A Lateral Line Specific Mucin Involved in Cupula Growth and Vibration Detection in Zebrafish
Abstract
1. Introduction
2. Results
2.1. Structure of Zebrafish Mucin-5AC
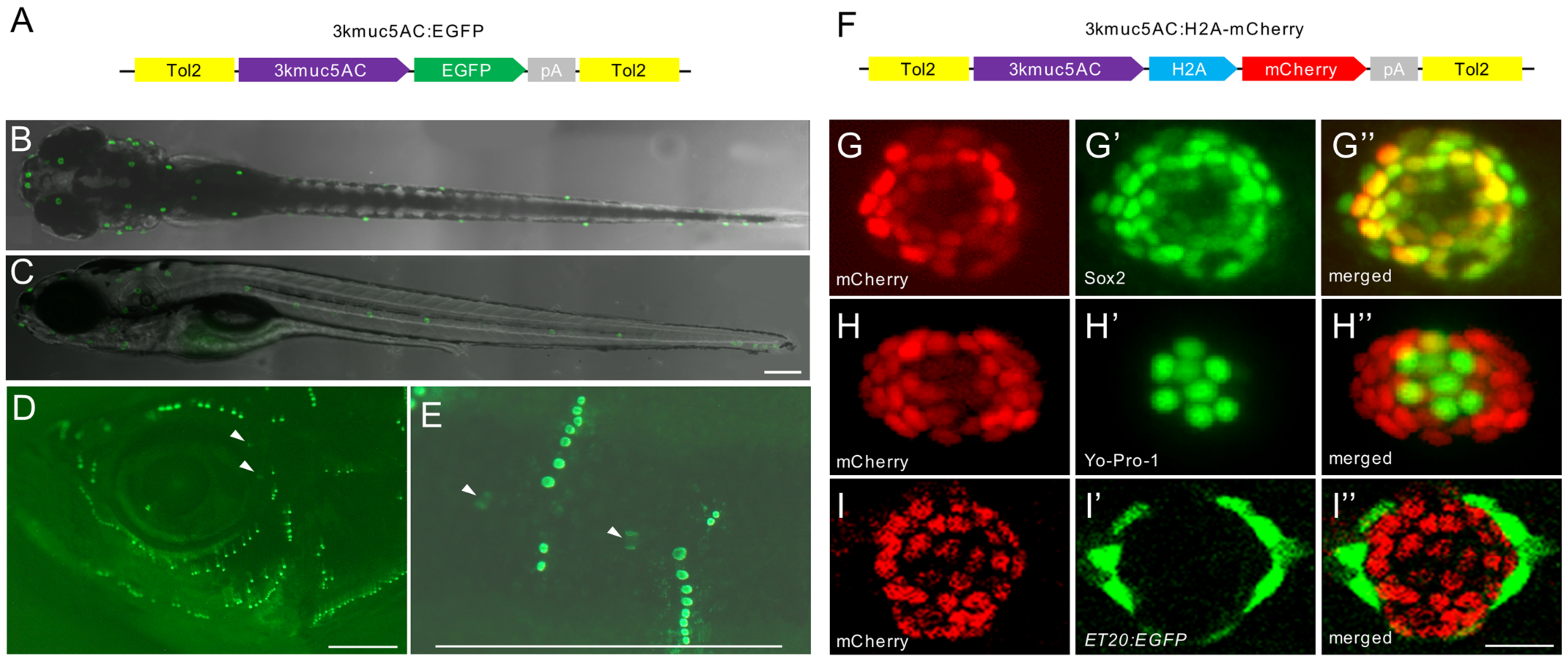
2.2. muc5AC Is Exclusively Expressed in Neuromasts in Zebrafish
2.3. muc5AC Is Specifically Expressed in the Support Cells in Neuromast
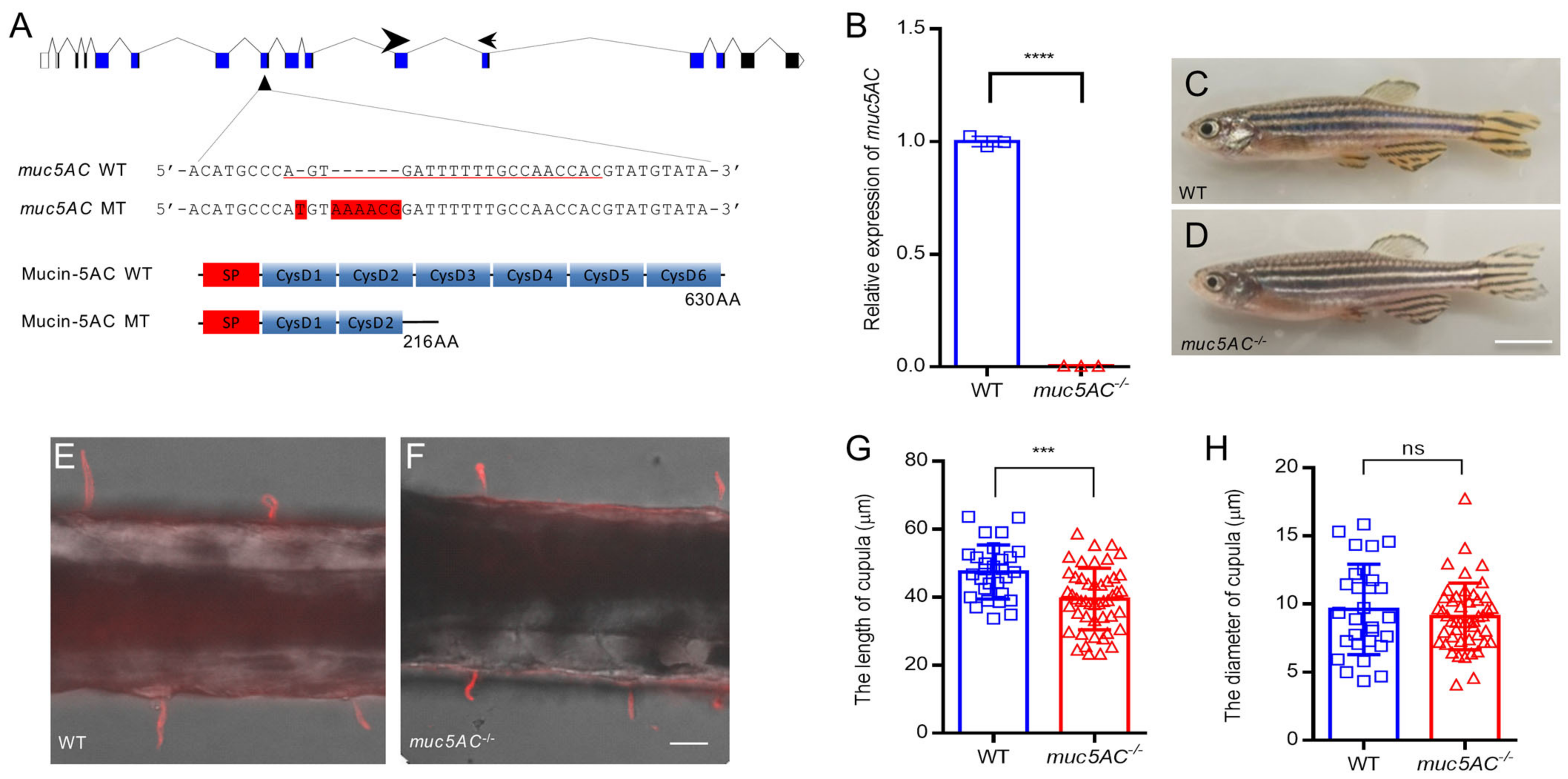
2.4. Deficiency of muc5AC Reduces Cupula Length
2.5. The Startle Response to Vibration Stimuli Is Compromised in muc5AC Mutants
3. Discussion
4. Materials and Methods
4.1. Zebrafish Maintenance
4.2. Sequence Alignment, Phylogenetics, and Structural Analysis
4.3. Whole-Mount In Situ Hybridization
4.4. Transgenic Zebrafish Lines Generation
4.5. Gene Knockdown with Antisense Morpholino
4.6. Gene Knockout Using CRISPR/Cas9
4.7. Quantitative RT-PCR (qRT-PCR)
4.8. Hair Cell Staining with YO-PRO-1
4.9. Cupula Staining with Fluorescent Microspheres
4.10. Immunofluorescence
4.11. Vibration-Induced Startle Response Assay
4.12. Data Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bleckmann, H.; Zelick, R. Lateral Line System of Fish. Integr. Zool. 2009, 4, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Dijkgraaf, S. The Functioning and Significance of the Lateral-Line Organs. Biol. Rev. Camb. Philos. Soc. 1963, 38, 51–105. [Google Scholar] [CrossRef] [PubMed]
- Mogdans, J. Sensory Ecology of the Fish Lateral-Line System: Morphological and Physiological Adaptations for the Perception of Hydrodynamic Stimuli. J. Fish Biol. 2019, 95, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.F. Lateral Line Morphology and Development and Implications for the Ontogeny of Flow Sensing in Fishes. In Flow Sensing in Air and Water: Behavioral, Neural and Engineering Principles of Operation; Bleckmann, H., Mogdans, J., Coombs, S.L., Eds.; Springer: Berlin, Heidelberg, 2014; pp. 247–270. ISBN 978-3-642-41446-6. [Google Scholar]
