Daily Lipolysis Gene Expression in Male Rat Mesenteric Adipose Tissue: Obesity and Melatonin Effects
Abstract
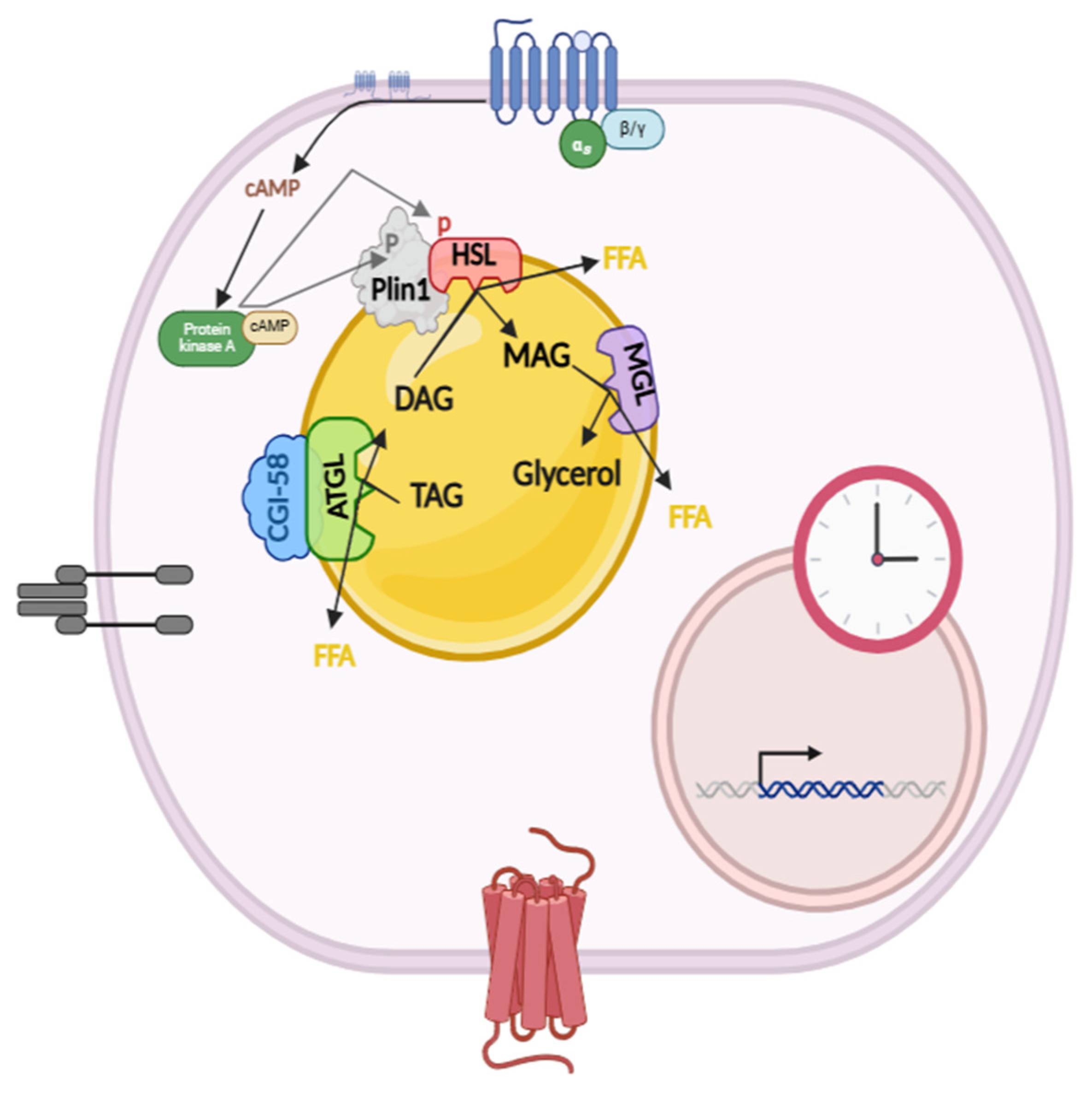
1. Introduction
2. Results
2.1. Rhythmicity of Lipolysis Protein Gene Expression and Plasma Glycerol and FFA Levels
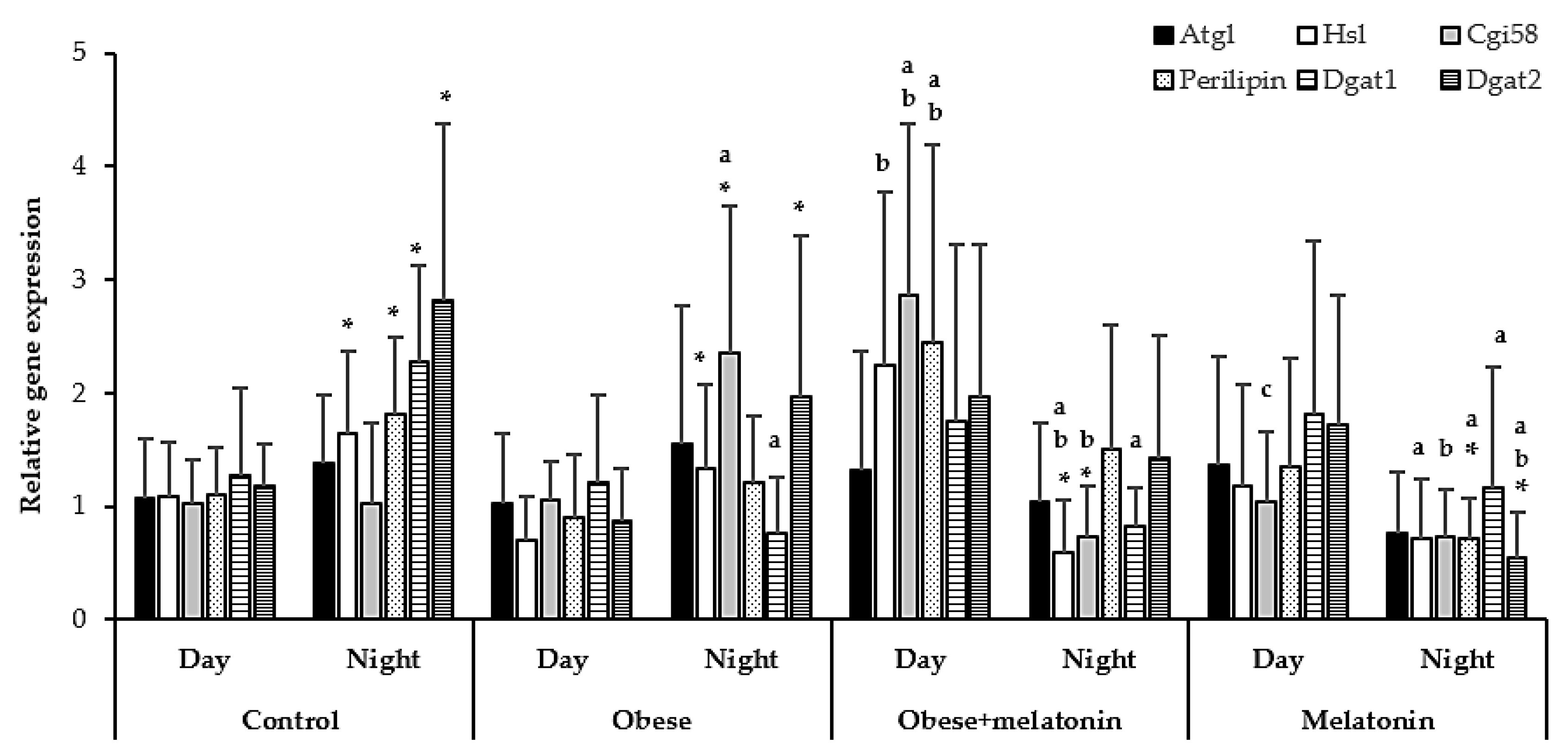
2.2. Melatonin Effects in Lipolysis mRNA Levels in Rat Mesenteric Adipose Tissue Are Diet and Photoperiod Dependent
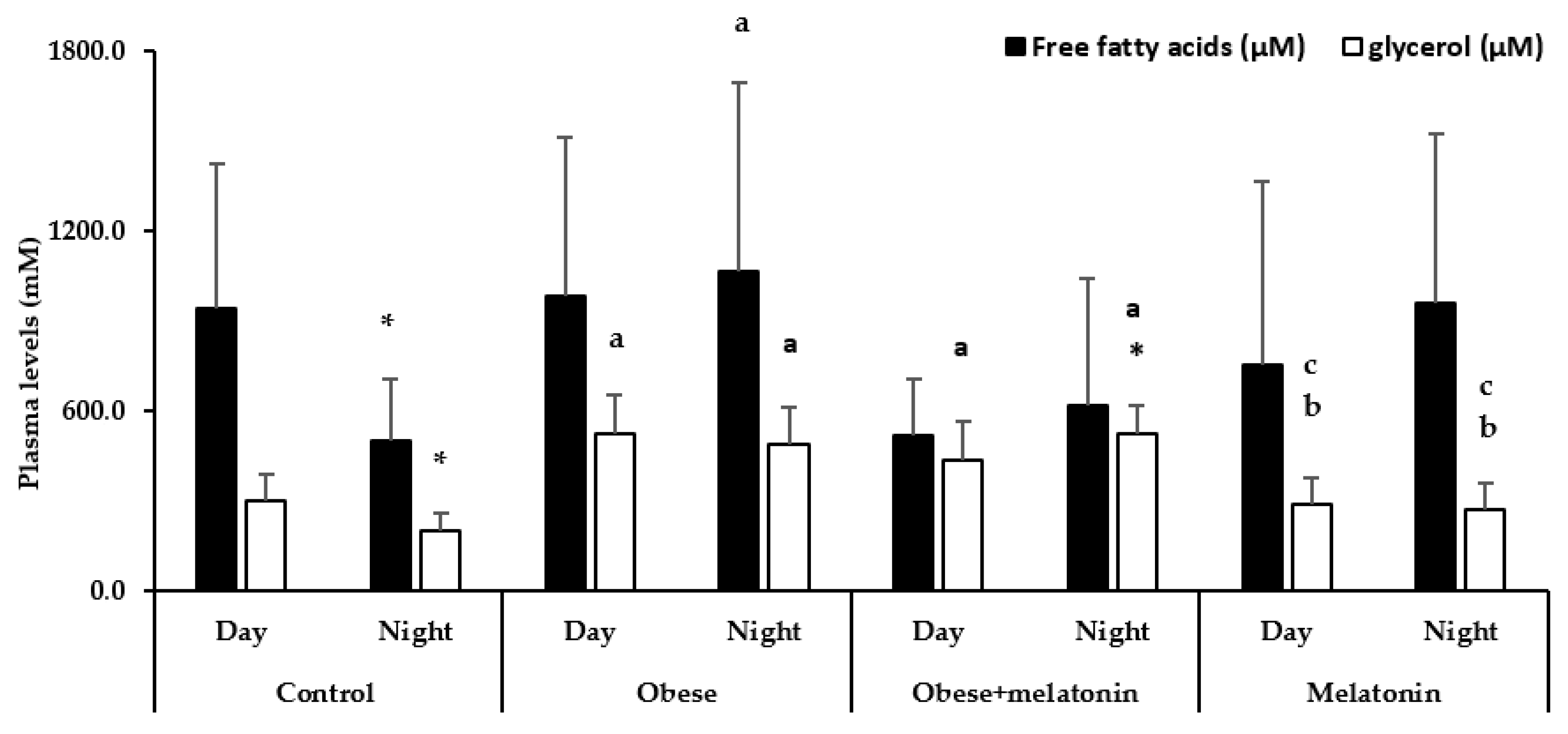
2.3. Melatonin Effects in Plasma FFA and Glycerol Levels in Rats Are Diet and Photoperiod Dependent
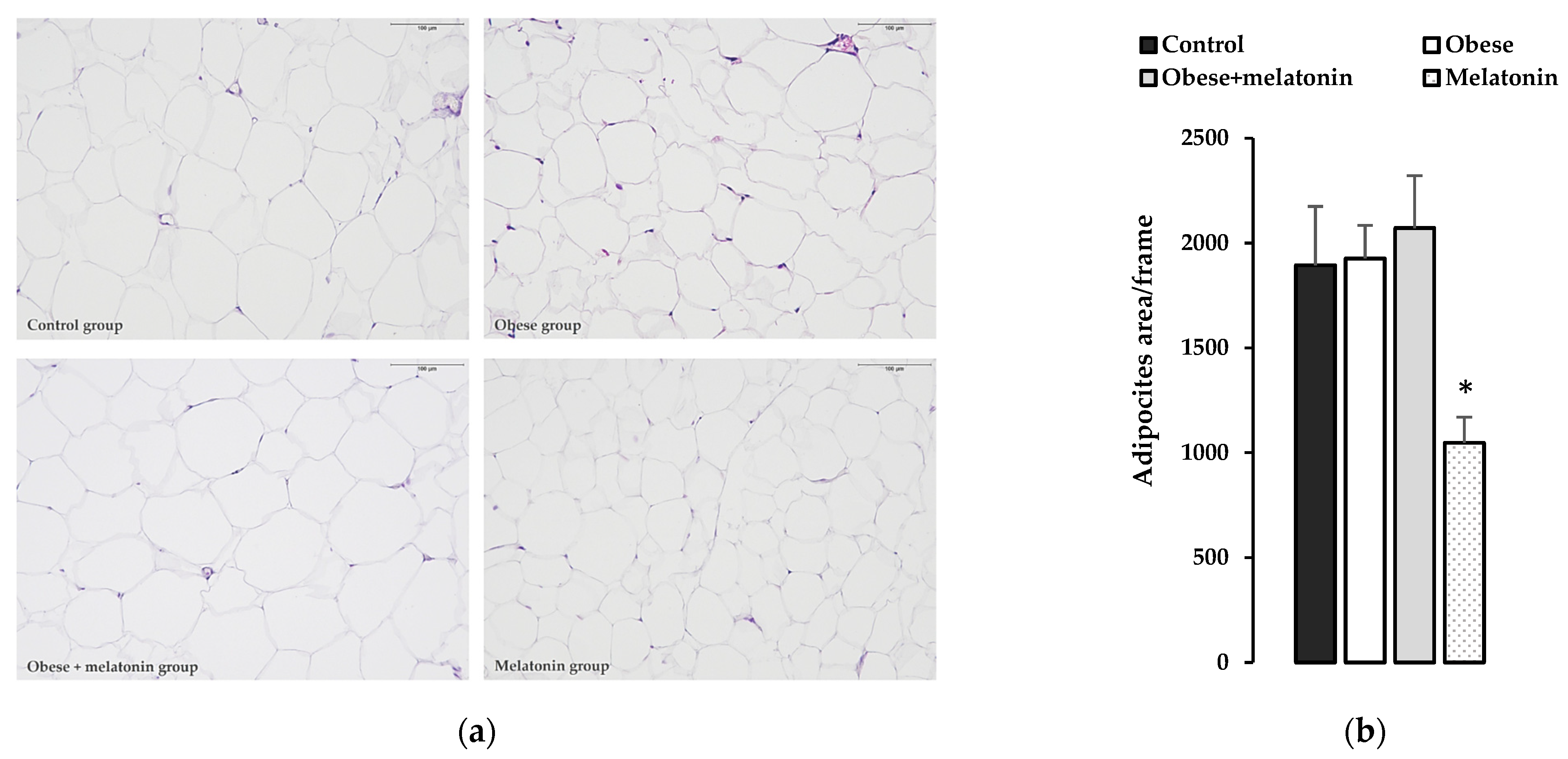
2.4. Morphological and Morphometric Study of the Effects of Melatonin on Rat Mesenteric Adipose Tissue
3. Discussion
3.1. Rhythmic Gene Expression of Lipolysis Proteins in Rat Mesenteric Adipose Tissue
3.2. Effect of HFD on Daily Expression of Lipolysis Genes in Rat Mesenteric Adipose Tissue
3.3. Effects of Melatonin on the Daily Expression of Lipolysis Genes in Mesenteric Adipose Tissue of Rats Fed a High-Fat Diet or Control Diet
- Cgi58, Perilipin and Dgat1 relative gene expression in rat mesenteric adipose tissue and plasma FFA and glycerol levels describe daily rhythms patterns.
- HFD disrupts lipolysis protein relative gene expression rhythms patterns and elevates plasma levels of that metabolism pathway’s products.
- Melatonin administration induces circadian rhythms in Atgl, Hsl, Cgi58, Perilipin, and Dgat1 relative gene expression in rat mesenteric adipose tissue, with an effect that is diet dependent.
- This treatment prevented fatty acid levels increase during nighttime in obese animals, suggesting its involvement in the regulation of cellular metabolism in rat mesenteric adipose tissue.
Limitations of the Study
4. Materials and Methods
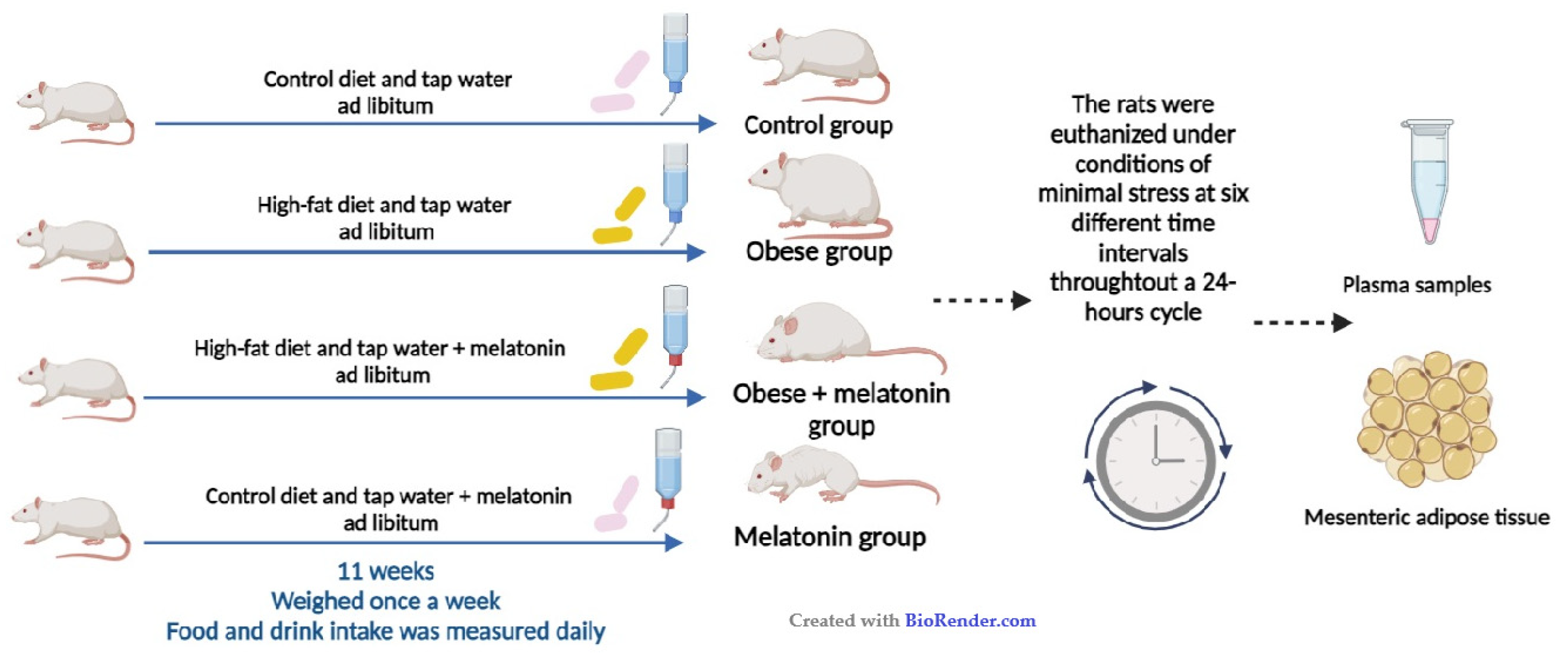
4.1. Animals and Experimental Design
4.2. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
4.3. Biochemical Assays
4.4. Sample Preparation for Histological Analysis Using Adiposoft
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, M.-J.; Wu, Y.; Fried, S.K. Adipose Tissue Heterogeneity: Implication of Depot Differences in Adipose Tissue for Obesity Complications. Mol. Aspects Med. 2013, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Thomou, T.; Zhu, Y.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and Metabolic Implications of Regional Differences among Fat Depots. Cell Metab. 2013, 17, 644–656. [Google Scholar] [CrossRef]
- Perez-Miguelsanz, J.; Jiménez-Ortega, V.; Cano-Barquilla, P.; Garaulet, M.; Esquifino, A.I.; Varela-Moreiras, G.; Fernández-Mateos, P. Early Appearance of Epicardial Adipose Tissue through Human Development. Nutrients 2021, 13, 2906. [Google Scholar] [CrossRef]
