Starch-Assisted Eco-Friendly Synthesis of ZnO Nanoparticles: Enhanced Photocatalytic, Supercapacitive, and UV-Driven Antioxidant Properties with Low Cytotoxic Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of ZnO NPs
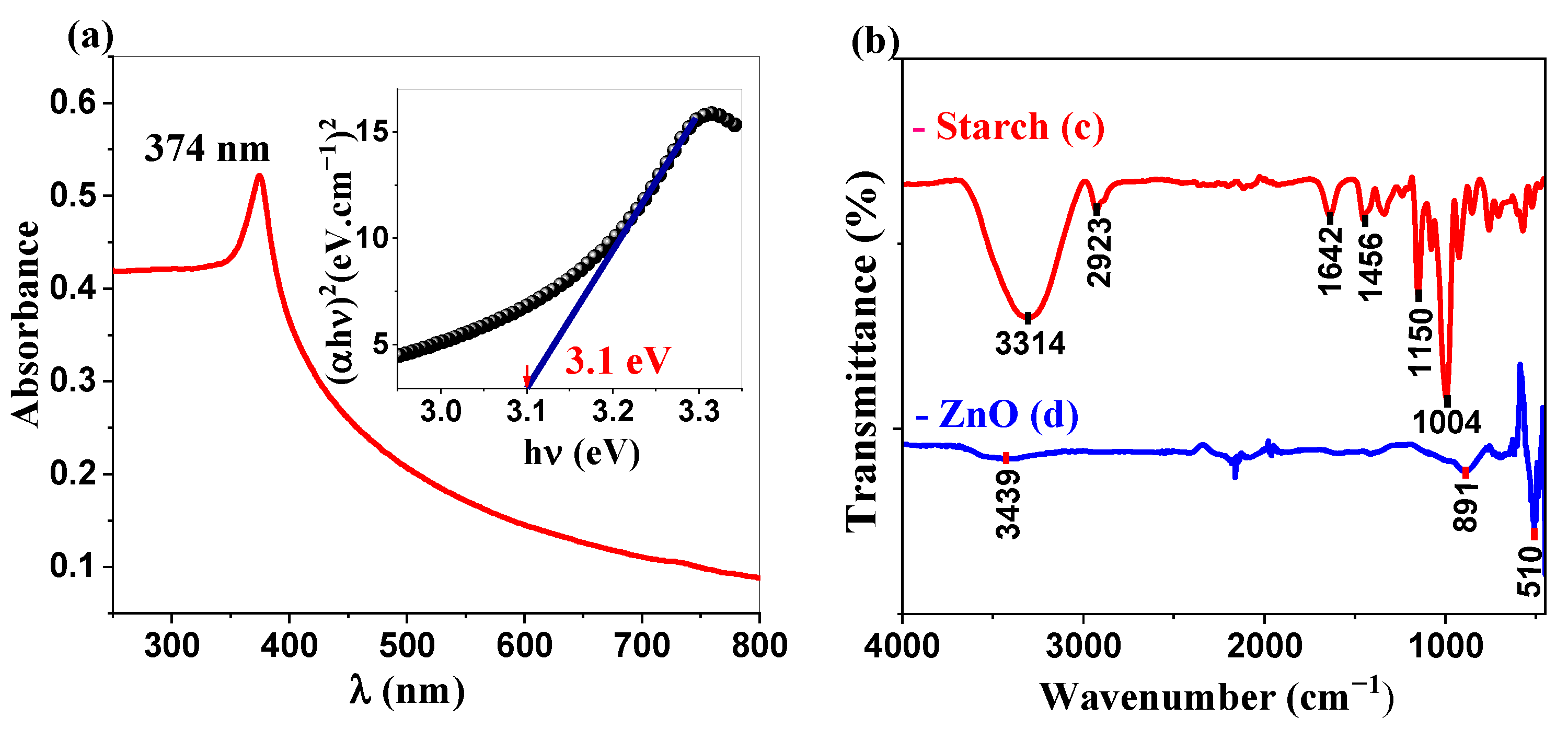
2.1.1. UV-Visible and IR Analysis
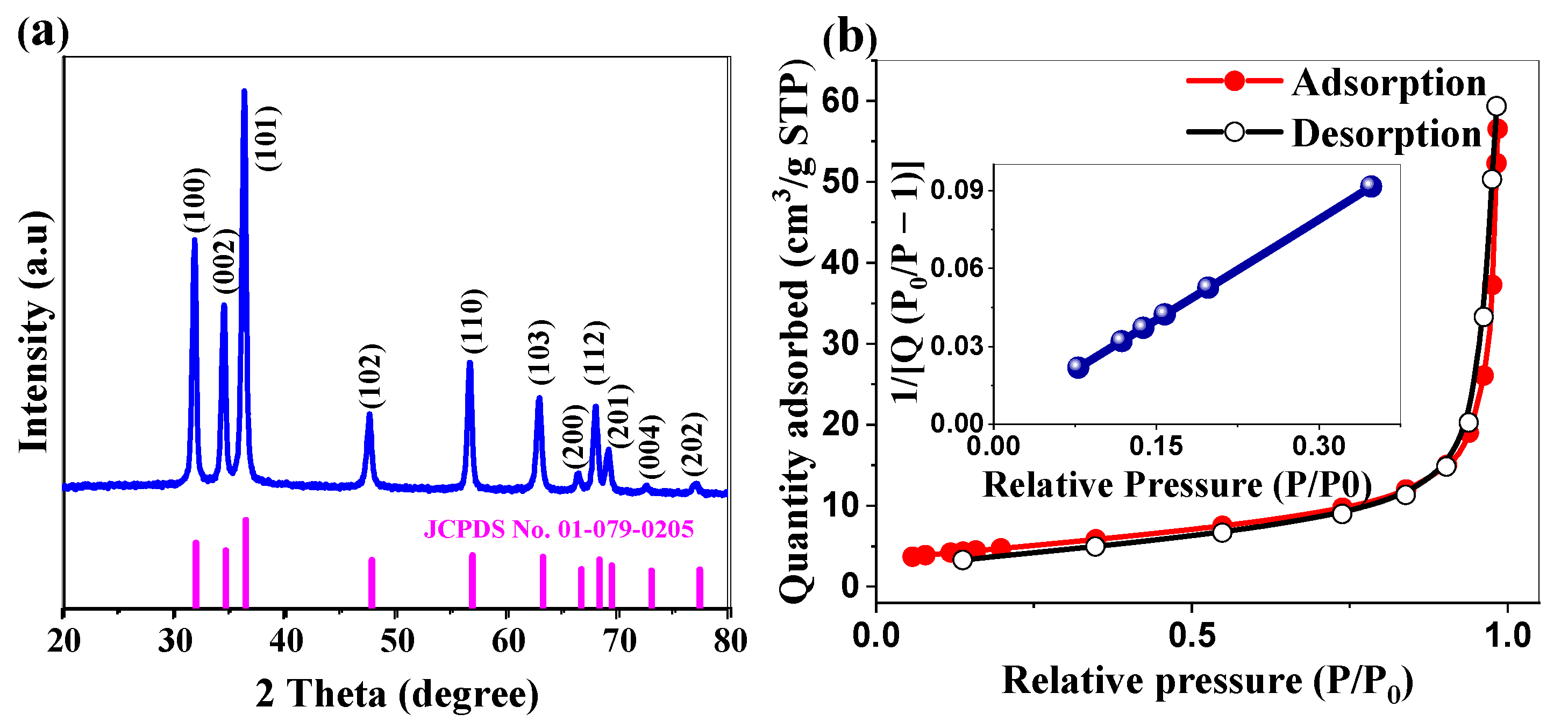
2.1.2. Crystallographic Structure and Surface Area Analysis
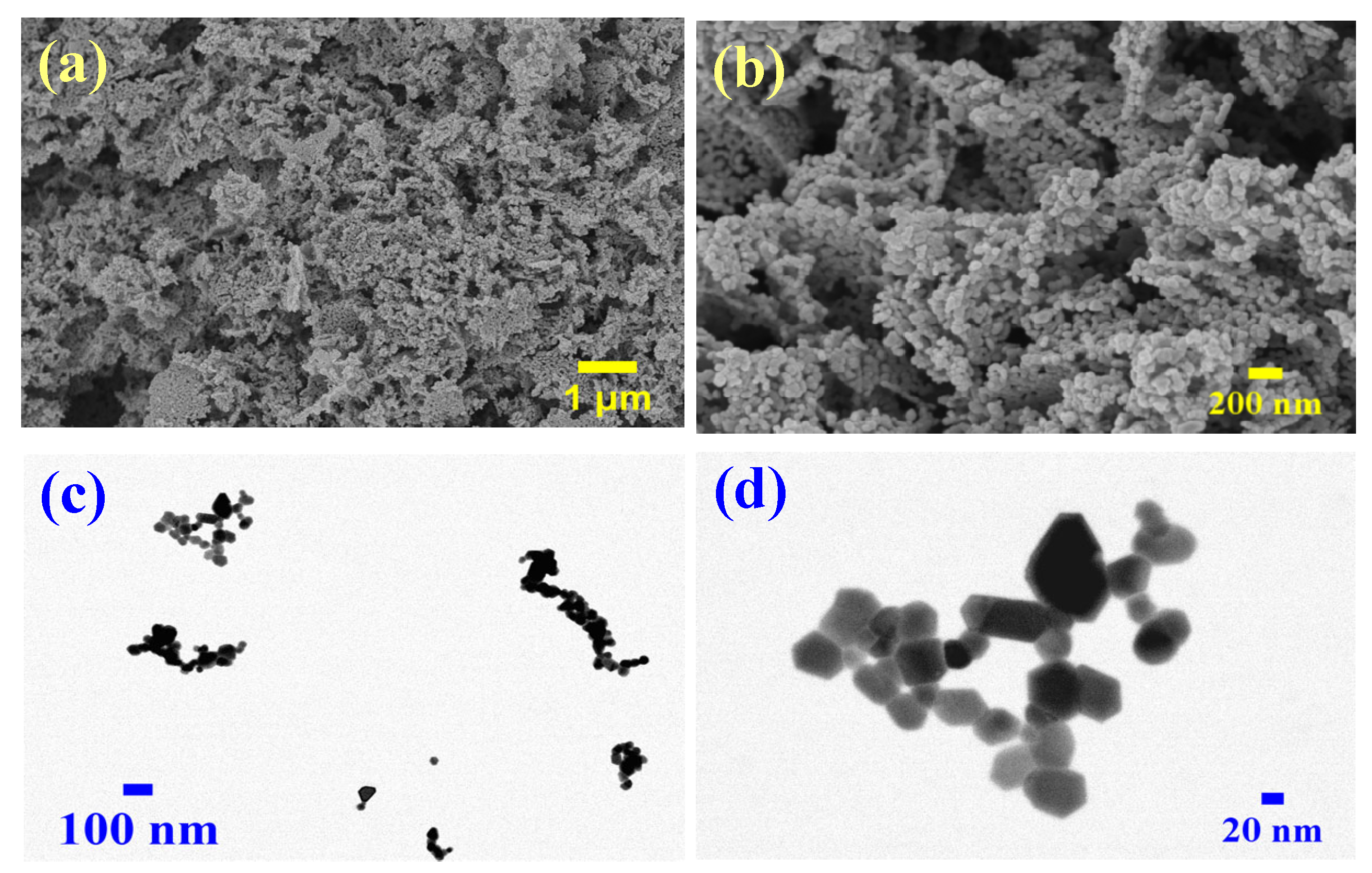
2.1.3. Morphological and Elemental Characterization of ZnO Nanoparticles
2.2. Formation Mechanism of ZnO Nanoparticles
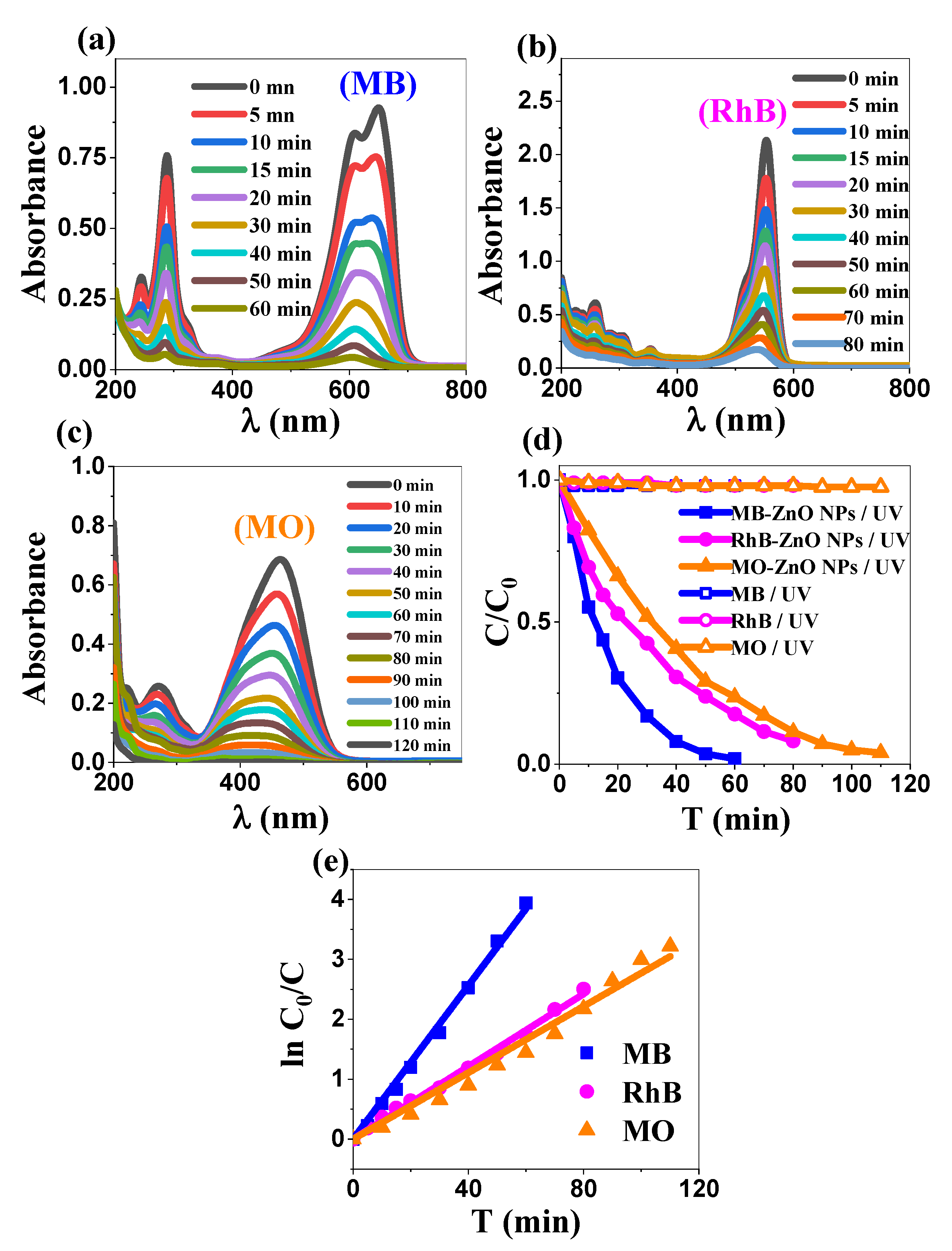
2.3. Photocatalytic Study
2.3.1. Comparative Dye Degradation
2.3.2. Enhanced Photocatalytic Performance Under Alkaline Conditions and Comparative Analysis
2.3.3. The Impact of Isopropanol on Photocatalytic Dye Degradation Rate: Mechanistic Insights
- (1)
- Photoexcitation and charge carrier generation, in the conduction band and valence band, respectively:ZnO + hν → e−(CB) + h⁺ (VB)
- (2)
- Formation of reactive oxygen species (ROS):h⁺ + H2O → •OH + H⁺e− + O2 → O2•−2O2•− + 2H⁺ → H2O2 + O2H2O2 + e− → •OH + OH−
- (3)
- Dye degradation pathways:
- Primary pathway (>90%): (•OH-mediated);
- Secondary pathways (<10%) (O2•−, h⁺, and H2O2 mediated processes).
2.3.4. Kinetics’ Analysis: Applicability of the Langmuir–Hinshelwood Model
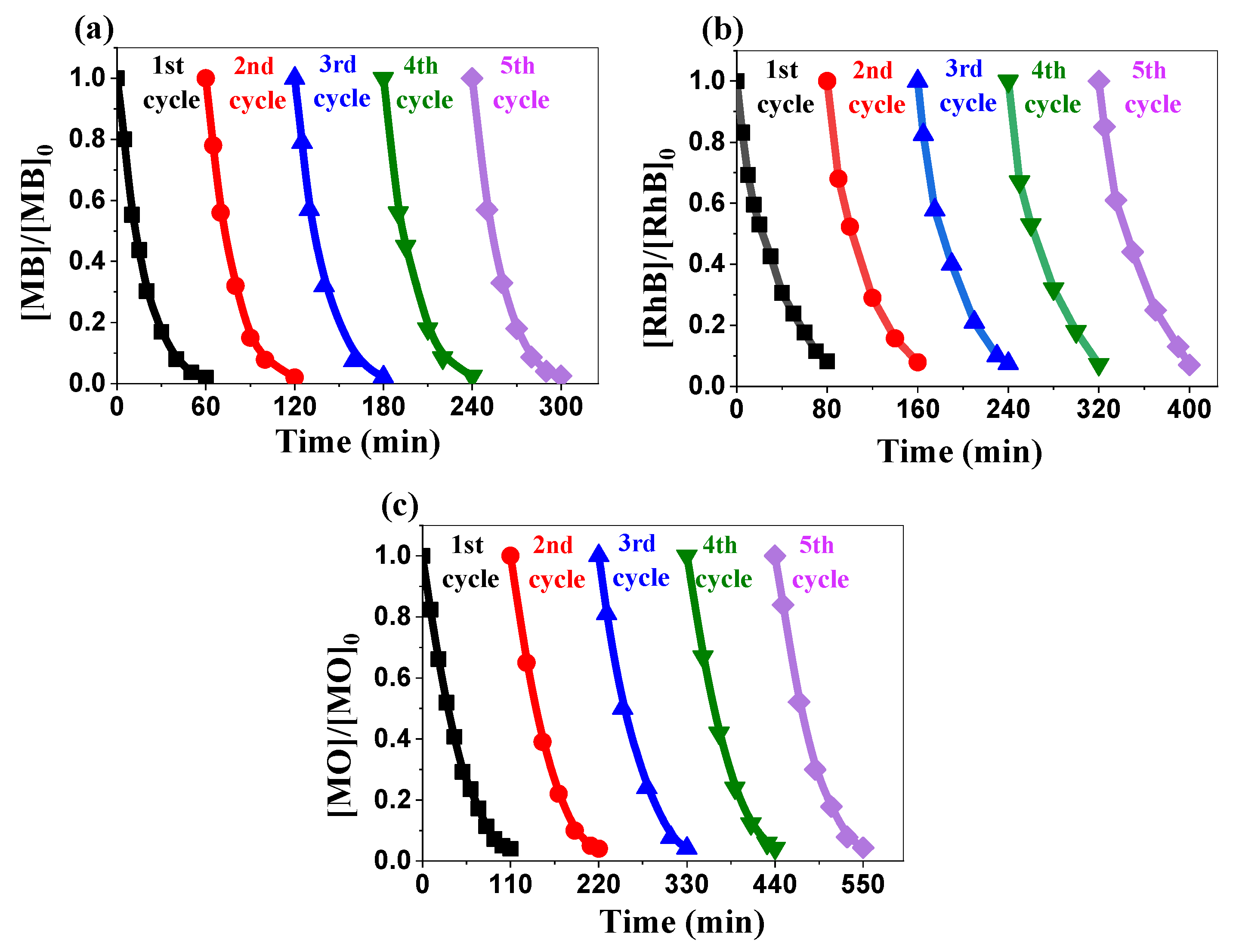
2.3.5. Recyclability Performance Evaluation
2.4. DPPH Radical Scavenging Activity of Biosynthesized ZnO Nanoparticles
2.5. Electrochemical Study
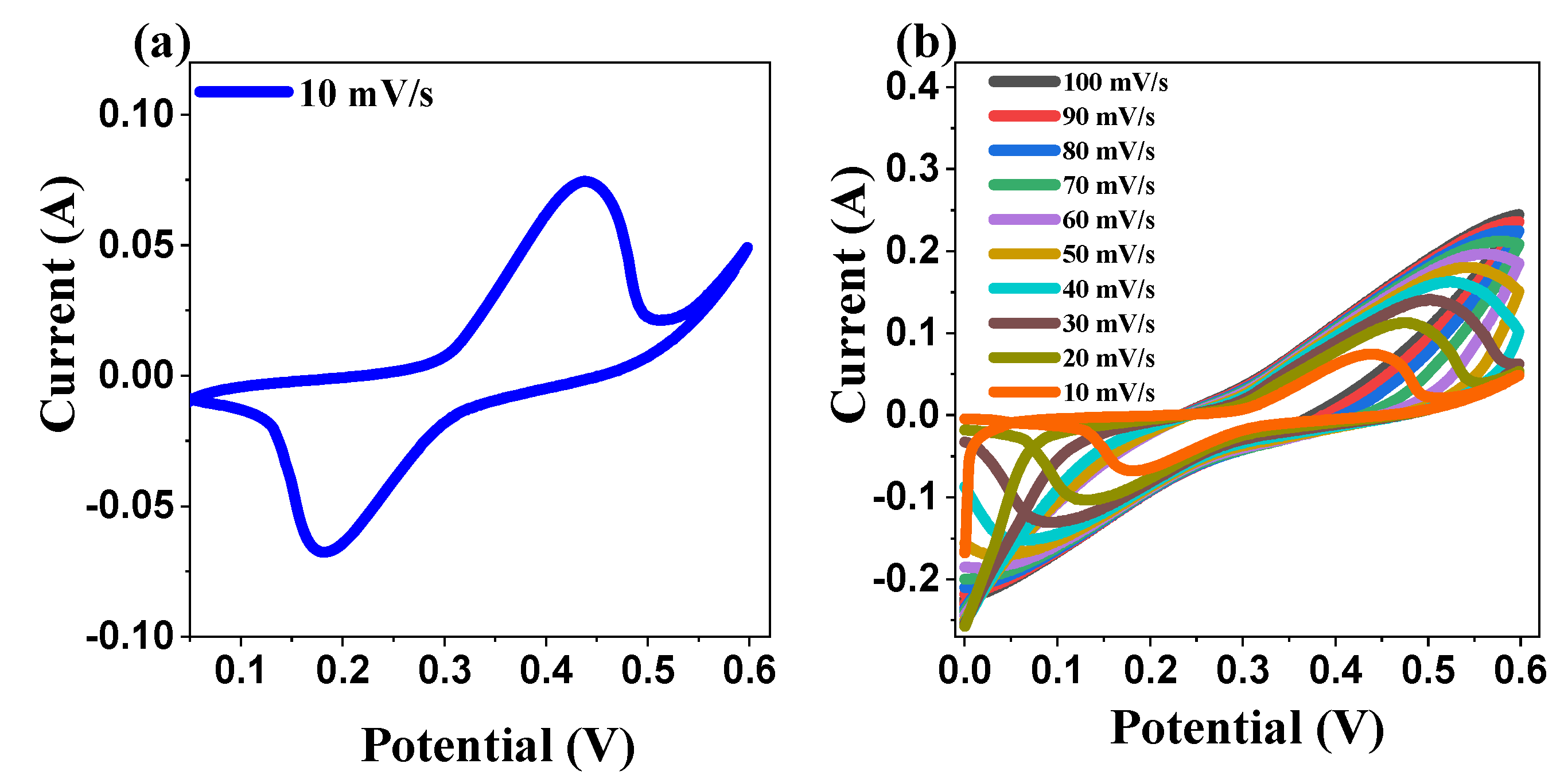
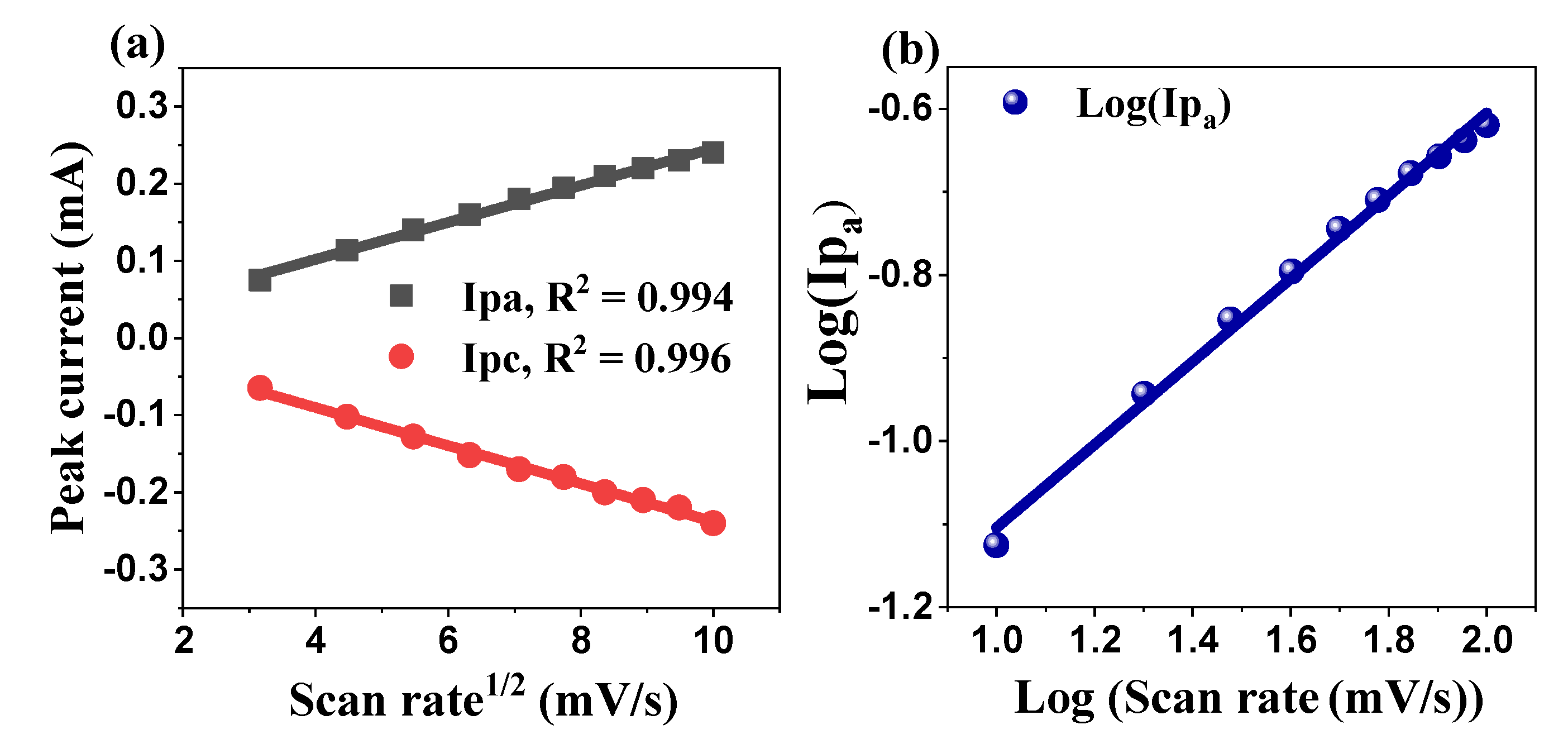
2.5.1. Cyclic Voltammetry
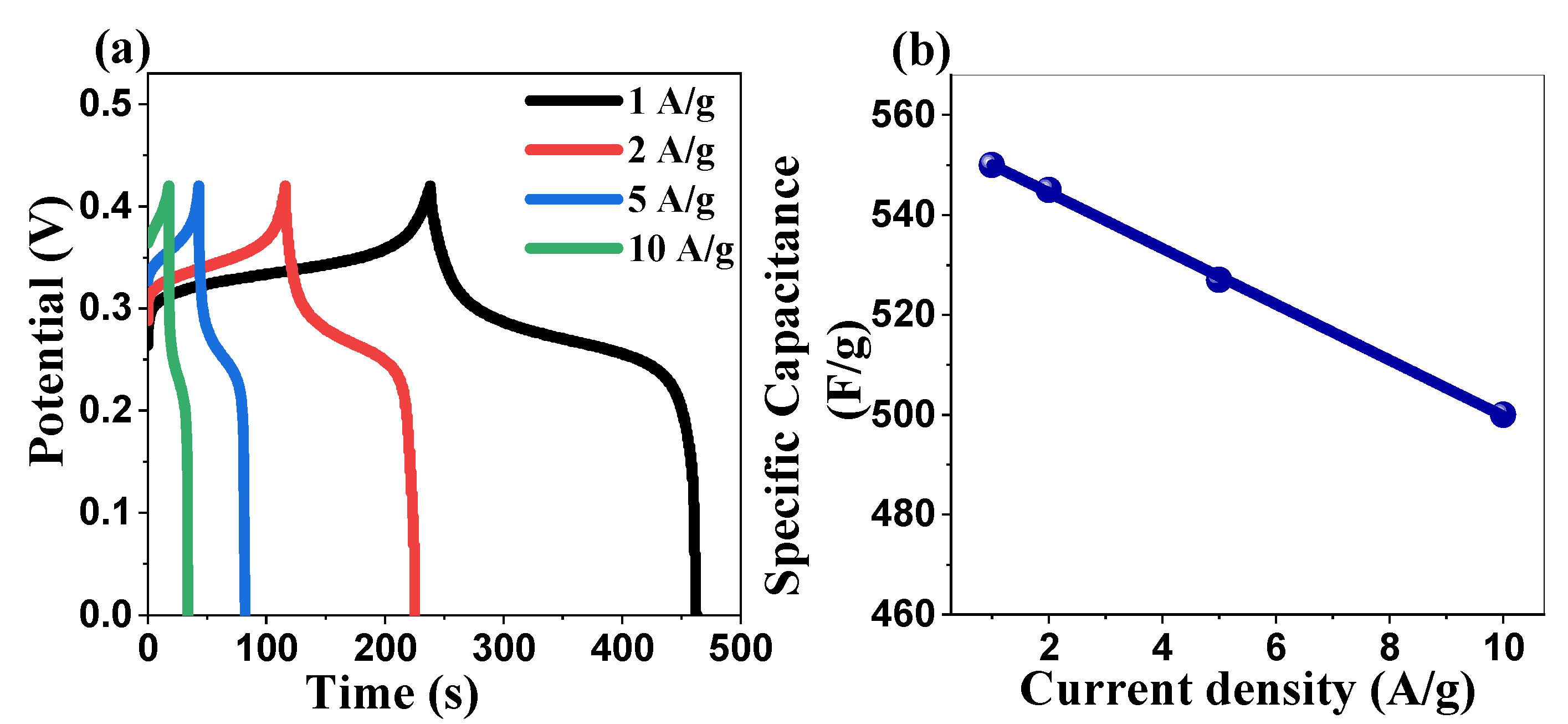
2.5.2. Galvanostatic Charge–Discharge Studies
2.5.3. Electrochemical Impedance Spectroscopy Investigation
2.5.4. Comparative Analysis and Discussion
2.6. Cytotoxicity Assessment of ZnO NPs
3. Methods and Materials
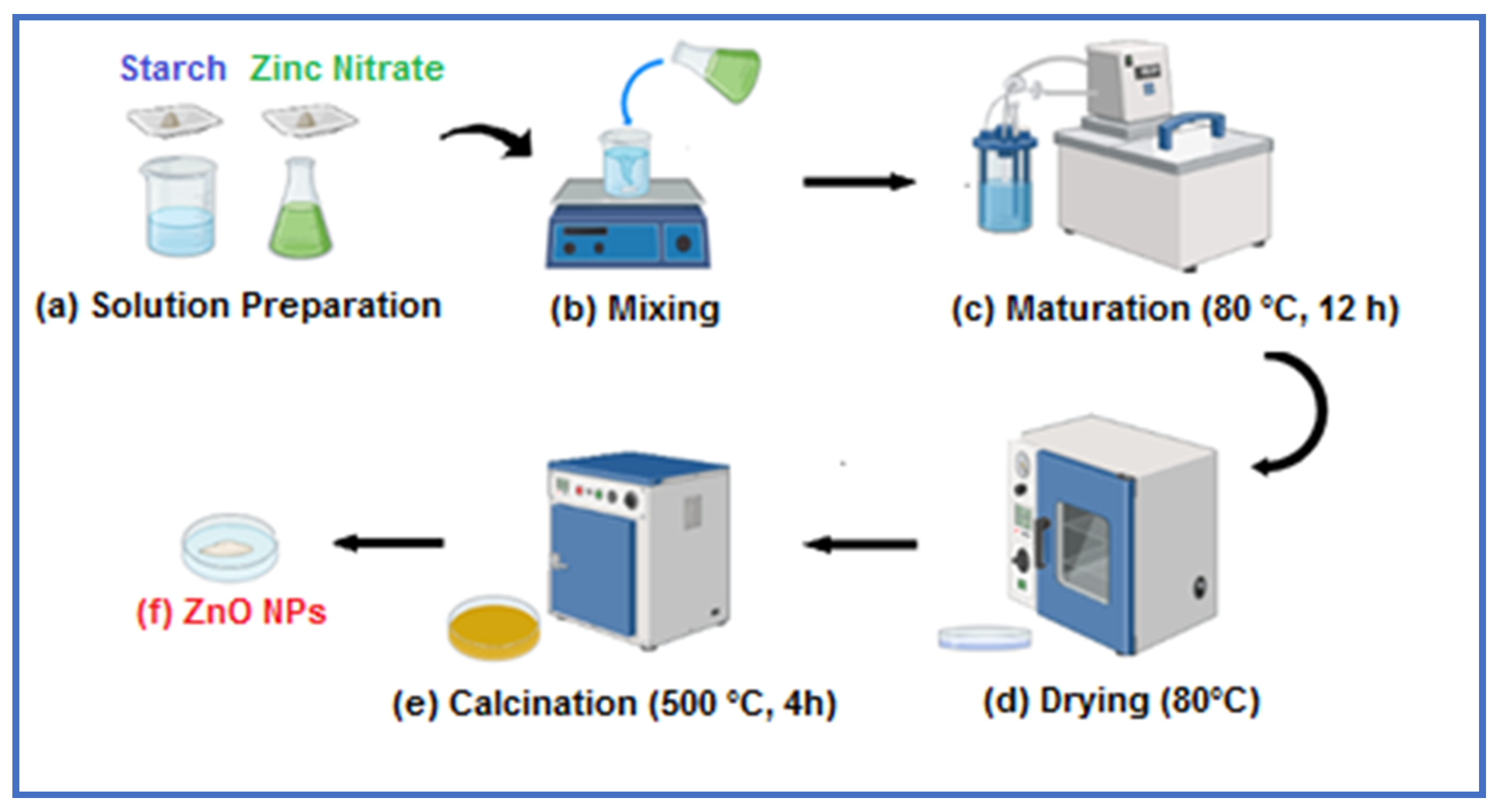
3.1. Zinc Oxide Nanoparticle Synthesis
3.2. Point of Zero Charge Measurement of ZnO Nanoparticles
3.3. Characterization Methods
3.4. Photocatalytic Degradation
3.5. Recyclability Testing Procedure
3.6. DPPH Radical Scavenging Assay and EC50 Determination
3.7. Electrode Preparation
3.8. Electrochemical Characterization
3.9. Brine Shrimp Lethality Assay (BSLA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousa, S.A.; Wissa, D.A.; Hassan, H.H.; Ebnalwaled, A.A.; Khairy, S.A. Enhanced photocatalytic activity of green synthesized zinc oxide nanoparticles using low-cost plant extracts. Sci. Rep. 2024, 14, 16713. [Google Scholar] [CrossRef]
- Roy, N.; Chakraborty, S. ZnO as photocatalyst: An approach to waste water treatment. Mater. Today Proc. 2021, 46, 6399–6403. [Google Scholar] [CrossRef]
- Di Mari, G.M.; Mineo, G.; Franzò, G.; Mirabella, S.; Bruno, E.; Strano, V. Low-Cost, High-Yield ZnO Nanostars Synthesis for Pseudocapacitor Applications. Nanomaterials 2022, 12, 2588. [Google Scholar] [CrossRef] [PubMed]
- Tonpe, D.A.; Gattu, K.P.; Kutwade, V.V.; Han, S.-H.; Sathe, B.R.; Sharma, R. ZnO-PANI nanocomposite: Enhanced electrochemical performance towards energy storage. J. Energy Storage 2024, 81, 110434. [Google Scholar] [CrossRef]
- Kuhn, R.; Bryant, I.M.; Jensch, R.; Böllmann, J. Applications of Environmental Nanotechnologies in Remediation, Wastewater Treatment, Drinking Water Treatment, and Agriculture. Appl. Nano 2022, 3, 54–90. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Marwani, H.M.; Asiri, A.M.; Alamry, K.A.; Rub, M.A.; Khan, A.; Khan, A.A.P.; Qusti, A.H. Low dimensional Ni-ZnO nanoparticles as marker of toxic lead ions for environmental remediation. J. Ind. Eng. Chem. 2014, 20, 1071–1078. [Google Scholar] [CrossRef]
- Shojaee, N.; Ebadzadeh, T.; Aghaei, A. Effect of concentration and heating conditions on microwave-assisted hydrothermal synthesis of ZnO nanorods. Mater. Charact. 2010, 61, 1418–1423. [Google Scholar] [CrossRef]
