Two- and Three-Dimensional In Vitro Models of Parkinson’s and Alzheimer’s Diseases: State-of-the-Art and Applications
Abstract
:1. Introduction
2. Parkinson’s and Alzheimer’s Disease: The Most Prevalent Neurodegenerative Diseases
3. Two-Dimensional In Vitro Models to Study Parkinson’s and Alzheimer’s Disease
3.1. Immortalised Cell Lines
3.1.1. SH-SY5Y Cells
3.1.2. PC12 Cells
3.1.3. LUHMES Cells
3.2. iPSC-Derived Cells
3.2.1. iPSC-Derived Neurons
3.2.2. iPSC-Derived Glial Cells
3.3. Neurons Directly Derived from Somatic Cells
3.4. Advantages and Disadvantages of 2D In Vitro Models
4. Three-Dimensional In Vitro Models of Parkinson’s and Alzheimer’s Disease
4.1. Organoid Models
4.1.1. Midbrain Organoids for Parkinson’s Disease
4.1.2. Brain Organoids for Alzheimer’s Disease
4.1.3. Advantages and Disadvantages of Organoid Models
4.2. Engineering-Based 3D Models
5. Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | amyloid-β |
| ACh | cholinergic |
| AD | Alzheimer’s disease |
| APP | amyloid precursor protein |
| DA | dopaminergic |
| GSK-3β | glycogen synthase kinase-3β |
| iNs | induced neurons |
| iPSCs | induced pluripotent stem cells |
| ipsNSCs | neural stem cells differentiated from iPSCs |
| LRRK2 | leucine rich repeat kinase 2 |
| LUHMES | Lund human mesencephalic |
| MO | midbrain organoid |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydroperydine |
| NFT | neurofibrillary tangles |
| ND | neurodegenerative disease |
| PINK1 | PTEN-induced kinase 1 |
| PRKN | Parkin |
| PD | Parkinson’s disease |
| PP1 | phosphatase 1 |
| PSEN | presenilin |
| RA | retinoic acid |
| SNCA | synuclein alpha |
| VPS35 | vacuolar sorting protein 35 |
| 6-OHDA | 6-hydroxydopamine |
References
- Heemels, M.-T. Neurodegenerative diseases. Nature 2016, 539, 179. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Cookson, M.R.; Bosch, L.V.D.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Kesidou, E.; Theotokis, P.; Damianidou, O.; Boziki, M.; Konstantinidou, N.; Taloumtzis, C.; Sintila, S.-A.; Grigoriadis, P.; Evangelopoulos, M.E.; Bakirtzis, C.; et al. CNS Ageing in Health and Neurodegenerative Disorders. J. Clin. Med. 2023, 12, 2255. [Google Scholar] [CrossRef]
- Dejanovic, B.; Sheng, M.; Hanson, J.E. Targeting synapse function and loss for treatment of neurodegenerative diseases. Nat. Rev. Drug Discov. 2023, 23, 23–42. [Google Scholar] [CrossRef]
- Toader, C.; Dobrin, N.; Brehar, F.-M.; Popa, C.; Covache-Busuioc, R.-A.; Glavan, L.A.; Costin, H.P.; Bratu, B.-G.; Corlatescu, A.D.; Popa, A.A.; et al. From Recognition to Remedy: The Significance of Biomarkers in Neurodegenerative Disease Pathology. Int. J. Mol. Sci. 2023, 24, 16119. [Google Scholar] [CrossRef] [PubMed]
- Damianidou, E.; Mouratidou, L.; Kyrousi, C. Research models of neurodevelopmental disorders: The right model in the right place. Front. Neurosci. 2022, 16, 1031075. [Google Scholar] [CrossRef]
- Solana-Manrique, C.; Moltó, M.D.; Calap-Quintana, P.; Sanz, F.J.; Llorens, J.V.; Paricio, N. Drosophila as a Model System for the Identification of Pharmacological Therapies in Neurodegenerative Diseases. In Insights into Human Neurodegeneration: Lessons Learnt from Drosophila; Springer: Singapore, 2019; pp. 433–467. [Google Scholar] [CrossRef]
- Dovonou, A.; Bolduc, C.; Linan, V.S.; Gora, C.; Iii, M.R.P.; Lévesque, M. Animal models of Parkinson’s disease: Bridging the gap between disease hallmarks and research questions. Transl. Neurodegener. 2023, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Pingale, T.; Gupta, G.L. Classic and evolving animal models in Parkinson’s disease. Pharmacol. Biochem. Behav. 2020, 199, 173060. [Google Scholar] [CrossRef]
- Polis, B.; Samson, A.O. Addressing the Discrepancies Between Animal Models and Human Alzheimer’s Disease Pathology: Implications for Translational Research. J. Alzheimer’s Dis. 2024, 98, 1199–1218. [Google Scholar] [CrossRef]
- Pereira, I.; Lopez-Martinez, M.J.; Samitier, J. Advances in current in vitro models on neurodegenerative diseases. Front. Bioeng. Biotechnol. 2023, 11, 1260397. [Google Scholar] [CrossRef] [PubMed]
- Silva-Pedrosa, R.; Salgado, A.J.; Ferreira, P.E. Revolutionizing Disease Modeling: The Emergence of Organoids in Cellular Systems. Cells 2023, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- di Domenico, A.; Carola, G.; Calatayud, C.; Pons-Espinal, M.; Muñoz, J.P.; Richaud-Patin, Y.; Fernandez-Carasa, I.; Gut, M.; Faella, A.; Parameswaran, J.; et al. Patient-Specific iPSC-Derived Astrocytes Contribute to Non-Cell-Autonomous Neurodegeneration in Parkinson’s Disease. Stem Cell Rep. 2019, 12, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ha, S.; Abekura, F.; Lim, H.; Chang, Y.; Lee, M.; Lee, M.; Lee, Y.; Kim, C. 4-O-carboxymethylascochlorin protected against microglial-mediated neurotoxicity in SH-SY5Y and BV2 cocultured cells from LPS–induced neuroinflammation and death by inhibiting MAPK, NF-κB, and Akt pathways. J. Cell Biochem. 2018, 120, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Roodveldt, C.; Bernardino, L.; Oztop-Cakmak, O.; Dragic, M.; E Fladmark, K.; Ertan, S.; Aktas, B.; Pita, C.; Ciglar, L.; Garraux, G.; et al. The immune system in Parkinson’s disease: What we know so far. Brain 2024, 147, 3306–3324. [Google Scholar] [CrossRef]
