An FGF2-Derived Short Peptide Attenuates Bleomycin-Induced Pulmonary Fibrosis by Inhibiting Collagen Deposition and Epithelial–Mesenchymal Transition via the FGFR/MAPK Signaling Pathway
Abstract
1. Introduction
2. Results
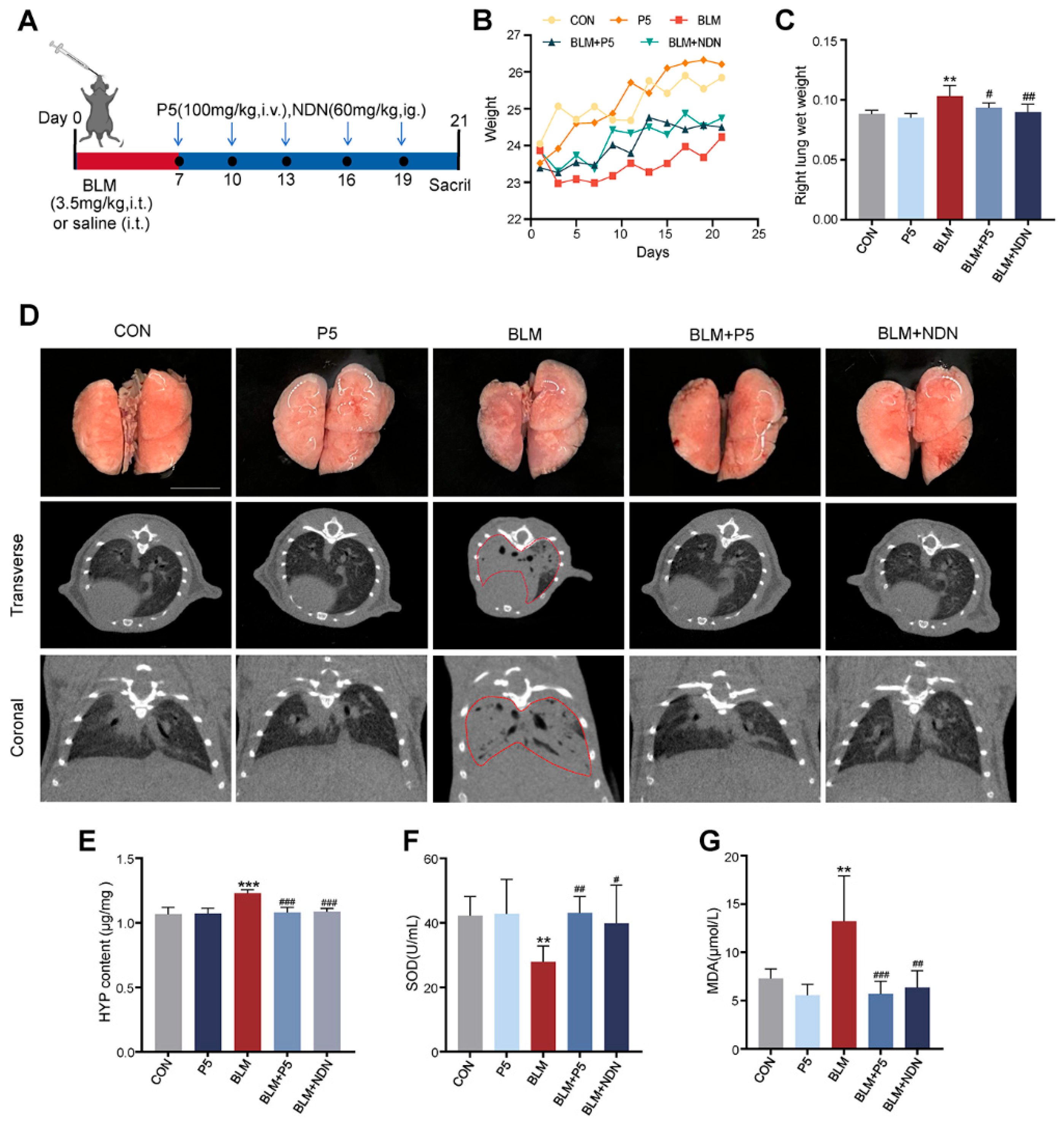
2.1. Therapeutic Effects of P5 on BLM-Induced Pulmonary Fibrosis in Mice
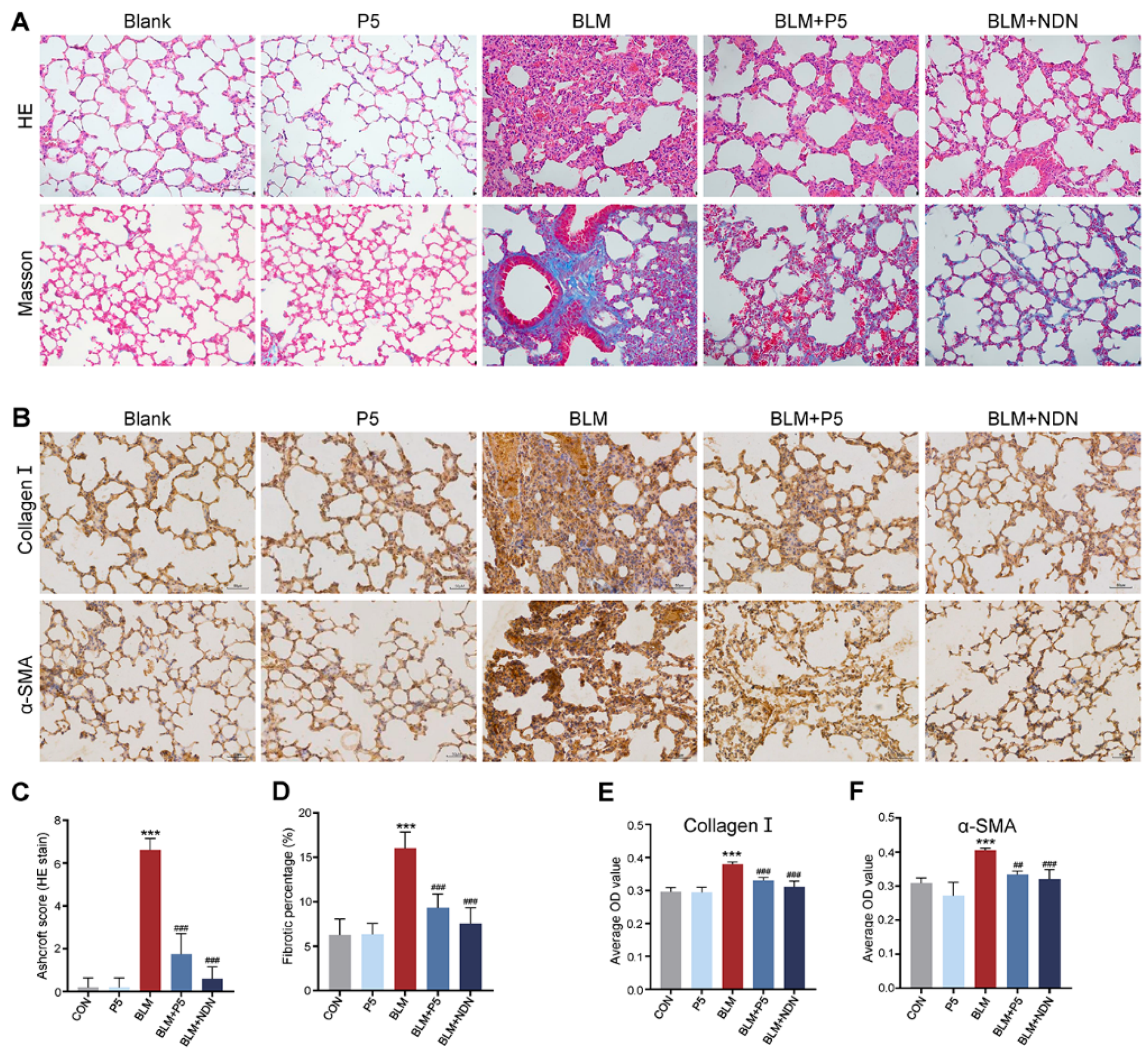
2.2. P5 Ameliorates BLM-Induced Lung Tissue Damage in Mice
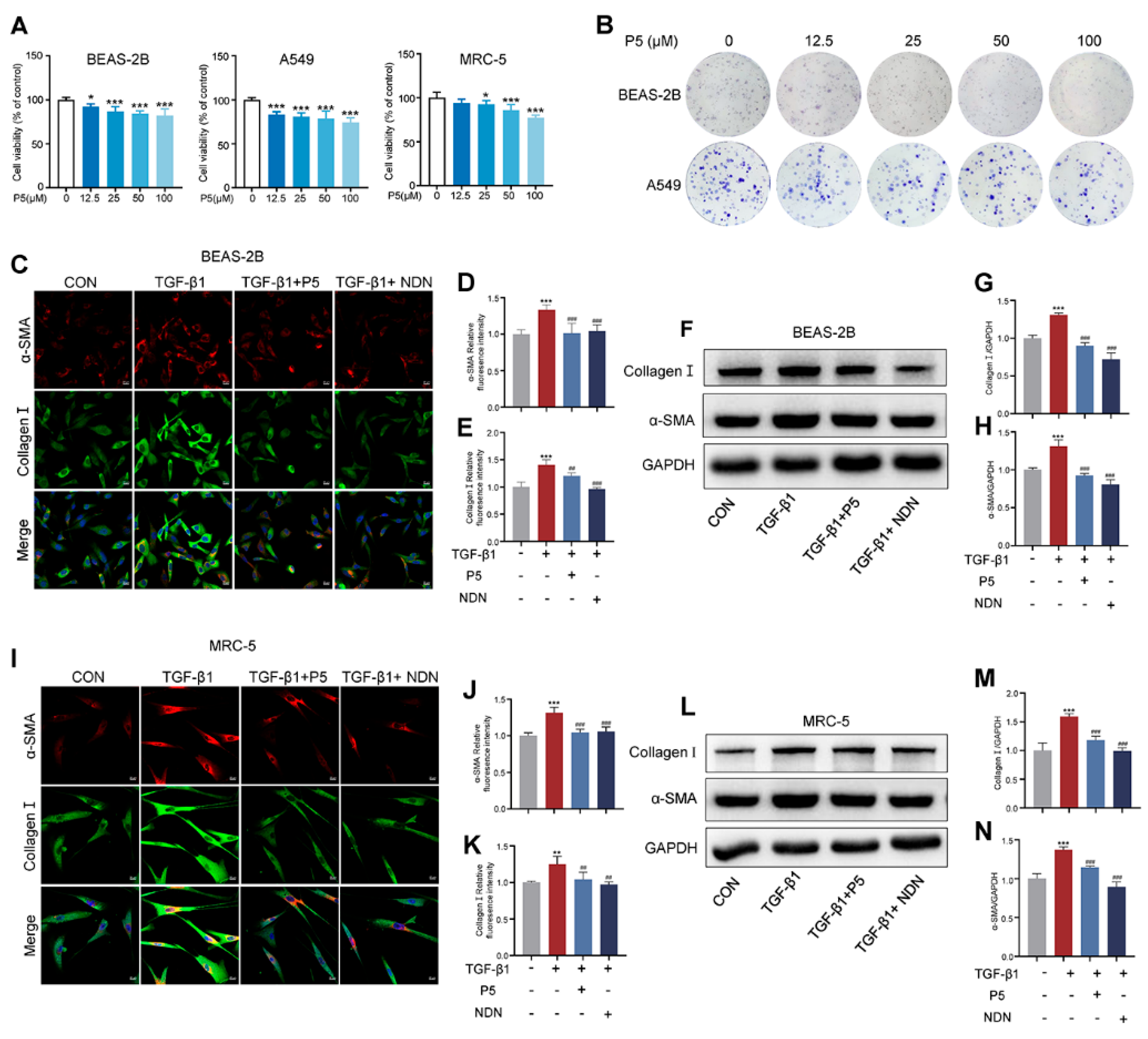
2.3. P5 Reduces α-SMA and Collagen I in TGF-β1-Activated Cells
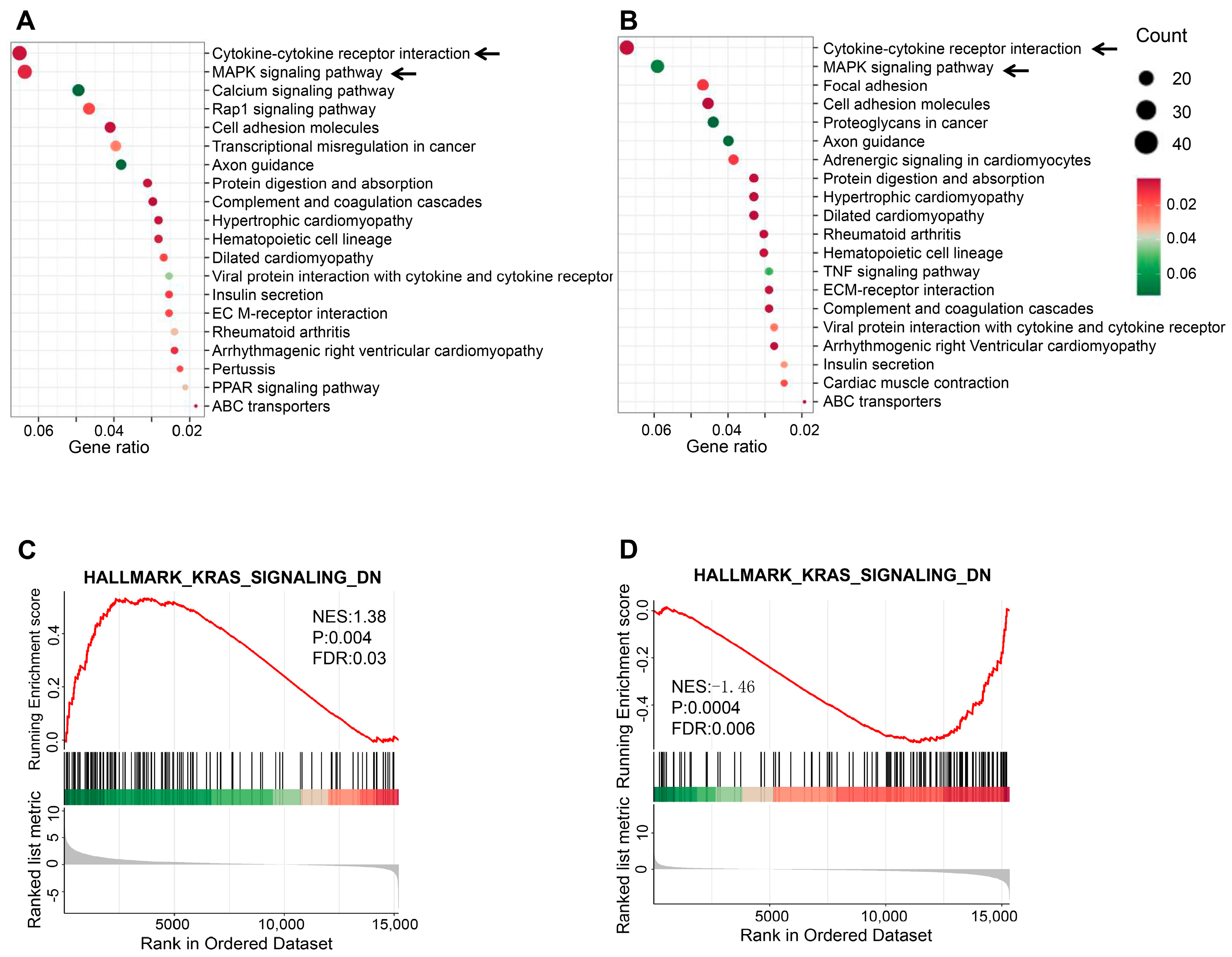
2.4. Transcriptomic Analysis of TGF-β1-Induced BEAS-2B Cells
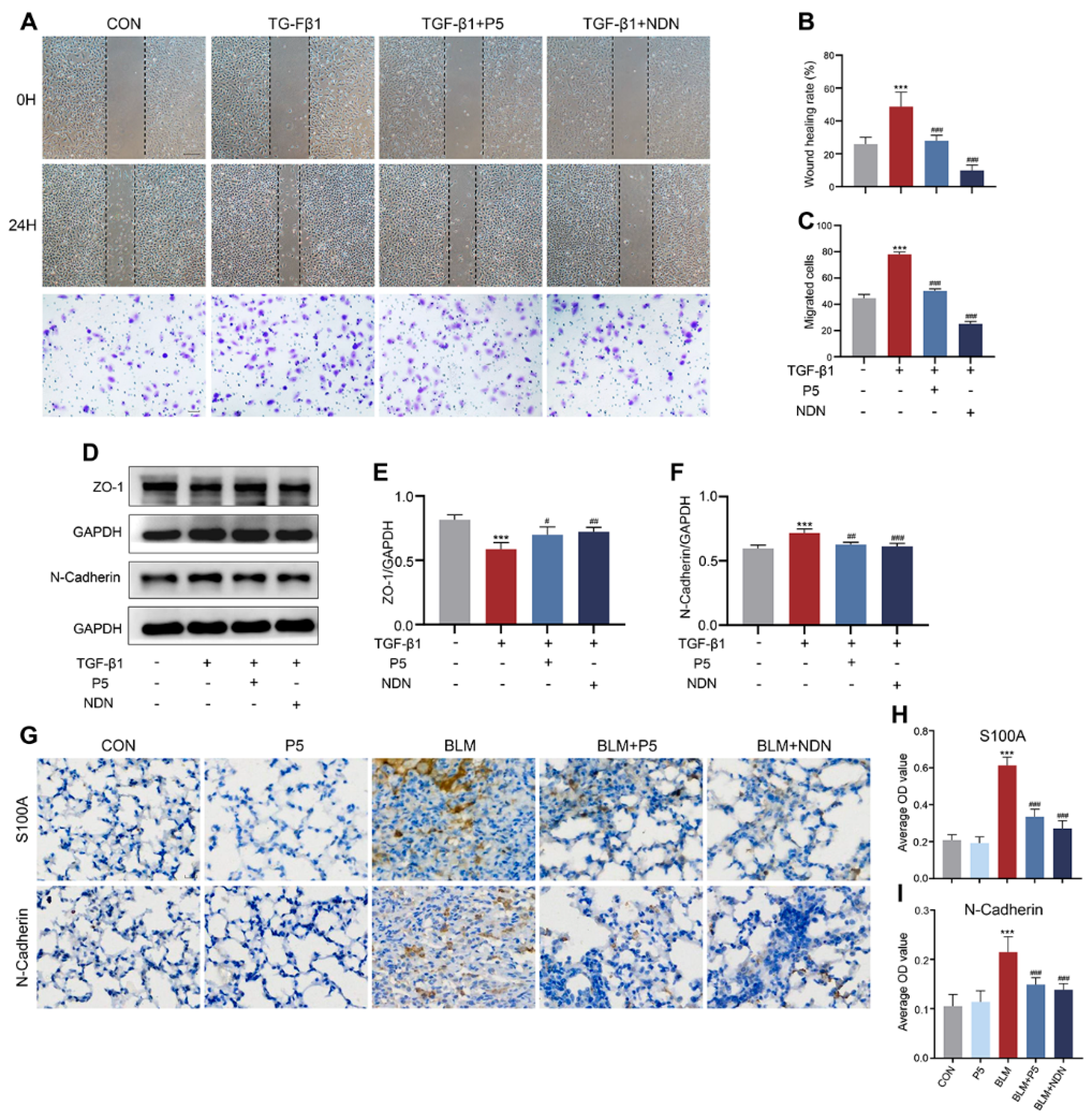
2.5. P5 Inhibits EMT Induced by TGF-β1 and BLM, Reducing Cell Migration and Mesenchymal Transition
2.6. P5 Modulates TGF-β1-Induced Signaling Pathways and Gene Expression Changes
2.7. P5 Regulates Bleomycin-Induced Pulmonary Fibrosis Through the FGFR/MAPK Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Experimental Animal Groups and Treatments
4.3. Micro-CT Imaging
4.4. Assessment of Hydroxyproline Levels
4.5. Serum Antioxidant Enzyme Activity Determination (MDA, SOD)
4.6. Cell Culture and Cell Viability Assay
4.7. Immunofluorescence (IF) Staining
4.8. Histology
4.9. Immunohistochemical (IHC) Staining
4.10. Western Blot
4.11. Colony Formation Assay
4.12. Wound Healing Assay
4.13. Transwell Migration Assay
4.14. Molecular Docking
4.15. RNA Sequencing (RNA-Seq) Transcript Profiling
4.16. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Summer, R.; Chun, P. Pressed for Understanding: Interstitial lung disease in Dry-Cleaning Workers. Am. J. Med Sci. 2024, 369, 122–125. [Google Scholar] [CrossRef]
- Yatera, K.; Nishida, C. Contemporary Concise Review 2023: Environmental and occupational lung diseases. Respirology 2024, 29, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.A.; Zamora-Sifuentes, J.L.; Vecillas, L.D.L.; Quirce, S. Respiratory Diseases Associated with Organic Dust Exposure. J. Allergy Clin. Immunol. Pract. 2024, 12, 1960–1971. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.I.; Ascherman, D.P. Rheumatoid Arthritis-Associated Interstitial Lung Disease (RA-ILD): Update on Prevalence, Risk Factors, Pathogenesis, and Therapy. Curr. Rheumatol. Rep. 2024, 26, 431–449. [Google Scholar] [CrossRef]