- Kottapalli, A.G.P.; Bora, M.; Kanhere, E.; Asadnia, M.; Miao, J.; Triantafyllou, M.S. Cupula-Inspired Hyaluronic Acid-Based Hydrogel Encapsulation to Form Biomimetic MEMS Flow Sensors. Sensors 2017, 17, 1728. [Google Scholar] [CrossRef]
- Kelly, J.P.; van Netten, S.M. Topography and Mechanics of the Cupula in the Fish Lateral Line. I. Variation of Cupular Structure and Composition in Three Dimensions. J. Morphol. 1991, 207, 23–36. [Google Scholar] [CrossRef]
- Windsor, S.P.; McHenry, M.J. The Influence of Viscous Hydrodynamics on the Fish Lateral-Line System. Integr. Comp. Biol. 2009, 49, 691–701. [Google Scholar] [CrossRef]
- Teyke, T. Morphological Differences in Neuromasts of the Blind Cave Fish Astyanax Hubbsi and the Sighted River Fish Astyanax Mexicanus. Brain Behav. Evol. 1990, 35, 23–30. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Jeffery, W.R.; van Netten, S.M.; McHenry, M.J. The Sensitivity of Lateral Line Receptors and Their Role in the Behavior of Mexican Blind Cavefish (Astyanax mexicanus). J. Exp. Biol. 2014, 217, 886–895. [Google Scholar] [CrossRef]
- Mukai, Y.; Yoshikawa, H.; Kobayashi, H. The Relationship Between the Length of the Cupulae of Free Neuromasts and Feeding Ability in Larvae of the Willow Shiner Gnathopogon Elongatus caerulescens (Teleostei, Cyprinidae). J. Exp. Biol. 1994, 197, 399–403. [Google Scholar] [CrossRef]
- Sato, M. Studies on the Pit Organs of Fishes. V. The Structure and Polysaccharide Histochemistry of the Cupula of the Pit Organ. Annot. Zool. Jpn. 1962, 35, 80–88. [Google Scholar]
- Tester, A.L.; Kendall, J.I. Cupulae in Shark Neuromasts: Composition, Origin, Generation. Science 1968, 160, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Flock, Å.; Duvall, A. The Ultrastructure of the Kinocilium of the Sensory Cells in the Inner Ear and Lateral Line Organs. The J. Cell Biol. 1965, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Blaxter, J.H.S. Structure and Development of the Lateral Line. Biol. Rev. 1987, 62, 471–514. [Google Scholar] [CrossRef]