- Bakranova, D.; Nagel, D. ZnO for Photoelectrochemical Hydrogen Generation. Clean. Technol. 2023, 5, 1248–1268. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Truscă, R.-D.; Oprea, O.-C.; Surdu, V.-A.; Vasile, B.S.; Ficai, A.; Ficai, D.; Andronescu, E.; Ditu, L.-M. Zinc Oxide Nanoparticles for Water Purification. Materials 2021, 14, 4747. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Kumar, M.K.; Alinaghibeigi, M.; Moolayadukkam, S.; Eskandarinejad, S.; Mahmoudi, S.; Mirzamohammadi, S.; Rezaei-khamseh, M. A review on zinc oxide composites for energy storage applications: Solar cells, batteries, and supercapacitors. J. Compos. Compd. 2021, 3, 182–193. [Google Scholar]
- Mutukwa, D.; Taziwa, R.; Khotseng, L.E. A Review of the Green Synthesis of ZnO Nanoparticles Utilising Southern African Indigenous Medicinal Plants. Nanomaterials 2022, 12, 3456. [Google Scholar] [CrossRef] [PubMed]
- Majithia, M.; Barretto, D.A. Biocompatible green-synthesized nanomaterials for therapeutic applications. In Advances in Nano and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 12; pp. 285–367. [Google Scholar] [CrossRef]
- Nagar, V.; Singh, T.; Tiwari, Y.; Aseri, V.; Pandit, P.P.; Chopade, R.L.; Pandey, K.; Lodha, P.; Awasthi, G. ZnO Nanoparticles: Exposure, toxicity mechanism and assessment. Mater. Today Proc. 2022, 69, 56–63. [Google Scholar] [CrossRef]
- Shafiee, P.; Mahmoudi, S.; Eskandarinezhad, S.; Reisi Nafchi, M.; Ahmadi, E. Sol-gel zinc oxide nanoparticles: Advances in synthesis and applications. Synth. Sinter. 2021, 1, 242–254. [Google Scholar] [CrossRef]
- Cao, Y.; Bian, X.; Luo, S.; Hu, X.; Liu, C. Synthesis and Characterization of the Starch-ZnO Hybrid Nanoparticles: Effect of the Amylose Content. Starch-Starke 2022, 75, 2100256. [Google Scholar] [CrossRef]
- Jadoun, S.; Sathish, M.; Jangid, N.K.; Yáñez, J.; Chinnam, S.; Aepuru, R. Recent advancements in sustainable synthesis of zinc oxide nanoparticles using various plant extracts for environmental remediation. Environ. Sci. Pollut. Res. Int. 2024, 31, 19123–19147. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, N.; Ji, Y.; Zhang, J.; Zhu, Y.; Yi, T. Nanosized zinc oxides-based materials for electrochemical energy storage and conversion: Batteries and supercapacitors. Chin. Chem. Lett. 2021, 33, 714–729. [Google Scholar] [CrossRef]
- Rahman, A.; Harunsani, M.H.; Tan, A.L.; Ahmad, N.; Hojamberdiev, M.; Mansoob Khan, M. Effect of Mg doping on ZnO fabricated using aqueous leaf extract of Ziziphus mauritiana Lam. for antioxidant and antibacterial studies. Bioprocess Biosyst. Eng. J. 2021, 44, 875–889. [Google Scholar] [CrossRef]
- Md Akhir, R.; Umbaidilah, S.Z.; Abdullah, N.A.; Alrokayan, S.A.H.; Khan, H.A.; Soga, T.; Rusop, M.; Khusaimi, Z. The potential of pandanus amaryllifolius leaves extract in fabrication of dense and uniform zno microrods. Micromachines 2020, 11, 299. [Google Scholar] [CrossRef]
- Coulter, J.B.; Birnie, D.P. Assessing tauc plot slope quantification: Zno thin films as a model system. Phys. Status Solidi B 2018, 255, 1700393. [Google Scholar] [CrossRef]
- Taha, K.K.; Mustafa, M.M.; Ahmed, H.A.M.; Talab, S. Selenium zinc oxide (Se/zno) nanoparticles: Synthesis, characterization, and photocatalytic activity. Z. Für Naturforschung A 2019, 74, 1043–1056. [Google Scholar] [CrossRef]
- Ponsanti, K.; Tangnorawich, B.; Ngernyuang, N.; Pechyen, C. A flower shape-green synthesis and characterization of silver nanoparticles (AgNPs) with different starch as a reducing agent. J. Mater. Res. Technol. 2020, 9, 11003–11012. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using ft-raman and ftir spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, T.; Mohammed, A.; Mohamed, W.; Sobh, R.; Zahran, M. Optimized synthesis of biopolymer-based zinc oxide nanoparticles and evaluation of their antibacterial activity. Egypt. J. Chem. 2021, 64, 3767–3790. [Google Scholar] [CrossRef]
