Mendelian Randomization Study on hs-CRP and Dyslipidemia in Koreans: Identification of Novel SNP rs76400217
Abstract
:1. Introduction
2. Results
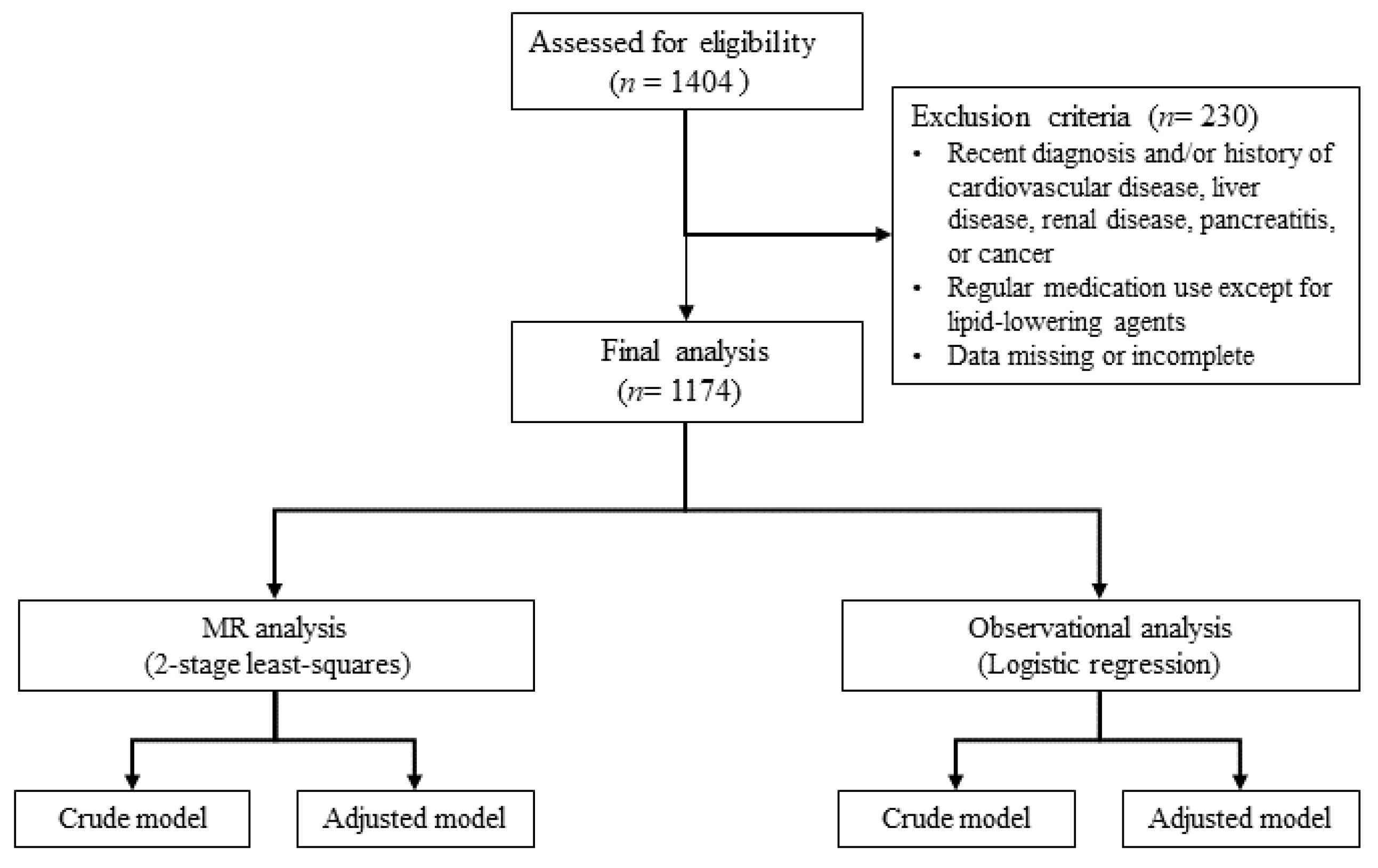
2.1. Demographic and Clinical Characteristics According to the Presence of Dyslipidemia
2.2. Genotype Distribution
2.3. Association of rs76400217 with Clinical and Metabolic Parameters
2.4. Association Between the hs-CRP and Dyslipidemia
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Definition of Disease
4.3. Lifestyle and Anthropometric Assessments
4.4. Biochemical Assessments
4.5. SNP Genotyping and Selection
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berberich, A.J.; Hegele, R.A. A modern approach to dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef]
- Jeong, S.M.; Choi, S.; Kim, K.; Kim, S.M.; Lee, G.; Park, S.Y.; Kim, Y.Y.; Son, J.S.; Yun, J.M.; Park, S.M. Effect of change in total cholesterol levels on cardiovascular disease among young adults. J. Am. Heart Assoc. 2018, 7, e008819. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Lelong, H.; Lopez-Sublet, M.; Topouchian, J.; Safar, M.E.; Blacher, J. Association between different lipid parameters and aortic stiffness: Clinical and therapeutic implication perspectives. J. Hypertens. 2019, 37, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Chung, B.; Sohn, C. A relationship between dietary patterns and dyslipidemia in urban-dwelling middle-aged Korean men: Using Korean Genome and Epidemiology Study (KoGES). Clin. Nutr. Res. 2019, 8, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.S.; Shim, J.S.; Kim, S.E.; Bae, J.H.; Kang, S.; Won, J.C.; Shin, M.J.; Jin, H.Y.; Moon, J.; Lee, H.; et al. Dyslipidemia fact sheet in South Korea, 2022. J. Lipid Atheroscler. 2023, 12, 237–251. [Google Scholar] [CrossRef]
- Cho, S.M.J.; Lee, H.; Lee, H.H.; Baek, J.; Heo, J.E.; Joo, H.J.; Hong, S.J.; Kim, H.C. Dyslipidemia fact sheets in Korea 2020: An analysis of nationwide population-based data. J. Lipid Atheroscler. 2021, 10, 202–209. [Google Scholar] [CrossRef]
- Eklund, C.M. Proinflammatory cytokines in CRP baseline regulation. Adv. Clin. Chem. 2009, 48, 111–136. [Google Scholar] [PubMed]
- Wang, A.; Liu, J.; Li, C.; Gao, J.; Li, X.; Chen, S.; Wu, S.; Ding, H.; Fan, H.; Hou, S. Cumulative exposure to high-sensitivity c-reactive protein predicts the risk of cardiovascular disease. J. Am. Heart Assoc. 2017, 6, e005610. [Google Scholar] [CrossRef]
