Zongertinib, a Novel HER2 Tyrosine Kinase Inhibitor, Maintains an Anticancer Activity for Trastuzumab Deruxtecan-Resistant Cancers Harboring HER2-Overexpression
Abstract
1. Introduction
2. Results
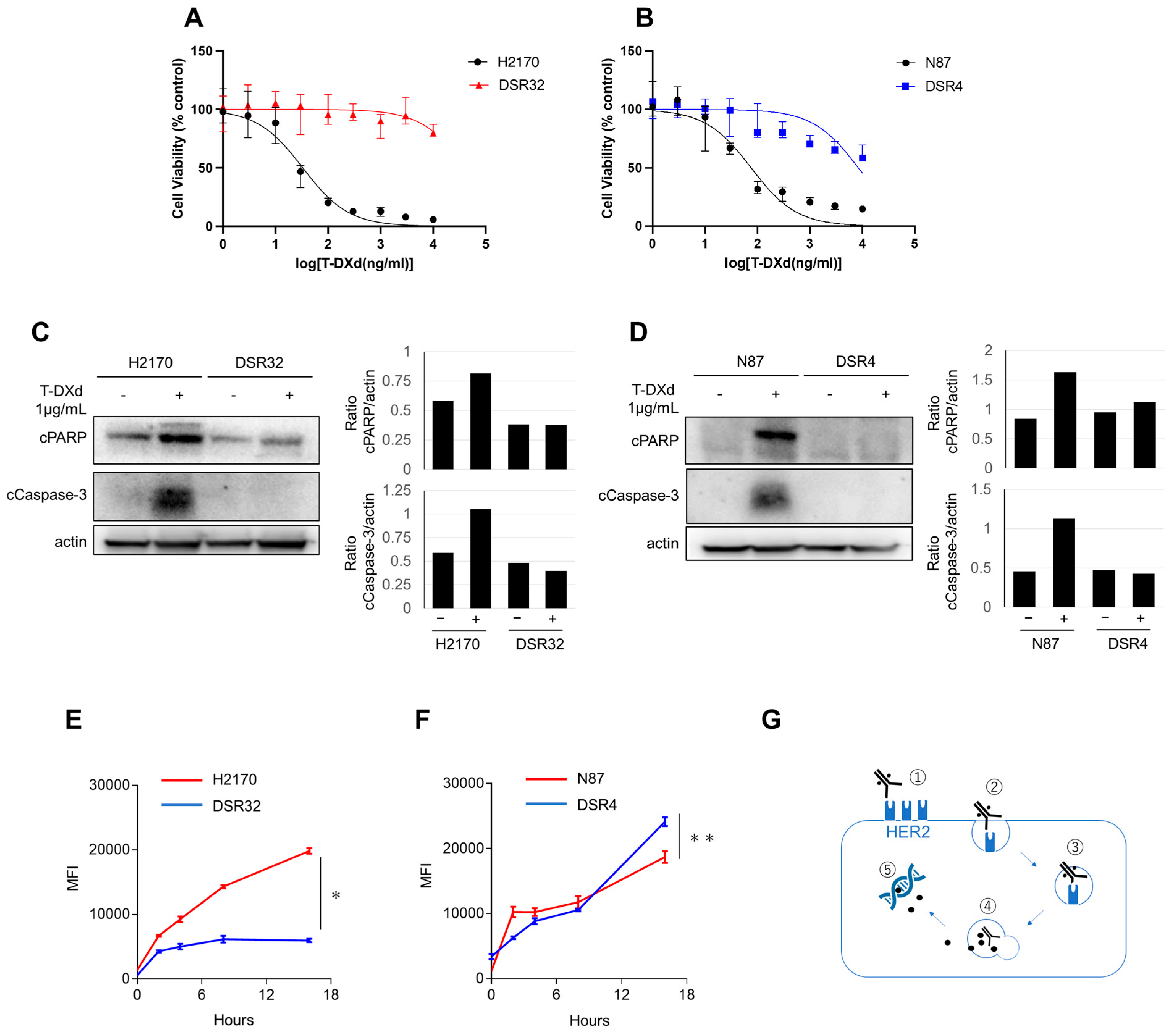
2.1. T-DXd-Resistance and Its Relationship to Internalization and Lysosomal Transport
2.2. HER2 Gene Copy Number Reduction Leads to Impaired T-DXd Internalization in Resistant Cells
2.3. Resistance to T-DXd May Not Be Solely Due to Impaired Internalization or Transport to Lysosomes
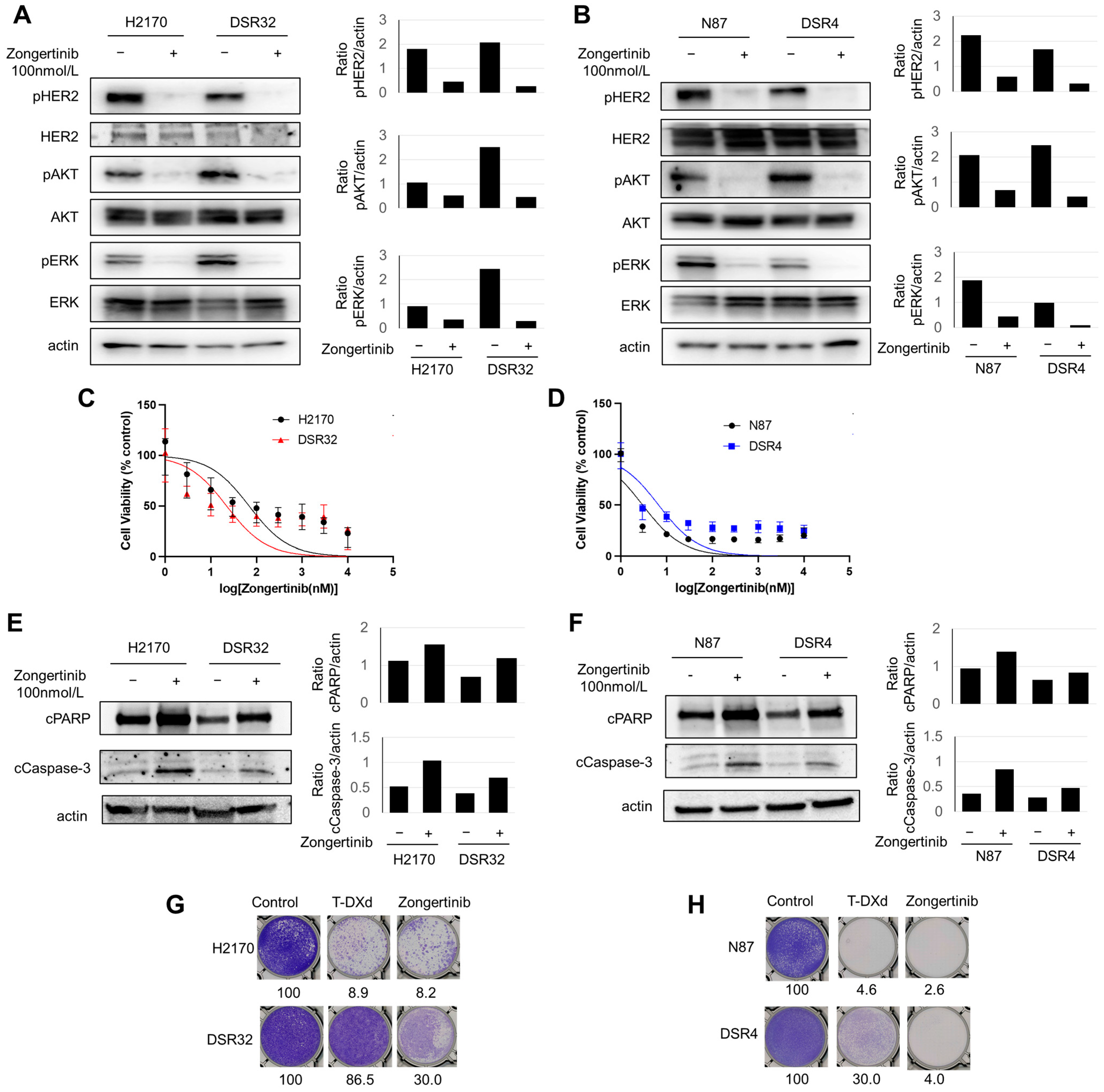
2.4. T-DXd-Resistant Cells Demonstrated Susceptibility to Zongertinib
3. Discussion
4. Materials and Methods
4.1. Cells and Reagents
4.2. In Vitro Growth Inhibition Assay
4.3. ADC Internalization Detection Assay
4.4. Cell Surface HER2 Measuring Assay
4.5. Colony-Formation Assay
4.6. Western Blotting
4.7. NGS
4.8. Microarray
4.9. In Vivo Tumor Growth Inhibition Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EGFR | epidermal growth factor receptor |
| ADC | Antibody–drug conjugate |
| PBS | Phosphate-buffered saline |
| FBS | Fetal bovine serum |
| NGS | Next-generation sequencing |
| IC50 | Inhibitory concentration value |
| TKI | Tyrosine kinase inhibitor |
| T-DXd | Trastuzumab deruxtecan |
| T-DM1 | trastuzumab emtansine |
| CI | confidence interval |
| MFI | mean fluorescence intensity |
| SD | standard deviation |
| SEM | standard error of the mean |
References
- Rubin, I.; Yarden, Y. The basic biology of HER2. Ann. Oncol. 2001, 12 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Oh, D.Y. HER2-targeted therapies beyond breast cancer—An update. Nat. Rev. Clin. Oncol. 2024, 21, 675–700. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, P.; Li, F.; Lai, H.; Qi, T.; Wang, Y. Advances in the study of marketed antibody-drug conjugates (ADCs) for the treatment of breast cancer. Front. Pharmacol. 2024, 14, 1332539. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.F.; Felip, E.F.; Uprety, D.; Nagasaka, M.; Nakagawa, K.; Rodríguez, P.A.; Pacheco, J.M.; Li, B.T.; Planchard, D.; Baik, C.; et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): Primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 2024, 25, 439–454. [Google Scholar] [CrossRef]