- Santillana, N.; Astudillo-Guerrero, C.; D’Espessailles, A.; Cruz, G. White Adipose Tissue Dysfunction: Pathophysiology and Emergent Measurements. Nutrients 2023, 15, 1722. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Blüher, M. Adipokines in Health and Disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Unger, R.H.; Scherer, P.E. Gluttony, Sloth and the Metabolic Syndrome: A Roadmap to Lipotoxicity. Trends Endocrinol. Metab. TEM 2010, 21, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Langin, D.; Dicker, A.; Tavernier, G.; Hoffstedt, J.; Mairal, A.; Rydén, M.; Arner, E.; Sicard, A.; Jenkins, C.M.; Viguerie, N.; et al. Adipocyte Lipases and Defect of Lipolysis in Human Obesity. Diabetes 2005, 54, 3190–3197. [Google Scholar] [CrossRef] [PubMed]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular Mechanisms for Lipid Mobilization from Fat Stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Raben, D.M.; Baldassare, J.J. A New Lipase in Regulating Lipid Mobilization: Hormone-Sensitive Lipase Is Not Alone. Trends Endocrinol. Metab. TEM 2005, 16, 35–36. [Google Scholar] [CrossRef]
- Lass, A.; Zimmermann, R.; Haemmerle, G.; Riederer, M.; Schoiswohl, G.; Schweiger, M.; Kienesberger, P.; Strauss, J.G.; Gorkiewicz, G.; Zechner, R. Adipose Triglyceride Lipase-Mediated Lipolysis of Cellular Fat Stores Is Activated by CGI-58 and Defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006, 3, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Cerk, I.K.; Wechselberger, L.; Oberer, M. Adipose Triglyceride Lipase Regulation: An Overview. Curr. Protein Pept. Sci. 2018, 19, 221–233. [Google Scholar] [CrossRef]
- Krintel, C.; Mörgelin, M.; Logan, D.T.; Holm, C. Phosphorylation of Hormone-Sensitive Lipase by Protein Kinase A in Vitro Promotes an Increase in Its Hydrophobic Surface Area. FEBS J. 2009, 276, 4752–4762. [Google Scholar] [CrossRef] [PubMed]
- Recazens, E.; Mouisel, E.; Langin, D. Hormone-Sensitive Lipase: Sixty Years Later. Prog. Lipid Res. 2021, 82, 101084. [Google Scholar] [CrossRef] [PubMed]
- Chitraju, C.; Walther, T.C.; Farese, R.V. The Triglyceride Synthesis Enzymes DGAT1 and DGAT2 Have Distinct and Overlapping Functions in Adipocytes. J. Lipid Res. 2019, 60, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Dong, H.H. FoxO Integration of Insulin Signaling with Glucose and Lipid Metabolism. J. Endocrinol. 2017, 233, R67–R79. [Google Scholar] [CrossRef]
- Xin, H.; Zhang, J.; Huang, R.; Li, L.; Lam, S.M.; Shui, G.; Deng, F.; Zhang, Z.; Li, M.-D. Circadian Signatures of Adipose Tissue in Diet-Induced Obesity. Front. Physiol. 2022, 13, 953237. [Google Scholar] [CrossRef]
- Cho, C.H.; Patel, S.; Rajbhandari, P. Adipose Tissue Lipid Metabolism: Lipolysis. Curr. Opin. Genet. Dev. 2023, 83, 102114. [Google Scholar] [CrossRef]
- Shostak, A.; Meyer-Kovac, J.; Oster, H. Circadian Regulation of Lipid Mobilization in White Adipose Tissues. Diabetes 2013, 62, 2195–2203. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133. [Google Scholar] [CrossRef] [PubMed]
- Civelek, E.; Ozturk Civelek, D.; Akyel, Y.K.; Kaleli Durman, D.; Okyar, A. Circadian Dysfunction in Adipose Tissue: Chronotherapy in Metabolic Diseases. Biology 2023, 12, 1077. [Google Scholar] [CrossRef]
