Exploring How Adipose Tissue, Obesity, and Gender Influence the Immune Response to Vaccines: A Comprehensive Narrative Review
Abstract
:1. Introduction
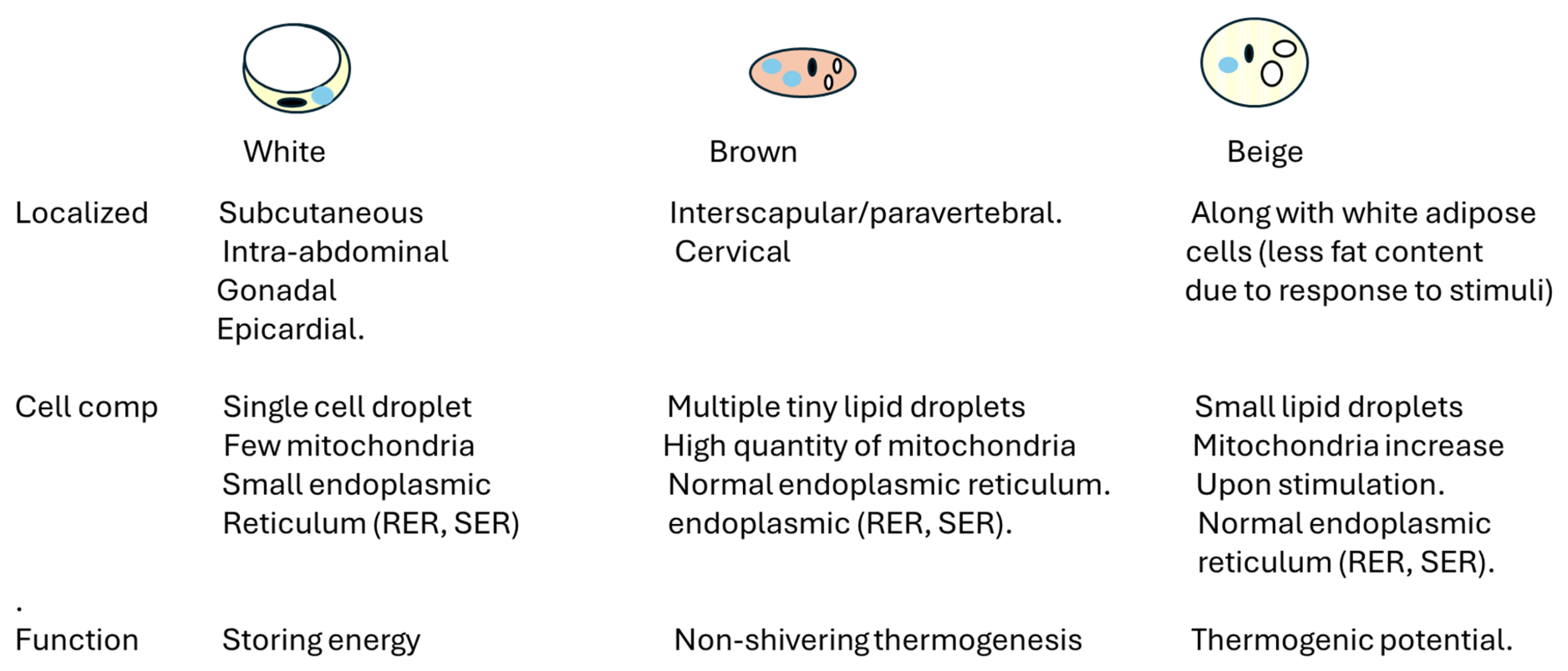
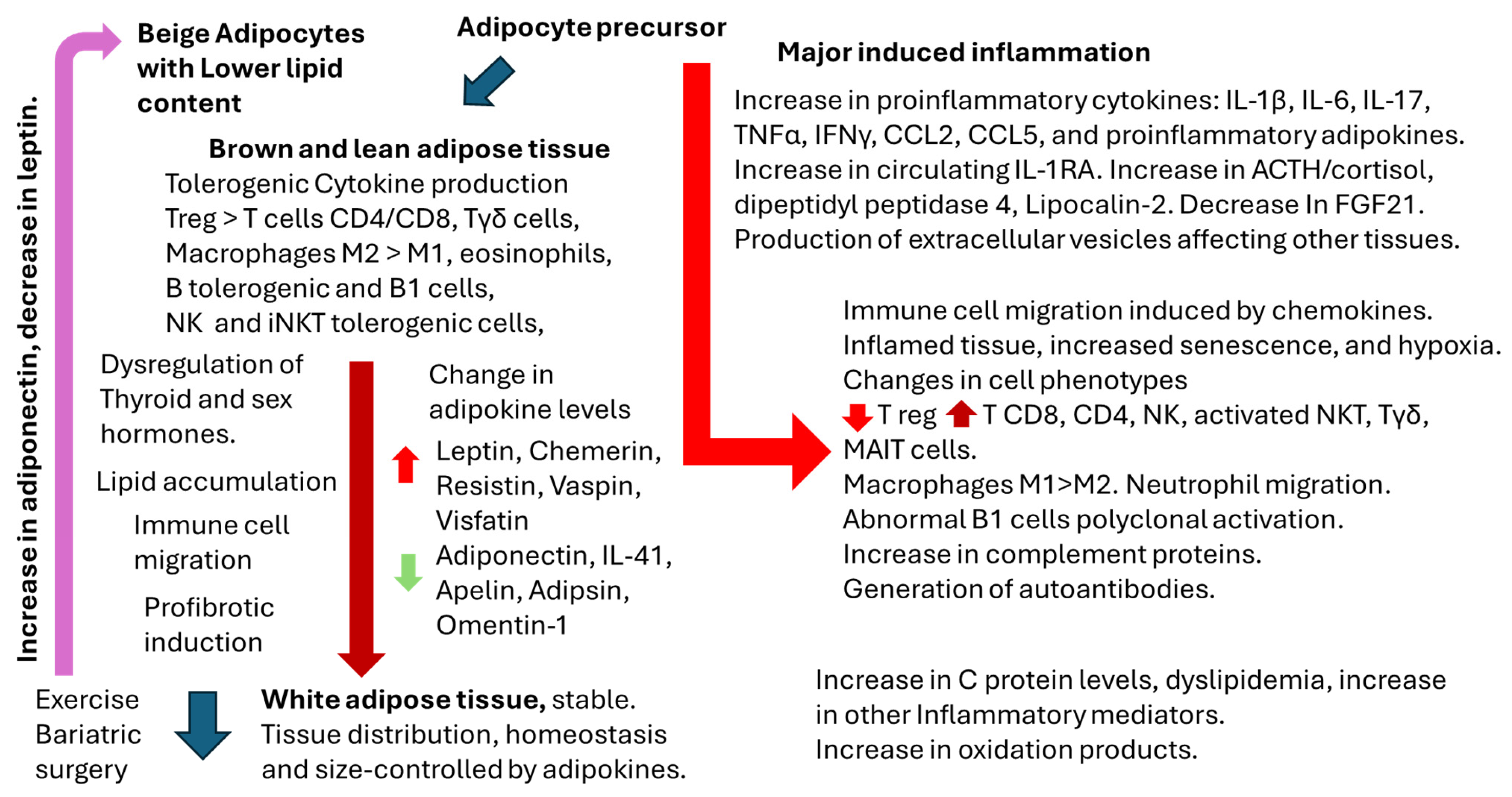
2. Overview of Adipose Tissue Physiology and Physiopathology
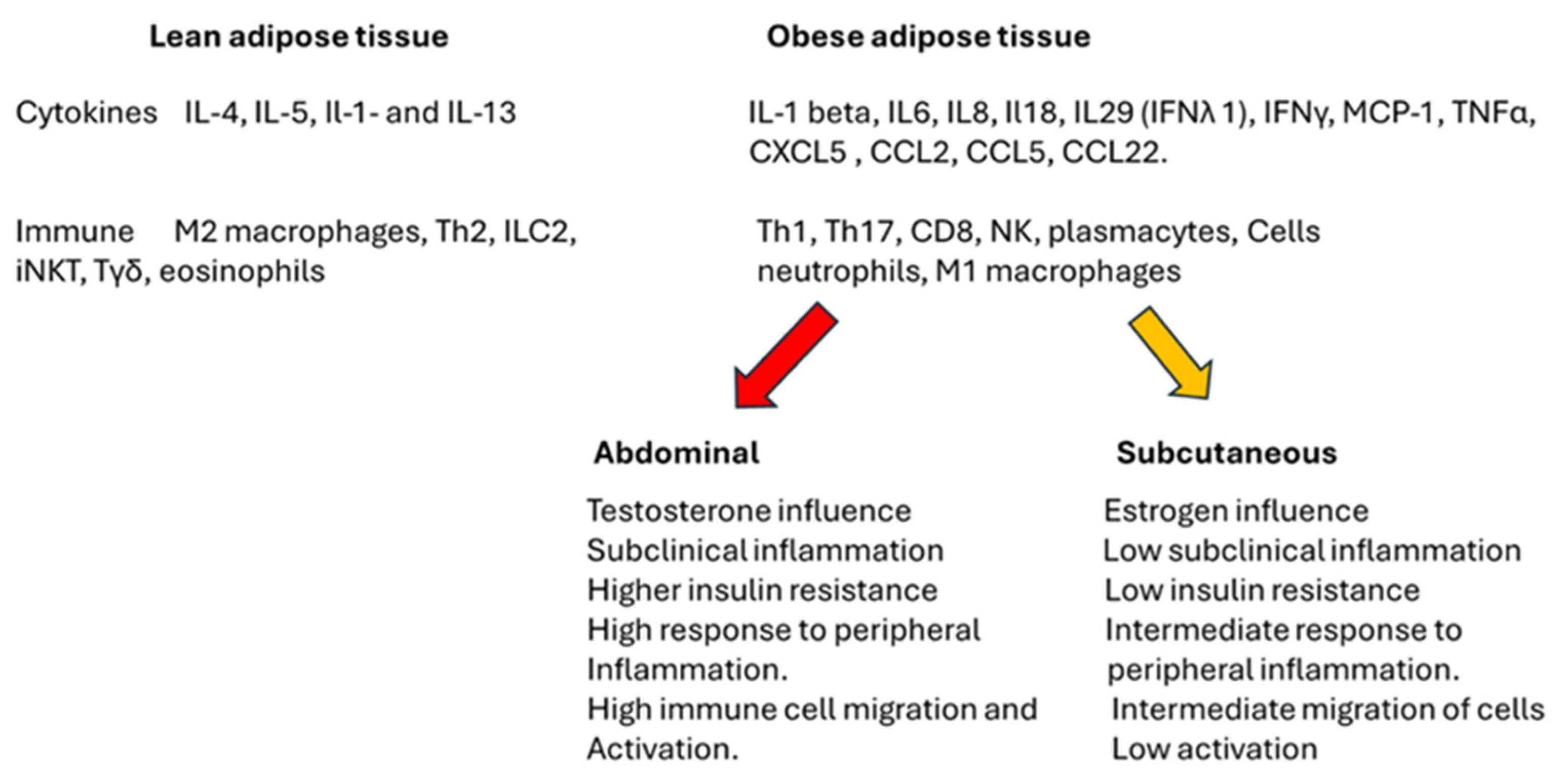
2.1. Adipose Tissue, Gender, and Immune Response
2.2. Thyroid Hormones, Gender, and Immune Response
3. Adipocytes as Antigen-Presenting Cells
Adipocyte-Derived Extracellular Vesicles
4. Obesity and Infectious Diseases
5. Impact of Obesity on Vaccination Response
5.1. Inactivated or Subunit Vaccines
5.2. Live-Attenuated Vaccines
5.3. RNA and Recombinant Vaccines
5.4. Heterleogous Vaccination: COVID-19 Vaccines
5.5. Gender, Thyroid Function, and Vaccine Response
6. Microbiota
7. Limitations of the Studies Involving Overweight and Obesity
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Explaining How Vaccines Work. Available online: https://www.cdc.gov/vaccines/basics/explaining-how-vaccines-work.html (accessed on 8 December 2024).