- Munirah, M.; Khan, Z.R.; Aziz, A.; Khan, M.S.; Khandaker, M.U. Influence of zinc concentration on band gap and sub-band gap absorption on ZnO nanocrystalline thin films sol-gel grown. Mater. Sci. Pol. 2017, 35, 246–253. [Google Scholar] [CrossRef]
- Shahabuddin, A.K.M.; Jewena, N.; Das, S.K.; Khandaker, J.I.; Ahmed, F. Low-temperature growth of ZnO nanoparticles by using autoclave. Nanosistemi Nanomater. Nanotehnologii 2021, 19, 177–188. [Google Scholar] [CrossRef]
- Khorsand Zak, A.; Abd-Majid, W.H.; Mahmoudian, M.R.; Darroudi, M.; Yousefi, R. Starch-stabilized synthesis of ZnO nanopowders at low temperature and optical properties study. Adv. Powder Technol. 2013, 24, 618–624. [Google Scholar] [CrossRef]
- Nezamabadi, V.; Akhgar, M.R.; Tahamipour, B.; Rajaei, P. Biosynthesis and antibacterial activity of ZnO nanoparticles by artemisia aucheri extract. Iran. J. Biotechnol. 2020, 18, e2426. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Mohammed, A.K.; Sumaila, A. Comparative study of crystallite size using Williamson-Hall and Debye-Scherrer plots for ZnO nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045013. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Feng, F.; Yin, H.; Si, N.; Hao, C. Solid-Phase Synthesis of Mesoporous ZnO Using Lignin-Amine Template and Its Photocatalytic Properties. Ind. Eng. Chem. Res. 2014, 53, 6585–6592. [Google Scholar] [CrossRef]
- Innocenzi, P.; Malfatti, L. Mesoporous ordered titania films: An advanced platform for photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2023, 58, 100646. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, G.; Huang, L.; Zhao, Y.; Zhang, Y. Synthesis of Mesoporous ZnO Nanosheets via Facile Solvothermal Method as the Anode Materials for Lithium-ion Batteries. Nanoscale Res. Lett. 2016, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chetia, T.R.; Qureshi, M.; Ansari, M.S. Rational design of hierarchical ZnO superstructures for efficient charge transfer: Mechanistic and photovoltaic studies of hollow, mesoporous, cage-like nanostructures with compacted 1D building blocks. Phys. Chem. Chem. Phys. 2016, 18, 5344–5357. [Google Scholar] [CrossRef] [PubMed]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Pauporté, T.; Rathouský, J. Electrodeposited Mesoporous ZnO Thin Films as Efficient Photocatalysts for the Degradation of Dye Pollutants. J. Phys. Chem. C 2007, 111, 7639–7644. [Google Scholar] [CrossRef]
- Lin, S.-T.; Thirumavalavan, M.; Lee, J.-F. A comprehensive study on the mechanism for controlled synthesis of ZnO-based nanomaterials via various polysaccharides as chelates. Results Phys. 2018, 9, 1596–1601. [Google Scholar] [CrossRef]
- Carp, O.; Tirsoaga, A.; Jurca, B.; Ene, R.; Somacescu, S.; Ianculescu, A. Biopolymer starch mediated synthetic route of multi-spheres and donut ZnO structures. Carbohydr. Polym. 2015, 115, 285–293. [Google Scholar] [CrossRef]
- Kržišnik, N.; Mladenovič, A.; Škapin, A.S.; Škrlep, L.; Ščančar, J.; Milačič, R. Nanoscale zero-valent iron for the removal of Zn2+, Zn(Ii)–EDTA and Zn(Ii)–citrate from aqueous solutions. Sci. Total Environ. 2014, 476, 20–28. [Google Scholar] [CrossRef]
- Nawal, H.M.; Abdulqadier, H.A.K. Synthesis and study of zinc oxide nanoparticles and their nanocomposites. J. Pharm. Negat. Results 2022, 13, 790–797. [Google Scholar] [CrossRef]
- Zhang, G.; Shen, X.; Yang, Y. Facile synthesis of monodisperse porous ZnO spheres by a soluble starch-assisted method and their photocatalytic activity. J. Phys. Chem. C 2011, 115, 7145–7152. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Choi, Y.I.; Jung, H.J.; Shin, W.G.; Sohn, Y. Band gap-engineered ZnO and Ag/ZnO by ball-milling method and their photocatalytic and Fenton-like photocatalytic activities. Appl. Surf. Sci. 2015, 356, 615–625. [Google Scholar] [CrossRef]
- Gowthambabu, V.; Balamurugan, A.; Dhivya Bharathy, R.; Satheeshkumar, S.; Kanmani, S.S. ZnO nanoparticles as efficient sunlight driven photocatalyst prepared by solution combustion method involved lime juice as biofuel. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 258, 119857. [Google Scholar] [CrossRef] [PubMed]