- Banait, T.; Wanjari, A.; Danade, V.; Banait, S.; Jain, J. Role of high-sensitivity c-reactive protein (Hs-CRP) in non-communicable diseases: A review. Cureus 2022, 14, e30225. [Google Scholar] [CrossRef]
- Wang, X.; Bao, W.; Liu, J.; Ouyang, Y.Y.; Wang, D.; Rong, S.; Xiao, X.; Shan, Z.L.; Zhang, Y.; Yao, P.; et al. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2013, 36, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Boarescu, P.M.; Boarescu, I.; Pop, R.M.; Roşian, Ş.H.; Bocșan, I.C.; Rus, V.; Mada, R.O.; Popa, I.D.; Neagu, N.; Bulboacă, A.E.; et al. Evaluation of oxidative stress biomarkers, pro-inflammatory cytokines, and histological changes in experimental hypertension, dyslipidemia, and type 1 diabetes mellitus. Int. J. Mol. Sci. 2022, 23, 1438. [Google Scholar] [CrossRef] [PubMed]
- Holven, K.B.; Narverud, I.; Lindvig, H.W.; Halvorsen, B.; Langslet, G.; Nenseter, M.S.; Ulven, S.M.; Ose, L.; Aukrust, P.; Retterstøl, K. Subjects with familial hypercholesterolemia are characterized by an inflammatory phenotype despite long-term intensive cholesterol lowering treatment. Atherosclerosis 2014, 233, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Huang, X.; Han, Y.; Kim, M. NPC1L1 rs217434 A > G as a novel single nucleotide polymorphism related to dyslipidemia in a Korean population. Biochem. Genet. 2024, 62, 4103–4119. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Davey Smith, G.; Kundu, D.; Bruckdorfer, K.R.; Ebrahim, S. Those confounded vitamins: What can we learn from the differences between observational versus randomised trial evidence? Lancet 2004, 363, 1724–1727. [Google Scholar] [CrossRef]
- Schooling, C.M.; Chow, C.K.; Au Yeung, S.L. Causality and causal inference in epidemiology: We need also to address causes of effects. Int. J. Epidemiol. 2016, 45, 2200–2201. [Google Scholar] [CrossRef] [PubMed]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef] [PubMed]
- Haycock, P.C.; Burgess, S.; Wade, K.H.; Bowden, J.; Relton, C.; Davey Smith, G. Best (but oft-forgotten) practices: The design, analysis, and interpretation of Mendelian randomization studies. Am. J. Clin. Nutr. 2016, 103, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Walia, G.K.; Sachdeva, M.P. ‘Mendelian randomization’: An approach for exploring causal relations in epidemiology. Public Health 2017, 145, 113–119. [Google Scholar] [CrossRef]
- Sarwar, N.; Sandhu, M.S.; Ricketts, S.L.; Butterworth, A.S.; Di Angelantonio, E.; Boekholdt, S.M.; Ouwehand, W.; Watkins, H.; Samani, N.J.; Saleheen, D.; et al. Triglyceride-mediated pathways and coronary disease: Collaborative analysis of 101 studies. Lancet 2010, 375, 1634–1639. [Google Scholar]