- Petrakis, D.; Margină, D.; Tsarouhas, K.; Tekos, F.; Stan, M.; Nikitovic, D.; Kouretas, D.; Spandidos, D.A.; Tsatsakis, A. Obesity—A risk factor for increased COVID-19 prevalence, severity and lethality (Review). Mol. Med. Rep. 2020, 22, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Pisaturo, M.; Zollo, V.; Martini, S.; Maggi, P.; Numis, F.G.; Gentile, I.; Sangiovanni, N.; Rossomando, A.M.; Bianco, V.; et al. Obesity as a Risk Factor of Severe Outcome of COVID-19: A Pair-Matched 1:2 Case–Control Study. J. Clin. Med. 2023, 12, 4055. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.-J.C.; Geerling, E.; Pinto, A.K. Impact of Obesity on Vaccination to SARS-CoV-2. Front. Endocrinol. 2022, 13, 898810. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, C.; Retnakumar, S.V.; Bayry, J. Obesity negatively impacts maintenance of antibody response to COVID-19 vaccines. Cell Rep. Med. 2023, 4, 101117. [Google Scholar] [CrossRef]
- van der Klaauw, A.A.; Horner, E.C.; Pereyra-Gerber, P.; Agrawal, U.; Foster, W.S.; Spencer, S.; Vergese, B.; Smith, M.; Henning, E.; Ramsay, I.D.; et al. Accelerated waning of the humoral response to COVID-19 vaccines in obesity. Nat. Med. 2023, 29, 1146–1154. [Google Scholar] [CrossRef]
- D’souza, M.; Keeshan, A.; Gravel, C.A.; Langlois, M.-A.; Cooper, C.L. Obesity does not influence SARS-CoV-2 humoral vaccine immunogenicity. NPJ Vaccines 2024, 9, 226. [Google Scholar] [CrossRef]
- Zwick, R.K.; Guerrero-Juarez, C.F.; Horsley, V.; Plikus, M.V. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018, 27, 68–83. [Google Scholar] [CrossRef]
- Hagberg, C.E.; Spalding, K.L. White adipocyte dysfunction and obesity-associated pathologies in humans. Nat. Rev. Mol. Cell Biol. 2024, 25, 270–289. [Google Scholar] [CrossRef]
- Richard, A.J.; White, U.; Elks, C.M.; Stephens, J.M. Adipose Tissue: Physiology to Metabolic Dysfunction. [Updated 2020 Apr 4]. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555602/ (accessed on 10 December 2024).
- Gavin, K.M.; Bessesen, D.H. Sex Differences in Adipose Tissue Function. Endocrinol. Metab. Clin. North Am. 2020, 49, 215–228. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adiponectin: Friend or foe in obesity and inflammation. Med. Rev. 2022, 2, 349–362. [Google Scholar] [CrossRef]
- Baldelli, S.; Aiello, G.; Di Martino, E.M.; Campaci, D.; Muthanna, F.M.S.; Lombardo, M. The Role of Adipose Tissue and Nutrition in the Regulation of Adiponectin. Nutrients 2024, 16, 2436. [Google Scholar] [CrossRef] [PubMed]
- Dare, A.; Chen, S.-Y. Adipsin in the pathogenesis of cardiovascular diseases. Vasc. Pharmacol. 2024, 154, 107270. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Huang, H.; Zhu, J.; Jin, X.; Wang, Y.; Xu, Y.; Xia, Z. Adipokines and their potential impacts on susceptibility to myocardial ischemia/reperfusion injury in diabetes. Lipids Health Dis. 2024, 23, 372. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigné, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpéné, C.; et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Lu, X.; Danser, A.H.J.; Verdonk, K. The Role of Chemerin in Metabolic and Cardiovascular Disease: A Literature Review of Its Physiology and Pathology from a Nutritional Perspective. Nutrients 2023, 15, 2878. [Google Scholar] [CrossRef]
- Münzberg, H.; Heymsfield, S.B.; Berthoud, H.-R.; Morrison, C.D. History and future of leptin: Discovery, regulation and signaling. Metab. Clin. Exp. 2024, 161, 156026. [Google Scholar] [CrossRef]
- Perakakis, N.; Mantzoros, C.S. Evidence from clinical studies of leptin: Current and future clinical applications in humans. Metab. Clin. Exp. 2024, 161, 156053. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Z.; Sun, T.; Zhang, S.; Yang, S.; Zheng, M.; Shen, H. Meteorin-like/Metrnl, a novel secreted protein implicated in inflammation, immunology, and metabolism: A comprehensive review of preclinical and clinical studies. Front. Immunol. 2023, 14, 1098570. [Google Scholar] [CrossRef]
- Shi, R.; He, M.; Peng, Y.; Xia, X. Homotherapy for heteropathy: Interleukin-41 and its biological functions. Immunology 2024, 173, 1–13. [Google Scholar] [CrossRef]
- Sena, C.M. Omentin: A Key Player in Glucose Homeostasis, Atheroprotection, and Anti-Inflammatory Potential for Cardiovascular Health in Obesity and Diabetes. Biomedicines 2024, 12, 284. [Google Scholar] [CrossRef]
- Tripathi, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in metabolism, inflammation, and disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Radzik-Zając, J.; Wytrychowski, K.; Wiśniewski, A.; Barg, W. The role of the novel adipokines vaspin and omentin in chronic inflammatory diseases. Pediatr. Endocrinol. Diabetes Metab. 2023, 29, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R.; Tankova, T. The role of vaspin in the development of metabolic and glucose tolerance disorders and atherosclerosis. BioMed Res. Int. 2015, 2015, 823481. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E. Visfatin: Structure, function and relation to diabetes mellitus and other dysfunctions. Curr. Med. Chem. 2008, 15, 1851–1862. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, Y. CCL2-CCR2 signaling axis in obesity and metabolic diseases. J. Cell. Physiol. 2024, 239, e31192. [Google Scholar] [CrossRef]
- Chan, P.-C.; Lu, C.-H.; Chien, H.-C.; Tian, Y.-F.; Hsieh, P.-S. Adipose Tissue-Derived CCL5 Enhances Local Pro-Inflammatory Monocytic MDSCs Accumulation and Inflammation via CCR5 Receptor in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2022, 23, 14226. [Google Scholar] [CrossRef]
- Yuan, Y.; Hu, R.; Park, J.; Xiong, S.; Wang, Z.; Qian, Y.; Shi, Z.; Wu, R.; Han, Z.; Ong, S.-G.; et al. Macrophage-derived chemokine CCL22 establishes local LN-mediated adaptive thermogenesis and energy expenditure. Sci. Adv. 2024, 10, eadn5229. [Google Scholar] [CrossRef]
- Wueest, S.; Konrad, D. The role of adipocyte-specific IL-6-type cytokine signaling in FFA and leptin release. Adipocyte 2018, 7, 226–228. [Google Scholar] [CrossRef]
- Huang, L.-Y.; Chiu, C.-J.; Hsing, C.-H.; Hsu, Y.-H. Interferon Family Cytokines in Obesity and Insulin Sensitivity. Cells 2022, 11, 4041. [Google Scholar] [CrossRef]
- Sewter, C.; Digby, J.; Blows, F.; Prins, J.; O’Rahilly, S. Regulation of tumour necrosis factor-alpha release from human adipose tissue in vitro. J. Endocrinol. 1999, 163, 33–38. [Google Scholar] [CrossRef]
- Engin, A. Reappraisal of Adipose Tissue Inflammation in Obesity. Adv. Exper. Med. Biol. 2024, 1460, 297–327. [Google Scholar] [CrossRef]
- Ghanbari, M.; Momen Maragheh, S.; Aghazadeh, A.; Mehrjuyan, S.R.; Hussen, B.M.; Abdoli Shadbad, M.; Dastmalchi, N.; Safaralizadeh, R. Interleukin-1 in obesity-related low-grade inflammation: From molecular mechanisms to therapeutic strategies. Int. Immunopharmacol. 2021, 96, 107765. [Google Scholar] [CrossRef] [PubMed]
- Hofwimmer, K.; Souza, J.d.P.; Subramanian, N.; Vujičić, M.; Rachid, L.; Méreau, H.; Zhao, C.; Dror, E.; Barreby, E.; Björkström, N.K.; et al. IL-1β promotes adipogenesis by directly targeting adipocyte precursors. Nat. Commun. 2024, 15, 7957. [Google Scholar] [CrossRef] [PubMed]
- Juge-Aubry, C.E.; Somm, E.; Giusti, V.; Pernin, A.; Chicheportiche, R.; Verdumo, C.; Rohner-Jeanrenaud, F.; Burger, D.; Dayer, J.-M.; Meier, C.A. Adipose tissue is a major source of interleukin-1 receptor antagonist: Upregulation in obesity and inflammation. Diabetes 2003, 52, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Ramírez, B.; Valentí, V.; Becerril, S.; Rodríguez, A.; Moncada, R.; Baixauli, J.; Silva, C.; Escalada, J.; et al. Serum Levels of IL-1 RA Increase with Obesity and Type 2 Diabetes in Relation to Adipose Tissue Dysfunction and are Reduced After Bariatric Surgery in Parallel to Adiposity. J. Inflamm. Res. 2022, 15, 1331–1345. [Google Scholar] [CrossRef]
- Barchetta, I.; Cimini, F.A.; Dule, S.; Cavallo, M.G. Dipeptidyl Peptidase 4 (DPP4) as A Novel Adipokine: Role in Metabolism and Fat Homeostasis. Biomedicines 2022, 10, 2306. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef]