- Poewe, W. Parkinson disease Primer—A true team effort. Nat. Rev. Dis. Primers 2020, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.; Dawson, V.L.; Dawson, T.M. Trumping neurodegeneration: Targeting common pathways regulated by autosomal recessive Parkinson’s disease genes. Exp. Neurol. 2017, 298, 191–201. [Google Scholar] [CrossRef]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The pathogenesis of Parkinson’s disease. Lancet 2024, 403, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Blood–Brain Barrier Dysfunction as a Cause and Consequence of Alzheimer’s Disease. J. Cereb. Blood Flow Metab. 2013, 33, 1500–1513. [Google Scholar] [CrossRef]
- Niikura, T.; Tajima, H.; Kita, Y. Neuronal Cell Death in Alzheimer’s Disease and a Neuroprotective Factor, Humanin. Curr. Neuropharmacol. 2006, 4, 139–147. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Craft, S.; Cholerton, B.; Baker, L.D. Insulin and Alzheimer’s Disease: Untangling the Web. J. Alzheimer’s Dis. 2012, 33, S263–S275. [Google Scholar] [CrossRef] [PubMed]
- Sędzikowska, A.; Szablewski, L. Insulin and Insulin Resistance in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhi, W.; Wang, L. Role of Tau Protein in Neurodegenerative Diseases and Development of Its Targeted Drugs: A Literature Review. Molecules 2024, 29, 2812. [Google Scholar] [CrossRef]
- Galizzi, G.; Di Carlo, M. Mitochondrial DNA and Inflammation in Alzheimer’s Disease. Curr. Issues Mol. Biol. 2023, 45, 8586–8606. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Leonoudakis, D.; Rane, A.; Angeli, S.; Lithgow, G.J.; Andersen, J.K.; Chinta, S.J. Anti-Inflammatory and Neuroprotective Role of Natural Product Securinine in Activated Glial Cells: Implications for Parkinson’s Disease. Mediat. Inflamm. 2017, 2017, 8302636. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.; Clouaire, T.; Bao, X.X.; Kemp, S.E.; Xenophontos, M.; de Las Heras, J.I.; Stancheva, I. Immortality, but not oncogenic transformation, of primary human cells leads to epigenetic reprogramming of DNA methylation and gene expression. Nucleic Acids Res. 2014, 42, 3529–3541. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Graf, T.; Enver, T. Forcing cells to change lineages. Nature 2009, 462, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Meng, X.; Yu, X.; Wang, G.; Dong, Z.; Zhou, Z.; Qi, M.; Yu, X.; Ji, T.; Wang, F. From 2D to 3D Co-Culture Systems: A Review of Co-Culture Models to Study the Neural Cells Interaction. Int. J. Mol. Sci. 2022, 23, 13116. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y Cell Line in Parkinson’s Disease Research: A Systematic Review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Ioghen, O.C.; Ceafalan, L.C.; Popescu, B.O. SH-SY5Y Cell Line In Vitro Models for Parkinson Disease Research-Old Practice for New Trends. J. Integr. Neurosci. 2023, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, X.; Zhao, L.; Yang, C.; Pan, L.; Li, C.; Liu, K.; Bai, G.; Gao, H.; Yan, Z. Metabolic Disturbances in the Striatum and Substantia Nigra in the Onset and Progression of MPTP-Induced Parkinsonism Model. Front. Neurosci. 2018, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Sanz, F.J.; Solana-Manrique, C.; Muñoz-Soriano, V.; Calap-Quintana, P.; Moltó, M.D.; Paricio, N. Identification of potential therapeutic compounds for Parkinson’s disease using Drosophila and human cell models. Free Radic. Biol. Med. 2017, 108, 683–691. [Google Scholar] [CrossRef]
- Bell, M.; Zempel, H. SH-SY5Y-derived neurons: A human neuronal model system for investigating TAU sorting and neuronal subtype-specific TAU vulnerability. Prog. Neurobiol. 2021, 33, 1–15. [Google Scholar] [CrossRef]
- de Medeiros, L.M.; De Bastiani, M.A.; Rico, E.P.; Schonhofen, P.; Pfaffenseller, B.; Wollen-haupt-Aguiar, B.; Grun, L.; Barbé-Tuana, F.; Zimmer, E.R.; Castro, M.A.A.; et al. Cholinergic Differentiation of Human Neuroblastoma SH-SY5Y Cell Line and Its Potential Use as an In Vitro Model for Alzheimer’s Disease Studies. Mol. Neurobiol. 2019, 56, 7355–7367. [Google Scholar] [CrossRef]
- Chiu, Y.-J.; Lin, C.-H.; Lee, M.-C.; Hsieh-Li, H.M.; Chen, C.-M.; Wu, Y.-R.; Chang, K.-H.; Lee-Chen, G.-J. Formulated Chinese medicine Shaoyao Gancao Tang reduces NLRP1 and NLRP3 in Alzheimer’s disease cell and mouse models for neuroprotection and cognitive improvement. Aging 2021, 13, 15620–15637. [Google Scholar] [CrossRef]
- Maiuolo, J.; Costanzo, P.; Masullo, M.; D’errico, A.; Nasso, R.; Bonacci, S.; Mollace, V.; Oliverio, M.; Arcone, R. Hydroxytyrosol–Donepezil Hybrids Play a Protective Role in an In Vitro Induced Alzheimer’s Disease Model and in Neuronal Differentiated Human SH-SY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2023, 24, 13461. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, S.; Alsanie, W.F.; Khan, Z.N.; I Albalawi, H.; I Felimban, R.; Moretti, M.; Steiner, N.; Chaudhary, A.G.; A E Hauser, C. A Parkinson’s disease model composed of 3D bioprinted dopaminergic neurons within a biomimetic peptide scaffold. Biofabrication 2022, 14, 044103. [Google Scholar] [CrossRef]