- Albrecht, U. Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Markussen, L.K.; Rondini, E.A.; Johansen, O.S.; Madsen, J.G.S.; Sustarsic, E.G.; Marcher, A.-B.; Hansen, J.B.; Gerhart-Hines, Z.; Granneman, J.G.; Mandrup, S. Lipolysis Regulates Major Transcriptional Programs in Brown Adipocytes. Nat. Commun. 2022, 13, 3956. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, L.; Cai, Y.; Wang, X.; Wang, S.; Li, F. The Role of Circadian Clock in Regulating Cell Functions: Implications for Diseases. MedComm 2024, 5, e504. [Google Scholar] [CrossRef] [PubMed]
- Shostak, A.; Husse, J.; Oster, H. Circadian Regulation of Adipose Function. Adipocyte 2013, 2, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Klein, S. A Novel Link Between Circadian Clocks and Adipose Tissue Energy Metabolism. Diabetes 2013, 62, 2175–2177. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. Misalignment of Circadian Rhythms in Diet-Induced Obesity. Adv. Exp. Med. Biol. 2024, 1460, 27–71. [Google Scholar] [CrossRef]
- Hardeland, R.; Madrid, J.A.; Tan, D.-X.; Reiter, R.J. Melatonin, the Circadian Multioscillator System and Health: The Need for Detailed Analyses of Peripheral Melatonin Signaling. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Wang, M.; Jiang, Y.; Ge, Q.; Li, T.; Hou, Z.; Shi, P.; Yao, K.; Yin, J. Meta-Analysis and Machine Learning Reveal the Antiobesity Effects of Melatonin on Obese Rodents. Obes. Rev. 2024, 25, e13701. [Google Scholar] [CrossRef]
- Owino, S.; Buonfiglio, D.D.C.; Tchio, C.; Tosini, G. Melatonin Signaling a Key Regulator of Glucose Homeostasis and Energy Metabolism. Front. Endocrinol. 2019, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mateos, P.; Cano-Barquilla, P.; Jiménez-Ortega, V.; Virto, L.; Pérez-Miguelsanz, J.; Esquifino, A.I. Effect of Melatonin on Redox Enzymes Daily Gene Expression in Perirenal and Subcutaneous Adipose Tissue of a Diet Induced Obesity Model. Int. J. Mol. Sci. 2023, 24, 960. [Google Scholar] [CrossRef] [PubMed]
- Challet, E.; Pévet, P. Melatonin in Energy Control: Circadian Time-Giver and Homeostatic Monitor. J. Pineal Res. 2024, 76, e12961. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Lugo, M.J.; Cano, P.; Jiménez-Ortega, V.; Fernández-Mateos, M.P.; Scacchi, P.A.; Cardinali, D.P.; Esquifino, A.I. Melatonin Effect on Plasma Adiponectin, Leptin, Insulin, Glucose, Triglycerides and Cholesterol in Normal and High Fat–Fed Rats. J. Pineal Res. 2010, 49, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, Energy Metabolism, and Obesity: A Review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.A.P.; Gallo, C.C.; de Camargo, L.S.; de Carvalho, P.V.V.; Olesçuck, I.F.; Macedo, F.; da Cunha, F.M.; Cipolla-Neto, J.; do Amaral, F.G. Melatonin Multiple Effects on Brown Adipose Tissue Molecular Machinery. J. Pineal Res. 2019, 66, e12549. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Mechanisms of Melatonin in Obesity: A Review. Int. J. Mol. Sci. 2021, 23, 218. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; McFadden, J.W.; Yang, G.; Zhu, H.; Lian, H.; Fu, T.; Sun, Y.; Gao, T.; Li, M. Effect of Melatonin on Visceral Fat Deposition, Lipid Metabolism and Hepatic Lipo-Metabolic Gene Expression in Male Rats. J. Anim. Physiol. Anim. Nutr. 2021, 105, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, D.; Li, H.; Zhang, O.; Huang, Y.; Shao, H.; Wang, Y.; Cai, S.; Zhu, Y.; Jin, S.; et al. Suppression of Obesity by Melatonin through Increasing Energy Expenditure and Accelerating Lipolysis in Mice Fed a High-Fat Diet. Nutr. Diabetes 2022, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Brydon, L.; Petit, L.; Delagrange, P.; Strosberg, A.D.; Jockers, R. Functional Expression of MT2 (Mel1b) Melatonin Receptors in Human PAZ6 Adipocytes. Endocrinology 2001, 142, 4264–4271. [Google Scholar] [CrossRef]
- Boutin, J.A.; Jockers, R. Melatonin Controversies, an Update. J. Pineal Res. 2021, 70, e12702. [Google Scholar] [CrossRef]
- Garaulet, M.; Qian, J.; Florez, J.C.; Arendt, J.; Saxena, R.; Scheer, F.A.J.L. Melatonin Effects on Glucose Metabolism: Time to Unlock the Controversy. Trends Endocrinol. Metab. 2020, 31, 192–204. [Google Scholar] [CrossRef]
- Dollet, L.; Zierath, J.R. Interplay between Diet, Exercise and the Molecular Circadian Clock in Orchestrating Metabolic Adaptations of Adipose Tissue. J. Physiol. 2019, 597, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Cases, S.; Stone, S.J.; Zhou, P.; Yen, E.; Tow, B.; Lardizabal, K.D.; Voelker, T.; Farese, R.V. Cloning of DGAT2, a Second Mammalian Diacylglycerol Acyltransferase, and Related Family Members. J. Biol. Chem. 2001, 276, 38870–38876. [Google Scholar] [CrossRef] [PubMed]
- Ghibaudi, L.; Cook, J.; Farley, C.; van Heek, M.; Hwa, J.J. Fat Intake Affects Adiposity, Comorbidity Factors, and Energy Metabolism of Sprague-Dawley Rats. Obes. Res. 2002, 10, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Groop, L.C.; Saloranta, C.; Shank, M.; Bonadonna, R.C.; Ferrannini, E.; Defronzo, R.A. The Role of Free Fatty Acid Metabolism in the Pathogenesis of Insulin Resistance in Obesity and Noninsulin-Dependent Diabetes Mellitus. J. Clin. Endocrinol. Metab. 1991, 72, 96–107. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, J.; Chi, Y.; Zhang, F.; Xu, J.; Song, Y.; Cong, X.; Wu, N.; Liu, Y. Mesenteric Adipose Tissue Contributes to Intestinal Barrier Integrity and Protects against Nonalcoholic Fatty Liver Disease in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G659–G670. [Google Scholar] [CrossRef] [PubMed]
- Cano, P.; Cardinali, D.P.; Ríos-Lugo, M.J.; Fernández-Mateos, M.P.; Toso, C.F.R.; Esquifino, A.I. Effect of a High-Fat Diet on 24-Hour Pattern of Circulating Adipocytokines in Rats. Obesity 2009, 17, 1866–1871. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus—An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef] [PubMed]
- Bastías-Pérez, M.; Serra, D.; Herrero, L. Dietary Options for Rodents in the Study of Obesity. Nutrients 2020, 12, 3234. [Google Scholar] [CrossRef]