- Rathika, A.; Irine, T.M. Synthesis of silver (Ag) doped zinc oxide (ZnO) nanoparticles as efficient photocatalytic activity for degradation methylene blue dye. J. Adv. Sci. Res. 2022, 13, 129–135. [Google Scholar] [CrossRef]
- Rao, G.T.; Ravikumar, R.V.S.S.N.; Reddy, N.B.; Zyryanov, G.V. Hydrothermal synthesis of ZnO nanopowder and its photocatalytic performance under UV and visible light irradiation. AIP Conf. Proc. 2019, 2063, 040044. [Google Scholar] [CrossRef]
- Wouters, R.D.; Muraro, P.C.L.; Druzian, D.M.; Viana, A.R.; De Oliveira Pinto, E.; Da Silva, J.K.L.; Vizzotto, B.S.; Ruiz, Y.P.M.; Galembeck, A.; Pavoski, G.; et al. Zinc oxide nanoparticles: Biosynthesis, characterization, biological activity and photocatalytic degradation for tartrazine yellow dye. J. Mol. Liq. 2022, 371, 121090. [Google Scholar] [CrossRef]
- Alharthi, M.N.; Ismail, I.; Bellucci, S.; Khdary, N.H.; Abdel Salam, M. Biosynthesis microwave-assisted of zinc oxide nanoparticles with ziziphus jujuba leaves extract: Characterization and photocatalytic application. Nanomaterials 2021, 11, 1682. [Google Scholar] [CrossRef]
- Tăbăcaru, A.; Buşilă, M.; Pleşcan, V.; Muşat, V. Photocatalytic study of organosilane-modified zinc oxide nanoparticles. Ovidius Univ. Ann. Chem. 2015, 26, 61–66. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K.; Sibhatu, A.K. Photocatalytic activity of biosynthesized α-Fe2O3 nanoparticles for the degradation of methylene blue and methyl orange dyes. Optik 2021, 241, 167226. [Google Scholar] [CrossRef]
- Luque-Morales, P.A.; Garrafa-Galvez, H.E.; Orozco-Carmona, V.M.; Baez-Lopez, Y.A.; Nava-Olivas, O.J.; Lopez-Peraza, A.; Chinchillas-Chinchillas, M.D.J.; Amaya-Parra, G. ZnO Semiconductor Nanoparticles and Their Application in Photocatalytic Degradation of Various Organic Dyes. Materials 2021, 14, 7537. [Google Scholar] [CrossRef]
- Maro, C.A.G.; Flores, H.G.; Morales, M.L.; Hernández, D.V.; Gálvez, H.E.G.; Chinchillas, M.D.J.C.; Carmona, V.M.O.; Olivas, O.D.J.N. Peumus boldus Used in the Synthesis of ZnO Semiconductor Nanoparticles and Their Evaluation in Organic Contaminants. Materials 2023, 16, 4344. [Google Scholar] [CrossRef]
- Mohamed, Z.H.; Riyad, Y.M.; Hendawy, H.A.; Abdelbary, H.M.H. Enhanced photocatalytic degradation of the antidepressant sertraline in aqueous solutions by zinc oxide nanoparticles. Water 2023, 15, 2074. [Google Scholar] [CrossRef]
- Pham, T.A.T.; Tran, V.A.; Le, V.D.; Nguyen, M.V.; Truong, D.D.; Do, X.T.; Vu, A.-T. Facile preparation of zno nanoparticles and ag/zno nanocomposite and their photocatalytic activities under visible light. Int. J. Photoenergy 2020, 2020, 8897667. [Google Scholar] [CrossRef]
- Sukri, S.N.A.M.; Shameli, K.; Isa, E.D.M. Photocatalytic Degradation of Malachite Green Dye by Plant-mediated Biosynthesized Zinc Oxide Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 808, 012034. [Google Scholar] [CrossRef]
- Ranjbari, A.; Demeestere, K.; Kim, K.-H.; Heynderickx, P.M. Oxygen vacancy modification of commercial ZnO by hydrogen reduction for the removal of thiabendazole: Characterization and kinetic study. Appl. Catal. B Environ. 2022, 324, 122265. [Google Scholar] [CrossRef]
- Sulistyo Rini, A.; Rati, Y.; Nabilla, A. Microwave-assisted biosynthesis and characterization of ZnO film for photocatalytic application in methylene blue degradation. Commun. Sci. Technol. 2021, 6, 69–73. [Google Scholar] [CrossRef]
- Jarvin, M.; Rosaline, D.R.; Dotto, G.L.; Kumar, S.A.; Foletto, E.L.; Inbanathan, S.S.R. Remarkable sunlight-driven photocatalytic performance of Ag-doped ZnO nanoparticles prepared by green synthesis for degradation of emerging pollutants in water. Environ. Sci. Pollut. Res. 2022, 29, 57330–57344. [Google Scholar] [CrossRef]