- Flores-Cortez, Y.A.; Barragán-Bonilla, M.I.; Mendoza-Bello, J.M.; González-Calixto, C.; Flores-Alfaro, E.; Espinoza-Rojo, M. Interplay of retinol binding protein 4 with obesity and associated chronic alterations (Review). Mol. Med. Rep. 2022, 26, 244. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Zhang, Y.; LeRoith, D.; Bernlohr, D.A.; Chen, X. The Role of Lipocalin 2 in the Regulation of Inflammation in Adipocytes and Macrophages. Mol. Endocrinol. 2008, 22, 1416–1426. [Google Scholar] [CrossRef]
- Moschen, A.R.; Adolph, T.E.; Gerner, R.R.; Wieser, V.; Tilg, H. Lipocalin-2: A master mediator of intestinal and metabolic inflammation. Trends Endocrinol. Metab. 2017, 28, 388–397. [Google Scholar] [CrossRef]
- Lee, M.-J. Transforming growth factor beta superfamily regulation of adipose tissue biology in obesity. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2018, 1864, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Verde, L.; Vetrani, C.; Barrea, L.; Savastano, S.; Colao, A. Obesity: A gender-view. J. Endocrinol. Investig. 2023, 47, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.-F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]
- Guerra, B.; Fuentes, T.; Delgado-Guerra, S.; Guadalupe-Grau, A.; Olmedillas, H.; Santana, A.; Ponce-Gonzalez, J.G.; Dorado, C.; Calbet, J.A.L. Gender dimorphism in skeletal muscle leptin receptors, serum leptin and insulin sensitivity. PLoS ONE 2008, 3, e3466. [Google Scholar] [CrossRef]
- Rak, A.; Mellouk, N.; Froment, P.; Dupont, J. Adiponectin and resistin: Potential metabolic signals affecting hypothalamo-pituitary gonadal axis in females and males of different species. Reproduction 2017, 153, R215–R226. [Google Scholar] [CrossRef]
- Sanchez-Rebordelo, E.; Cunarro, J.; Perez-Sieira, S.; Seoane, L.M.; Diéguez, C.; Nogueiras, R.; Tovar, S. Regulation of Chemerin and CMKLR1 Expression by Nutritional Status, Postnatal Development, and Gender. Int. J. Mol. Sci. 2018, 19, 2905. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Leutner, M.; Harreiter, J. Sex differences in type 2 diabetes. Diabetologia 2023, 66, 986–1002. [Google Scholar] [CrossRef]
- Koceva, A.; Herman, R.; Janez, A.; Rakusa, M.; Jensterle, M. Sex- and Gender-Related Differences in Obesity: From Pathophysiological Mechanisms to Clinical Implications. Int. J. Mol. Sci. 2024, 25, 7342. [Google Scholar] [CrossRef]
- Luo, L.; Chen, L.; Song, J.; Ma, X.; Wang, X. Association between systemic immune-inflammatory index and systemic inflammatory response index with body mass index in children and adolescents: A population-based study based on the National Health and Nutrition Examination Survey 2017–2020. Front. Endocrinol. 2024, 15, 1426404. [Google Scholar] [CrossRef]
- Silva, J.; Iwasaki, A. Sex differences in postacute infection syndromes. Sci. Transl. Med. 2024, 16, eado2102. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Ning, Z.; Huang, K.; Yuan, Y.; Tan, X.; Pan, Y.; Zhang, R.; Tian, L.; Lu, Y.; Wang, X.; et al. Analysis of sex-biased gene expression in a Eurasian admixed population. Brief. Bioinform. 2024, 25, bbae451. [Google Scholar] [CrossRef] [PubMed]
- Persons, P.A.; Williams, L.; Fields, H.; Mishra, S.; Mehta, R. Weight gain during midlife: Does race/ethnicity influence risk? Maturitas 2024, 185, 108013. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, N.M.; Chen, H.-C.; Lechner, M.G.; Su, M.A. Sex Differences in Immunity. Annu. Rev. Immunol. 2022, 40, 75–94. [Google Scholar] [CrossRef]
- Popotas, A.; Casimir, G.J.; Corazza, F.; Lefèvre, N. Sex-related immunity: Could Toll-like receptors be the answer in acute inflammatory response? Front. Immunol. 2024, 15, 1379754. [Google Scholar] [CrossRef]
- Wang, P.; Yang, X.; Zhang, L.; Sha, S.; Huang, J.; Peng, J.; Gu, J.; Pearson, J.A.; Hu, Y.; Zhao, H.; et al. Tlr9 deficiency in B cells leads to obesity by promoting inflammation and gut dysbiosis. Nat. Commun. 2024, 15, 4232. [Google Scholar] [CrossRef]
- Hamerman, J.A.; Barton, G.M. The path ahead for understanding Toll-like receptor-driven systemic autoimmunity. Curr. Opin. Immunol. 2024, 91, 102482. [Google Scholar] [CrossRef]
- Layug, P.J.; Vats, H.; Kannan, K.; Arsenio, J. Sex differences in CD8+ T cell responses during adaptive immunity. WIREs Mech. Dis. 2024, 16, e1645. [Google Scholar] [CrossRef]
- Forsyth, K.S.; Jiwrajka, N.; Lovell, C.D.; Toothacre, N.E.; Anguera, M.C. The conneXion between sex and immune responses. Nat. Rev. Immunol. 2024, 24, 487–502. [Google Scholar] [CrossRef]
- Hoffmann, J.P.; Liu, J.A.; Seddu, K.; Klein, S.L. Sex hormone signaling and regulation of immune function. Immunity 2023, 56, 2472–2491. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, D.; Raychaudhuri, M. Hypothyroidism and obesity: An intriguing link. Indian J. Endocrinol. Metab. 2016, 20, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; del Prado, S.S.N.; Celi, F.S. Thyroid Hormone Action and Energy Expenditure. J. Endocr. Soc. 2019, 3, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Sror-Turkel, O.; El-Khatib, N.; Sharabi-Nov, A.; Avraham, Y.; Merchavy, S. Low TSH and low T3 hormone levels as a prognostic for mortality in COVID-19 intensive care patients. Front. Endocrinol. 2024, 15, 1322487. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Nemati, M.; Jafarzadeh, S.; Nozari, P.; Mortazavi, S.M.J. Thyroid dysfunction following vaccination with COVID-19 vaccines: A basic review of the preliminary evidence. J. Endocrinol. Investig. 2022, 45, 1835–1863. [Google Scholar] [CrossRef]
- Ovčariček, P.P.; Görges, R.; Giovanella, L. Autoimmune Thyroid Diseases. Semin. Nucl. Med. 2024, 54, 219–236. [Google Scholar] [CrossRef]
- Yang, P.; Shen, G.; Zhang, H.; Zhang, C.; Li, J.; Zhao, F.; Li, Z.; Liu, Z.; Wang, M.; Zhao, J.; et al. Incidence of thyroid dysfunction caused by immune checkpoint inhibitors combined with chemotherapy: A systematic review and meta-analysis. Int. Immunopharmacol. 2024, 133, 111961. [Google Scholar] [CrossRef]
- Barbagallo, F.; Cannarella, R.; Condorelli, R.A.; Cucinella, L.; La Vignera, S.; Nappi, R.; Calogero, A.E. Thyroid diseases and female sexual dysfunctions. Sex. Med. Rev. 2024, 12, 321–333. [Google Scholar] [CrossRef]
- Zierau, O.; Zenclussen, A.C.; Jensen, F. Role of female sex hormones, estradiol and progesterone, in mast cell behavior. Front. Immunol. 2012, 3, 25406. [Google Scholar] [CrossRef]
- Kadel, S.; Kovats, S. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front. Immunol. 2018, 9, 1653. [Google Scholar] [CrossRef]
- Buendía-González, F.O.; Legorreta-Herrera, M. The Similarities and Differences between the Effects of Testosterone and DHEA on the Innate and Adaptive Immune Response. Biomolecules 2022, 12, 1768. [Google Scholar] [CrossRef] [PubMed]
- Foyle, K.L.; A Robertson, S. Gamma delta (γδ) T cells in the female reproductive tract: Active participants or indifferent bystanders in reproductive success? Discov. Immunol. 2024, 3, kyae004. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, M.d.M.; Pellizas, C.G. Thyroid Hormone Action on Innate Immunity. Front. Endocrinol. 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Laurino, A.; Cinci, L.; Gencarelli, M.; Raimondi, L. Thyroid Hormone, Thyroid Hormone Metabolites and Mast Cells: A Less Explored Issue. Front. Cell. Neurosci. 2019, 13, 79. [Google Scholar] [CrossRef]
- Adamska-Fita, E.; Śliwka, P.W.; Karbownik-Lewińska, M.; Lewiński, A.; Stasiak, M. The Absence of Thyroid-Stimulating Hormone Receptor Expression on Natural Killer T Cells: Implications for the Immune–Endocrine Interaction. Int. J. Mol. Sci. 2024, 25, 11434. [Google Scholar] [CrossRef]
- Azimnasab-Sorkhabi, P.; Soltani-Asl, M.; Ekhtiyari, M.S.; Junior, J.R.K. Landscape of unconventional γδ T cell subsets in cancer. Mol. Biol. Rep. 2024, 51, 238. [Google Scholar] [CrossRef]
- Wenzek, C.; Boelen, A.; Westendorf, A.M.; Engel, D.R.; Moeller, L.C.; Führer, D. The interplay of thyroid hormones and the immune system—Where we stand and why we need to know about it. Eur. J. Endocrinol. 2022, 186, R65–R77. [Google Scholar] [CrossRef]
- Santana-Sánchez, P.; Vaquero-García, R.; Legorreta-Haquet, M.V.; Chávez-Sánchez, L.; Chávez-Rueda, A.K. Hormones and B-cell development in health and autoimmunity. Front. Immunol. 2024, 15, 1385501. [Google Scholar] [CrossRef]