- Engin, A. Circadian Rhythms in Diet-Induced Obesity. Adv. Exp. Med. Biol. 2017, 960, 19–52. [Google Scholar] [CrossRef]
- Lewis, P.; Oster, H.; Korf, H.W.; Foster, R.G.; Erren, T.C. Food as a Circadian Time Cue—Evidence from Human Studies. Nat. Rev. Endocrinol. 2020, 16, 213–223. [Google Scholar] [CrossRef]
- Rodrigues, L.G.F.; de Araujo, L.D.; Roa, S.L.R.; Bueno, A.C.; Uchoa, E.T.; Antunes-Rodrigues, J.; Moreira, A.C.; Elias, L.L.K.; de Castro, M.; Martins, C.S. Restricted Feeding Modulates Peripheral Clocks and Nutrient Sensing Pathways in Rats. Arch. Endocrinol. Metab. 2021, 65, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.L.; Patel, V.R.; de Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag, S.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the Circadian Clock by Nutritional Challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Ngandiri, D.A.; Llerins Perez, M.; Wolf, A.; Wang, Y. The Molecular Brakes of Adipose Tissue Lipolysis. Front. Physiol. 2022, 13, 826314. [Google Scholar] [CrossRef] [PubMed]
- Wilfling, F.; Wang, H.; Haas, J.T.; Krahmer, N.; Gould, T.J.; Uchida, A.; Cheng, J.-X.; Graham, M.; Christiano, R.; Fröhlich, F.; et al. Triacylglycerol Synthesis Enzymes Mediate Lipid Droplet Growth by Relocalizing from the ER to Lipid Droplets. Dev. Cell 2013, 24, 384–399. [Google Scholar] [CrossRef]
- Amin, N.B.; Saxena, A.R.; Somayaji, V.; Dullea, R. Inhibition of Diacylglycerol Acyltransferase 2 Versus Diacylglycerol Acyltransferase 1: Potential Therapeutic Implications of Pharmacology. Clin. Ther. 2023, 45, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Sadie-Van Gijsen, H.; Kotzé-Hörstmann, L. Rat Models of Diet-Induced Obesity and Metabolic Dysregulation: Current Trends, Shortcomings and Considerations for Future Research. Obes. Res. Clin. Pract. 2023, 17, 449–457. [Google Scholar] [CrossRef]
- Yang, W.; Tang, K.; Wang, Y.; Zhang, Y.; Zan, L. Melatonin Promotes Triacylglycerol Accumulation via MT2 Receptor during Differentiation in Bovine Intramuscular Preadipocytes. Sci. Rep. 2017, 7, 15080. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Vale, M.I.C.; Andreotti, S.; Mukai, P.Y.; Borges-Silva, C.d.N.; Peres, S.B.; Cipolla-Neto, J.; Lima, F.B. Melatonin and the Circadian Entrainment of Metabolic and Hormonal Activities in Primary Isolated Adipocytes. J. Pineal Res. 2008, 45, 422–429. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Luo, D.; Sun, C. Melatonin Promotes Circadian Rhythm-Induced Proliferation through Clock/Histone Deacetylase 3/c-Myc Interaction in Mouse Adipose Tissue. J. Pineal Res. 2017, 62, e12383. [Google Scholar] [CrossRef] [PubMed]