- Obi, O.N.; Saketkoo, L.A.; Maier, L.A.; Baughman, R.P. Developmental drugs for sarcoidosis. J. Autoimmun. 2024, 149, 103179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Shao, C. Cytological changes in radiation-induced lung injury. Life Sci. 2024, 358, 123188. [Google Scholar] [CrossRef]
- Borie, R.; Berteloot, L.; Kannengiesser, C.; Griese, M.; Cazes, A.; Crestani, B.; Hadchouel, A.; Debray, M.P. Rare genetic interstitial lung diseases: A pictorial essay. Eur. Respir. Rev. 2024, 33, 240101. [Google Scholar] [CrossRef]
- Borie, R.; Ba, I.; Debray, M.-P.; Kannengiesser, C.; Crestani, B. Syndromic genetic causes of pulmonary fibrosis. Curr. Opin. Pulm. Med. 2024, 30, 473–483. [Google Scholar] [CrossRef]
- Giriyappagoudar, M.; Vastrad, B.; Horakeri, R.; Vastrad, C. Study on Potential Differentially Expressed Genes in Idiopathic Pulmonary Fibrosis by Bioinformatics and Next-Generation Sequencing Data Analysis. Biomedicines 2023, 11, 3109. [Google Scholar] [CrossRef] [PubMed]
- Koudstaal, T.; Wijsenbeek, M.S. Idiopathic pulmonary fibrosis. Presse Med. 2023, 52, 104166. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Koteci, A.; Morgan, A.; George, P.M.; Quint, J.K. Incidence and prevalence of interstitial lung diseases worldwide: A systematic literature review. BMJ Open Respir. Res. 2023, 10, e001291. [Google Scholar] [CrossRef]
- Podolanczuk, A.J.; Thomson, C.C.; Remy-Jardin, M.; Richeldi, L.; Martinez, F.J.; Kolb, M.; Raghu, G. Idiopathic pulmonary fibrosis: State of the art for 2023. Eur. Respir. J. 2023, 61, 2200957. [Google Scholar] [CrossRef]
- Ruaro, B.; Pozzan, R.; Confalonieri, P.; Tavano, S.; Hughes, M.; Matucci Cerinic, M.; Baratella, E.; Zanatta, E.; Lerda, S.; Geri, P.; et al. Gastroesophageal Reflux Disease in Idiopathic Pulmonary Fibrosis: Viewer or Actor? To Treat or Not to Treat? Pharmaceuticals 2022, 15, 1033. [Google Scholar] [CrossRef]
- Chanda, D.; Otoupalova, E.; Smith, S.R.; Volckaert, T.; De Langhe, S.P.; Thannickal, V.J. Developmental pathways in the pathogenesis of lung fibrosis. Mol. Asp. Med. 2019, 65, 56–69. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, F.; Zheng, D.; Wang, D.; Li, X.; Zhao, C.; Huang, X. FGF/FGFR signaling: From lung development to respiratory diseases. Cytokine Growth Factor. Rev. 2021, 62, 94–104. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Wei, L.; Wang, Z.; Ma, W.; Liu, F.; Qian, Y. FGF2 and FGFR2 in patients with idiopathic pulmonary fibrosis and lung cancer. Oncol. Lett. 2018, 16, 2490–2494. [Google Scholar] [CrossRef]