- Münz, H. Morphology and Innervation of the Lateral Line System in Sarotherodon niloticus (L.) (Cichlidae, Teleostei). Zoomorphologie 1979, 93, 73–86. [Google Scholar] [CrossRef]
- Rouse, G.W.; Pickles, J.O. Ultrastructure of Free Neuromasts of Bathygobius fuscus (Gobiidae) and Canal Neuromasts of Apogon cyanosoma (Apogonidae). J. Morphol. 1991, 209, 111–120. [Google Scholar] [CrossRef]
- Ghysen, A.; Dambly-Chaudière, C. The Lateral Line Microcosmos. Genes Dev. 2007, 21, 2118–2130. [Google Scholar] [CrossRef]
- Wagner, C.E.; Wheeler, K.M.; Ribbeck, K. Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annu. Rev. Cell. Dev. Biol. 2018, 34, 189–215. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. Mucin Structure, Aggregation, Physiological Functions and Biomedical Applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Perez-Vilar, J.; Randell, S.H.; Boucher, R.C. C-Mannosylation of MUC5AC and MUC5B Cys Subdomains. Glycobiology 2004, 14, 325–337. [Google Scholar] [CrossRef]
- Silver, R.B.; Reeves, A.P.; Steinacker, A.; Highstein, S.M. Examination of the Cupula and Stereocilia of the Horizontal Semicircular Canal in the Toadfish Opsanus Tau. J. Comp. Neurol. 1998, 402, 48–61. [Google Scholar] [CrossRef]
- Melrose, J. Mucin-like Glycopolymer Gels in Electrosensory Tissues Generate Cues Which Direct Electrolocation in Amphibians and Neuronal Activation in Mammals. Neural Regen. Res. 2019, 14, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Salmon, M.; El-Amraoui, A.; Leibovici, M.; Petit, C. Otogelin: A Glycoprotein Specific to the Acellular Membranes of the Inner Ear. Proc. Natl. Acad. Sci. USA 1997, 94, 14450–14455. [Google Scholar] [CrossRef] [PubMed]
- Ganaha, A.; Kaname, T.; Yanagi, K.; Tono, T.; Higa, T.; Suzuki, M. Clinical Characteristics with Long-Term Follow-up of Four Okinawan Families with Moderate Hearing Loss Caused by an OTOG Variant. Hum. Genome Var. 2019, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Thisse, B.; Heyer, V.; Lux, A.; Alunni, V.; Degrave, A.; Seiliez, I.; Kirchner, J.; Parkhill, J.