- Greene, L.A.; Tischler, A. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef] [PubMed]
- Brodaty, H.; Breteler, M.M.B.; DeKosky, S.T.; Dorenlot, P.; Fratiglioni, L.; Hock, C.; Kenigsberg, P.-A.; Scheltens, P.; De Strooper, B. The World of Dementia Beyond 2020. J. Am. Geriatr. Soc. 2011, 59, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Abud, E.M.; Ramirez, R.N.; Martinez, E.S.; Healy, L.M.; Nguyen, C.H.H.; Newman, S.A.; Yeromin, A.V.; Scarfone, V.M.; Marsh, S.E.; Fimbres, C.; et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 2017, 94, 278–293.e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, Q.; Cao, B.; Yuan, Y.; Wen, S.; Liu, Z. Cadmium induces mitophagy via AMP-activated protein kinases activation in a PINK1/Parkin-dependent manner in PC12 cells. Cell Prolif. 2020, 53, e12817. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Xiao, J.; Yang, L.; Ye, F.; Cao, J.; Sai, Y. Mutual Antagonism of PINK1/Parkin and PGC-1α Contributes to Maintenance of Mitochondrial Homeostasis in Rotenone-Induced Neurotoxicity. Neurotox. Res. 2019, 35, 331–343. [Google Scholar] [CrossRef]
- Migheli, R.; Del Giudice, M.G.; Spissu, Y.; Sanna, G.; Xiong, Y.; Dawson, T.M.; Dawson, V.L.; Galioto, M.; Rocchitta, G.; Biosa, A.; et al. LRRK2 Affects Vesicle Trafficking, Neurotransmitter Extracellular Level and Membrane Receptor Localization. PLoS ONE 2013, 8, e77198. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, K.; Amirfazli, M.; Moghimi, P.; Safari, F.; Takhshid, M.A. The role of neuron-like cell lines and primary neuron cell models in unraveling the complexity of neurodegenerative diseases: A comprehensive review. Mol. Biol. Rep. 2024, 51, 1024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Yin, M.; Zhang, M.-H. Cell-based assays for Parkinson’s disease using differentiated human LUHMES cells. Acta Pharmacol. Sin. 2014, 35, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Lotharius, J.; Falsig, J.; van Beek, J.; Payne, S.; Dringen, R.; Brundin, P.; Leist, M. Progressive Degeneration of Human Mesencephalic Neuron-Derived Cells Triggered by Dopamine-Dependent Oxidative Stress Is Dependent on the Mixed-Lineage Kinase Pathway. J. Neurosci. 2005, 25, 6329–6342. [Google Scholar] [CrossRef]
- Schildknecht, S.; Pöltl, D.; Nagel, D.M.; Matt, F.; Scholz, D.; Lotharius, J.; Schmieg, N.; Salvo-Vargas, A.; Leist, M. Requirement of a dopaminergic neuronal phenotype for toxicity of low concentrations of 1-methyl-4-phenylpyridinium to human cells. Toxicol. Appl. Pharmacol. 2009, 241, 23–35. [Google Scholar] [CrossRef]
- Beliakov, S.V.; Blokhin, V.; Surkov, S.A.; Ugrumov, M.V. LUHMES Cells: Phenotype Refinement and Development of an MPP+-Based Test System for Screening Antiparkinsonian Drugs. Int. J. Mol. Sci. 2023, 24, 733. [Google Scholar] [CrossRef]
- Lal, R.; Singh, A.; Watts, S.; Chopra, K. Experimental models of Parkinson’s disease: Challenges and Opportunities. Eur. J. Pharmacol. 2024, 980, 176819. [Google Scholar] [CrossRef] [PubMed]
- Scholz, D.; Chernyshova, Y.; Leist, M. Control of Aβ release from human neurons by differentiation status and RET signaling. Neurobiol. Aging 2012, 34, 184–199. [Google Scholar] [CrossRef]
- Ratcliffe, L.E.; Villaseñor, I.V.; Jennings, L.; Heath, P.R.; Mortiboys, H.; Schwartzentruber, A.; Karyka, E.; Simpson, J.E.; Ince, P.G.; Garwood, C.J.; et al. Loss of IGF1R in Human Astrocytes Alters Complex I Activity and Support for Neurons. Neuroscience 2018, 390, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Haake, K.; Ackermann, M.; Lachmann, N. Concise Review: Towards the Clinical Translation of Induced Pluripotent Stem Cell-Derived Blood Cells—Ready for Take-Off. Stem Cells Transl. Med. 2018, 8, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Morizane, A.; Doi, D.; Magotani, H.; Onoe, H.; Hayashi, T.; Mizuma, H.; Takara, S.; Takahashi, R.; Inoue, H.; et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 2017, 548, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Shaltouki, A.; Sivapatham, R.; Pei, Y.; Gerencser, A.A.; Momčilović, O.; Rao, M.S.; Zeng, X. Mitochondrial Alterations by PARKIN in Dopaminergic Neurons Using PARK2 Patient-Specific and PARK2 Knockout Isogenic iPSC Lines. Stem Cell Rep. 2015, 4, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Santiago, R.; Carballo-Carbajal, I.; Castellano, G.; Torrent, R.; Richaud, Y.; Sánchez-Danés, A.; Vilarrasa-Blasi, R.; Sánchez-Pla, A.; Mosquera, J.L.; Soriano, J.; et al. Aberrant epigenome in iPSC -derived dopaminergic neurons from Parkinson’s disease patients. EMBO Mol. Med. 2015, 7, 1529–1546. [Google Scholar] [CrossRef]
- Liu, G.-H.; Qu, J.; Suzuki, K.; Nivet, E.; Li, M.; Montserrat, N.; Yi, F.; Xu, X.; Ruiz, S.; Zhang, W.; et al. Progressive degeneration of human neural stem cells caused by pathogenic LRRK2. Nature 2012, 491, 603–607. [Google Scholar] [CrossRef]