- Shimbori, C.; Bellaye, P.-S.; Xia, J.; Gauldie, J.; Ask, K.; Ramos, C.; Becerril, C.; Pardo, A.; Selman, M.; Kolb, M. Fibroblast growth factor-1 attenuates TGF-β1-induced lung fibrosis. J. Pathol. 2016, 240, 197–210. [Google Scholar] [CrossRef]
- Ramos, C.; Becerril, C.; Montaño, M.; García-De-Alba, C.; Ramírez, R.; Checa, M.; Pardo, A.; Selman, M. FGF-1 reverts epithelial-mesenchymal transition induced by TGF-β1 through MAPK/ERK kinase pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L222–L231. [Google Scholar] [CrossRef]
- Ramos, C.; Montaño, M.; Becerril, C.; Cisneros-Lira, J.; Barrera, L.; Ruíz, V.; Pardo, A.; Selman, M. Acidic fibroblast growth factor decreases alpha-smooth muscle actin ex pression and induces apoptosis in human normal lung fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L871–L879. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, S.; Scotton, C.J.; McNulty, K.; Nye, E.; Stamp, G.; Laurent, G.; Bonnet, D.; Janes, S.M. Bone marrow stem cells expressing keratinocyte growth factor via an in ducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS ONE 2009, 4, e8013. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, B.; Henneke, I.; Hezel, S.; Al Alam, D.; El Agha, E.; Chao, C.-M.; Quantius, J.; Wilhelm, J.; Jones, M.; Goth, K.; et al. Attenuating endogenous Fgfr2b ligands during bleomycin-induced lung fi brosis does not compromise murine lung repair. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 308, L1014–L1024. [Google Scholar] [CrossRef]
- Schütz, K.; Schmidt, A.; Schwerk, N.; Renz, D.M.; Gerard, B.; Schaefer, E.; Antal, M.C.; Peters, S.; Griese, M.; Rapp, C.K.; et al. Variants in FGF10 cause early onset of severe childhood interstitial l ung disease: A detailed description of four affected children. Pediatr. Pulmonol. 2023, 58, 3095–3105. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-Q.; Cai, G.-F.; Zeng, P.-P.; Dhlamini, Q.; Chen, L.-F.; Chen, J.-J.; Lyu, H.-D.; Mossahebi-Mohammadi, M.; Ahmadvand, N.; Bellusci, S.; et al. FGF10 Therapeutic Administration Promotes Mobilization of Injury-Activ ated Alveolar Progenitors in a Mouse Fibrosis Model. Cells 2022, 11, 2396. [Google Scholar] [CrossRef]