- Heymach, J.V.; Opdam, F.; Barve, M.; Tu, H.Y.; Wu, H.Y.; Berz, D.; Schröter, L.; Botilde, Y.; Sadrolhefazi, B.; Serra, J.; et al. HER2-selective tyrosine kinase inhibitor, zongertinib (BI 1810631), in patients with advanced/metastatic solid tumors with HER2 alterations: A phase Ia dose-escalation study. J. Clin. Oncol. 2025, 43, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Wilding, B.; Woelflingseder, L.; Baum, A.; Chylinski, K.; Vainorius, G.; Gibson, N.; Waizenegger, I.C.; Gerlach, D.; Augsten, M.; Spreitzer, F.; et al. Zongertinib (BI 1810631), an irreversible HER2 TKI, spares EGFR signaling and improves therapeutic response in preclinical models and patients with HER2-driven cancers. Cancer Discov. 2025, 15, 119–138. [Google Scholar] [CrossRef]
- Yonesaka, K.; Zejnullahu, K.; Okamoto, I.; Satoh, T.; Cappuzzo, F.; Souglakos, J.; Ercan, D.; Rogers, A.; Roncalli, M.; Takeda, M.; et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci. Transl. Med. 2011, 3, 99ra86. [Google Scholar] [CrossRef]
- Suzuki, S.; Yonesaka, K.; Teramura, T.; Takehara, T.; Kato, R.; Sakai, H.; Haratani, K.; Tanizaki, J.; Kawakami, H.; Hayashi, H.; et al. KRAS inhibitor resistance in MET-amplified KRASG12C non-small cell lung cancer induced by RAS- and non-RAS-mediated cell signaling mechanisms. Clin. Cancer Res. 2021, 27, 5697–5707, Erratum in Clin. Cancer Res. 2022, 28, 428. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.; Desnoyers, S.H.; Ottaviano, Y.; Davidson, N.E.; Poirier, G.G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: An early marker of chemotherapy-induced apoptosis. Cancer Res. 1993, 53, 3976–3985. [Google Scholar]
- Liu, X.; Zou, H.; Slaughter, C.; Wang, X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 1997, 89, 175–184. [Google Scholar] [CrossRef]
- Nakagawa, H.; Saito, H.; Ikegami, Y.; Aida-Hyugaji, S.; Sawada, S.; Ishikawa, T. Molecular modeling of new camptothecin analogues to circumvent ABCG2-mediated drug resistance in cancer. Cancer Lett. 2006, 234, 81–89. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Grinda, T.; Rassy, E.; Pistilli, B. Antibody-drug conjugate revolution in breast cancer: The road ahead. Curr. Treat. Options Oncol. 2023, 24, 442–465, Erratum in Curr. Treat. Options Oncol. 2023, 24, 466–467. [Google Scholar] [CrossRef]
