancatelli, R.M.L.; Catravas, J.D. Post-treatment with a heat shock protein 90 inhibitor prevents chronic lung injury and pulmonary fibrosis, following acute exposure of mice to HCl. Exp. Lung Res. 2020, 46, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Tzilas, V.; Bouros, D. Usual interstitial pneumonia pattern in the diagnosis of idiopathic pulmonary fibrosis? Lancet Respir. Med. 2016, 4, 770–772. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Madala, S.K.; Schmidt, S.; Davidson, C.; Ikegami, M.; Wert, S.; Hardie, W.D. MEK-ERK pathway modulation ameliorates pulmonary fibrosis associated with epidermal growth factor receptor activation. Am. J. Respir. Cell Mol. Biol. 2012, 46, 380–388. [Google Scholar] [CrossRef]
- Lake, D.; Corrêa, S.A.; Müller, J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell. Mol. Life Sci. 2016, 73, 4397–4413. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Solopov, P.; Gregory, B.; Catravas, J.D. HSP90 Inhibition and Modulation of the Proteome: Therapeutical Implications for Idiopathic Pulmonary Fibrosis (IPF). Int. J. Mol. Sci. 2020, 21, 5286. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Tang, T.; Liu, Y.; Ma, Y.; Wang, Z.; Tao, H.; Zhang, Y.; Qi, Z. Inducible HSP70 antagonizes cisplatin-induced cell apoptosis through inhibition of the MAPK signaling pathway in HGC-27 cells. Int. J. Mol. Med. 2018, 42, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Utsugi, T. New challenges and inspired answers for anticancer drug discovery and development. Jpn. J. Clin. Oncol. 2013, 43, 945–953. [Google Scholar] [CrossRef]
- Suzuki, R.; Hideshima, T.; Mimura, N.; Minami, J.; Ohguchi, H.; Kikuchi, S.; Yoshida, Y.; Gorgun, G.; Cirstea, D.; Cottini, F.; et al. Anti-tumor activities of selective HSP90α/β inhibitor, TAS-116, in combination with bortezomib in multiple myeloma. Leukemia 2015, 29, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S. Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol. Biol. Evol. 1995, 12, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Toft, D.O. Regulation of Signaling Protein Function and Trafficking by the hsp90/hsp70-Based Chaperone Machinery. Exp. Biol. Med. 2003, 228, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Subbarao Sreedhar, A.; Kalmár, É.; Csermely, P.; Shen, Y.-F. Hsp90 isoforms: Functions, expression and clinical importance. FEBS Lett. 2004, 562, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Cambiazo, V.; González, M.; Isamit, C.; Maccioni, R.B. The β-isoform of heat shock protein hsp-90 is structurally related with human microtubule-interacting protein Mip-90. FEBS Lett. 1999, 457, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Czar, M.J.; Welsh, M.J.; Pratt, W.B. Immunofluorescence localization of the 90-kDa heat-shock protein to cytoskeleton. Eur. J. Cell Biol. 1996, 70, 322–330. [Google Scholar] [PubMed]
- Wu, Y.P.; Kita, K.; Suzuki, N. Involvement of human heat shock protein 90 alpha in nicotine-induced apoptosis. Int. J. Cancer 2002, 100, 37–42. [Google Scholar] [CrossRef]
- Sontake, V.; Wang, Y.; Kasam, R.K.; Sinner, D.; Reddy, G.B.; Naren, A.P.; McCormack, F.X.; White, E.S.; Jegga, A.G.; Madala, S.K. Hsp90 regulation of fibroblast activation in pulmonary fibrosis. JCI Insight 2017, 2, e91454. [Google Scholar] [CrossRef]
- Belenichev, I.F.; Aliyeva, O.G.; Popazova, O.O.; Bukhtiyarova, N.V. Involvement of heat shock proteins HSP70 in the mechanisms of endogenous neuroprotection: The prospect of using HSP70 modulators. Front. Cell. Neurosci. 2023, 17, 1131683. [Google Scholar] [CrossRef] [PubMed]
- Sellares, J.; Veraldi, K.L.; Thiel, K.J.; Cárdenes, N.; Alvarez, D.; Schneider, F.; Pilewski, J.M.; Rojas, M.; Feghali-Bostwick, C.A. Intracellular Heat Shock Protein 70 Deficiency in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2019, 60, 629–636. [Google Scholar] [CrossRef]
- Ahookhosh, K.; Vanoirbeek, J.; Vande Velde, G. Lung function measurements in preclinical research: What has been done and where is it headed? Front. Physiol. 2023, 14, 1130096. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colunga Biancatelli, R.M.L.; Solopov, P.A.; Day, T.; Austin, D.E., Jr.; Murray, L.E.; Catravas, J.D. Combination of HSP90 Inhibitors and HSP70 Inducers Prevent Hydrochloric Acid-Induced Pulmonary Fibrosis in Rabbits. Int. J. Mol. Sci. 2025, 26, 441. https://doi.org/10.3390/ijms26020441
Colunga Biancatelli RML, Solopov PA, Day T, Austin DE Jr., Murray LE, Catravas JD. Combination of HSP90 Inhibitors and HSP70 Inducers Prevent Hydrochloric Acid-Induced Pulmonary Fibrosis in Rabbits. International Journal of Molecular Sciences. 2025; 26(2):441. https://doi.org/10.3390/ijms26020441
Chicago/Turabian StyleColunga Biancatelli, Ruben M. L., Pavel A. Solopov, Tierney Day, Dan E. Austin, Jr., Len E. Murray, and John D. Catravas. 2025. "Combination of HSP90 Inhibitors and HSP70 Inducers Prevent Hydrochloric Acid-Induced Pulmonary Fibrosis in Rabbits" International Journal of Molecular Sciences 26, no. 2: 441. https://doi.org/10.3390/ijms26020441
APA StyleColunga Biancatelli, R. M. L., Solopov, P. A., Day, T., Austin, D. E., Jr., Murray, L. E., & Catravas, J. D. (2025). Combination of HSP90 Inhibitors and HSP70 Inducers Prevent Hydrochloric Acid-Induced Pulmonary Fibrosis in Rabbits. International Journal of Molecular Sciences, 26(2), 441. https://doi.org/10.3390/ijms26020441