- Israel, M.A.; Yuan, S.H.; Bardy, C.; Reyna, S.M.; Mu, Y.; Herrera, C.; Hefferan, M.P.; Van Gorp, S.; Nazor, K.L.; Boscolo, F.S.; et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 2012, 482, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Dolatabadi, N.; Trudler, D.; Zhang, X.; Wu, Y.; Mohata, M.; Ambasudhan, R.; Talantova, M.; ALipton, S. Mechanisms of hyperexcitability in Alzheimer’s disease hiPSC-derived neurons and cerebral organoids vs. isogenic controls. eLife 2019, 8, e50333. [Google Scholar] [CrossRef]
- Marei, H.E.; Khan, M.U.A.; Hasan, A. Potential use of iPSCs for disease modeling, drug screening, and cell-based therapy for Alzheimer’s disease. Cell. Mol. Biol. Lett. 2023, 28, 98. [Google Scholar] [CrossRef]
- Lagomarsino, V.N.; Pearse, R.V.; Liu, L.; Hsieh, Y.-C.; Fernandez, M.A.; Vinton, E.A.; Paull, D.; Felsky, D.; Tasaki, S.; Gaiteri, C.; et al. Stem cell-derived neurons reflect features of protein networks, neuropathology, and cognitive outcome of their aged human donors. Neuron 2021, 109, 3402–3420.e9. [Google Scholar] [CrossRef] [PubMed]
- Hanslik, K.L.; Marino, K.M.; Ulland, T.K. Modulation of Glial Function in Health, Aging, and Neurodegenerative Disease. Front. Cell. Neurosci. 2021, 15, 718324. [Google Scholar] [CrossRef] [PubMed]
- Gerasimova, T.; Stepanenko, E.; Novosadova, L.; Arsenyeva, E.; Shimchenko, D.; Tarantul, V.; Grivennikov, I.; Nenasheva, V.; Novosadova, E. Glial Cultures Differentiated from iPSCs of Patients with PARK2-Associated Parkinson’s Disease Demonstrate a Pro-Inflammatory Shift and Reduced Response to TNFα Stimulation. Int. J. Mol. Sci. 2023, 24, 2000. [Google Scholar] [CrossRef]
- Yarkova, E.S.; Grigor’eva, E.V.; Medvedev, S.P.; Pavlova, S.V.; Zakian, S.M.; Malakhova, A.A. IPSC-Derived Astrocytes Contribute to In Vitro Modeling of Parkinson’s Disease Caused by the GBA1 N370S Mutation. Int. J. Mol. Sci. 2023, 25, 327. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Wang, Y. TREM2 variants: New keys to decipher Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 2016, 17, 201–207. [Google Scholar] [CrossRef]
- Speicher, A.M.; Wiendl, H.; Meuth, S.G.; Pawlowski, M. Generating microglia from human pluripotent stem cells: Novel in vitro models for the study of neurodegeneration. Mol. Neurodegener. 2019, 14, 46. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.-L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 2018, 98, 1141–1154.e7. [Google Scholar] [CrossRef] [PubMed]
- Haenseler, W.; Rajendran, L. Concise Review: Modeling Neurodegenerative Diseases with Human Pluripotent Stem Cell-Derived Microglia. Stem Cells 2019, 37, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.D.; Ulland, T.K.; Colonna, M.; Holtzman, D.M. Elucidating the Role of TREM2 in Alzheimer’s Disease. Neuron 2017, 94, 237–248. [Google Scholar] [CrossRef]
- Aversano, S.; Caiazza, C.; Caiazzo, M. Induced Pluripotent Stem Cell-Derived and Directly Reprogrammed Neurons to Study Neurodegenerative Diseases: The Impact of Aging Signatures. Front. Aging Neurosci. 2022, 14, 1069482. [Google Scholar] [CrossRef]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Südhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Brennand, K.J.; Simone, A.; Tran, N.; Gage, F.H. Modeling psychiatric disorders at the cellular and network levels. Mol. Psychiatry 2012, 17, 1239–1253. [Google Scholar] [CrossRef]
- Wang, C.; Ward, M.E.; Chen, R.; Liu, K.; Tracy, T.E.; Chen, X.; Xie, M.; Sohn, P.D.; Ludwig, C.; Meyer-Franke, A.; et al. Scalable Production of iPSC-Derived Human Neurons to Identify Tau-Lowering Compounds by High-Content Screening. Stem Cell Rep. 2017, 9, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Cerneckis, J.; Bu, G.; Shi, Y. Pushing the boundaries of brain organoids to study Alzheimer’s disease. Trends Mol. Med. 2023, 29, 659–672. [Google Scholar] [CrossRef]
- Agholme, L.; Lindström, T.; Kågedal, K.; Marcusson, J.; Hallbeck, M. An In Vitro Model for Neuroscience: Differentiation of SH-SY5Y Cells into Cells with Morphological and Biochemical Characteristics of Mature Neurons. J. Alzheimer’s Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef]
- Barmpa, K.; Saraiva, C.; Gomez-Giro, G.; Gabassi, E.; Spitz, S.; Brandauer, K.; Gatica, J.E.R.; Antony, P.; Robertson, G.; Papastefanaki, F.; et al. Age-Induced Midbrain-Striatum Assembloids Model Early Phenotypes of Parkinson’s Disease. bioRxiv 2023, 2023.10.28.564305. [Google Scholar] [CrossRef]
- Bolognin, S.; Fossépré, M.; Qing, X.; Jarazo, J.; Ščančar, J.; Moreno, E.L.; Nickels, S.L.; Wasner, K.; Ouzren, N.; Walter, J.; et al. 3D Cultures of Parkinson’s Disease-Specific Dopaminergic Neurons for High Content Phenotyping and Drug Testing. Adv. Sci. 2018, 6, 1800927. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.S.; Corvace, F.; Faustmann, P.M.; Faustmann, T.J. Pharmacological Investigations in Glia Culture Model of Inflammation. Front. Cell. Neurosci. 2021, 15, 805755. [Google Scholar] [CrossRef] [PubMed]