- Hunter, F.W.; Barker, H.R.; Lipert, B.; Rothé, F.; Gebhart, G.; Piccart-Gebhart, M.J.; Sotiriou, C.; Jamieson, S.M.F. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br. J. Cancer 2020, 122, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.L.; Schwettmann, B.; McArthur, H.L.; Chan, I.S. Antibody-drug conjugates in breast cancer: Overcoming resistance and boosting immune response. J. Clin. Investig. 2023, 133, e172156. [Google Scholar] [CrossRef]
- Yamashita-Kashima, Y.; Yoshimura, Y.; Fujimura, T.; Shu, S.; Yanagisawa, M.; Yorozu, K.; Furugaki, K.; Higuchi, R.; Shoda, J.; Harada, N. Molecular targeting of HER2-overexpressing biliary tract cancer cells with trastuzumab emtansine, an antibody-cytotoxic drug conjugate. Cancer Chemother. Pharmacol. 2019, 83, 659–671. [Google Scholar] [CrossRef]
- Vasalou, C.; Proia, T.A.; Kazlauskas, L.; Przybyla, A.; Sung, M.; Mamidi, S.; Maratea, K.; Griffin, M.; Sargeant, R.; Urosevic, J.; et al. Quantitative evaluation of trastuzumab deruxtecan pharmacokinetics and pharmacodynamics in mouse models of varying degrees of HER2 expression. CPT Pharmacomet. Syst. Pharmacol. 2024, 13, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, S.; He, W.; Song, M. Emerging insights into mechanisms of trastuzumab resistance in HER2-positive cancers. Int. Immunopharmacol. 2023, 122, 110602. [Google Scholar] [CrossRef] [PubMed]
- Loganzo, F.; Tan, X.; Sung, M.; Jin, G.; Myers, J.S.; Melamud, E.; Wang, F.; Diesl, V.; Follettie, M.T.; Musto, S.; et al. Tumor cells chronically treated with a trastuzumab-maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol. Cancer Ther. 2015, 14, 952–963. [Google Scholar] [CrossRef]
- Sabbaghi, M.; Gil-Gómez, G.; Guardia, C.; Servitja, S.; Arpí, O.; García-Alonso, S.; Menendez, S.; Arumi-Uria, M.; Serrano, L.; Salido, M.; et al. Defective cyclin B1 induction in trastuzumab-emtansine (T-DM1) acquired resistance in HER2-positive breast cancer. Clin. Cancer Res. 2017, 23, 7006–7019. [Google Scholar] [CrossRef]