- Joannes, A.; Brayer, S.; Besnard, V.; Marchal-Sommé, J.; Jaillet, M.; Mordant, P.; Mal, H.; Borie, R.; Crestani, B.; Mailleux, A.A. FGF9 and FGF18 in idiopathic pulmonary fibrosis promote survival and m igration and inhibit myofibroblast differentiation of human lung fibro blasts in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 310, L615–L629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, D.; Wang, M.; Huang, T.; Wu, H.; Zhang, Y.; Zhang, T.; Wang, W.; Yin, J.; Ren, G.; et al. FGF21 attenuates pulmonary fibrogenesis through ameliorating oxidative stress in vivo and in vitro. Biomed. Pharmacother. 2018, 103, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, B.; Korfei, M.; Henneke, I.; Sibinska, Z.; Tian, X.; Hezel, S.; Dilai, S.; Wasnick, R.; Schneider, B.; Wilhelm, J.; et al. Increased FGF1-FGFRc expression in idiopathic pulmonary fibrosis. Respir. Res. 2015, 16, 83. [Google Scholar] [CrossRef]
- Landi, C.; Bergantini, L.; Cameli, P.; d’Alessandro, M.; Carleo, A.; Shaba, E.; Rottoli, P.; Bini, L.; Bargagli, E. Idiopathic Pulmonary Fibrosis Serum proteomic analysis before and after nintedanib therapy. Sci. Rep. 2020, 10, 9378. [Google Scholar] [CrossRef]
- Easter, M.; Hirsch, M.J.; Harris, E.; Howze, P.H.t.; Matthews, E.L.; Jones, L.I.; Bollenbecker, S.; Vang, S.; Tyrrell, D.J.; Sanders, Y.Y.; et al. FGF receptors mediate cellular senescence in the cystic fibrosis airwa y epithelium. JCI Insight 2024, 9, e174888. [Google Scholar] [CrossRef]
- Yu, Z.-H.; Wang, D.-D.; Zhou, Z.-Y.; He, S.-L.; Chen, A.-A.; Wang, J. Mutant soluble ectodomain of fibroblast growth factor receptor-2 IIIc attenuates bleomycin-induced pulmonary fibrosis in mice. Biol. Pharm. Bull. 2012, 35, 731–736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, Z.; Zhang, Y.; Cao, J.; Sun, Y.; Cai, Y.; Zhang, B.; He, L.; Zhang, Z.; Xie, J.; Meng, Q.; et al. Hyaluronic acid-FGF2-derived peptide bioconjugates for suppression of FGFR2 and AR simultaneously as an acne antagonist. J. Nanobiotechnol. 2023, 21, 55. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, M.; Wang, H.; Zhang, B.; Guang, W.; Liu, R.; Li, X.; Shih, T.C.; Li, Z.; Cao, J.; et al. A cyclic peptide retards the proliferation of DU145 prostate cancer cells in vitro and in vivo through inhibition of FGFR2. MedComm 2020, 1, 362–375. [Google Scholar] [CrossRef]
- Katoh, M.; Loriot, Y.; Brandi, G.; Tavolari, S.; Wainberg, Z.A.; Katoh, M. FGFR-targeted therapeutics: Clinical activity, mechanisms of resistanc e and new directions. Nat. Rev. Clin. Oncol. 2024, 21, 312–329. [Google Scholar] [CrossRef]