- de Farias, T.d.S.M.; de Oliveira, A.C.; Andreotti, S.; do Amaral, F.G.; Chimin, P.; de Proença, A.R.A.; Torres Leal, F.L.; Sertié, R.A.L.; Campana, A.B.; Lopes, A.B.; et al. Pinealectomy Interferes with the Circadian Clock Genes Expression in White Adipose Tissue. J. Pineal Res. 2015, 58, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Tanaka, G.; Masuda, S.; Ogasawara, J.; Sakurai, T.; Kizaki, T.; Ohno, H.; Izawa, T. Melatonin Promotes Adipogenesis and Mitochondrial Biogenesis in 3T3-L1 Preadipocytes. J. Pineal Res. 2015, 59, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Liu, Q.; Xu, J.; Zeng, F.; Pang, X.; Jebessa, E.; Liang, S.; Nie, Q.; Zhang, X. Effects of Abhydrolase Domain Containing 5 Gene (ABHD5) Expression and Variations on Chicken Fat Metabolism. Poult. Sci. 2016, 95, 99–107. [Google Scholar] [CrossRef]
- de Farias, T.d.S.M.; Cruz, M.M.; de Sa, R.C.d.C.; Severi, I.; Perugini, J.; Senzacqua, M.; Cerutti, S.M.; Giordano, A.; Cinti, S.; Alonso-Vale, M.I.C. Melatonin Supplementation Decreases Hypertrophic Obesity and Inflammation Induced by High-Fat Diet in Mice. Front. Endocrinol. 2019, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Brasaemle, D.L. Thematic Review Series: Adipocyte Biology. The Perilipin Family of Structural Lipid Droplet Proteins: Stabilization of Lipid Droplets and Control of Lipolysis. J. Lipid Res. 2007, 48, 2547–2559. [Google Scholar] [CrossRef]
- Prunet-Marcassus, B.; Desbazeille, M.; Bros, A.; Louche, K.; Delagrange, P.; Renard, P.; Casteilla, L.; Pénicaud, L. Melatonin Reduces Body Weight Gain in Sprague Dawley Rats with Diet-Induced Obesity. Endocrinology 2003, 144, 5347–5352. [Google Scholar] [CrossRef] [PubMed]
- Terrón, M.P.; Delgado-Adámez, J.; Pariente, J.A.; Barriga, C.; Paredes, S.D.; Rodríguez, A.B. Melatonin Reduces Body Weight Gain and Increases Nocturnal Activity in Male Wistar Rats. Physiol. Behav. 2013, 118, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Seo, I.; Shim, H.; Cho, H. Melatonin Ameliorates SGLT2 Inhibitor-Induced Diabetic Ketoacidosis by Inhibiting Lipolysis and Hepatic Ketogenesis in Type 2 Diabetic Mice. J. Pineal Res. 2020, 68, e12623. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Cano Barquilla, P.; Pagano, E.S.; Jiménez-Ortega, V.; Fernández-Mateos, P.; Esquifino, A.I.; Cardinali, D.P. Melatonin Normalizes Clinical and Biochemical Parameters of Mild Inflammation in Diet-Induced Metabolic Syndrome in Rats. J. Pineal Res. 2014, 57, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Lugo, M.J.; Jiménez-Ortega, V.; Cano-Barquilla, P.; Mateos, P.F.; Spinedi, E.J.; Cardinali, D.P.; Esquifino, A.I. Melatonin Counteracts Changes in Hypothalamic Gene Expression of Signals Regulating Feeding Behavior in High-Fat Fed Rats. Horm. Mol. Biol. Clin. Investig. 2015, 21, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, M.; Campión, J.; Muñoz-Barrutia, A.; Boqué, N.; Moreno, H.; Martínez, J.A.; Milagro, F.; Ortiz-de-Solórzano, C. Adiposoft: Automated Software for the Analysis of White Adipose Tissue Cellularity in Histological Sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Serrano, A.; Arola-Arnal, A.; Suárez-García, S.; Bravo, F.I.; Suárez, M.; Arola, L.; Bladé, C. Grape Seed Proanthocyanidin Supplementation Reduces Adipocyte Size and Increases Adipocyte Number in Obese Rats. Int. J. Obes. 2017, 41, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- van der Spek, R.; Fliers, E.; la Fleur, S.E.; Kalsbeek, A. Daily Gene Expression Rhythms in Rat White Adipose Tissue Do Not Differ Between Subcutaneous and Intra-Abdominal Depots. Front. Endocrinol. 2018, 9, 206. [Google Scholar] [CrossRef] [PubMed]