- Brown, E.D.L.; Obeng-Gyasi, B.; Hall, J.E.; Shekhar, S. The Thyroid Hormone Axis and Female Reproduction. Int. J. Mol. Sci. 2023, 24, 9815. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Platz, E.A.; Ladenson, P.W.; Mondul, A.M.; Menke, A.; de González, A.B. Body fatness and markers of thyroid function among U.S. men and women. PLoS ONE 2012, 7, e34979. [Google Scholar] [CrossRef]
- Morenas, R.; Singh, D.; Hellstrom, W.J.G. Thyroid disorders and male sexual dysfunction. Int. J. Impot. Res. 2024, 36, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef] [PubMed]
- Trim, W.V.; Lynch, L. Immune and non-immune functions of adipose tissue leukocytes. Nat. Rev. Immunol. 2021, 22, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yang, X.; Lin, Y.; Li, S.; Jiang, J.; Qian, S.; Tang, Q.; He, R.; Li, X. Large adipocytes function as antigen-presenting cells to activate CD4+ T cells via upregulating MHCII in obesity. Int. J. Obes. 2015, 40, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.; Damen, M.S.; Alarcon, P.C.; Sanchez-Gurmaches, J.; Divanovic, S. Inflammation and Immunity: From an Adipocyte’s Perspective. J. Interf. Cytokine Res. 2019, 39, 459–471. [Google Scholar] [CrossRef]
- Castoldi, A.; Sanin, D.E.; Bakker, N.v.T.; Aguiar, C.F.; Monteiro, L.d.B.; Rana, N.; Grzes, K.M.; Kabat, A.M.; Curtis, J.; Cameron, A.M.; et al. Metabolic and functional remodeling of colonic macrophages in response to high-fat diet-induced obesity. iScience 2023, 26, 107719. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Huang, Y.; Zhao, X.; Jia, X.; Meng, G.; Zheng, Q.; Zhang, M.; Wu, Y.; Wang, L. Obesity Reshapes Visceral Fat-Derived MHC I Associated-Immunopeptidomes and Generates Antigenic Peptides to Drive CD8+ T Cell Responses. iScience 2020, 23, 100977. [Google Scholar] [CrossRef]
- Satoh, M.; Iizuka, M.; Majima, M.; Ohwa, C.; Hattori, A.; Van Kaer, L.; Iwabuchi, K. Adipose invariant NKT cells interact with CD1d-expressing macrophages to regulate obesity-related inflammation. Immunology 2022, 165, 414–427. [Google Scholar] [CrossRef]
- Satoh, M.; Iwabuchi, K. Contribution of NKT cells and CD1d-expressing cells in obesity-associated adipose tissue inflammation. Front. Immunol. 2024, 15, 1365843. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Physiol. 2020, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Valentine, Y.; Nikolajczyk, B.S. T cells in obesity-associated inflammation: The devil is in the details. Immunol. Rev. 2024, 324, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Lund, P.K. Role of intestinal inflammation as an early event in obesity and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Brotfain, E.; Hadad, N.; Shapira, Y.; Avinoah, E.; Zlotnik, A.; Raichel, L.; Levy, R. Neutrophil functions in morbidly obese subjects. Clin. Exp. Immunol. 2015, 181, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Casado, G.; Jimenez-Gonzalez, A.; Rodriguez-Muñoz, A.; Tinahones, F.J.; González-Mesa, E.; Murri, M.; Ortega-Gomez, A. Neutrophils as indicators of obesity-associated inflammation: A systematic review and meta-analysis. Obes. Rev. 2024, e13868. [Google Scholar] [CrossRef]
- Shantaram, D.; Hoyd, R.; Blaszczak, A.M.; Antwi, L.; Jalilvand, A.; Wright, V.P.; Liu, J.; Smith, A.J.; Bradley, D.; Lafuse, W.; et al. Obesity-associated microbiomes instigate visceral adipose tissue inflammation by recruitment of distinct neutrophils. Nat. Commun. 2024, 15, 5434. [Google Scholar] [CrossRef]
- Hu, Y.; Chakarov, S. Eosinophils in obesity and obesity-associated disorders. Discov. Immunol. 2023, 2, kyad022. [Google Scholar] [CrossRef]
- Divoux, A.; Moutel, S.; Poitou, C.; Lacasa, D.; Veyrie, N.; Aissat, A.; Arock, M.; Guerre-Millo, M.; Clément, K. Mast cells in human adipose tissue: Link with morbid obesity, inflammatory status, and diabetes. J. Clin. Endocrinol. Metab. 2012, 97, E1677–E1685. [Google Scholar] [CrossRef]
- Mukherjee, S.; Skrede, S.; Haugstøyl, M.; López, M.; Fernø, J. Peripheral and central macrophages in obesity. Front. Endocrinol. 2023, 14, 1232171. [Google Scholar] [CrossRef]
- Wilkin, C.; Piette, J.; Legrand-Poels, S. Unravelling metabolic factors impacting iNKT cell biology in obesity. Biochem. Pharmacol. 2024, 228, 116436. [Google Scholar] [CrossRef]
- Cui, G.; Abe, S.; Kato, R.; Ikuta, K. Insights into the heterogeneity of iNKT cells: Tissue-resident and circulating subsets shaped by local microenvironmental cues. Front. Immunol. 2024, 15, 1349184. [Google Scholar] [CrossRef] [PubMed]
- Canter, R.J.; Judge, S.J.; Collins, C.P.; Yoon, D.J.; Murphy, W.J. Suppressive effects of obesity on NK cells: Is it time to incorporate obesity as a clinical variable for NK cell-based cancer immunotherapy regimens? J. Immunother. Cancer 2024, 12, e008443. [Google Scholar] [CrossRef] [PubMed]
- De Barra, C.; O’Shea, D.; Hogan, A.E. NK cells vs. obesity: A tale of dysfunction & redemption. Clin. Immunol. 2023, 255, 109744. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Shchukina, I.; Asher, J.L.; Sidorov, S.; Artyomov, M.N.; Dixit, V.D. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat. Metab. 2020, 2, 50–61. [Google Scholar] [CrossRef]
- Frasca, D.; Romero, M.; Blomberg, B.B. Similarities in B Cell Defects Between Aging and Obesity. J. Immunol. 2024, 213, 1407–1413. [Google Scholar] [CrossRef]
- Gao, F.; Litchfield, B.; Wu, H. Adipose tissue lymphocytes and obesity. J. Cardiovasc. Aging 2024, 4, 5. [Google Scholar] [CrossRef]
- Meher, A.K.; McNamara, C.A. B-1 lymphocytes in adipose tissue as innate modulators of inflammation linked to cardiometabolic disease. Immunol. Rev. 2024, 324, 95–103. [Google Scholar] [CrossRef]
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef]
- McLaughlin, T.; Liu, L.-F.; Lamendola, C.; Shen, L.; Morton, J.; Rivas, H.; Winer, D.; Tolentino, L.; Choi, O.; Zhang, H.; et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arter. Thromb. Vasc. Biol. 2014, 34, 2637–2643. [Google Scholar] [CrossRef]
- Zi, C.; Wang, D.; Gao, Y.; He, L. The role of Th17 cells in endocrine organs: Involvement of the gut, adipose tissue, liver and bone. Front. Immunol. 2023, 13, 1104943. [Google Scholar] [CrossRef]
- Kochumon, S.; Hasan, A.; Al-Rashed, F.; Sindhu, S.; Thomas, R.; Jacob, T.; Al-Sayyar, A.; Arefanian, H.; Al Madhoun, A.; Al-Ozairi, E.; et al. Increased Adipose Tissue Expression of IL-23 Associates with Inflammatory Markers in People with High LDL Cholesterol. Cells 2022, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Cella, M.; Mccartney, S.A.; Fuchs, A.; Abumrad, N.A.; Pietka, T.A.; Chen, Z.; Finck, B.N.; Han, D.H.; Magkos, F.; et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology 2013, 145, 366–374.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Xu, D. The roles of T cells in obese adipose tissue inflammation. Adipocyte 2021, 10, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Delacher, M.; Schmidleithner, L.; Simon, M.; Stüve, P.; Sanderink, L.; Hotz-Wagenblatt, A.; Wuttke, M.; Schambeck, K.; Ruhland, B.; Hofmann, V.; et al. The effector program of human CD8 T cells supports tissue remodeling. J. Exp. Med. 2024, 221, e20230488. [Google Scholar] [CrossRef]
- Magalhaes, I.; Pingris, K.; Poitou, C.; Bessoles, S.; Venteclef, N.; Kiaf, B.; Beaudoin, L.; Da Silva, J.; Allatif, O.; Rossjohn, J.; et al. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J. Clin. Investig. 2015, 125, 1752–1762. [Google Scholar] [CrossRef]
- Kedia-Mehta, N.; Hogan, A.E. MAITabolism2—The emerging understanding of MAIT cell metabolism and their role in metabolic disease. Front. Immunol. 2022, 13, 1108071. [Google Scholar] [CrossRef]
- Sage, P.T.; Sharpe, A.H. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 2015, 36, 410–418. [Google Scholar] [CrossRef]
- Hildreth, A.D.; Ma, F.; Wong, Y.Y.; Sun, R.; Pellegrini, M.; O’sullivan, T.E. Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat. Immunol. 2021, 22, 639–653. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Vazquez, T.; Blomberg, B.B. Obesity induces pro-inflammatory B cells and impairs B cell function in old mice. Mech. Ageing Dev. 2017, 162, 91–99. [Google Scholar] [CrossRef]
- Park, M.-J.; Kwok, S.-K.; Lee, S.-H.; Kim, E.-K.; Park, S.-H.; Cho, M.-L. Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplant. 2015, 24, 2367–2377. [Google Scholar] [CrossRef]