- Li, G.; Guo, J.; Shen, B.Q.; Yadav, B.Q.; Sliwkowski, M.X.; Crocker, L.M.; Lacap, J.A.; Phillips, G.D.L. Mechanisms of acquired resistance to trastuzumab emtansine in breast cancer cells. Mol. Cancer Ther. 2018, 17, 1441–1453. [Google Scholar] [CrossRef]
- Mosele, F.; Deluche, E.; Lusque, A.; Le Bescond, L.; Filleron, T.; Pradat, Y.; Ducoulombier, A.; Pistilli, B.; Bachelot, T.; Viret, F.; et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: The phase 2 DAISY trial. Nat. Med. 2023, 29, 2110–2120. [Google Scholar] [CrossRef]
- Tsurutani, J.; Iwata, H.; Krop, I.; Jänne, P.A.; Doi, T.; Takahashi, S.; Park, H.; Redfern, C.; Tamura, K.; Wise-Draper, T.M.; et al. Targeting HER2 with trastuzumab deruxtecan: A dose-expansion, Phase I study in multiple advanced solid tumors. Cancer Discov. 2020, 10, 688–701, Erratum in Cancer Discov. 2020, 10, 1078. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: Primary results from the DESTINY-PanTumor02 Phase II trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Gouda, M.A.; Gonugunta, A.; Dumbrava, E.E.; Yap, T.A.; Rodon, J.; Piha-Paul, S.A.; Pohlmann, P.R.; Damodaran, S.; Murthy, R.; Valero, V.; et al. Human epidermal growth factor receptor 2 loss following treatment with trastuzumab deruxtecan in patients with metastatic breast cancer. Clin. Cancer Res. 2025, 31, 1268–1274. [Google Scholar] [CrossRef]
- Shiose, Y.; Ochi, Y.; Kuga, H.; Yamashita, F.; Hashida, M. Relationship between drug release of DE-310, macromolecular prodrug of DX-8951f, and cathepsins activity in several tumors. Biol. Pharm. Bull. 2007, 30, 2365–2370. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.D.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef] [PubMed]
- Le Du, F.; Diéras, V.; Curigliano, G. The role of tyrosine kinase inhibitors in the treatment of HER2+ metastatic breast cancer. Eur. J. Cancer 2021, 154, 175–189, Erratum in Eur. J. Cancer 2021, 158, 255. [Google Scholar] [CrossRef]