- Tarricone, G.; Carmagnola, I.; Chiono, V. Tissue-Engineered Models of the Human Brain: State-of-the-Art Analysis and Challenges. J. Funct. Biomater. 2022, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Simão, D.; Pinto, C.; Piersanti, S.; Weston, A.; Peddie, C.J.; Bastos, A.E.; Licursi, V.; Schwarz, S.C.; Collinson, L.M.; Salinas, S.; et al. Modeling Human Neural Functionality In Vitro: Three-Dimensional Culture for Dopaminergic Differentiation. Tissue Eng. Part A 2015, 21, 654–668. [Google Scholar] [CrossRef]
- Brito, C.; Simão, D.; Costa, I.; Malpique, R.; Pereira, C.I.; Fernandes, P.; Serra, M.; Schwarz, S.C.; Schwarz, J.; Kremer, E.J.; et al. Generation and genetic modification of 3D cultures of human dopaminergic neurons derived from neural progenitor cells. Methods 2012, 56, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kim, Y.H.; Hebisch, M.; Sliwinski, C.; Lee, S.; D’avanzo, C.; Chen, H.; Hooli, B.; Asselin, C.; Muffat, J.; et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 2014, 515, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Sun, A.X.; An, J.; Chua, C.K.; Chew, S.Y. 3D neural tissue models: From spheroids to bioprinting. Biomaterials 2018, 154, 113–133. [Google Scholar] [CrossRef]
- Kadoshima, T.; Sakaguchi, H.; Nakano, T.; Soen, M.; Ando, S.; Eiraku, M.; Sasai, Y. Self-Organization of Axial Polarity, inside-out Layer Pattern, and Species-Specific Progenitor Dynamics in Human ES Cell-Derived Neocortex. Proc. Natl. Acad. Sci. USA 2013, 110, 20284–20289. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Coppola, G.; Zhang, P.; Abyzov, A.; Provini, L.; Tomasini, L.; Amenduni, M.; Szekely, A.; Palejev, D.; Wilson, M.; et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 2015, 162, 375–390. [Google Scholar] [CrossRef]
- Jo, J.; Xiao, Y.; Sun, A.X.; Cukuroglu, E.; Tran, H.-D.; Göke, J.; Tan, Z.Y.; Saw, T.Y.; Tan, C.-P.; Lokman, H.; et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 2016, 19, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.J.; Choi, H.; Chang, Y.; Park, H.; Shin, J.; Kim, J.; Lengner, C.J.; Lee, Y.K.; Kim, J. Modeling G2019S-LRRK2 Sporadic Parkinson’s Disease in 3D Midbrain Organoids. Stem Cell Rep. 2019, 12, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Monzel, A.S.; Smits, L.M.; Hemmer, K.; Hachi, S.; Moreno, E.L.; van Wuellen, T.; Jarazo, J.; Walter, J.; Brüggemann, I.; Boussaad, I.; et al. Derivation of Human Midbrain-Specific Organoids from Neuroepithelial Stem Cells. Stem Cell Rep. 2017, 8, 1144–1154. [Google Scholar] [CrossRef]
- Smits, L.M.; Reinhardt, L.; Reinhardt, P.; Glatza, M.; Monzel, A.S.; Stanslowsky, N.; Rosato-Siri, M.D.; Zanon, A.; Antony, P.M.; Bellmann, J.; et al. Modeling Parkinson’s disease in midbrain-like organoids. npj Park. Dis. 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.S.Y.; Choo, X.Y.; Sun, A.X. Midbrain organoids—Development and applications in Parkinson’s disease. Oxf. Open Neurosci. 2023, 2, kvad009. [Google Scholar] [CrossRef] [PubMed]
- Kwak, T.H.; Kang, J.H.; Hali, S.; Kim, J.; Kim, K.-P.; Park, C.; Lee, J.-H.; Ryu, H.K.; Na, J.E.; Jo, J.; et al. Generation of homogeneous midbrain organoids with in vivo-like cellular composition facilitates neurotoxin-based Parkinson’s disease modeling. Stem Cells 2020, 38, 727–740. [Google Scholar] [CrossRef]
- Smeyne, R.J.; Jackson-Lewis, V. The MPTP model of Parkinson’s disease. Mol. Brain Res. 2005, 134, 57–66. [Google Scholar] [CrossRef]
- Yao, X.; Kang, J.H.; Kim, K.-P.; Shin, H.; Jin, Z.-L.; Guo, H.; Xu, Y.-N.; Li, Y.-H.; Hali, S.; Kwon, J.; et al. Production of Highly Uniform Midbrain Organoids from Human Pluripotent Stem Cells. Stem Cells Int. 2023, 2023, 3320211. [Google Scholar] [CrossRef]
- Fiorenzano, A.; Sozzi, E.; Birtele, M.; Kajtez, J.; Giacomoni, J.; Nilsson, F.; Bruzelius, A.; Sharma, Y.; Zhang, Y.; Mattsson, B.; et al. Single-cell transcriptomics captures features of human midbrain development and dopamine neuron diversity in brain organoids. Nat. Commun. 2021, 12, 7302. [Google Scholar] [CrossRef]
- Fu, C.-L.; Dong, B.-C.; Jiang, X.; Li, D.; Yao, J. A cell therapy approach based on iPSC-derived midbrain organoids for the restoration of motor function in a Parkinson’s disease mouse model. Heliyon 2024, 10, e24234. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Takanashi, M.; Oyama, G.; Yoritaka, A.; Hatano, T.; Shiba-Fukushima, K.; Nagai, M.; Nishiyama, K.; Hasegawa, K.; Inoshita, T.; et al. Reduced astrocytic reactivity in human brains and midbrain organoids with PRKN mutations. npj Park. Dis. 2020, 6, 33. [Google Scholar] [CrossRef]
- Mohamed, N.-V.; Sirois, J.; Ramamurthy, J.; Mathur, M.; Lépine, P.; Deneault, E.; Maussion, G.; Nicouleau, M.; Chen, C.X.-Q.; Abdian, N.; et al. Midbrain organoids with anSNCAgene triplication model key features of synucleinopathy. Brain Commun. 2021, 3, fcab223. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, G.M.; Coccia, E.; Goldman, C.; Whitney, K.; Reyes, R.; Sarrafha, L.; Nam, K.H.; Sohail, S.; Jones, D.R.; Crary, J.F.; et al. Disruption of lysosomal proteolysis in astrocytes facilitates midbrain organoid proteostasis failure in an early onset Parkinson’s disease model. Nat. Commun. 2024, 15, 447. [Google Scholar] [CrossRef]