- Hong, C.; Li, X.; Zhang, K.; Huang, Q.; Li, B.; Xin, H.; Hu, B.; Meng, F.; Zhu, X.; Tang, D.; et al. Novel perspectives on autophagy-oxidative stress-inflammation axis in the orchestration of adipogenesis. Front. Endocrinol. 2024, 15, 1404697. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, F.; Chen, H.; Hu, Y.; Yang, N.; Yang, W.; Wang, J.; Yang, Y.; Xu, R.; Xu, C. The differentiation courses of the Tfh cells: A new perspective on autoimmune disease pathogenesis and treatment. Biosci. Rep. 2024, 44, BSR20231723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chua, S., Jr. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, K.; MacIver, N.J. The Role of the Adipokine Leptin in Immune Cell Function in Health and Disease. Front. Immunol. 2021, 11, 622468. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Chen, Q.; Chen, Z.; Liang, K.; Gao, X.; Wang, X.; Makota, F.V.; Ong, H.S.; Wan, Y.; Luo, K.; et al. The metabolic hormone leptin promotes the function of TFH cells and supports vaccine responses. Nat. Commun. 2021, 12, 3073. [Google Scholar] [CrossRef]
- Park, J.; Sohn, J.H.; Han, S.M.; Park, Y.J.; Huh, J.Y.; Choe, S.S.; Kim, J.B. Adipocytes Are the Control Tower That Manages Adipose Tissue Immunity by Regulating Lipid Metabolism. Front. Immunol. 2021, 11, 598566. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Beck, M.A.; Alwarawrah, Y.; MacIver, N.J. Emerging mechanisms of obesity-associated immune dysfunction. Nat. Rev. Endocrinol. 2023, 20, 136–148. [Google Scholar] [CrossRef]
- Soták, M.; Clark, M.; Suur, B.E.; Börgeson, E. Inflammation and resolution in obesity. Nat. Rev. Endocrinol. 2025, 21, 45–61. [Google Scholar] [CrossRef]
- Lee, M.-J.; Kim, J. The pathophysiology of visceral adipose tissues in cardiometabolic diseases. Biochem. Pharmacol. 2024, 222, 116116. [Google Scholar] [CrossRef]
- McTavish, P.V.; Mutch, D.M. Omega-3 fatty acid regulation of lipoprotein lipase and FAT/CD36 and its impact on white adipose tissue lipid uptake. Lipids Health Dis. 2024, 23, 386. [Google Scholar] [CrossRef]
- Lima, G.B.; Figueiredo, N.; Kattah, F.M.; Oliveira, E.S.; Horst, M.A.; Dâmaso, A.R.; Oyama, L.M.; Whitton, R.G.M.; de Souza, G.I.M.H.; Lima, G.C.; et al. Serum Fatty Acids and Inflammatory Patterns in Severe Obesity: A Preliminary Investigation in Women. Biomedicines 2024, 12, 2248. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.-M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, K.; Sano, M. T Cell Immunosenescence in Aging, Obesity, and Cardiovascular Disease. Cells 2021, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, C.; Han, Y.; Gu, Z.; Sun, C. Immunosenescence, aging and successful aging. Front. Immunol. 2022, 13, 942796. [Google Scholar] [CrossRef]
- Shimi, G.; Sohouli, M.H.; Ghorbani, A.; Shakery, A.; Zand, H. The interplay between obesity, immunosenescence, and insulin resistance. Immun. Ageing 2024, 21, 13. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Garcia, D.; Blomberg, B.B. B Cell Immunosenescence. Annu. Rev. Cell Dev. Biol. 2020, 36, 551–574. [Google Scholar] [CrossRef]
- Garmendia, J.V.; Moreno, D.; Garcia, A.H.; De Sanctis, J.B. Metabolic syndrome and asthma. Recent Pat. Endocr. Metab. Immune Drug Discov. 2014, 8, 60–66. [Google Scholar] [CrossRef]
- Kudlova, N.; De Sanctis, J.B.; Hajduch, M. Cellular Senescence: Molecular Targets, Biomarkers, and Senolytic Drugs. Int. J. Mol. Sci. 2022, 23, 4168. [Google Scholar] [CrossRef]
- Valentino, T.R.; Chen, N.; Makhijani, P.; Khan, S.; Winer, S.; Revelo, X.S.; Winer, D.A. The role of autoantibodies in bridging obesity, aging, and immunosenescence. Immun. Ageing 2024, 21, 85. [Google Scholar] [CrossRef]
- Zhou, Z.; Tao, Y.; Zhao, H.; Wang, Q. Adipose Extracellular Vesicles: Messengers from and to Macrophages in Regulating Immunometabolic Homeostasis or Disorders. Front. Immunol. 2021, 12, 666344. [Google Scholar] [CrossRef] [PubMed]
- Kwan, H.Y.; Chen, M.; Xu, K.; Chen, B. The impact of obesity on adipocyte-derived extracellular vesicles. Cell. Mol. Life Sci. 2021, 78, 7275–7288. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, J.; Berg, V.; Vaittinen, M.; Impola, U.; Mustonen, A.-M.; Männistö, V.; Malinen, M.; Luukkonen, V.; Rosso, N.; Turunen, T.; et al. Increased secretion of adipocyte-derived extracellular vesicles is associated with adipose tissue inflammation and the mobilization of excess lipid in human obesity. J. Transl. Med. 2024, 22, 623. [Google Scholar] [CrossRef] [PubMed]
- Rakib, A.; Kiran, S.; Mandal, M.; Singh, U.P. MicroRNAs: A crossroad that connects obesity to immunity and aging. Immun. Ageing 2022, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Mendivil-Alvarado, H.; Sosa-León, L.A.; Carvajal-Millan, E.; Astiazaran-Garcia, H. Malnutrition and Biomarkers: A Journey through Extracellular Vesicles. Nutrients 2022, 14, 1002. [Google Scholar] [CrossRef]
- Leocádio, P.C.L.; Oriá, R.B.; Crespo-Lopez, M.E.; Alvarez-Leite, J.I. Obesity: More Than an Inflammatory, an Infectious Disease? Front. Immunol. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Pugliese, G.; Liccardi, A.; Graziadio, C.; Barrea, L.; Muscogiuri, G.; Colao, A. Obesity and infectious diseases: Pathophysiology and epidemiology of a double pandemic condition. Int. J. Obes. 2022, 46, 449–465. [Google Scholar] [CrossRef]
- Cristancho, C.; Mogensen, K.M.; Robinson, M.K. Malnutrition in patients with obesity: An overview perspective. Nutr. Clin. Pract. 2024, 39, 1300–1316. [Google Scholar] [CrossRef]
- Crespo, F.I.; Mayora, S.J.; De Sanctis, J.B.; Martínez, W.Y.; Zabaleta-Lanz, M.E.; Toro, F.I.; Deibis, L.H.; García, A.H. SARS-CoV-2 Infection in Venezuelan Pediatric Patients—A Single Center Prospective Observational Study. Biomedicines 2023, 11, 1409. [Google Scholar] [CrossRef]
- García, A.H.; Crespo, F.I.; Mayora, S.J.; Martinez, W.Y.; Belisario, I.; Medina, C.; De Sanctis, J.B. Role of Micronutrients in the Response to SARS-CoV-2 Infection in Pediatric Patients. Immuno 2024, 4, 211–225. [Google Scholar] [CrossRef]
- Cordeiro, A.; Luna, M.; Pereira, S.E.; Saboya, C.J.; Ramalho, A. Impairment of Vitamin D Nutritional Status and Metabolic Profile Are Associated with Worsening of Obesity According to the Edmonton Obesity Staging System. Int. J. Mol. Sci. 2022, 23, 14705. [Google Scholar] [CrossRef] [PubMed]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.-F. Vitamin D and Obesity/Adiposity—A Brief Overview of Recent Studies. Nutrients 2022, 14, 2049. [Google Scholar] [CrossRef] [PubMed]
- Keto, J.; Feuth, T.; Linna, M.; Saaresranta, T. Lower respiratory tract infections among newly diagnosed sleep apnea patients. BMC Pulm. Med. 2023, 23, 332. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.A.; Yang, C.-A.; Ojuri, V.; Buckley, K.; Bedi, B.; Musonge-Effoe, J.; Soibi-Harry, A.; Lahiri, C.D. Sex Differences in Metabolic Disorders of Aging and Obesity in People with HIV. Curr. HIV/AIDS Rep. 2024, 22, 3. [Google Scholar] [CrossRef]
- Cancelier, A.C.L.; Schuelter-Trevisol, F.; Trevisol, D.J.; Atkinson, R.L. Adenovirus 36 infection and obesity risk: Current understanding and future therapeutic strategies. Expert Rev. Endocrinol. Metab. 2022, 17, 143–152. [Google Scholar] [CrossRef]
- Hameed, M.; Geerling, E.; Pinto, A.K.; Miraj, I.; Weger-Lucarelli, J. Immune response to arbovirus infection in obesity. Front. Immunol. 2022, 13, 968582. [Google Scholar] [CrossRef]
- Tian, Y.; Jennings, J.; Gong, Y.; Sang, Y. Viral Infections and Interferons in the Development of Obesity. Biomolecules 2019, 9, 726. [Google Scholar] [CrossRef]
- Gallagher, P.; Chan, K.R.; Rivino, L.; Yacoub, S. The association of obesity and severe dengue: Possible pathophysiological mechanisms. J. Infect. 2020, 81, 10–16. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chiu, Y.-Y.; Chen, Y.-C.; Huang, C.-H.; Wang, W.-H.; Chen, Y.-H.; Lin, C.-Y. Obesity as a clinical predictor for severe manifestation of dengue: A systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 502. [Google Scholar] [CrossRef]