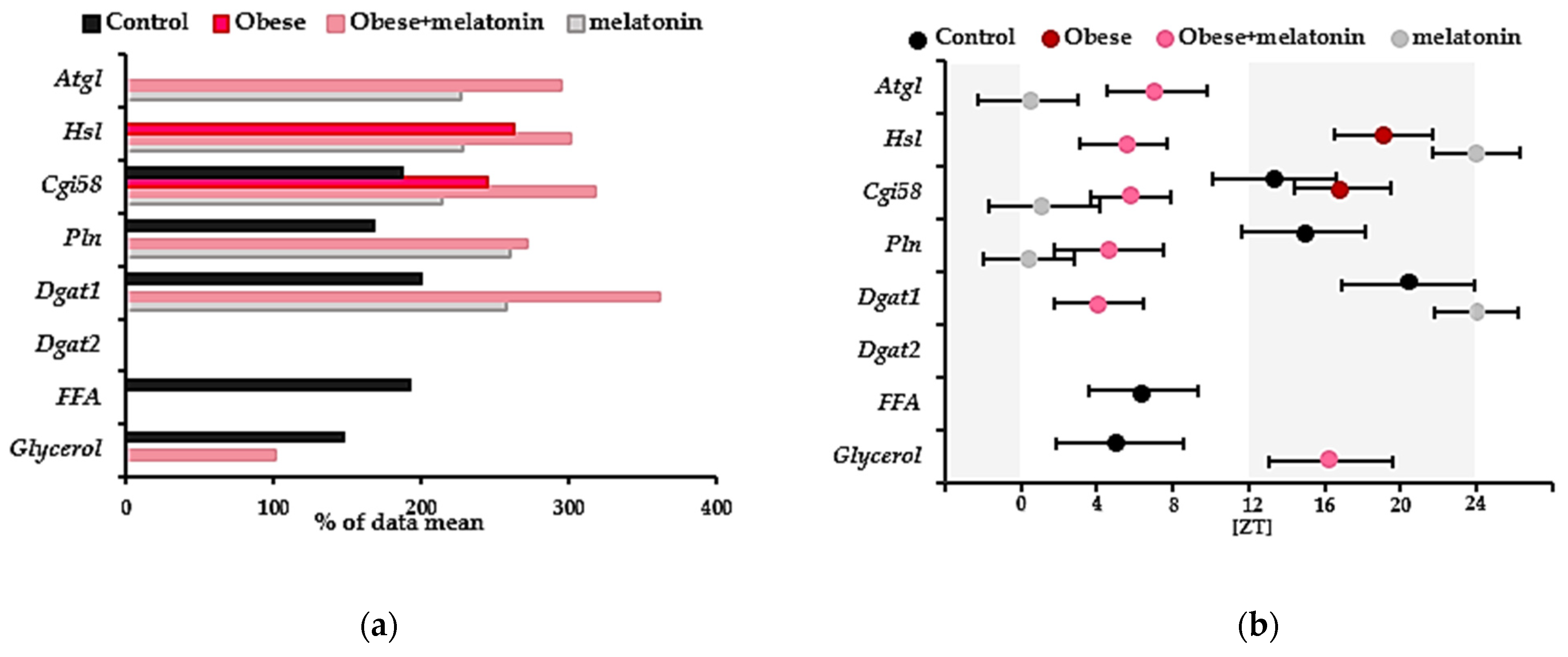
| Group | Analysis | Data Mean ± SD | ANOVA | CircWave | (CoG ± SD) Zeitgeber Time [ZT] | Amplitude (% of Data Mean) |
|---|---|---|---|---|---|---|
| Control | Atgl (mWAT) | 1.22 ± 0.57 (relative gene expression) | NS | NS | ||
| Hsl (mWAT) | 1.31 ± 0.677 (relative gene expression) | NS | NS | |||
| Cgi58 (mWAT) | 1.02 + 0.56 (relative gene expression) | F = 4.763 p = 0.006 | F = 4.916 p = 0.006 r2 = 0.508 * | 13.38 ± 3.27 | 186.27 | |
| Perilipin (mWAT) | 1.485 ± 0.66 (relative gene expression) | F = 2.915 p = 0.044 | F = 4.704 p = 0.021 r2 = 0.319 | 14.92 ± 3.25 | 167.68 | |
| Dgat1 (mWAT) | 1.70 ± 0.94 (relative gene expression) | F = 3.652 p = 0.019 | F = 5.736 p = 0.01 r2 = 0.364 | 20.41 ± 3.49 | 199.41 | |
| Dgat2 (mWAT) | 1.95 ± 1.35 (relative gene expression) | NS | NS | |||
| FFA (plasma) | 772.02 ± 446.70 (μM) | F = 3.086 p = 0.0438 | F = 4.910 p = 0.020 r2 = 0.366 | 6.46 ± 2.92 | 192.57 | |
| Glycerol (plasma) | 240.4 ± 80.5 (μM) | F = 5.975 p = 0.001 | F = 6.253 p = 0.006 r2 = 0.333 | 5.23 ± 3.34 | 147.46 | |
| Obese | Atgl (mWAT) | 1.30 ± 1.01 (relative gene expression) | NS | NS | ||
| Hsl (mWAT) | 1.02 ± 0.65 (relative gene expression) | F = 2.788 p = 0.049 | F = 6.497 p = 0.006 r2 = 0.382 | 19.08 ± 2.60 | 262.61 | |
| Cgi58 (mWAT) | 1.76 + 1.16 (relative gene expression) | F = 3.16 p = 0.031 | F = 6.660 p = 0.005 r2 = 0.388 | 16.93 ± 2.57 | 245.13 | |
| Perilipin (mWAT) | 1.07 ± 0.58 (relative gene expression) | NS | NS | |||
| Dgat1 (mWAT) | 9.72 ± 7.49 (relative gene expression) | NS | NS | |||
| Dgat2 (mWAT) | 1.44 ± 1.18 (relative gene expression) | NS | NS | |||
| FFA (plasma) | 1033.83 ± 586.04 (μM) | NS | NS | |||
| Glycerol (plasma) | 515.6 ± 124.3 (μM) | NS | NS | |||
| Obese + melatonin | Atgl (mWAT) | 1.13 ± 0.88 (relative gene expression) | F = 7.32 p = 0.0003 | F = 4.480 p = 0.021 r2 = 0.263 | 7.19 ± 2.78 | 294.07 |
| Hsl (mWAT) | 1.44 ± 1.36 (relative gene expression) | F = 5.844 p = 0.001 | F = 11.27 p = 0.0002 r2 = 0.464 | 5.42 ± 2.18 | 300.38 | |
| Cgi58 (mWAT) | 1.76 + 1.52 (relative gene expression) | F = 7.557 p = 0.0002 r2 = 0.621 | F = 20.006 p = 0.00005 r2 = 0.606 | 5.78 ± 2.12 | 317.98 | |
| Perilipin (mWAT) | 1.95 ± 1.50 (relative gene expression) | F = 4.182 p = 0.007 | F = 3.982 p = 0.031 r2 = 0.234 | 4.60 ± 2.88 | 271.44 | |
| Dgat1 (mWAT) | 1.31 + 1.24 (relative gene expression) | F = 5.97 p = 0.002 | F = 4.178 p = 0.029 r2 = 0.284 | 4.13 + 2.34 | 360.61 | |
| Dgat2 (mWAT) | 10.24 ± 7.52 (relative gene expression) | NS | NS | |||
| FFA (plasma) | 579.21 ± 341.31 (μM) | NS | NS | |||
| Glycerol (plasma) | 481.4 ± 110.2 (μM) | F = 3.492 p = 0.018 | F = 3.659 p = 0.040 r2 = 0.233 | 16.325 ± 3.23 | 100.50 | |