- Abbasi, A.; Deetman, P.E.; Corpeleijn, E.; Gansevoort, R.T.; Gans, R.O.; Hillege, H.L.; van der Harst, P.; Stolk, R.P.; Navis, G.; Alizadeh, B.Z.; et al. Bilirubin as a potential causal factor in type 2 diabetes risk: A Mendelian randomization study. Diabetes 2015, 64, 1459–1469. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Wade, K.H.; Richmond, R.C.; Langdon, R.J.; Bull, C.J.; Tilling, K.M.; Relton, C.L.; Lewis, S.J.; Davey Smith, G.; Martin, R.M. Causal inference in cancer epidemiology: What is the role of Mendelian randomization? Cancer Epidemiol. Biomark. Prev. 2018, 27, 995–1010. [Google Scholar] [CrossRef]
- Jin, D.; Zhu, D.M.; Hu, H.L.; Yao, M.N.; Yin, W.J.; Tao, R.X.; Zhu, P. Vitamin D status affects the relationship between lipid profile and high-sensitivity C-reactive protein. Nutr. Metab. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Heidari-Bakavoli, A.R.; Shoeibi, S.; Mirhafez, S.R.; Moohebati, M.; Esmaily, H.; Ghazavi, H.; Saberi Karimian, M.; Parizadeh, S.M.; Mohammadi, M.; et al. Association of serum hs-CRP levels with the presence of obesity, diabetes mellitus, and other cardiovascular risk factors. J. Clin. Lab. Anal. 2016, 30, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; He, Y.; Liu, L.; Ou, Q.; Lin, J. Association of serum lipids with autoantibodies and inflammatory markers in rheumatoid arthritis patients. Clin. Chim. Acta 2018, 486, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Toms, T.E.; Panoulas, V.F.; Douglas, K.M.; Nightingale, P.; Smith, J.P.; Griffiths, H.; Sattar, N.; Symmons, D.P.; Kitas, G.D. Are lipid ratios less susceptible to change with systemic inflammation than individual lipid components in patients with rheumatoid arthritis? Angiology 2011, 62, 167–175. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Wang, R.; Jiang, J.; Hu, Y.; Wang, W.; Wang, Y.; Li, H. The predictive value of the hs-CRP/HDL-C ratio, an inflammation-lipid composite marker, for cardiovascular disease in middle-aged and elderly people: Evidence from a large national cohort study. Lipids Health Dis. 2024, 23, 66. [Google Scholar] [CrossRef]