-P.; Thisse, C. Spatial and Temporal Expression of the Zebrafish Genome by Large-Scale in Situ Hybridization Screening. Methods Cell. Biol. 2004, 77, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Elkon, R.; Milon, B.; Morrison, L.; Shah, M.; Vijayakumar, S.; Racherla, M.; Leitch, C.C.; Silipino, L.; Hadi, S.; Weiss-Gayet, M.; et al. RFX Transcription Factors Are Essential for Hearing in Mice. Nat. Commun. 2015, 6, 8549. [Google Scholar] [CrossRef] [PubMed]
- Kozak, E.L.; Palit, S.; Miranda-Rodríguez, J.R.; Janjic, A.; Böttcher, A.; Lickert, H.; Enard, W.; Theis, F.J.; López-Schier, H. Epithelial Planar Bipolarity Emerges from Notch-Mediated Asymmetric Inhibition of Emx2. Curr. Biol. 2020, 30, 1142–1151.e6. [Google Scholar] [CrossRef]
- McHenry, M.J.; Feitl, K.E.; Strother, J.A.; Van Trump, W.J. Larval Zebrafish Rapidly Sense the Water Flow of a Predator’s Strike. Biol. Lett. 2009, 5, 477–479. [Google Scholar] [CrossRef]
- Buck, L.M.J.; Winter, M.J.; Redfern, W.S.; Whitfield, T.T. Ototoxin-Induced Cellular Damage in Neuromasts Disrupts Lateral Line Function in Larval Zebrafish. Hear. Res. 2012, 284, 67–81. [Google Scholar] [CrossRef]
- Burgess, H.A.; Granato, M. Sensorimotor Gating in Larval Zebrafish. J. Neurosci. 2007, 27, 4984–4994. [Google Scholar] [CrossRef]
- Roberts, A.C.; Reichl, J.; Song, M.Y.; Dearinger, A.D.; Moridzadeh, N.; Lu, E.D.; Pearce, K.; Esdin, J.; Glanzman, D.L. Habituation of the C-Start Response in Larval Zebrafish Exhibits Several Distinct Phases and Sensitivity to NMDA Receptor Blockade. PLoS ONE 2011, 6, e29132. [Google Scholar] [CrossRef]
- van Netten, S.M.; McHenry, M.J. The Biophysics of the Fish Lateral Line. In The Lateral Line System; Coombs, S., Bleckmann, H., Fay, R.R., Popper, A.N., Eds.; Springer: New York, NY, USA, 2014; pp. 99–119. ISBN 978-1-4614-8851-4. [Google Scholar]