| Melatonin | Atgl (mWAT) | 1.03 ± 0.80 (relative gene expression) | F = 3.555 p = 0.017 | F = 4.489 p = 0.022 r2 = 0.272 | 0.37 ± 2.64 | 227 |
| Hsl (mWAT) | 0.93 ± 0.73 (relative gene expression) | F = 3.64 p = 0.016 | F = 6.397 p = 0.006 r2 = 0.357 | 23.97 ± 2.28 | 228.34 | |
| Cgi58 (mWAT) | 0.87 + 0.55 (relative gene expression) | F = 3.996 p = 0.012 | F = 5.254 p = 0.004 r2 = 0.512 * | 1.20 + 2.92 | 213.56 | |
| Perilipin (mWAT) | 1.006 ± 0.75 (relative gene expression) | F = 5.756 p = 0.001 | F = 6.060 p = 0.007 r2 = 0.345 | 0.41 ± 2.40 | 259.64 | |
| Dgat1 (mWAT) | 1.46 ± 1.31 (relative gene expression) | F = 4.05 p = 0.01 | F = 9.651 p = 0.0009 r2 = 0.456 | 23.97 ± 2.19 | 257.95 | |
| Dgat2 (mWAT) | 1.12 ± 1.03 (relative gene expression) | NS | NS | |||
| FFA (plasma) | 861.53 ± 587.06 (μM) | NS | NS | |||
| Glycerol (plasma) | 283.2 ± 82.4 (μM) | F = 6.8 p = 0.0005 | NS |
| Gene | Primers |
|---|---|
| b-actina | Forward 5′-ccagatcatgttgagaccttcaa-3′ |
| Backward 5′-ccagaggcgtacaggatagc-3′ | |
| Atgl | Forward 5′-ggatgaaggagcagacagct-3′ |
| Backward 5′-ggcacagacggcagagac-3′ | |
| Hsl | Forward 5′-aacactacaaacgcaacgaga-3′ |
| Backward 5′-attcagccccacgcaact-3′ | |
| Cgi58 | Forward 5′-cactgcgacccaagtcatac-3′ |
| Backward 5′-ttgaactcctctggctggt-3′ | |
| Perilipin | Forward 5′-atctcctgccaccagacaag-3′ |
| Backward 5′-gtgctgaccctcctcaaaag-3′ | |
| Dgat1 | Forward 5′-catcggcgggttcttgag-3′ |
| Backward 5′-tagggaccatccactgctg-3′ | |
| Dgat2 | Forward 5′-gcagcgagaacaagaataaagg-3′ |
| Backward 5′-ttgagccaggtgacagagaa-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Barquilla, P.; Jiménez-Ortega, V.; Fernández-Mateos, P.; Virto, L.; Maldonado Bautista, E.; Perez-Miguelsanz, J.; Esquifino, A.I. Daily Lipolysis Gene Expression in Male Rat Mesenteric Adipose Tissue: Obesity and Melatonin Effects. Int. J. Mol. Sci. 2025, 26, 577. https://doi.org/10.3390/ijms26020577
Cano-Barquilla P, Jiménez-Ortega V, Fernández-Mateos P, Virto L, Maldonado Bautista E, Perez-Miguelsanz J, Esquifino AI. Daily Lipolysis Gene Expression in Male Rat Mesenteric Adipose Tissue: Obesity and Melatonin Effects. International Journal of Molecular Sciences. 2025; 26(2):577. https://doi.org/10.3390/ijms26020577
Chicago/Turabian StyleCano-Barquilla, Pilar, Vanesa Jiménez-Ortega, Pilar Fernández-Mateos, Leire Virto, Estela Maldonado Bautista, Juliana Perez-Miguelsanz, and Ana I. Esquifino. 2025. "Daily Lipolysis Gene Expression in Male Rat Mesenteric Adipose Tissue: Obesity and Melatonin Effects" International Journal of Molecular Sciences 26, no. 2: 577. https://doi.org/10.3390/ijms26020577
APA StyleCano-Barquilla, P., Jiménez-Ortega, V., Fernández-Mateos, P., Virto, L., Maldonado Bautista, E., Perez-Miguelsanz, J., & Esquifino, A. I. (2025). Daily Lipolysis Gene Expression in Male Rat Mesenteric Adipose Tissue: Obesity and Melatonin Effects. International Journal of Molecular Sciences, 26(2), 577. https://doi.org/10.3390/ijms26020577