- Kuppa, A.; Tripathi, H.; Al-Darraji, A.; Tarhuni, W.M.; Abdel-Latif, A. C-reactive protein levels and risk of cardiovascular diseases: A two-sample bidirectional Mendelian randomization study. Int. J. Mol. Sci. 2023, 24, 9129. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Chambers, J.C.; Zhang, W.; Clarke, R.; Hopewell, J.C.; Peden, J.F.; Erdmann, J.; Braund, P.; Engert, J.C.; Bennett, D.; et al. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA 2009, 302, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Du, L.; Ma, Z.; Xie, J.; Huang, Y.; Wei, X.; Jiang, X.; Xu, J.; Yin, R.; Wang, Y.; et al. Circulating C-reactive protein increases lung cancer risk: Results from a prospective cohort of UK Biobank. Int. J. Cancer 2022, 150, 47–55. [Google Scholar] [CrossRef]
- Si, S.; Li, J.; Tewara, M.A.; Xue, F. Genetically determined chronic low-grade inflammation and hundreds of health outcomes in the UK Biobank and the FinnGen population: A phenome-wide Mendelian randomization study. Front. Immunol. 2021, 12, 720876. [Google Scholar] [CrossRef]
- Markozannes, G.; Koutsioumpa, C.; Cividini, S.; Monori, G.; Tsilidis, K.K.; Kretsavos, N.; Theodoratou, E.; Gill, D.; Ioannidis, J.P.; Tzoulaki, I. Global assessment of C-reactive protein and health-related outcomes: An umbrella review of evidence from observational studies and Mendelian randomization studies. Eur. J. Epidemiol. 2021, 36, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Nambi, V. Markers of inflammation and their clinical significance. Atheroscler. Suppl. 2005, 6, 21–29. [Google Scholar] [CrossRef]
- Raman, K.; Chong, M.; Akhtar-Danesh, G.G.; D’Mello, M.; Hasso, R.; Ross, S.; Xu, F.; Paré, G. Genetic markers of inflammation and their role in cardiovascular disease. Can. J. Cardiol. 2013, 29, 67–74. [Google Scholar] [CrossRef]
- Fonseca, F.A.; Izar, M.C. High-sensitivity C-reactive protein and cardiovascular disease across countries and ethnicities. Clinics 2016, 71, 235–242. [Google Scholar] [CrossRef]
- National Library of Medicine. Reference SNP (rs) Report. Available online: https://www.ncbi.nlm.nih.gov/snp/rs76400217 (accessed on 5 August 2024).
- The Human ncRNA Gene Database. LOC105370802 Gene-Uncharacterized LOC105370802. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=LOC105370802 (accessed on 5 August 2024).
- Ma, B.; Geng, Y.; Meng, F.; Yan, G.; Song, F. Identification of a sixteen-gene prognostic biomarker for lung adenocarcinoma using a machine learning method. J. Cancer 2020, 11, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, H.R.; Lee, M.H.; Kim, N.H.; Wang, K.M.; Lee, S.H.; Park, O.; Hong, E.J.; Youn, J.W.; Kim, Y.Y. Smoking-related DNA methylation is differentially associated with cadmium concentration in blood. Biochem. Genet. 2020, 58, 617–630. [Google Scholar] [CrossRef] [PubMed]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef] [PubMed]