- Azmina, M.S.; Md Nor, R.; Osman, Z.; Sani, S.F.A.; Razak, N.S.A.; Rafaie, H.A. Enhanced photocatalytic activity of ZnO nanoparticles grown on porous silica microparticles. Appl. Nanosci. 2017, 7, 885–892. [Google Scholar] [CrossRef]
- Ghaffar, S.; Hanbashi, A.; Alhazmi, H.A.; Najmi, A.; Amin, H.M.A.; Zoghebi, K.; Makeen, H.A.; Ullah, S.; Assad, N.; Naeem-Ul-Hassan, M.; et al. Improved Photocatalytic and Antioxidant Activity of Olive Fruit Extract-Mediated ZnO Nanoparticles. Antioxidants 2023, 12, 1201. [Google Scholar] [CrossRef]
- Abdul Rahman, I.; Ayob, M.T.M.; Radiman, S. Enhanced photocatalytic performance of nio-decorated zno nanowhiskers for methylene blue degradation. J. Nanotechnol. 2014, 2014, 212694. [Google Scholar] [CrossRef]
- Jeyachitra, R.; Kalpana, S.; Senthil, T.S.; Kang, M. Electrical behavior and enhanced photocatalytic activity of (Ag, Ni) co-doped ZnO nanoparticles synthesized from co-precipitation technique. Water Sci. Technol. 2020, 81, 1296–1307. [Google Scholar] [CrossRef]
- Isai, K.; Shrivastava, V. Photocatalytic degradation of methylene blue using ZnO and 2%Fe-ZnO semiconductor nanomaterials synthesized by sol-gel method: A comparative study. SN Appl. Sci. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Purba, F.J.; Sitorus, Z.; Tarigan, K.; Siregar, N. Enhanced photocatalytic activity of Cu2O/ZnO/GO nanocomposites on the methylene blue degradation. Baghdad Sci. J. 2023, 21, 62–71. [Google Scholar] [CrossRef]
- Acedo-Mendoza, A.G.; Infantes-Molina, A.; Vargas-Hernández, D.; Chávez-Sánchez, C.A.; Rodríguez-Castellón, E.; Tánori-Córdova, J.C. Photodegradation of methylene blue and methyl orange with CuO supported on ZnO photocatalysts: The effect of copper loading and reaction temperature. Mater. Sci. Semicond. Process. 2020, 119, 105257. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Mater. Lett. 2013, 91, 170–174. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.K.; Kaushik, R.D.; Purohit, L.P. Chalcogen-doped zinc oxide nanoparticles for photocatalytic degradation of Rhodamine B under the irradiation of ultraviolet light. Mater. Today Chem. 2021, 20, 100464. [Google Scholar] [CrossRef]
- Ajil, A.H.; Ahmed, N.M.; Yam, F.K.; Zango, Z.U.; Wadi, I.A.; Binzowaimil, A.M.; Aldaghri, O.; Ibnaouf, K.H.; Cabrera, H. Enhancing methyl orange degradation with laser-generated zno and ce-doped zno nanoparticles. Appl. Sci. 2023, 13, 11857. [Google Scholar] [CrossRef]
- Richard, C.; Bosquet, F.; Pilichowski, J.-F. Photocatalytic transformation of aromatic compounds in aqueous zinc oxide suspensions: Effect of substrate concentration on the distribution of products. J. Photochem. Photobiol. A Chem. 1997, 108, 45–49. [Google Scholar] [CrossRef]
- Medina-Acosta, M.; Chinchillas-Chinchillas, M.J.; Garrafa-Gálvez, H.E.; Garcia-Maro, C.A.; Rosas-Casarez, C.A.; Lugo-Medina, E.; Luque-Morales, P.A.; Soto-Robles, C.A. Photocatalytic Degradation of Four Organic Dyes Present in Water Using ZnO Nanoparticles Synthesized with Green Synthesis Using Ambrosia ambrosioides Leaf and Root Extract. Processes 2024, 12, 2456. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, C.; Zhu, Z.; Lu, J.; Manohari, A.G.; Shi, Z. Self-assembled ZnO/Ag hollow spheres for effective photocatalysis and bacteriostasis. Mater. Res. Bull. 2017, 98, 64–69. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; He, Y.; Zhang, Y.; Ti, M.; Wu, L. Comparative study on the removal of different-type organic pollutants on three-dimensional hexagonal-tungsten trioxide/reduced graphene oxide nanorod arrays: Adsorption and visible light photodegradation. J. Mater. Sci. Mater. Electron. 2021, 32, 2268–2282. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Mohite, B.M.; Yadav, A.A. Basics of Photocatalysis and Different Strategy for Enhancing the Photocatalytic Efficiency. Am. J. Eng. Appl. Sci. 2020, 13, 265–268. [Google Scholar] [CrossRef]
- Villarreal, R.C.; Luque-Morales, M.; Chinchillas-Chinchillas, M.J.; Luque, P.A. Langmuir-Hinshelwood-Hougen-Watson model for the study of photodegradation properties of zinc oxide semiconductor nanoparticles synthetized by Peumus boldus. Results Phys. 2022, 36, 105421. [Google Scholar] [CrossRef]