- Molokwu, J.C.; Penaranda, E.; Lopez, D.S.; Dwivedi, A.; Dodoo, C.; Shokar, N. Association of Metabolic Syndrome and Human Papillomavirus Infection in Men and Women Residing in the United States. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1321–1327. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Q.; Yang, P.; Li, Y.; Yuan, H.; Wu, L.; Chen, Z. Metabolic Syndrome and Risk of Cervical Human Papillomavirus Incident and Persistent Infection. Medicine 2016, 95, e2905. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Jun, B.G.; Yi, S.-W. Impact of diabetes, obesity, and dyslipidemia on the risk of hepatocellular carcinoma in patients with chronic liver diseases. Clin. Mol. Hepatol. 2022, 28, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Markakis, K.; Tsachouridou, O.; Georgianou, E.; Pilalas, D.; Nanoudis, S.; Metallidis, S. Weight Gain in HIV Adults Receiving Antiretroviral Treatment: Current Knowledge and Future Perspectives. Life 2024, 14, 1367. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, C.; Castillo, M.; Carrillo, K.; Tapia, C.V.; Valderrama, G.; Maquilón, C.; Toro-Ascuy, D.; Zorondo-Rodríguez, F.; Fuenzalida, L.F. Overnutrition as a risk factor for more serious respiratory viral infections in children: A retrospective study in hospitalized patients. Endocrinol. Diabetes Nutr. 2023, 70, 476–483. [Google Scholar] [CrossRef]
- Ramaswamy, M.; Shi, L.; Monick, M.M.; Hunninghake, G.W.; Look, D.C. Specific inhibition of type I interferon signal transduction by respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 2004, 30, 893–900. [Google Scholar] [CrossRef]
- Mîndru, D.E.; Țarcă, E.; Adumitrăchioaiei, H.; Anton-Păduraru, D.T.; Ștreangă, V.; Frăsinariu, O.E.; Sidoreac, A.; Stoica, C.; Bernic, V.; Luca, A.-C. Obesity as a Risk Factor for the Severity of COVID-19 in Pediatric Patients: Possible Mechanisms—A Narrative Review. Children 2024, 11, 1203. [Google Scholar] [CrossRef]
- Jang, S.; Hong, W.; Moon, Y. Obesity-compromised immunity in post-COVID-19 condition: A critical control point of chronicity. Front. Immunol. 2024, 15, 1433531. [Google Scholar] [CrossRef]
- Miron, V.D.; Drăgănescu, A.C.; Pițigoi, D.; Aramă, V.; Streinu-Cercel, A.; Săndulescu, O. The Impact of Obesity on the Host–Pathogen Interaction with Influenza Viruses—Novel Insights: Narrative Review. Diabetes Metab. Syndr. Obes. 2024, 17, 769–777. [Google Scholar] [CrossRef]
- Chiang, C.-H. Association between metabolic factors and chronic hepatitis B virus infection. World J. Gastroenterol. 2014, 20, 7213–7216. [Google Scholar] [CrossRef]
- Hornung, F.; Rogal, J.; Loskill, P.; Löffler, B.; Deinhardt-Emmer, S. The Inflammatory Profile of Obesity and the Role on Pulmonary Bacterial and Viral Infections. Int. J. Mol. Sci. 2021, 22, 3456. [Google Scholar] [CrossRef]
- Hales, C.; Burnet, L.; Coombs, M.; Collins, A.M.; Ferreira, D.M. Obesity, leptin and host defence of Streptococcus pneumoniae: The case for more human research. Eur. Respir. Rev. 2022, 31, 220055. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, H.; Xia, Q.; Zhang, L. Correlation between body mass index and gender-specific 28-day mortality in patients with sepsis: A retrospective cohort study. Front. Med. 2024, 11, 1462637. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Rutala, W.A.; Samsa, G.P.; Santimaw, J.E.; Lemon, S.M. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA 1985, 254, 3187–3189. [Google Scholar] [CrossRef] [PubMed]
- CDC Pink Book. Available online: https://www.cdc.gov/pinkbook/site.html (accessed on 10 January 2025).
- Callahan, S.T.; Wolff, M.; Hill, H.R.; Edwards, K.M.; NIAID Vaccine and Treatment Evaluation Unit (VTEU) Pandemic H1N1 Vaccine Study Group. Impact of body mass index on immunogenicity of pandemic H1N1 vaccine in children and adults. J. Infect. Dis. 2014, 210, 1270–1274. [Google Scholar] [CrossRef]
- Clarke, M.; Mathew, S.M.; Giles, L.C.; Pena, A.S.; Barr, I.G.; Richmond, P.C.; Marshall, H.S. A Prospective Study Investigating the Impact of Obesity on the Immune Response to the Quadrivalent Influenza Vaccine in Children and Adolescents. Vaccines 2022, 10, 699. [Google Scholar] [CrossRef]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef]
- Huang, J.Y.; Kaur, B.P.; Seth, D.; Pansare, M.V.; Kamat, D.; McGrath, E.; Secord, E.A.; Poowuttikul, P. Can Obesity Alter the Immune Response to Childhood Vaccinations? J. Allergy Clin. Immunol. 2019, 143, AB299. [Google Scholar] [CrossRef]
- Huang, J.; Kaur, B.; Farooqi, A.; Miah, T.; McGrath, E.; Seth, D.; Secord, E.; Poowuttikul, P. Elevated Glycated Hemoglobin Is Associated with Reduced Antibody Responses to Vaccinations in Children. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 193–198. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef]
- Vashishtha, V.M.; Kumar, P. The durability of vaccine-induced protection: An overview. Expert Rev. Vaccines 2024, 23, 389–408. [Google Scholar] [CrossRef]
- Dumrisilp, T.; Wongpiyabovorn, J.; Buranapraditkun, S.; Tubjaroen, C.; Chaijitraruch, N.; Prachuapthunyachart, S.; Sintusek, P.; Chongsrisawat, V. Impact of Obesity and Being Overweight on the Immunogenicity to Live Attenuated Hepatitis A Vaccine in Children and Young Adults. Vaccines 2021, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Soponkanabhorn, T.; Suratannon, N.; Buranapraditkun, S.; Tubjareon, C.; Prachuapthunyachart, S.; Eiamkulbutr, S.; Chongsrisawat, V. Cellular immune response to a single dose of live attenuated hepatitis a virus vaccine in obese children and adolescents. Heliyon 2000, 10, e36610. [Google Scholar] [CrossRef] [PubMed]
- Fonzo, M.; Nicolli, A.; Maso, S.; Carrer, L.; Trevisan, A.; Bertoncello, C. Body Mass Index and Antibody Persistence after Measles, Mumps, Rubella and Hepatitis B Vaccinations. Vaccines 2022, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Kara, Z.; Akçin, R.; Demir, A.N.; Dinç, H.; Taşkın, H.E.; Kocazeybek, B.; Yumuk, V.D. Antibody Response to SARS-CoV-2 Vaccines in People with Severe Obesity. Obes. Surg. 2022, 32, 2987–2993. [Google Scholar] [CrossRef]
- Drożdżyńska, J.; Jakubowska, W.; Kemuś, M.; Krokowska, M.; Karpezo, K.; Wiśniewska, M.; Bogdański, P.; Skrypnik, D. SARS-CoV-2 and Influenza Vaccines in People with Excessive Body Mass—A Narrative Review. Life 2022, 12, 1617. [Google Scholar] [CrossRef]
- Frasca, D.; Romero, M.; Diaz, A.; Blomberg, B.B. Obesity accelerates age defects in B cells, and weight loss improves B cell function. Immun. Ageing 2023, 20, 35. [Google Scholar] [CrossRef]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef]
- Xue, P.; Merikanto, I.; Delale, E.A.; Bjelajac, A.; Yordanova, J.; Chan, R.N.Y.; Korman, M.; Mota-Rolim, S.A.; Landtblom, A.-M.; Matsui, K.; et al. Associations between obesity, a composite risk score for probable long COVID, and sleep problems in SARS-CoV-2 vaccinated individuals. Int. J. Obes. 2024, 48, 1300–1306. [Google Scholar] [CrossRef]
- Ou, X.; Jiang, J.; Lin, B.; Liu, Q.; Lin, W.; Chen, G.; Wen, J. Antibody responses to COVID-19 vaccination in people with obesity: A systematic review and meta-analysis. Influ. Other Respir. Viruses 2023, 17, e13078. [Google Scholar] [CrossRef]
- Faizo, A.A.; Qashqari, F.S.; El-Kafrawy, S.A.; Barasheed, O.; Almashjary, M.N.; Alfelali, M.; Bawazir, A.A.; Albarakati, B.M.; Khayyat, S.A.; Hassan, A.M.; et al. A potential association between obesity and reduced effectiveness of COVID-19 vaccine-induced neutralizing humoral immunity. J. Med. Virol. 2022, 95, e28130. [Google Scholar] [CrossRef]
- Shaw, R.H.; Greenland, M.; Stuart, A.S.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; Darton, T.; et al. Persistence of immune response in heterologous COVID vaccination schedules in the Com-COV2 study—A single-blind, randomised trial incorporating mRNA, viral-vector and protein-adjuvant vaccines. J. Infect. 2023, 86, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.; Ardizzone, C.M.; Khanna, M.; Trauth, A.J.; Hagensee, M.E.; Ramsay, A.J. Dynamics of Serum-Neutralizing Antibody Responses in Vaccinees through Multiple Doses of the BNT162b2 Vaccine. Vaccines 2023, 11, 1720. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Marriott, I.; Fish, E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.; Wang, N.; Shea, P.J.; Rosser, E.N.; Kuo, H.; Shapiro, J.R.; Fenstermacher, K.Z.; Pekosz, A.; Rothman, R.E.; Klein, S.L.; et al. Sex and gender differences in adverse events following influenza and COVID-19 vaccination. Biol. Sex Differ. 2024, 15, 50. [Google Scholar] [CrossRef]