| Normal (n = 581) | Dyslipidemia (n = 593) | p | p a | p b | p c | |
|---|---|---|---|---|---|---|
| Male sex (n, %) | 223 (38.4) | 188 (34.7) | 0.016 | - | - | - |
| Alcohol drinker (n, %) | 376 (64.7) | 329 (55.5) | 0.001 | 0.069 | - | - |
| Current smoker (n, %) | 85 (14.6) | 82 (13.8) | 0.366 | 0.367 | 0.267 | 0.233 |
| Prediabetes or T2DM (n, %) | 191 (32.9) | 248 (41.8) | 0.002 | 0.014 | 0.012 | - |
| Age (year) | 48.0 ± 0.47 | 50.5 ± 0.43 | <0.001 | - | - | - |
| Waist (cm) | 82.7 ± 0.31 | 82.0 ± 0.30 | 0.138 | 0.121 | 0.108 | - |
| BMI (kg/m2) | 23.6 ± 0.11 | 23.9 ± 0.10 | 0.032 | 0.014 | 0.016 | - |
| Systolic BP (mmHg) | 118.7 ± 0.63 | 119.9 ± 0.60 | 0.169 | 0.337 | 0.345 | 0.877 |
| Diastolic BP (mmHg) | 73.7 ± 0.47 | 74.7 ± 0.40 | 0.081 | 0.190 | 0.172 | 0.476 |
| Glucose (mg/dL) ∮ | 95.3 ± 0.72 | 97.0 ± 0.95 | 0.258 | 0.430 | 0.385 | 0.372 |
| Insulin (μIU/dL) ∮ | 8.99 ± 0.24 | 9.21 ± 0.17 | 0.034 | 0.028 | 0.034 | 0.140 |
| HOMA-IR ∮ | 2.15 ± 0.08 | 2.20 ± 0.05 | 0.018 | 0.022 | 0.025 | 0.274 |
| HbA1c (%) ∮ | 6.02 ± 0.05 | 6.26 ± 0.07 | 0.006 | 0.001 | 0.001 | 0.053 |
| Free fatty acids (μEq/L) ∮ | 554.6 ± 11.0 | 583.2 ± 10.7 | 0.013 | 0.102 | 0.073 | 0.076 |
| Triglycerides (mg/dL) ∮ | 80.8 ± 1.23 | 127.6 ± 3.23 | <0.001 | <0.001 | <0.001 | <0.001 |
| Total-cholesterol (mg/dL) ∮ | 172.0 ± 0.77 | 203.2 ± 1.38 | <0.001 | <0.001 | <0.001 | <0.001 |
| HDL-cholesterol (mg/dL) ∮ | 56.4 ± 0.46 | 55.8 ± 0.61 | 0.033 | 0.011 | 0.019 | 0.069 |
| LDL-cholesterol (mg/dL) ∮ | 99.4 ± 0.76 | 121.9 ± 1.31 | <0.001 | <0.001 | <0.001 | <0.001 |
| hs-CRP (mg/L) ∮ | 1.40 ± 0.14 | 1.53 ± 0.11 | <0.001 | <0.001 | <0.001 | <0.001 |
| Malondialdehyde (nmol/mL) ∮ | 8.81 ± 0.14 | 9.75 ± 0.18 | <0.001 | <0.001 | <0.001 | <0.001 |
| Ox-LDL (U/L) ∮ | 35.7 ± 0.73 | 41.3 ± 0.91 | <0.001 | 0.002 | 0.001 | 0.007 |
| 8-epi-PGF2α (pg/mg creatinine) ∮ | 1072.2 ± 36.0 | 1638.8 ± 29.9 | <0.001 | <0.001 | <0.001 | <0.001 |
| T Allele (n = 85) | CC (n = 1089) | p | |
|---|---|---|---|
| Male sex (n, %) | 24 (28.2) | 387 (35.5) | 0.174 |
| Alcohol drinker (n, %) | 47 (55.3) | 658 (60.4) | 0.353 |
| Current smoker (n, %) | 12 (14.1) | 155 (14.2) | 0.551 |
| Prediabetes or T2DM (n, %) | 34 (40.0) | 405 (37.2) | 0.606 |
| Dyslipidemia (n, %) | 38 (44.7) | 555 (51.0) | 0.267 |
| Age (year) | 48.8 ± 1.24 | 49.3 ± 0.33 | 0.695 |
| Waist (cm) | 80.9 ± 0.92 | 82.5 ± 0.22 | 0.052 |
| BMI (kg/m2) | 23.3 ± 0.28 | 23.7 ± 0.08 | 0.140 |
| Systolic BP (mmHg) | 118.1 ± 1.63 | 119.4 ± 0.45 | 0.451 |
| Diastolic BP (mmHg) | 73.7 ± 1.11 | 74.3 ± 0.32 | 0.625 |