- Torroba, M.; Barrutia, M.G.; Zapata, A.G. Morphological, Histochemical, and Ultrastructural Characterization of the Accessory Cells of Neuromasts in the Salamander Pleurodeles waltlii. Cell Tissue Res. 1988, 254, 233–240. [Google Scholar] [CrossRef]
- McHenry, M.J.; Strother, J.A.; van Netten, S.M. Mechanical Filtering by the Boundary Layer and Fluid-Structure Interaction in the Superficial Neuromast of the Fish Lateral Line System. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2008, 194, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Lush, M.E.; Diaz, D.C.; Koenecke, N.; Baek, S.; Boldt, H.; St Peter, M.K.; Gaitan-Escudero, T.; Romero-Carvajal, A.; Busch-Nentwich, E.M.; Perera, A.G.; et al. scRNA-Seq Reveals Distinct Stem Cell Populations That Drive Hair Cell Regeneration after Loss of Fgf and Notch Signaling. eLife 2019, 8, e44431. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Cheng, A.G.; Cunningham, L.L.; MacDonald, G.; Raible, D.W.; Rubel, E.W. Neomycin-Induced Hair Cell Death and Rapid Regeneration in the Lateral Line of Zebrafish (Danio rerio). J. Assoc. Res. Otolaryngol. 2003, 4, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, E.; Slevin, C.C.; Colón-Cruz, L.; Burgess, S.M. Vestibular and Auditory Hair Cell Regeneration Following Targeted Ablation of Hair Cells with Diphtheria Toxin in Zebrafish. Front. Cell. Neurosci. 2021, 15, 721950. [Google Scholar] [CrossRef]
- Peloggia, J.; Münch, D.; Meneses-Giles, P.; Romero-Carvajal, A.; Lush, M.E.; Lawson, N.D.; McClain, M.; Pan, Y.A.; Piotrowski, T. Adaptive Cell Invasion Maintains Lateral Line Organ Homeostasis in Response to Environmental Changes. Dev. Cell. 2021, 56, 1296–1312.e7. [Google Scholar] [CrossRef]
- Steiner, A.B.; Kim, T.; Cabot, V.; Hudspeth, A.J. Dynamic Gene Expression by Putative Hair-Cell Progenitors during Regeneration in the Zebrafish Lateral Line. Proc. Natl. Acad. Sci. USA 2014, 111, E1393–1401. [Google Scholar] [CrossRef]