- Arora, M.; Lakshmi, R. Vaccines–safety in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 23–40. [Google Scholar] [CrossRef]
- Feng, Z.; Liao, M.; Zhang, L. Sex differences in disease: Sex chromosome and immunity. J. Transl. Med. 2024, 22, 1150. [Google Scholar] [CrossRef]
- Tadount, F.; Kiely, M.; Assi, A.; Rafferty, E.; Sadarangani, M.; E MacDonald, S.; Quach, C. Sex Differences in the Immunogenicity and Efficacy of Seasonal Influenza Vaccines: A Meta-analysis of Randomized Controlled Trials. Open Forum Infect. Dis. 2024, 11, ofae222. [Google Scholar] [CrossRef]
- Lindsey, N.P.; Schroeder, B.A.; Miller, E.R.; Braun, M.M.; Hinckley, A.F.; Marano, N.; Slade, B.A.; Barnett, E.D.; Brunette, G.W.; Horan, K.; et al. Adverse event reports following yellow fever vaccination. Vaccine 2008, 26, 6077–6082. [Google Scholar] [CrossRef]
- Querec, T.D.; Akondy, R.S.; Lee, E.K.; Cao, W.; Nakaya, H.I.; Teuwen, D.; Pirani, A.; Gernert, K.; Deng, J.; Marzolf, B.; et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009, 10, 116–125. [Google Scholar] [CrossRef]
- Gaucher, D.; Therrien, R.; Kettaf, N.; Angermann, B.R.; Boucher, G.; Filali-Mouhim, A.; Moser, J.M.; Mehta, R.S.; Drake, D.R., 3rd; Castro, E.; et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 2008, 205, 3119–3131. [Google Scholar] [CrossRef] [PubMed]
- Peer, V.; Schwartz, N.; Green, M.S. A multi-country, multi-year, meta-analytic evaluation of the sex differences in age-specific pertussis incidence rates. PLoS ONE 2020, 15, e0231570. [Google Scholar] [CrossRef] [PubMed]
- Boef, A.G.; van der Klis, F.R.; Berbers, G.A.; Buisman, A.-M.; Sanders, E.A.; Kemmeren, J.M.; van der Ende, A.; de Melker, H.E.; Rots, N.Y.; Knol, M.J. Differences by sex in IgG levels following infant and childhood vaccinations: An individual participant data meta-analysis of vaccination studies. Vaccine 2018, 36, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Yen, P.M. Metabolic Messengers: Thyroid Hormones. Nat. Metab. 2024, 6, 639–650. [Google Scholar] [CrossRef]
- Paschou, S.A.; Karalis, V.; Psaltopoulou, T.; Vasileiou, V.; Charitaki, I.; Bagratuni, T.; Ktena, V.; Papandroulaki, F.; Gumeni, S.; Kassi, G.N.; et al. Patients With Autoimmune Thyroiditis Present Similar Immunological Response to COVID-19 BNT162b2 mRNA Vaccine With Healthy Subjects, While Vaccination May Affect Thyroid Function: A Clinical Study. Front. Endocrinol. 2022, 13, 840668. [Google Scholar] [CrossRef]
- Polymeris, A.; Papapetrou, P.D.; Psachna, S.; Ioannidis, D.; Lilis, D.; Drakou, M.; Vaiopoulos, A.; Polymerou, V.; Spanos, G. Patients with Hashimoto’s thyroiditis present higher immune response to COVID-19 mRNA vaccine compared to normal individuals. Hormones 2024, 23, 89–95. [Google Scholar] [CrossRef]
- Lynn, D.J.; Benson, S.C.; Lynn, M.A.; Pulendran, B. Modulation of immune responses to vaccination by the microbiota: Implications and potential mechanisms. Nat. Rev. Immunol. 2021, 22, 33–46. [Google Scholar] [CrossRef]
- Rio, P.; Caldarelli, M.; Chiantore, M.; Ocarino, F.; Candelli, M.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Immune Cells, Gut Microbiota, and Vaccines: A Gender Perspective. Cells 2024, 13, 526. [Google Scholar] [CrossRef]
- Syromyatnikov, M.; Nesterova, E.; Gladkikh, M.; Smirnova, Y.; Gryaznova, M.; Popov, V. Characteristics of the Gut Bacterial Composition in People of Different Nationalities and Religions. Microorganisms 2022, 10, 1866. [Google Scholar] [CrossRef]
- World Health Organization. Obesity Epidemiological Data. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-overweight-among-adults-bmi--25-(age-standardized-estimate)-(-) (accessed on 10 January 2025).
- Health Statistics of the National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity#:~:text=the%20above%20table- (accessed on 10 January 2025).
- Lofton, H.; Ard, J.D.; Hunt, R.R.; Knight, M.G. Obesity among African American people in the United States: A review. Obesity 2023, 31, 306–315. [Google Scholar] [CrossRef]
- Zare, H.; Aazami, A.; Shalby, N.; Gilmore, D.R.; Thorpe, R.J. Measuring Racial Differences in Obesity Risk Factors in Non-Hispanic Black and White Men Aged 20 Years or Older. Am. J. Men’s Health 2023, 17, 15579883231205845. [Google Scholar] [CrossRef] [PubMed]
- Stanislawski, M.A.; Dabelea, D.; Lange, L.A.; Wagner, B.D.; Lozupone, C.A. Gut microbiota phenotypes of obesity. NPJ Biofilms Microbiomes 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Norton, T.; Lynn, M.A.; Rossouw, C.; Abayasingam, A.; Perkins, G.; Hissaria, P.; Bull, R.A.; Lynn, D.J. B and T cell responses to the BNT162b2 COVID-19 mRNA vaccine are not impaired in germ-free or antibiotic-treated mice. Gut 2023, 73, 1222–1224. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.; Tunbridge, M.J.; Shi, B.; Perkins, G.B.; Chai, C.S.; Salehi, T.; Sim, B.Z.; Kireta, S.; Johnston, J.K.; Akerman, A.; et al. Dietary Inulin to Improve SARS-CoV-2 Vaccine Response in Kidney Transplant Recipients: The RIVASTIM-Inulin Randomised Controlled Trial. Vaccines 2024, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Hitch, T.C.; Hall, L.J.; Walsh, S.K.; Leventhal, G.E.; Slack, E.; de Wouters, T.; Walter, J.; Clavel, T. Microbiome-based interventions to modulate gut ecology and the immune system. Mucosal Immunol. 2022, 15, 1095–1113. [Google Scholar] [CrossRef]
- Jiang, W.; Lu, G.; Gao, D.; Lv, Z.; Li, D. The relationships between the gut microbiota and its metabolites with thyroid diseases. Front. Endocrinol. 2022, 13, 943408. [Google Scholar] [CrossRef]
- Yan, K.; Sun, X.; Fan, C.; Wang, X.; Yu, H. Unveiling the Role of Gut Microbiota and Metabolites in Autoimmune Thyroid Diseases: Emerging Perspectives. Int. J. Mol. Sci. 2024, 25, 10918. [Google Scholar] [CrossRef]
- Mendoza-León, M.J.; Mangalam, A.K.; Regaldiz, A.; González-Madrid, E.; Rangel-Ramírez, M.A.; Álvarez-Mardonez, O.; Vallejos, O.P.; Méndez, C.; Bueno, S.M.; Melo-González, F.; et al. Gut microbiota short-chain fatty acids and their impact on the host thyroid function and diseases. Front. Endocrinol. 2023, 14, 1192216. [Google Scholar] [CrossRef]
- García, A.; De Sanctis, J.B. An overview of adjuvant formulations and delivery systems. APMIS 2013, 122, 257–267. [Google Scholar] [CrossRef]
- White, S.J.; Taylor, M.J.; Hurt, R.T.; Jensen, M.D.; Poland, G.A. Leptin-based adjuvants: An innovative approach to improve vaccine response. Vaccine 2013, 31, 1666–1672. [Google Scholar] [CrossRef]
- Ben Nasr, M.; Usuelli, V.; Dellepiane, S.; Seelam, A.J.; Fiorentino, T.V.; D’addio, F.; Fiorina, E.; Xu, C.; Xie, Y.; Balasubramanian, H.B.; et al. Glucagon-like peptide 1 receptor is a T cell-negative costimulatory molecule. Cell Metab. 2024, 36, 1302–1319.e12. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, G.; Coelmont, L.; Alpizar, Y.A.; Kelchtermans, L.; Broeckhoven, E.; Dallmeier, K. GLP-1R agonist therapy and vaccine response: Neglected implications. Cytokine Growth Factor Rev. 2024, 78, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, J.V.; García, A.H.; De Sanctis, C.V.; Hajdúch, M.; De Sanctis, J.B. Autoimmunity and Immunodeficiency in Severe SARS-CoV-2 Infection and Prolonged COVID-19. Curr. Issues Mol. Biol. 2022, 45, 33–50. [Google Scholar] [CrossRef] [PubMed]
- García, A.H.; De Sanctis, J.B. Exploring the Contrasts and Similarities of Dengue and SARS-CoV-2 Infections During the COVID-19 Era. Int. J. Mol. Sci. 2024, 25, 11624. [Google Scholar] [CrossRef]
- Jha, S.K.; Imran, M.; Jha, L.A.; Hasan, N.; Panthi, V.K.; Paudel, K.R.; Almalki, W.H.; Mohammed, Y.; Kesharwani, P. A Comprehensive review on Pharmacokinetic Studies of Vaccines: Impact of delivery route, carrier-and its modulation on immune response. Environ. Res. 2023, 236 Pt 2, 116823. [Google Scholar] [CrossRef]
- Jiang, G.; Zou, Y.; Zhao, D.; Yu, J. Optimising vaccine immunogenicity in ageing populations: Key strategies. Lancet Infect. Dis. 2025, 25, e23–e33. [Google Scholar] [CrossRef]
| Adipokine | Pro-Inflammatory | Anti-Inflammatory | Reference |
|---|---|---|---|
| Adiponectin | No | Yes | [12,13] |
| Adipsin (complement factor-D) | No | Yes | [14,15] |
| Apelin | No | Yes | [16] |
| Chemerin | Yes | No | [17] |
| Leptin | Yes | Yes | [18,19] |