- Birtele, M.; Storm, P.; Sharma, Y.; Kajtez, J.; Wahlestedt, J.N.; Sozzi, E.; Nilsson, F.; Stott, S.; He, X.L.; Mattsson, B.; et al. Single-Cell Transcriptional and Functional Analysis of Dopaminergic Neurons in Organoid-like Cultures Derived from Human Fetal Midbrain. Development 2022, 149, dev200504. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Gao, W.; Saiyin, H.; Li, Y.; Zeng, Y.; Zhang, Z.; Li, X.; Liu, Z.; Gao, Q.; An, P.; et al. MLKL deficiency alleviates neuroinflammation and motor deficits in the α-synuclein transgenic mouse model of Parkinson’s disease. Mol. Neurodegener. 2023, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zhou, S.; Wang, N.; Wang, J.; Wu, Y.; Duan, Y.; Ni, P.; Zhang, J.; Yu, S. Cerebral-Organoid-Derived Exosomes Alleviate Oxidative Stress and Promote LMX1A-Dependent Dopaminergic Differentiation. Int. J. Mol. Sci. 2023, 24, 11048. [Google Scholar] [CrossRef]
- Zagare, A.; Barmpa, K.; Smajic, S.; Smits, L.M.; Grzyb, K.; Grünewald, A.; Skupin, A.; Nickels, S.L.; Schwamborn, J.C. Midbrain organoids mimic early embryonic neurodevelopment and recapitulate LRRK2-p.Gly2019Ser-associated gene expression. Am. J. Hum. Genet. 2022, 109, 311–327. [Google Scholar] [CrossRef]
- Pamies, D.; Barreras, P.; Block, K.; Makri, G.; Kumar, A.; Wiersma, D.; Smirnova, L.; Zhang, C.; Bressler, J.; Christian, K.M.; et al. A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. Altex 2017, 34, 362–376. [Google Scholar] [CrossRef]
- Kanton, S.; Pasça, S.P. Human Assembloids. Development 2022, 149, dev201120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, J.; Xu, Z.; Yan, H.; Tang, B.; Liu, C.; Chen, C.; Meng, Q. Microglia-containing human brain organoids for the study of brain development and pathology. Mol. Psychiatry 2022, 28, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Sabate-Soler, S.; Nickels, S.L.; Saraiva, C.; Berger, E.; Dubonyte, U.; Barmpa, K.; Lan, Y.J.; Kouno, T.; Jarazo, J.; Robertson, G.; et al. Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality. Glia 2022, 70, 1267–1288. [Google Scholar] [CrossRef] [PubMed]
- Kälvälä, S.; Abushik, P.; Dougalis, A.; Malm, T.; Pelkonen, A.; Lehtonen, Š. Air-Liquid Interface Culture of Midbrain Organoids Improves Neuronal Functionality and Integration of Microglia. bioRxiv 2023, 2023.10.10.561672. [Google Scholar] [CrossRef]
- Hernández-Sapiéns, M.A.; Reza-Zaldívar, E.E.; Cevallos, R.R.; Márquez-Aguirre, A.L.; Gazarian, K.; Canales-Aguirre, A.A. A Three-Dimensional Alzheimer’s Disease Cell Culture Model Using IPSC-Derived Neurons Carrying A246E Mutation in PSEN1. Front. Cell Neurosci. 2020, 14, 516039. [Google Scholar] [CrossRef]
- Young, J.E.; Goldstein, L.S.B. Human-Induced Pluripotent Stem Cell (hiPSC)-Derived Neurons and Glia for the Elucidation of Pathogenic Mechanisms in Alzheimer’s Disease. In Alzheimer’s Disease; Methods in Molecular Biology; Humana: New York, NY, USA, 2022; Volume 2561, pp. 105–133. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.-J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.-S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef]
- Pavoni, S.; Jarray, R.; Nassor, F.; Guyot, A.-C.; Cottin, S.; Rontard, J.; Mikol, J.; Mabondzo, A.; Deslys, J.-P.; Yates, F. Small-molecule induction of Aβ-42 peptide production in human cerebral organoids to model Alzheimer’s disease associated phenotypes. PLoS ONE 2018, 13, e0209150. [Google Scholar] [CrossRef] [PubMed]
- Karmirian, K.; Holubiec, M.; Goto-Silva, L.; Fernandez Bessone, I.; Vitória, G.; Mello, B.; Alloatti, M.; Vanderborght, B.; Falzone, T.L.; Rehen, S. Modeling Alzheimer’s Disease Using Human Brain Organoids. Methods Mol. Biol. 2023, 2561, 135–158. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Le, J.; He, J.; Alcorn, J.; Mousseau, D.D. Fundamental Neurochemistry Review: Incorporating a greater diversity of cell types, including microglia, in brain organoid cultures improves clinical translation. J. Neurochem. 2022, 164, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Holubiec, M.I.; Alloatti, M.; Bianchelli, J.; Greloni, F.; Arnaiz, C.; Prinz, M.G.; Bessone, I.F.; Devoto, V.P.; Falzone, T.L. Mitochondrial vulnerability to oxidation in human brain organoids modelling Alzheimer’s disease. Free. Radic. Biol. Med. 2023, 208, 394–401. [Google Scholar] [CrossRef]
- Rueda-Carrasco, J.; Sokolova, D.; Lee, S.; Childs, T.; Jurčáková, N.; Crowley, G.; De Schepper, S.; Ge, J.Z.; Lachica, J.I.; Toomey, C.E.; et al. Microglia-synapse Engulfment via PtdSer-TREM2 Ameliorates Neuronal Hyperactivity in Alzheimer’s Disease Models. EMBO J. 2023, 42, e113246. [Google Scholar] [CrossRef] [PubMed]
- Linsley, J.W.; Reisine, T.; Finkbeiner, S. Three Dimensional and Four Dimensional Live Im-aging to Study Mechanisms of Progressive Neurodegeneration. J. Biol. Chem. 2024, 300, 107433. [Google Scholar] [CrossRef] [PubMed]