- Heiman, M.; Kulicke, R.; Fenster, R.J.; Greengard, P.; Heintz, N. Cell Type-Specific mRNA Purification by Translating Ribosome Affinity Purification (TRAP). Nat. Protoc. 2014, 9, 1282–1291. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Duan, Q.; Zheng, H.; Qin, Y.; Yan, J.; Wang, J.; Burgess, S.M.; Fan, C. Stat3 Has a Different Role in Axon Growth During Development Than It Does in Axon Regeneration After Injury. Mol. Neurobiol. 2024, 61, 1753–1768. [Google Scholar] [CrossRef] [PubMed]
- Kanther, M.; Sun, X.; Mühlbauer, M.; Mackey, L.C.; Flynn, E.J.; Bagnat, M.; Jobin, C.; Rawls, J.F. Microbial Colonization Induces Dynamic Temporal and Spatial Patterns of NF-κB Activation in the Zebrafish Digestive Tract. Gastroenterology 2011, 141, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Suster, M.L.; Kikuta, H.; Urasaki, A.; Asakawa, K.; Kawakami, K. Transgenesis in Zebrafish with the Tol2 Transposon System. Methods Mol. Biol. 2009, 561, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Nasevicius, A.; Ekker, S.C. Effective Targeted Gene “knockdown” in Zebrafish. Nat. Genet. 2000, 26, 216–220. [Google Scholar] [CrossRef]
- Varshney, G.K.; Carrington, B.; Pei, W.; Bishop, K.; Chen, Z.; Fan, C.; Xu, L.; Jones, M.; LaFave, M.C.; Ledin, J.; et al. A High-Throughput Functional Genomics Workflow Based on CRISPR/Cas9-Mediated Targeted Mutagenesis in Zebrafish. Nat. Protoc. 2016, 11, 2357–2375. [Google Scholar] [CrossRef]
- Carrington, B.; Varshney, G.K.; Burgess, S.M.; Sood, R. CRISPR-STAT: An Easy and Reliable PCR-Based Method to Evaluate Target-Specific sgRNA Activity. Nucleic Acids Res. 2015, 43, e157. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, Z.; Wang, J.; Fan, C. HIF Signaling Overactivation Inhibits Lateral Line Neuromast Development through Wnt in Zebrafish. Gene 2024, 898, 148077. [Google Scholar] [CrossRef]
- Zamora, L.Y.; Lu, Z. Alcohol-Induced Morphological Deficits in the Development of Octavolateral Organs of the Zebrafish (Danio rerio). Zebrafish 2013, 10, 52–61. [Google Scholar] [CrossRef]