| Meteorin like (IL41) | No | Yes | [20,21] |
| Omentin-1 | No | Yes | [22] |
| Resistin | Yes | No | [23] |
| Vaspin | Yes | Yes | [24,25] |
| Visfatin | Yes | No | [26] |
| Effect | Reference | |
|---|---|---|
| CCL2 (MCP-1) | Monocyte migration to adipose tissue. | [27] |
| CCL5 | Monocyte migration to adipose tissue. | [28] |
| CCL22 | Thermogenesis induction. | [29] |
| IL-6 | Local activation of immune cells. Metabolic dysregulation. | [30] |
| IFN | IFNα induces apoptosis in adipocytes. IFNβ regulates metabolism. IFNγ pro-inflammatory response; reduction in adipose tissue. IFNλ1 enhances inflammatory response. IFNτ reduces inflammatory response. | [31] |
| TNFα | Activation of tissue immune cells. Metabolic dysregulation. | [32,33] |
| IL-1 and IL-RA | IL-1 α hypertrophy of white adipose tissue. IL-1 β promotes adipogenesis in murine and human adipose-derived stem cells. IL-RA is upregulated in white adipose tissue, and high circulating levels in obesity. | [34,35,36,37] |
| Dipeptidyl peptidase 4 | Plays a role in metabolic homeostasis and inflammatory response. Inhibition of the enzyme, combined with metformin, induces a significant decrease in visceral adipose tissue. | [38] |
| Fibroblast growth factor 21 | Anti-inflammatory. | [39] |
| Retinol binding protein 4 | Induction of inflammatory response. Inhibition of insulin signaling. | [40] |
| Lipocalin-2 | Produced by white adipocytes. Increases adipose tissue. Involved in neutrophil chemoattraction. | [41,42] |
| TGFβ | Involved in tissue fibrosis and insulin resistance. | [43] |
| Immune Cells | Estrogens | Progestins | Androgens | Thyroid Hormones |
|---|---|---|---|---|
| Monocytes/macrophages | Inhibit pro-inflammatory cytokines. Increase phagocytosis | Inhibit inflammatory response and inhibit TLR4 and TLR9 activation | Enhance macrophage migration. Anti-inflammatory response | Increase phagocytosis (T3/T4). Increase M1 and decrease M2 differentiation (T3) |
| Dendritic cells | Promote cell differentiation. Promote pro-inflammatory cytokine production. Enhance T-cell activation | Decrease secretion of pro-inflammatory cytokines | Decrease pro-inflammatory cytokine production. Decrease T-cell stimulation | Promote maturation (T3/T4). Pro-inflammatory role (T3) |
| Neutrophils | Enhance cell activation and chemotaxis | Inhibition of neutrophil activation | Inhibition of neutrophil activation | Increase in oxidative burst and phagocytosis (T3/T4). |
| Mast cells | Increased inflammatory response | Decreased inflammatory response | Anti-inflammatory response | Mast cells store T3 and may impact thyroid function. T3 activates mast cells |
| Eosinophils | Enhanced cell activation | Decreased cell activation | No or low response | Not well defined. Activated cells affect the thyroid gland |
| NK cells | Activate NK cells | Modulate NK activity | No main effect on NK cells | Increased NK cytotoxic activity (T3/T4) |
| NKT cells | Decreased stimulation | Decreased stimulation | No response | No thyroid-stimulating hormone receptor is present |
| T γδ cells | Induce production of IL-17 and promote an increase in Th17 | Tolerogenic responses | Induce cell activation | Not well defined. Activated cells may affect the thyroid gland |
| T cells | Increase in Th1 and Th17 | Increase in Th2 and T reg cells | Decrease in Th17 cells | Increase in proliferative response and cytotoxicity |
| B cells | Increase the production of all types of antibodies, including IgE | Increase the production of IgG and IgA | Decrease in IgG secretion | Increase in proliferative and lymphopoiesis No defined role in antibody production |
| Cell Type | Effect | Reference |
|---|---|---|
| Neutrophils | Retain phagocytic activity, increase basal superoxide, and chemotaxis. Absolute neutrophil counts and neutrophil to lymphocyte ratio may indicate adipose tissue inflammation. Relationship of microbiota with neutrophil infiltration in adipose tissue. | [96,97,98] |
| Eosinophils | Protect adipose tissue from inflammation. | [99] |
| Mast cells | Mast cells are activated in human adipose tissue and localized preferentially in fibrosis depots. | [100] |
| Macrophages | M2 macrophages in lean tissue and M1 in inflammatory tissue. | [101] |
| iNKT cells | In lean adipose tissue, they can be activated by CD1 and can incorporate lipids, generating a local inflammatory response. | [91,103] |
| NK | Present in adipose tissue. Tolerogenic response in adipose tissue? Different responses depending on gender. | [104,105] |
| Tγδ | Inhibit inflammatory response. | [106] |
| B cells | Dysfunctional B cells in obese individuals. The lean adipose tissue contains B regulatory and B1 cells. B1 cells produce IgM antibodies for primary innate immunity. B2 cells usually generate protective antibodies in lymphoid organs. However, they participate in local inflammation and promote insulin resistance after migrating to white adipose tissue. | [107,108,109] |
| Th1 cells | Promote obesity-associated inflammation. | [108,111] |
| Th2 | Stabilize adipose tissue and induce M2 polarization. A decrease in Th2 cells in the tissue is due to increased local IFNγ and inflammation. | [108,111] |
| Th17 | Pro-inflammatory role. Related to IL-23 secretion in adipose tissue. | [112,113] |
| Th22 | IL-22 is produced by innate lymphocyte cells upon tissue inflammation. It is related to insulin resistance. | [114] |
| CD8 cells | Cytotoxic response. Adipose tissue inflammation. Tissue remodeling. | [115,116] |
| Mucosal-associated invariant T (MAIT) cells | Secrete IL-17, inducing local tissue inflammation. | [117,118] |
| T follicular (TF) cells. TFh helper and TFreg regulatory cells | Modulate the response of B cells in adipose tissue. Impairment of TF regulatory cells is related to autoimmunity. | [119,120] |
| Follicular B cells | In adipose tissue, they induce inflammation depending on the cytokine milieu. Mesenchymal adipose stem cells induce the expansion of IL-10-producing B cells—possible role in autoimmunity. | [121] |
| Mesenchymal stem cells | Anti-inflammatory in the presence of Treg and Th2 milieu. Pro-inflammatory in the presence of inflammatory cytokines. | [122,123] |
| Virus | Adipose Tissue Involvement | IFN Responses | Reference |
|---|---|---|---|
| Adenoviruses | Yes | Suppression. Chronic infection. Obesity-induced viral infection? | [156] |
| Arboviruses | Yes | Suppression. Chronic infection | [157] |
| Herpesviridae | Yes | HSV-1 suppression through miRNA CMV-multiple antagonistic mechanisms | [158] |
| Slow virus (Prion) | Yes | Inhibition of IFN signaling | [158] |
| Dengue | Yes | Inhibition of INF signaling | [159,160] |
| Papillomavirus | Yes | IFN signaling decreased | [161,162] |
| HCV | Yes | Antagonism of IFN signaling. Chronicity | [163] |
| HIV | Yes | Antagonism of IFN signaling. Chronicity | [164] |
| RSV | Yes | Inhibits IFN signaling | [165,166] |
| Coronavirus | Yes | IFN signaling is inhibited | [167,168] |
| Influenza | Yes | IFN signaling is inhibited | [169] |
| Hepatitis B virus | Yes | IFN response impaired | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Sanctis, J.B.; Balda Noria, G.; García, A.H. Exploring How Adipose Tissue, Obesity, and Gender Influence the Immune Response to Vaccines: A Comprehensive Narrative Review. Int. J. Mol. Sci. 2025, 26, 862. https://doi.org/10.3390/ijms26020862
De Sanctis JB, Balda Noria G, García AH. Exploring How Adipose Tissue, Obesity, and Gender Influence the Immune Response to Vaccines: A Comprehensive Narrative Review. International Journal of Molecular Sciences. 2025; 26(2):862. https://doi.org/10.3390/ijms26020862
Chicago/Turabian StyleDe Sanctis, Juan Bautista, Germán Balda Noria, and Alexis Hipólito García. 2025. "Exploring How Adipose Tissue, Obesity, and Gender Influence the Immune Response to Vaccines: A Comprehensive Narrative Review" International Journal of Molecular Sciences 26, no. 2: 862. https://doi.org/10.3390/ijms26020862
APA StyleDe Sanctis, J. B., Balda Noria, G., & García, A. H. (2025). Exploring How Adipose Tissue, Obesity, and Gender Influence the Immune Response to Vaccines: A Comprehensive Narrative Review. International Journal of Molecular Sciences, 26(2), 862. https://doi.org/10.3390/ijms26020862