- Kommalapati, A.; Tella, S.H.; Borad, M.; Javle, M.; Mahipal, A. FGFR Inhibitors in Oncology: Insight on the Management of Toxicities i n Clinical Practice. Cancers 2021, 13, 2968. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.J.; Binder, R.; Colbatzky, F.; Dallinger, C.; Schlenker-Herceg, R.; Hilberg, F.; Wollin, S.-L.; Kaiser, R. Nintedanib: From discovery to the clinic. J. Med. Chem. 2015, 58, 1053–1063. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. The role of fibroblast growth factor receptor (FGFR) protein-tyrosine kinase inhibitors in the treatment of cancers including those of the u rinary bladder. Pharmacol. Res. 2020, 151, 104567. [Google Scholar] [CrossRef]
- Ong, C.H.; Tham, C.L.; Harith, H.H.; Firdaus, N.; Israf, D.A. TGF-β-induced fibrosis: A review on the underlying mechanism and potential therapeutic strategies. Eur. J. Pharmacol. 2021, 911, 174510. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.A. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu. Rev. Physiol. 2011, 73, 413–435. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef]
- Bonella, F.; Spagnolo, P.; Ryerson, C. Current and Future Treatment Landscape for Idiopathic Pulmonary Fibros is. Drugs 2013, 83, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Moss, B.J.; Ryter, S.W.; Rosas, I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. 2022, 17, 515–546. [Google Scholar] [CrossRef]
- Ayilya, B.L.; Balde, A.; Ramya, M.; Benjakul, S.; Kim, S.-K.; Nazeer, R.A. Insights on the mechanism of bleomycin to induce lung injury and assoc iated in vivo models: A review. Int. Immunopharmacol. 2023, 121, 110493. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, J.; Rubio, G.A.; Limper, A.H.; Williams, K.; Elliot, S.J.; Ninou, I.; Aidinis, V.; Tzouvelekis, A.; Glassberg, M.K. Exploring Animal Models That Resemble Idiopathic Pulmonary Fibrosis. Front. Med. 2017, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Qin, Y.; Fu, X.; Yang, F.; Huo, F.; Liang, X.; Wang, S.; Cui, H.; Lin, P.; Zhou, G.; et al. Inhibition of ferroptosis and iron accumulation alleviates pulmonary f ibrosis in a bleomycin model. Redox Biol. 2022, 57, 102509. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, V.; Chaudhuri, N.; Torrisi, S.E.; Kahn, N.; Müller, V.; Kreuter, M. The therapy of idiopathic pulmonary fibrosis: What is next? Eur. Respir. Rev. 2019, 28, 190021. [Google Scholar] [CrossRef]