| Glucose (mg/dL) ∮ | 95.9 ± 2.37 | 96.1 ± 0.62 | 0.765 |
| Insulin (μIU/dL) ∮ | 9.90 ± 1.00 | 9.04 ± 0.13 | 0.953 |
| HOMA-IR ∮ | 2.35 ± 0.27 | 2.16 ± 0.04 | 0.963 |
| HbA1c (%) ∮ | 6.39 ± 0.17 | 6.14 ± 0.05 | 0.129 |
| Free fatty acids (μEq/L) ∮ | 563.0 ± 30.8 | 569.4 ± 7.94 | 0.484 |
| Triglycerides (mg/dL) ∮ | 94.2 ± 6.48 | 105.3 ± 1.95 | 0.062 |
| Total-cholesterol (mg/dL) ∮ | 185.7 ± 3.19 | 187.9 ± 0.96 | 0.591 |
| HDL-cholesterol (mg/dL) ∮ | 57.7 ± 1.60 | 56.0 ± 0.40 | 0.330 |
| LDL-cholesterol (mg/dL) ∮ | 109.2 ± 2.86 | 110.9 ± 0.86 | 0.642 |
| Malondialdehyde (nmol/mL) ∮ | 9.65 ± 0.52 | 9.26 ± 0.12 | 0.593 |
| Ox-LDL (U/L) ∮ | 38.2 ± 1.91 | 38.7 ± 0.62 | 0.234 |
| 8-epi-PGF2α (pg/mg creatinine) ∮ | 1430.1 ± 91.5 | 1352.8 ± 25.7 | 0.658 |
| Change in hs-CRP per Risk Allele | MR Analysis | Logistic Regression | ||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | F | p | OR (95% CI) | p | OR (95% CI) | p | |
| Model 1 | 0.688 | 0.120 | 32.7 | <0.001 | 1.44 (0.76–2.75) | 0.267 | 2.08 (1.81–2.39) | <0.001 |
| Model 2 | 0.667 | 0.119 | 19.4 | <0.001 | 1.50 (0.76–2.93) | 0.240 | 2.11 (1.83–2.43) | <0.001 |
| Model 3 | 0.672 | 0.119 | 16.3 | <0.001 | 1.51 (0.77–2.94) | 0.228 | 2.10 (1.82–2.42) | <0.001 |
| Model 4 | 0.635 | 0.117 | 28.5 | <0.001 | 1.48 (0.73–3.00) | 0.277 | 2.11 (1.82–2.44) | <0.001 |
| Model 5 | 0.640 | 0.117 | 24.1 | <0.001 | 1.49 (0.74–3.01) | 0.264 | 2.10 (1.82–2.44) | <0.001 |
| Model 6 | 0.644 | 0.117 | 20.3 | <0.001 | 1.52 (0.76–3.08) | 0.236 | 2.09 (1.81–2.43) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Han, Y.; Kim, M. Mendelian Randomization Study on hs-CRP and Dyslipidemia in Koreans: Identification of Novel SNP rs76400217. Int. J. Mol. Sci. 2025, 26, 506. https://doi.org/10.3390/ijms26020506
Huang X, Han Y, Kim M. Mendelian Randomization Study on hs-CRP and Dyslipidemia in Koreans: Identification of Novel SNP rs76400217. International Journal of Molecular Sciences. 2025; 26(2):506. https://doi.org/10.3390/ijms26020506
Chicago/Turabian StyleHuang, Ximei, Youngmin Han, and Minjoo Kim. 2025. "Mendelian Randomization Study on hs-CRP and Dyslipidemia in Koreans: Identification of Novel SNP rs76400217" International Journal of Molecular Sciences 26, no. 2: 506. https://doi.org/10.3390/ijms26020506
APA StyleHuang, X., Han, Y., & Kim, M. (2025). Mendelian Randomization Study on hs-CRP and Dyslipidemia in Koreans: Identification of Novel SNP rs76400217. International Journal of Molecular Sciences, 26(2), 506. https://doi.org/10.3390/ijms26020506