- Santos, F.; MacDonald, G.; Rubel, E.W.; Raible, D.W. Lateral Line Hair Cell Maturation Is a Determinant of Aminoglycoside Susceptibility in Zebrafish (Danio rerio). Hear. Res. 2006, 213, 25–33. [Google Scholar] [CrossRef]
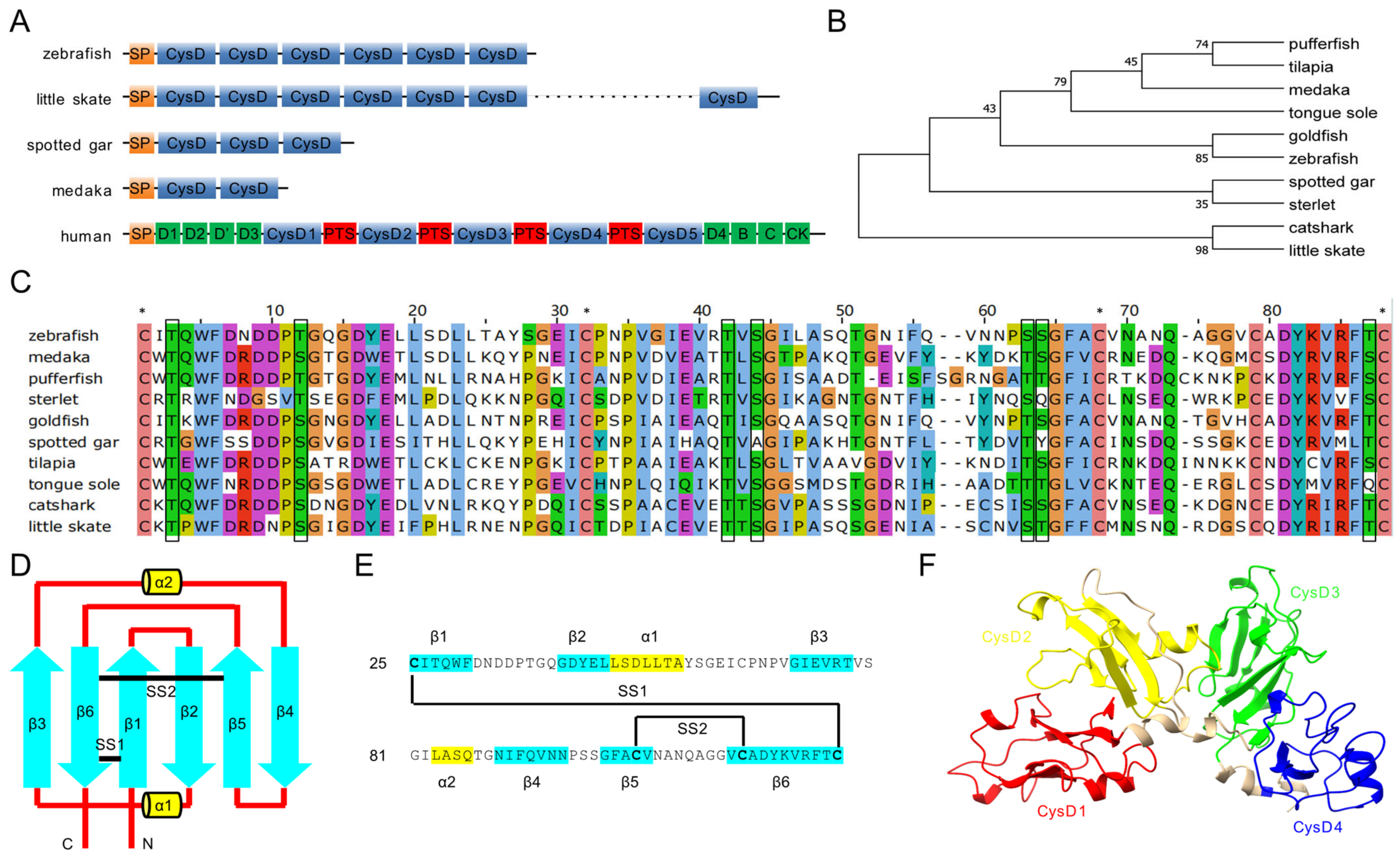




| Common Name | Species Name | Accession No. | CysD No. | Identity to Zebrafish |
|---|---|---|---|---|
| little skate | Amblyraja radiata | XP_032903886 | 27 | 48.75% |
| smaller spotted catshark | Scyliorhinus canicula | XP_038629501 | 19 | 47.45% |
| sterlet | Acipenser ruthenus | XP_058858610 | 4 | 50.38% |
| spotted gar | Lepisosteus oculatus | XP_015192309 | 3 | 42.53% |
| goldfish | Carassius auratus | XP_026092345 | 6 | 75.31% |
| tongue sole | Cynoglossus semilaevis | XP_008309417 | 7 | 42.23% |
| Japanese medaka | Oryzias latipes | XP_004085768 | 2 | 47.89% |
| Nile tilapia | Oreochromis niloticus | XP_025754833 | 2 | 42.86% |
| pufferfish | Takifugu rubripes | XP_029692542 | 2 | 47.49% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Tian, Y.; Wang, Y.; Wang, C.; Wang, J.; Fan, C. A Lateral Line Specific Mucin Involved in Cupula Growth and Vibration Detection in Zebrafish. Int. J. Mol. Sci. 2025, 26, 708. https://doi.org/10.3390/ijms26020708
Ma Z, Tian Y, Wang Y, Wang C, Wang J, Fan C. A Lateral Line Specific Mucin Involved in Cupula Growth and Vibration Detection in Zebrafish. International Journal of Molecular Sciences. 2025; 26(2):708. https://doi.org/10.3390/ijms26020708
Chicago/Turabian StyleMa, Ziyue, Yixuan Tian, Yingying Wang, Chenghao Wang, Jian Wang, and Chunxin Fan. 2025. "A Lateral Line Specific Mucin Involved in Cupula Growth and Vibration Detection in Zebrafish" International Journal of Molecular Sciences 26, no. 2: 708. https://doi.org/10.3390/ijms26020708
APA StyleMa, Z., Tian, Y., Wang, Y., Wang, C., Wang, J., & Fan, C. (2025). A Lateral Line Specific Mucin Involved in Cupula Growth and Vibration Detection in Zebrafish. International Journal of Molecular Sciences, 26(2), 708. https://doi.org/10.3390/ijms26020708