- Nisar, A.; Muneer, M.; Saeed, M.; Khan, I.; Akhtar, J.; Usman, M.; Adeel, M. Kinetic modeling of ZnO-rGO catalyzed degradation of methylene blue. Int. J. Chem. Kinet. 2020, 52, 645–654. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Zhan, H.; Zhou, P.; Guo, R.; Yuan, Y. Synthesis of Rectorite/Fe3O4/ZnO Composites and Their Application for the Removal of Methylene Blue Dye. Catalysts 2018, 8, 107. [Google Scholar] [CrossRef]
- Elsayed, M.S.; Hassan, A.F.; Bader, D.M.D.; Ahmed, I.A. Green Synthesis of Nano Zinc Oxide/Nanohydroxyapatite Composites Using Date Palm Pits Extract and Eggshells: Adsorption and Photocatalytic Degradation of Methylene Blue. Nanomaterials 2021, 12, 49. [Google Scholar] [CrossRef]
- Ollis, D.F. Kinetics of Photocatalyzed Reactions: Five Lessons Learned. Front. Chem. 2018, 6, 378. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, H.; Ma, H.; Zhao, L.; Guo, L.-H. Quantitative Analysis of Reactive Oxygen Species Photogenerated on Metal Oxide Nanoparticles and Their Bacteria Toxicity: The Role of Superoxide Radicals. Environ. Sci. Technol. 2017, 51, 10137–10145. [Google Scholar] [CrossRef]
- Siripireddy, B.; Mandal, B.K. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv. Powder Technol. 2017, 28, 785–797. [Google Scholar] [CrossRef]
- Suresh, D.; Udayabhanu; Nethravathi, P.C.; Lingaraju, K.; Rajanaika, H.; Sharma, S.C.; Nagabhushana, H. EGCG assisted green synthesis of ZnO nanopowders: Photodegradative, antimicrobial and antioxidant activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1467–1474. [Google Scholar] [CrossRef]
- Pairoj, S.; Damrongsak, P.; Damrongsak, B.; Jinawath, N.; Kaewkhaw, R.; Leelawattananon, T.; Ruttanasirawit, C.; Locharoenrat, K. Antiradical properties of chemo drug, carboplatin, in cooperation with ZnO nanoparticles under UV irradiation in putative model of cancer cells. Biocybern. Biomed. Eng. 2019, 39, 893–901. [Google Scholar] [CrossRef]
- Marin-Flores, C.A.; Rodríguez-Nava, O.; García-Hernández, M.; Ruiz-Guerrero, R.; Juárez-López, F.; Morales-Ramírez, A.D.J. Free-radical scavenging activity properties of ZnO sub-micron particles: Size effect and kinetics. J. Mater. Res. Technol. 2021, 13, 1665–1675. [Google Scholar] [CrossRef]
- Kumnoedauy, K.; Damrongsak, P.; Locharoenrat, K.; Damrongsak, B. Preparation, characterization and antiradical activity of zinc oxide nanoparticles. Suan Sunandha Sci. Technol. J. 2022, 9, 1–7. [Google Scholar] [CrossRef]
- Wang, H.; Liang, M.; Duan, D.; Shi, W.; Song, Y.; Sun, Z. Rose-like Ni3S4 as battery-type electrode for hybrid supercapacitor with excellent charge storage performance. Chem. Eng. J. 2018, 350, 523–533. [Google Scholar] [CrossRef]
- Peçenek, H.; Dokan, F.K.; Onses, M.S.; Yılmaz, E.; Sahmetlioglu, E. Outstanding supercapacitor performance with intertwined flower-like NiO/MnO2/CNT electrodes. Mater. Res. Bull. 2022, 149, 111745. [Google Scholar] [CrossRef]
- Yetiman, S.; Dokan, F.K.; Onses, M.S.; Huang, X.; Yılmaz, E.; Sahmetlioglu, E. Asymmetric and zinc-ion hybrid supercapacitors based on iron oxide and carbon dots. J. Energy Storage 2023, 68, 107608. [Google Scholar] [CrossRef]
- Pecenek, H.; Dokan, F.K.; Onses, M.S.; Yılmaz, E.; Sahmetlioglu, E. High energy density hybrid supercapacitors based on graphitic carbon nitride modified BiFeO3 and biomass-derived activated carbon. J. Energy Storage 2023, 64, 107075. [Google Scholar] [CrossRef]
- Jiao, Y.; Pei, J.; Chen, D.; Yan, C.; Hu, Y.; Zhang, Q.; Chen, G. Mixed-metallic MOF based electrode materials for high performance hybrid supercapacitors. J. Mater. Chem. A 2017, 5, 1094–1102. [Google Scholar] [CrossRef]
- Yetiman, S.; Dokan, F.K.; Onses, M.S.; Yılmaz, E.; Sahmetlioglu, E. Hybrid electrodes composed of graphitic carbon nitride and zeolitic imidazolate framework-67 for supercapacitor applications. Int. J. Energy Res. 2022, 46, 22730–22743. [Google Scholar] [CrossRef]