- Finnerty, J.P.; Ponnuswamy, A.; Dutta, P.; Abdelaziz, A.; Kamil, H. Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatme nt of progressive pulmonary fibrosis in both idiopathic pulmonary fibr osis (IPF) and non-IPF: A systematic review and meta-analysis. BMC Pulm. Med. 2021, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Karampitsakos, T.; Juan-Guardela, B.M.; Tzouvelekis, A.; Herazo-Maya, J.D. Precision medicine advances in idiopathic pulmonary fibrosis. EBioMedicine 2023, 95, 104766. [Google Scholar] [CrossRef] [PubMed]
- Varone, F.; Sgalla, G.; Iovene, B.; Bruni, T.; Richeldi, L. Nintedanib for the treatment of idiopathic pulmonary fibrosis. Expert Opin. Pharmacother. 2018, 19, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Tsujino, K.; Kijima, T.; Kumanogoh, A. Efficacy and safety of pirfenidone for idiopathic pulmonary fibrosis. Patient Prefer. Adherence 2014, 8, 361–370. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Liu, Y.; Wu, Y.; Wang, Q.; Jin, L.; Zhang, D. Therapeutic Peptides for Treatment of Lung Diseases: Infection, Fibros is, and Cancer. Int. J. Mol. Sci. 2023, 24, 8642. [Google Scholar] [CrossRef]
- Simon, K.S.; Coelho, L.C.; Veloso, P.H.H., Jr.; Melo-Silva, C.A.; Morais, J.A.V.; Longo, J.P.F.; Figueiredo, F.; Viana, L.; Silva Pereira, I.; Amado, V.M.; et al. Innovative Pre-Clinical Data Using Peptides to Intervene in the Evolution of Pulmonary Fibrosis. Int. J. Mol. Sci. 2023, 24, 1049. [Google Scholar] [CrossRef]
- Lan, Y.W.; Chen, Y.C.; Yen, C.C.; Chen, H.L.; Tung, M.C.; Fan, H.C.; Chen, C.M. Kefir peptides mitigate bleomycin-induced pulmonary fibrosis in mice through modulating oxidative stress, inflammation and gut microbiota. Biomed. Pharmacother. 2024, 174, 116431. [Google Scholar] [CrossRef] [PubMed]
- Keum, H.; Kim, J.; Yoo, D.; Kim, T.W.; Seo, C.; Kim, D.; Jon, S. Biomimetic lipid Nanocomplexes incorporating STAT3-inhibiting peptides effectively infiltrate the lung barrier and ameliorate pulmonary fibrosis. J. Control. Release Off. J. Control. Release Soc. 2021, 332, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Deng, R.; Gao, S.; Jiang, Q.; Liu, R.; Li, H.; Miao, Y.; Zhai, Y.; Zhang, S.; et al. Betulinic acid attenuated bleomycin-induced pulmonary fibrosis by effe ctively intervening Wnt/β-catenin signaling. Phytomedicine 2020, 81, 153428. [Google Scholar] [CrossRef] [PubMed]