- Bubnys, A.; Tsai, L.-H. Harnessing cerebral organoids for Alzheimer’s disease research. Curr. Opin. Neurobiol. 2022, 72, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Gerakis, Y.; Hetz, C. Brain organoids: A next step for humanized Alzheimer’s disease models? Mol. Psychiatry 2019, 24, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasamurthy, S.; Laul, M.; Zhao, N.; Kim, T.; Zhu, D. Current progress of cerebral organoids for modeling Alzheimer’s disease origins and mechanisms. Bioeng. Transl. Med. 2022, 8, e10378. [Google Scholar] [CrossRef] [PubMed]
- Yanakiev, M.; Soper, O.; Berg, D.A.; Kang, E. Modelling Alzheimer’s disease using human brain organoids: Current progress and challenges. Expert Rev. Mol. Med. 2022, 25, e3. [Google Scholar] [CrossRef]
- Zagare, A.; Gobin, M.; Monzel, A.S.; Schwamborn, J.C. A robust protocol for the generation of human midbrain organoids. STAR Protoc. 2021, 2, 100524. [Google Scholar] [CrossRef]
- Ham, O.; Jin, Y.B.; Kim, J.; Lee, M.-O. Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2019, 521, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, L.; Wang, M.; Liu, J.; Zhong, S.; Li, R.; Li, P.; Guo, L.; Fang, A.; Chen, R.; et al. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 2020, 18, e3000705. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Ju, X.-C.; Li, Y.; Zeng, P.-M.; Wu, J.; Zhou, Y.-Y.; Shen, L.-B.; Dong, J.; Chen, Y.-J.; Luo, Z.-G.; et al. Generation of vascularized brain organoids to study neurovascular interactions. eLife 2022, 11, e76707. [Google Scholar] [CrossRef]
- Sun, X.; Kofman, S.; Ogbolu, V.C.; Karch, C.M.; Ibric, L.; Qiang, L. Vascularized Brain Assembloids with Enhanced Cellular Complexity Provide Insights Into the Cellular Deficits of Tauopathy. Stem Cells 2023, 42, 107–115. [Google Scholar] [CrossRef]
- ALancaster, M.; AKnoblich, J. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.; Rooney, L.A.; Daniszewski, M.; Gulluyan, L.; Liang, H.H.; Cook, A.L.; Hewitt, A.W.; Pébay, A. Culture Variabilities of Human IPSC-Derived Cerebral Organoids Are a Major Issue for the Modelling of Phenotypes Observed in Alzheimer’s Disease. Stem Cell Rev. Rep. 2022, 18, 718–731. [Google Scholar] [CrossRef]
- Cerneckis, J.; Cai, H.; Shi, Y. Induced pluripotent stem cells (iPSCs): Molecular mechanisms of induction and applications. Signal Transduct. Target. Ther. 2024, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Cornacchia, D.; Studer, L. Back and forth in time: Directing age in iPSC-derived lineages. Brain Res. 2015, 1656, 14–26. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, S.; Tackenberg, C. Alzheimer’s in a dish—Induced pluripotent stem cell-based disease modeling. Transl. Neurodegener. 2019, 8, 21. [Google Scholar] [CrossRef]
- Carlson, A.L.; Bennett, N.K.; Francis, N.L.; Halikere, A.; Clarke, S.; Moore, J.C.; Hart, R.P.; Paradiso, K.; Wernig, M.; Kohn, J.; et al. Generation and transplantation of reprogrammed human neurons in the brain using 3D microtopographic scaffolds. Nat. Commun. 2016, 7, 10862. [Google Scholar] [CrossRef]
- Smirnova, L.; Harris, G.; Delp, J.; Valadares, M.; Pamies, D.; Hogberg, H.T.; Waldmann, T.; Leist, M.; Hartung, T. A LUHMES 3D dopaminergic neuronal model for neurotoxicity testing allowing long-term exposure and cellular resilience analysis. Arch. Toxicol. 2015, 90, 2725–2743. [Google Scholar] [CrossRef] [PubMed]
- Fiore, N.J.; Ganat, Y.M.; Devkota, K.; Batorsky, R.; Lei, M.; Lee, K.; Cowen, L.J.; Croft, G.; Noggle, S.A.; Nieland, T.J.F.; et al. Bioengineered models of Parkinson’s disease using patient-derived dopaminergic neurons exhibit distinct biological profiles in a 3D microenvironment. Cell. Mol. Life Sci. 2022, 79, 78. [Google Scholar] [CrossRef]
- Carballo-Molina, O.A.; Sánchez-Navarro, A.; López-Ornelas, A.; Lara-Rodarte, R.; Salazar, P.; Campos-Romo, A.; Ramos-Mejía, V.; Velasco, I. Semaphorin 3C Released from a Bio-compatible Hydrogel Guides and Promotes Axonal Growth of Rodent and Human Dopaminergic Neurons. Tissue Eng. Part A 2016, 22, 850–861. [Google Scholar] [CrossRef]
- Tejchman, A.; Znój, A.; Chlebanowska, P.; Frączek-Szczypta, A.; Majka, M. Carbon Fibers as a New Type of Scaffold for Midbrain Organoid Development. Int. J. Mol. Sci. 2020, 21, 5959. [Google Scholar] [CrossRef]
- Ranjan, V.D.; Qiu, L.; Lee, J.W.-L.; Chen, X.; Jang, S.E.; Chai, C.; Lim, K.-L.; Tan, E.-K.; Zhang, Y.; Huang, W.M.; et al. A microfiber scaffold-based 3Din vitrohuman neuronal culture model of Alzheimer’s disease. Biomater. Sci. 2020, 8, 4861–4874. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhai, Y.; Hao, Y.; Zhu, Z.; Cheng, G. The Regulatory Functionality of Exosomes Derived from hUMSCs in 3D Culture for Alzheimer’s Disease Therapy. Small 2019, 16, e1906273. [Google Scholar] [CrossRef] [PubMed]
- Miny, L.; Maisonneuve, B.G.C.; Quadrio, I.; Honegger, T. Modeling Neurodegenerative Diseases Using In Vitro Compartmentalized Microfluidic Devices. Front. Bioeng. Biotechnol. 2022, 10, 919646. [Google Scholar] [CrossRef]