- Peçenek, H.; Dokan, F.K.; Onses, M.S.; Yılmaz, E.; Sahmetlioglu, E. Highly compressible binder-free sponge supercapacitor electrode based on flower-like NiO/MnO2/CNT. J. Alloys Compd. 2022, 913, 165053. [Google Scholar] [CrossRef]
- Welegergs, G.G.; Gebretinsae, H.G.; Akoba, R.; Matinsie, N.; Nuru, Z.Y.; Maaza, M. Electrochemical properties of green synthesised Zinc oxide (Zno) Nanoparticles. MRS Adv. 2020, 5, 1103–1112. [Google Scholar] [CrossRef]
- Pradeeswari, K.; Venkatesan, A.; Pandi, P.; Karthik, K.; Hari Krishna, K.V.; Mohan Kumar, R. Study on the electrochemical performance of ZnO nanoparticles synthesized via non-aqueous sol-gel route for supercapacitor applications. Mater. Res. Express 2019, 6, 105525. [Google Scholar] [CrossRef]
- Chikere, C.; Faisal, N.H.; Lin, P.K.T.; Fernandez, C. Zinc oxide nanoparticles modified-carbon paste electrode used for the electrochemical determination of Gallic acid. J. Phys. Conf. Ser. 2019, 1310, 012008. [Google Scholar] [CrossRef]
- Sharafudheen, S.B.; Vijayakumar, C.; Anjana, P.M.; Rayar, S.L.; Rajakrishnan, R.; Arokiyaraj, S.; Bindhu, M.R. Synergistic effects of Curcuma amada functionalized ZnO nanostructures: Bioactivity, catalytic, photocatalytic, and supercapacitor application. Appl. Nanosci. 2024, 14, 891–916. [Google Scholar] [CrossRef]
- Gupta, M.K.; Kumar, Y.; Sonnathi, N.; Sharma, S.K. Hydrothermally grown zinc oxide nanostructures@carbon composites for supercapacitor application. Phys. Status Solidi A 2023, 220, 2200451. [Google Scholar] [CrossRef]
- AlFawaz, A.; Ahmad, A.; Ahmad, N.; Alharthi, F.A. Glycine based auto-combustion synthesis of ZnO nanoparticles as electrode material for supercapacitor. Phys. Scr. 2022, 97, 030009. [Google Scholar] [CrossRef]
- Bishwakarma, H.; Das, A.K. Synthesis of zinc oxide nanoparticles through hybrid machining process and their application in supercapacitors. J. Electron. Mater. 2020, 49, 1541–1549. [Google Scholar] [CrossRef]
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.; McLaughlin, J. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Eleryan, A.; Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Hassaan, M.A.; Elkatory, M.R.; Ragab, S.; Osibote, O.A.; Kusuma, H.S.; El Nemr, A. Adsorption of direct blue 106 dye using zinc oxide nanoparticles prepared via green synthesis technique. Environ. Sci. Pollut. Res. 2023, 30, 69666–69682. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Supraja, N.; Prasad, T.N.V.K.V.; Gandhi, A.D.; Anbumani, D.; Kavitha, P.; Babujanarthanam, R. Synthesis, characterization and evaluation of antimicrobial efficacy and brine shrimp lethality assay of Alstonia scholaris stem bark extract mediated ZnONPs. Biochem. Biophys. Rep. 2018, 14, 69–77. [Google Scholar] [CrossRef]

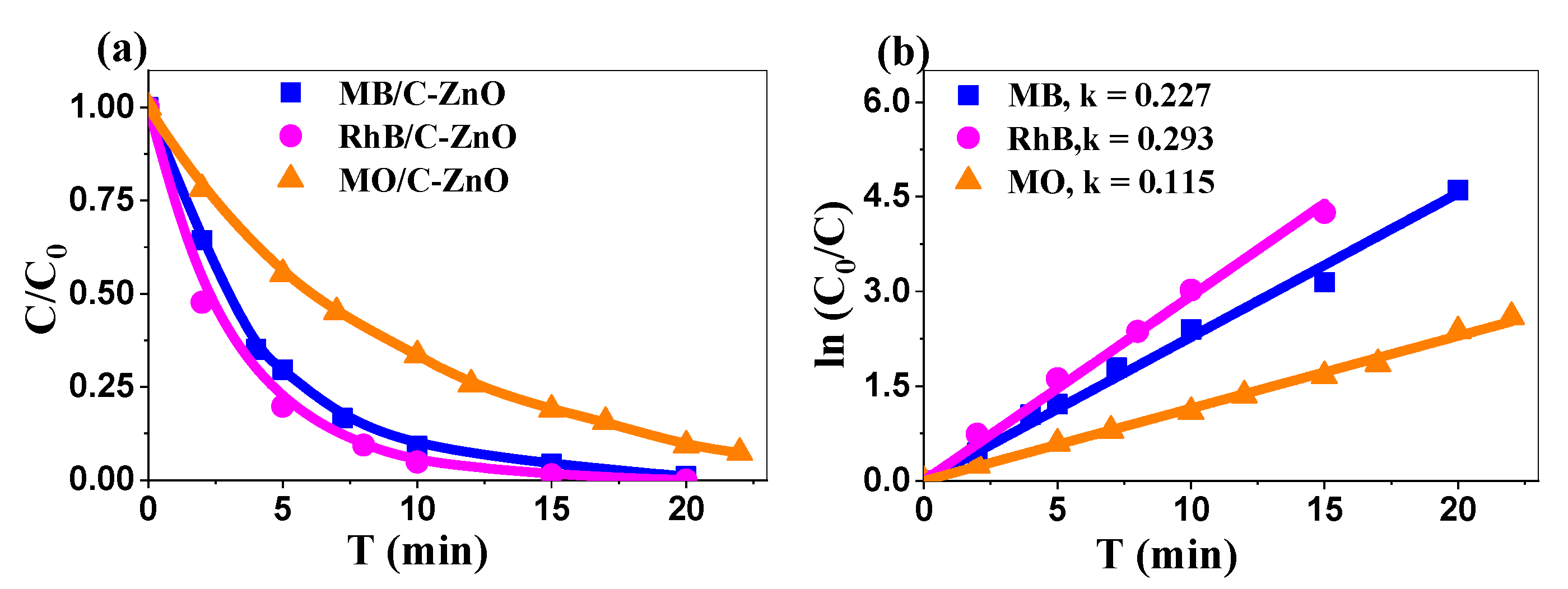
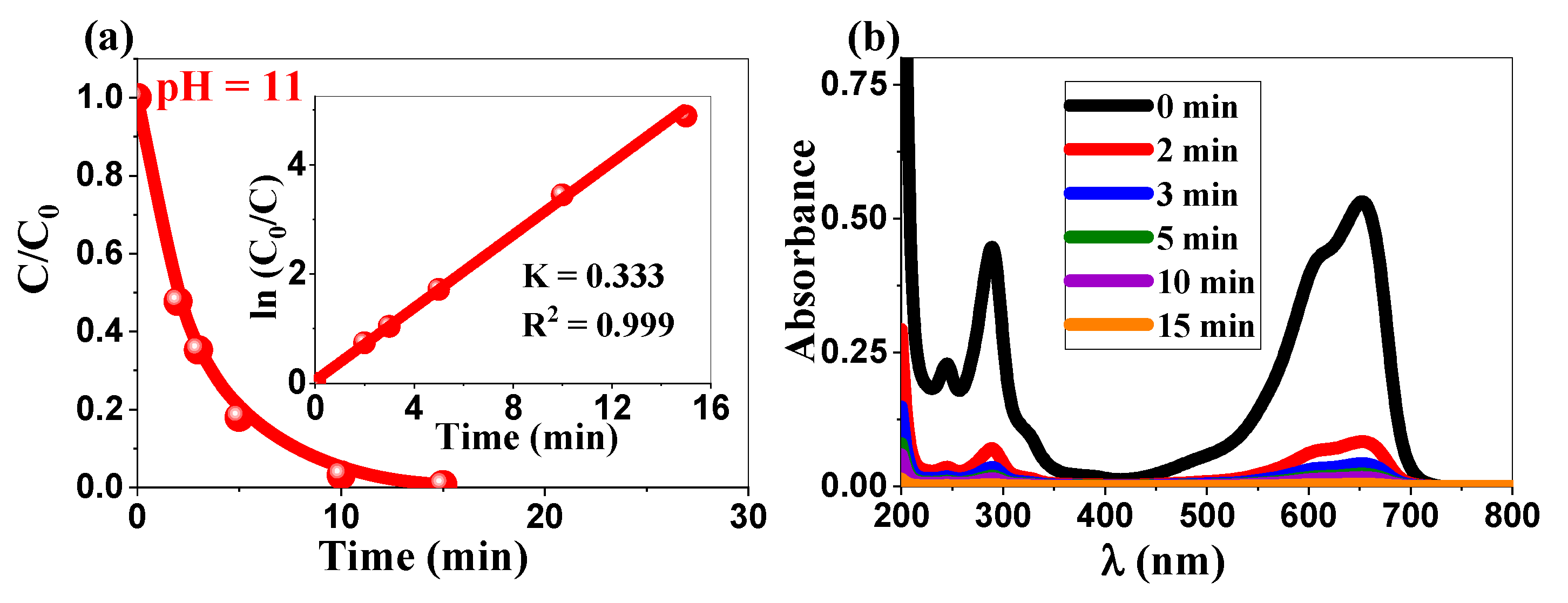
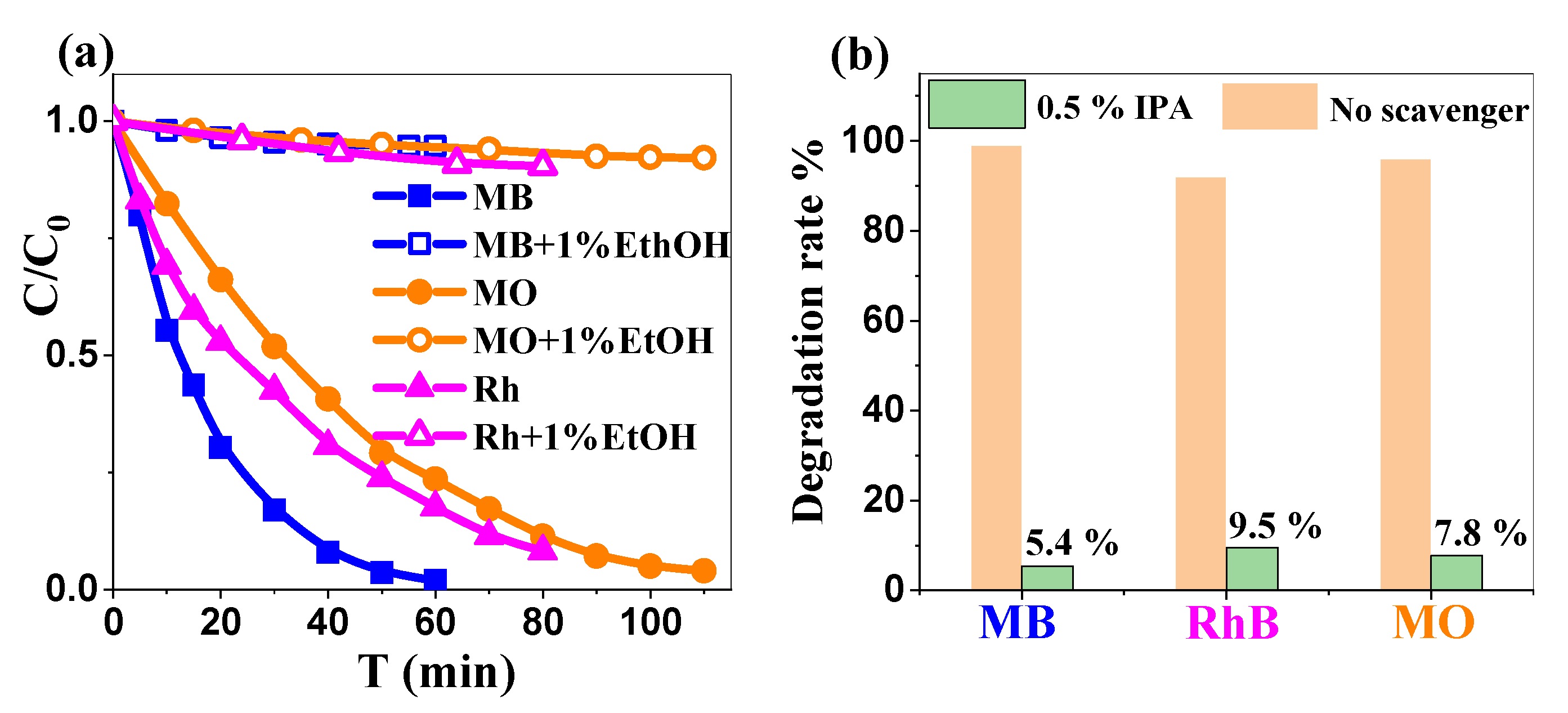
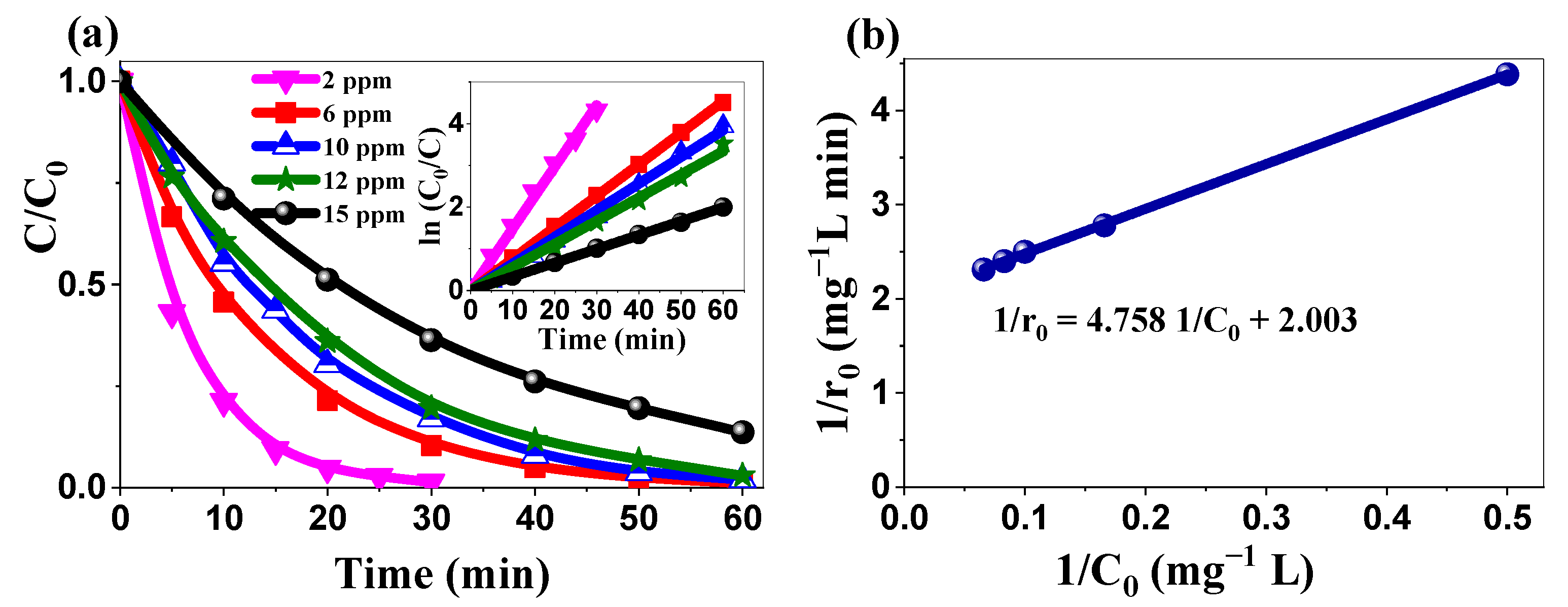



| 2θ (°) | (hkl) | FWHM (°) | a (nm) | c (nm) | D (nm) | Dmean (nm) |
|---|---|---|---|---|---|---|
| 31.932 | (100) | 0.37 | 0.323 | 22.332 | 22.2 ± 0.8 | |
| 34.578 | (002) | 0.359 | 0.518 | 23.176 | ||
| 36.415 | (101) | 0.394 | 21.226 |
| kapp (min−1) | |||
|---|---|---|---|
| Photocatalyst | MB | RhB | MO |
| C-ZnO | 0.227 | 0.293 | 0.115 |
| ZnO NPs | 0.064 | 0.03 | 0.027 |
| Dye | Catalyst | Synthesis Method | [Dye]0 (ppm) | kapp (min−1) | Deg (%) | Time (min) | [Ref] |
|---|---|---|---|---|---|---|---|
| MB | ZnO NPs | sol gel | 10 | 0.064 pH 7.3 | 98 | 60 | Present work |
| ZnO NPs | sol gel | 10 | 0.33 pH 11 | 100 | 15 | Present work | |
| ZnO | sol-gel | 10 | 0.0106 | 86 | 140 | [62] | |
| 2% Fe-ZnO | sol-gel | 10 | 0.0107 | 92 | 140 | [62] | |
| Cu₂O/ZnO/GO (5%) | coprecipitation and hydrothermal | 5 | - | 91.4 | 120 | [63] | |
| ZnO | sonication | 10 | 0.009 | 54 | 80 | [60] | |
| NiO-ZnO | sonication | 10 | 0.015 | 72 | 80 | [60] | |