- Boutanquoi, P.-M.; Burgy, O.; Beltramo, G.; Bellaye, P.-S.; Dondaine, L.; Marcion, G.; Pommerolle, L.; Vadel, A.; Spanjaard, M.; Demidov, O.; et al. TRIM33 prevents pulmonary fibrosis by impairing TGF-β1 signalling. Eur. Respir. J. 2019, 55, 1901346. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hou, R.; Liu, X.; Tian, Y.; Bai, Y.; Li, J.; Zhao, P. Yangqing Chenfei formula attenuates silica-induced pulmonary fibrosis by suppressing activation of fibroblast via regulating PI3K/AKT, JAK/S TAT, and Wnt signaling pathway. Phytomedicine 2022, 110, 154622. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guzy, R.; Ma, S.F.; Bonham, C.A.; Jou, J.; Schulte, J.J.; Kim, J.S.; Barros, A.J.; Espindola, M.S.; Husain, A.N.; et al. Central lung gene expression associates with myofibroblast features in idiopathic pulmonary fibrosis. BMJ Open Respir. Res. 2023, 10, e001391. [Google Scholar] [CrossRef]
- Amoakon, J.P.; Lee, J.; Liyanage, P.; Arora, K.; Karlstaedt, A.; Mylavarapu, G.; Amin, R.; Naren, A.P. Defective CFTR modulates mechanosensitive channels TRPV4 and PIEZO1 and drives endothelial barrier failure. iScience 2024, 27, 110703. [Google Scholar] [CrossRef]
- Fließer, E.; Jandl, K.; Lins, T.; Birnhuber, A.; Valzano, F.; Kolb, D.; Foris, V.; Heinemann, A.; Olschewski, H.; Evermann, M.; et al. Lung Fibrosis Is Linked to Increased Endothelial Cell Activation and D ysfunctional Vascular Barrier Integrity. Am. J. Respir. Cell. Mol. Biol. 2024, 71, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Yegen, C.H.; Marchant, D.; Bernaudin, J.F.; Planes, C.; Boncoeur, E.; Voituron, N. Chronic pulmonary fibrosis alters the functioning of the respiratory neural network. Front. Physiol. 2023, 14, 1205924. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudian, S.; Sajjadi, E.; Hadavi, N.; Soltani, M.; Karami, Z.; Abed Hamadi Al Qushawi, A.; Akrami, M.; Kalantari, F. Biomedical applications of peptide-gold nanoarchitectonics. Int. J. Pharm. 2024, 667, 124920. [Google Scholar] [CrossRef] [PubMed]
- Chawathe, A.; Ahire, V.; Luthra, K.; Patil, B.; Garkhal, K.; Sharma, N. Analytical and drug delivery strategies for short peptides: From manufacturing to market. Anal. Biochem. 2025, 696, 115699. [Google Scholar] [CrossRef] [PubMed]
- Martian, P.C.; Tertis, M.; Leonte, D.; Hadade, N.; Cristea, C.; Crisan, O. Cyclic peptides: A powerful instrument for advancing biomedical nanotechnologies and drug development. J. Pharm. Biomed. Anal. 2025, 252, 116488. [Google Scholar] [CrossRef]