- Duchnowska, R.; Loibl, S.; Jassem, J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer Treat. Rev. 2018, 67, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Esmaeili, H.; Minich, D.; Seitz, F.; Roessner, P.M.; Wind, S.; Grempler, R.; Gan, G.; Chan, T.S.; Mahmoudi, M.; et al. The effect of carbamazepine, a strong CYP3A inducer, on the pharmacokinetics of zongertinib in healthy male volunteers. Pharmacotherapy 2025, 45, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Yonesaka, K.; Kudo, K.; Nishida, S.; Takahama, T.; Iwasa, T.; Yoshida, T.; Tanaka, K.; Takeda, M.; Kaneda, H.; Okamoto, I.; et al. The pan-HER family tyrosine kinase inhibitor afatinib overcomes HER3 ligand heregulin-mediated resistance to EGFR inhibitors in non-small cell lung cancer. Oncotarget 2015, 6, 33602–33611. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurosaki, T.; Suzuki, S.; Yonesaka, K.; Kawanaka, Y.; Takehara, T.; Teramura, T.; Sakai, K.; Nishio, K.; Hayashi, H. Zongertinib, a Novel HER2 Tyrosine Kinase Inhibitor, Maintains an Anticancer Activity for Trastuzumab Deruxtecan-Resistant Cancers Harboring HER2-Overexpression. Int. J. Mol. Sci. 2025, 26, 10515. https://doi.org/10.3390/ijms262110515
Kurosaki T, Suzuki S, Yonesaka K, Kawanaka Y, Takehara T, Teramura T, Sakai K, Nishio K, Hayashi H. Zongertinib, a Novel HER2 Tyrosine Kinase Inhibitor, Maintains an Anticancer Activity for Trastuzumab Deruxtecan-Resistant Cancers Harboring HER2-Overexpression. International Journal of Molecular Sciences. 2025; 26(21):10515. https://doi.org/10.3390/ijms262110515
Chicago/Turabian StyleKurosaki, Takashi, Shinichiro Suzuki, Kimio Yonesaka, Yusuke Kawanaka, Toshiyuki Takehara, Takeshi Teramura, Kazuko Sakai, Kazuto Nishio, and Hidetoshi Hayashi. 2025. "Zongertinib, a Novel HER2 Tyrosine Kinase Inhibitor, Maintains an Anticancer Activity for Trastuzumab Deruxtecan-Resistant Cancers Harboring HER2-Overexpression" International Journal of Molecular Sciences 26, no. 21: 10515. https://doi.org/10.3390/ijms262110515
APA StyleKurosaki, T., Suzuki, S., Yonesaka, K., Kawanaka, Y., Takehara, T., Teramura, T., Sakai, K., Nishio, K., & Hayashi, H. (2025). Zongertinib, a Novel HER2 Tyrosine Kinase Inhibitor, Maintains an Anticancer Activity for Trastuzumab Deruxtecan-Resistant Cancers Harboring HER2-Overexpression. International Journal of Molecular Sciences, 26(21), 10515. https://doi.org/10.3390/ijms262110515