| 7.5% Cu-ZnO | impregnation | 20 | 0.137 (45 °C) | 99 | 15 | [64] | |
| RhB | ZnO NPs | sol-gel | 10 | 0.03 | 92 | 80 | Present work |
| ZnO NPs | solution phase | 10 | 0.034 | 95 | 70 | [65] | |
| 5% Se-ZnO NPs | sol-gel precipitation | 10 | 0.014 | 98.23 | 150 | [66] | |
| MO | ZnO NPs | sol gel | 10 | 0.027 | 96 | 110 | Present work |
| ZnO NPs | laser-assisted chemical bath | 20 | 0.00417 | 85 | 120 | [67] | |
| 1% Ce-ZnO NPs | laser-assisted chemical bath | 20 | 0.015 | 92 | 120 | [67] |
| C0 (mg/L) | 2 | 6 | 10 | 12 | 15 |
| Kapp (min−1) | 0.1481 | 0.075 | 0.064 | 0.055 | 0.033 |
| R2 | 0.998 | 0.999 | 0.997 | 0.997 | 0.999 |
| [ZnO] (µg/mL) | 6.25 | 12.5 | 25 | 50 | 100 | 200 | 400 | EC50 (µg/mL) |
|---|---|---|---|---|---|---|---|---|
| Inhibition (%) | 9.05 ± 0.29 | 22.56 ± 0.38 | 36.7 ± 0.11 | 64.24± 0.19 | 75.72 ± 0.38 | 89.36 ± 0.11 | 89.93 ± 0.11 | 32.21± 4.62 |
| Study | Material | EC50 (μg/mL) | Synthesis Method |
|---|---|---|---|
| Current study | ZnO NPs | 32.21 | Starch-mediated sol-gel |
| [82] | ZnO submicron particles | 97.65 | Precipitation method |
| [83] | ZnO NPs | 7.8 | Sol-gel, calcined at 300 °C |
| [83] | Commercial ZnO | 36.6 | Commercial (100 nm diameter) |
| Study | ZnO Material | Specific Capacitance (F/g) | Current Density (A/g) | Electrolyte | Synthesis Method |
|---|---|---|---|---|---|
| Current Study | ZnO NPs | 550 | 1 | KOH (2 M) | Starch sol-gel |
| [94] | Curcuma amada-functionalized ZnO | 457 | 1 | KOH (1 M) | Biosynthesis |
| [95] | ZnO NPs | 214 | 1 | Na2SO4 (1 M) | Hydrothermal |
| [96] | ZnO NPs | 170.6 | 1 | KOH (1 M) | Glycine-based auto-combustion |
| [97] | ZnO NPs | 708.75 | 1 | KOH (1 M) | Hybrid machining |
| [ZnO] (μg/mL) | Number of Dead Nauplii (After 24 h) | Mortality % | LC50 (μg/mL) | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Replication 1 | Replication 2 | Replication 3 | ||||
| 0 | 0 | 0 | 0 | 00.00 ± 00.00 | 1647.696 | [1127.548–3472.362] |
| 125 | 1 | 0 | 1 | 6.67 ± 5.77 | ||
| 250 | 2 | 1 | 3 | 20.00 ± 10.00 | ||
| 500 | 3 | 3 | 2 | 26.67 ± 05.77 | ||
| 1000 | 4 | 5 | 3 | 40.00 ± 10.00 | ||
| 2000 | 5 | 6 | 5 | 53.33 ± 05.77 | ||
| Compound | Methylene Blue (MB) | Rhodamine (RhB) | Methyl Orange (MO) |
|---|---|---|---|
| Structure | 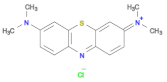 | 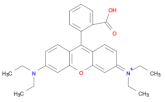 |  |
| Molecular formula | C16H18N3SCl | C28H31ClN2O3 | C14H14N3NaO3S |
| λmax | 652 nm | 553 nm | 462 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djafarou, R.; Brahmia, O.; Haya, S.; Sahmetlioglu, E.; Kılıç Dokan, F.; Hidouri, T. Starch-Assisted Eco-Friendly Synthesis of ZnO Nanoparticles: Enhanced Photocatalytic, Supercapacitive, and UV-Driven Antioxidant Properties with Low Cytotoxic Effects. Int. J. Mol. Sci. 2025, 26, 859. https://doi.org/10.3390/ijms26020859
Djafarou R, Brahmia O, Haya S, Sahmetlioglu E, Kılıç Dokan F, Hidouri T. Starch-Assisted Eco-Friendly Synthesis of ZnO Nanoparticles: Enhanced Photocatalytic, Supercapacitive, and UV-Driven Antioxidant Properties with Low Cytotoxic Effects. International Journal of Molecular Sciences. 2025; 26(2):859. https://doi.org/10.3390/ijms26020859
Chicago/Turabian StyleDjafarou, Roumaissa, Ouarda Brahmia, Soumia Haya, Ertugrul Sahmetlioglu, Fatma Kılıç Dokan, and Tarek Hidouri. 2025. "Starch-Assisted Eco-Friendly Synthesis of ZnO Nanoparticles: Enhanced Photocatalytic, Supercapacitive, and UV-Driven Antioxidant Properties with Low Cytotoxic Effects" International Journal of Molecular Sciences 26, no. 2: 859. https://doi.org/10.3390/ijms26020859
APA StyleDjafarou, R., Brahmia, O., Haya, S., Sahmetlioglu, E., Kılıç Dokan, F., & Hidouri, T. (2025). Starch-Assisted Eco-Friendly Synthesis of ZnO Nanoparticles: Enhanced Photocatalytic, Supercapacitive, and UV-Driven Antioxidant Properties with Low Cytotoxic Effects. International Journal of Molecular Sciences, 26(2), 859. https://doi.org/10.3390/ijms26020859