- Binder, U.; Skerra, A. Strategies for extending the half-life of biotherapeutics: Successes and complications. Expert. Opin. Biol. Ther. 2024, 25, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, F.; Ravanetti, F.; Essers, J.; Ridwan, Y.; Belenkov, S.; Vos, W.; Ferreira, F.; KleinJan, A.; van Heijningen, P.; Van Holsbeke, C.; et al. Longitudinal assessment of bleomycin-induced lung fibrosis by Micro-CT correlates with histological evaluation in mice. Multidiscip. Respir. Med. 2017, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, P.; Li, Z.; Yan, Y.; Cheng, X.; Wang, G.; Yang, X. Different cellular mechanisms from low- and high-dose zinc oxide nanoparticles-induced heart tube malformation during embryogenesis. Nanotoxicology 2022, 16, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Sun, Y.; Zhao, Y.; Jiang, X.; Wang, T.; Xie, J.; Yu, X.; Guo, S.; Zhang, Y.; Chen, X.; et al. An FGF2-Derived Short Peptide Attenuates Bleomycin-Induced Pulmonary Fibrosis by Inhibiting Collagen Deposition and Epithelial–Mesenchymal Transition via the FGFR/MAPK Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 517. https://doi.org/10.3390/ijms26020517
Wang M, Sun Y, Zhao Y, Jiang X, Wang T, Xie J, Yu X, Guo S, Zhang Y, Chen X, et al. An FGF2-Derived Short Peptide Attenuates Bleomycin-Induced Pulmonary Fibrosis by Inhibiting Collagen Deposition and Epithelial–Mesenchymal Transition via the FGFR/MAPK Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(2):517. https://doi.org/10.3390/ijms26020517
Chicago/Turabian StyleWang, Mengwei, Yuanmeng Sun, Yanzhi Zhao, Xinyi Jiang, Teng Wang, Junye Xie, Xiuling Yu, Shujun Guo, Yibo Zhang, Xiaojia Chen, and et al. 2025. "An FGF2-Derived Short Peptide Attenuates Bleomycin-Induced Pulmonary Fibrosis by Inhibiting Collagen Deposition and Epithelial–Mesenchymal Transition via the FGFR/MAPK Signaling Pathway" International Journal of Molecular Sciences 26, no. 2: 517. https://doi.org/10.3390/ijms26020517
APA StyleWang, M., Sun, Y., Zhao, Y., Jiang, X., Wang, T., Xie, J., Yu, X., Guo, S., Zhang, Y., Chen, X., & Hong, A. (2025). An FGF2-Derived Short Peptide Attenuates Bleomycin-Induced Pulmonary Fibrosis by Inhibiting Collagen Deposition and Epithelial–Mesenchymal Transition via the FGFR/MAPK Signaling Pathway. International Journal of Molecular Sciences, 26(2), 517. https://doi.org/10.3390/ijms26020517