- Kane, K.I.W.; Moreno, E.L.; Hachi, S.; Walter, M.; Jarazo, J.; Oliveira, M.A.P.; Hankemeier, T.; Vulto, P.; Schwamborn, J.C.; Thoma, M.; et al. Automated microfluidic cell culture of stem cell derived dopaminergic neurons. Sci. Rep. 2019, 9, 1796. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.L.; Hachi, S.; Hemmer, K.; Trietsch, S.J.; Baumuratov, A.S.; Hankemeier, T.; Vulto, P.; Schwamborn, J.C.; Fleming, R.M.T. Differentiation of neuroepithelial stem cells into functional dopaminergic neurons in 3D microfluidic cell culture. Lab A Chip 2015, 15, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Gribaudo, S.; Tixador, P.; Bousset, L.; Fenyi, A.; Lino, P.; Melki, R.; Peyrin, J.-M.; Perrier, A.L. Propagation of α-Synuclein Strains within Human Reconstructed Neuronal Network. Stem Cell Rep. 2019, 12, 230–244. [Google Scholar] [CrossRef]
- Tran, H.T.; Chung, C.H.-Y.; Iba, M.; Zhang, B.; Trojanowski, J.Q.; Luk, K.C.; Lee, V.M. α-Synuclein Immunotherapy Blocks Uptake and Templated Propagation of Misfolded α-Synuclein and Neurodegeneration. Cell Rep. 2014, 7, 2054–2065. [Google Scholar] [CrossRef]
- Park, J.; Wetzel, I.; Marriott, I.; Dréau, D.; D’avanzo, C.; Kim, D.Y.; Tanzi, R.E.; Cho, H. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 2018, 21, 941–951. [Google Scholar] [CrossRef]
- Kane, K.I.; Jarazo, J.; Moreno, E.L.; Fleming, R.M.; Schwamborn, J.C. Passive controlled flow for Parkinson’s disease neuronal cell culture in 3D microfluidic devices. Organs A Chip 2020, 2, 100005. [Google Scholar] [CrossRef]
- Jacquet, A.d.R.; Alpaugh, M.; Denis, H.L.; Tancredi, J.L.; Boutin, M.; Decaestecker, J.; Beauparlant, C.; Herrmann, L.; Saint-Pierre, M.; Parent, M.; et al. The contribution of inflammatory astrocytes to BBB impairments in a brain-chip model of Parkinson’s disease. Nat. Commun. 2023, 14, 3651. [Google Scholar] [CrossRef] [PubMed]
- Pediaditakis, I.; Kodella, K.R.; Manatakis, D.V.; Le, C.Y.; Hinojosa, C.D.; Tien-Street, W.; Manolakos, E.S.; Vekrellis, K.; Hamilton, G.A.; Ewart, L.; et al. Modeling alpha-synuclein pathology in a human brain-chip to assess blood–brain barrier disruption. Nat. Commun. 2021, 12, 5907. [Google Scholar] [CrossRef]
- Rueda-Gensini, L.; ASerna, J.; Rubio, D.; Orozco, J.C.; IBolaños, N.; Cruz, J.C.; Muñoz-Camargo, C. Three-dimensional neuroimmune co-culture system for modeling Parkinson’s disease microenvironments in vitro. Biofabrication 2023, 15, 45001. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Long, X.; Xu, T. Three-dimensional-engineered bioprinted in vitro human neural stem cell self-assembling culture model constructs of Alzheimer’s disease. Bioact. Mater. 2021, 11, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.M.D.; Kavanagh, E.; Allenby, G.; Vassey, M. Bioengineered 3D Glial Cell Culture Systems and Applications for Neurodegeneration and Neuroinflammation. SLAS Discov. Adv. Sci. Drug Discov. 2017, 22, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Peng, J.; Huang, X.; Liang, F.; Wang, L.; Shi, J.; Yamada, A.; Chen, Y. Generation of Interconnected Neural Clusters in Multiscale Scaffolds from Human-Induced Pluripo-tent Stem Cells. ACS Appl. Mater. Interfaces 2021, 13, 55939–55952. [Google Scholar] [CrossRef] [PubMed]
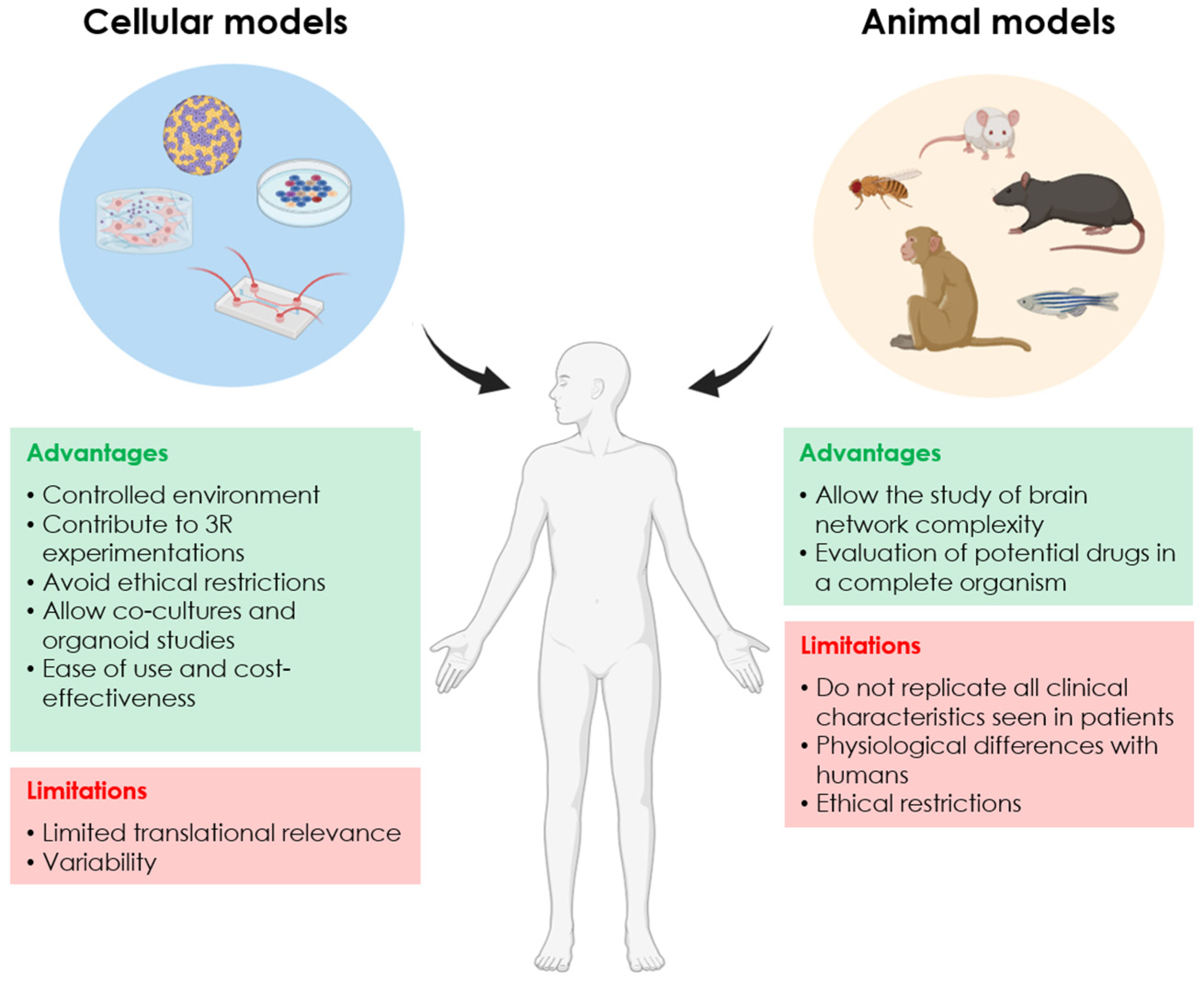



| Dimensions | Name | Species | Biological Origin | Advantages | Disadvantages |
|---|---|---|---|---|---|
| 2D | SH-SY5Y cells | Human | Neuroblastoma |
|
|
| PC12 cells | Rat | Pheochromocytoma tumour |
|
| |
| LUHMES cells | Human | Foetal mesencephalon |
|
| |
| iPSCs-derived cells | Human | (Usually) fibroblasts |
|
| |
| Induced neurons | Human | (Usually) fibroblasts |
|
| |
| 3D | Organoids | Human | (Usually) iPSCs |
|
|
| Engineering-based 3D models | Multiple (usually human) | Multiple cell types can be used |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solana-Manrique, C.; Sánchez-Pérez, A.M.; Paricio, N.; Muñoz-Descalzo, S. Two- and Three-Dimensional In Vitro Models of Parkinson’s and Alzheimer’s Diseases: State-of-the-Art and Applications. Int. J. Mol. Sci. 2025, 26, 620. https://doi.org/10.3390/ijms26020620
Solana-Manrique C, Sánchez-Pérez AM, Paricio N, Muñoz-Descalzo S. Two- and Three-Dimensional In Vitro Models of Parkinson’s and Alzheimer’s Diseases: State-of-the-Art and Applications. International Journal of Molecular Sciences. 2025; 26(2):620. https://doi.org/10.3390/ijms26020620
Chicago/Turabian StyleSolana-Manrique, Cristina, Ana María Sánchez-Pérez, Nuria Paricio, and Silvia Muñoz-Descalzo. 2025. "Two- and Three-Dimensional In Vitro Models of Parkinson’s and Alzheimer’s Diseases: State-of-the-Art and Applications" International Journal of Molecular Sciences 26, no. 2: 620. https://doi.org/10.3390/ijms26020620
APA StyleSolana-Manrique, C., Sánchez-Pérez, A. M., Paricio, N., & Muñoz-Descalzo, S. (2025). Two- and Three-Dimensional In Vitro Models of Parkinson’s and Alzheimer’s Diseases: State-of-the-Art and Applications. International Journal of Molecular Sciences, 26(2), 620. https://doi.org/10.3390/ijms26020620








