LRP4 and Agrin Are Modulated by Cartilage Degeneration and Involved in β-Catenin Signaling in Human Articular Chondrocytes
Abstract
:1. Introduction
2. Results
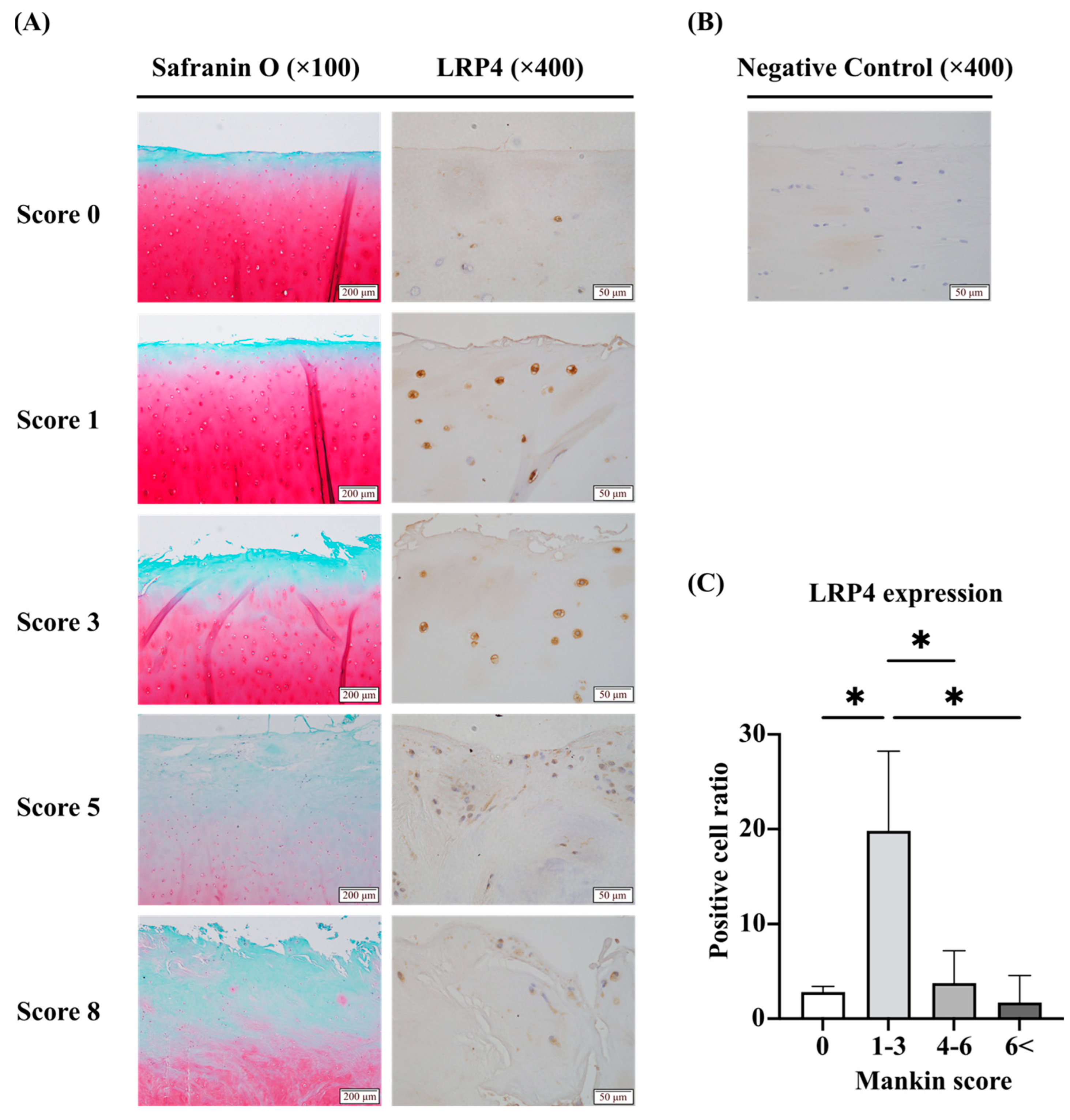
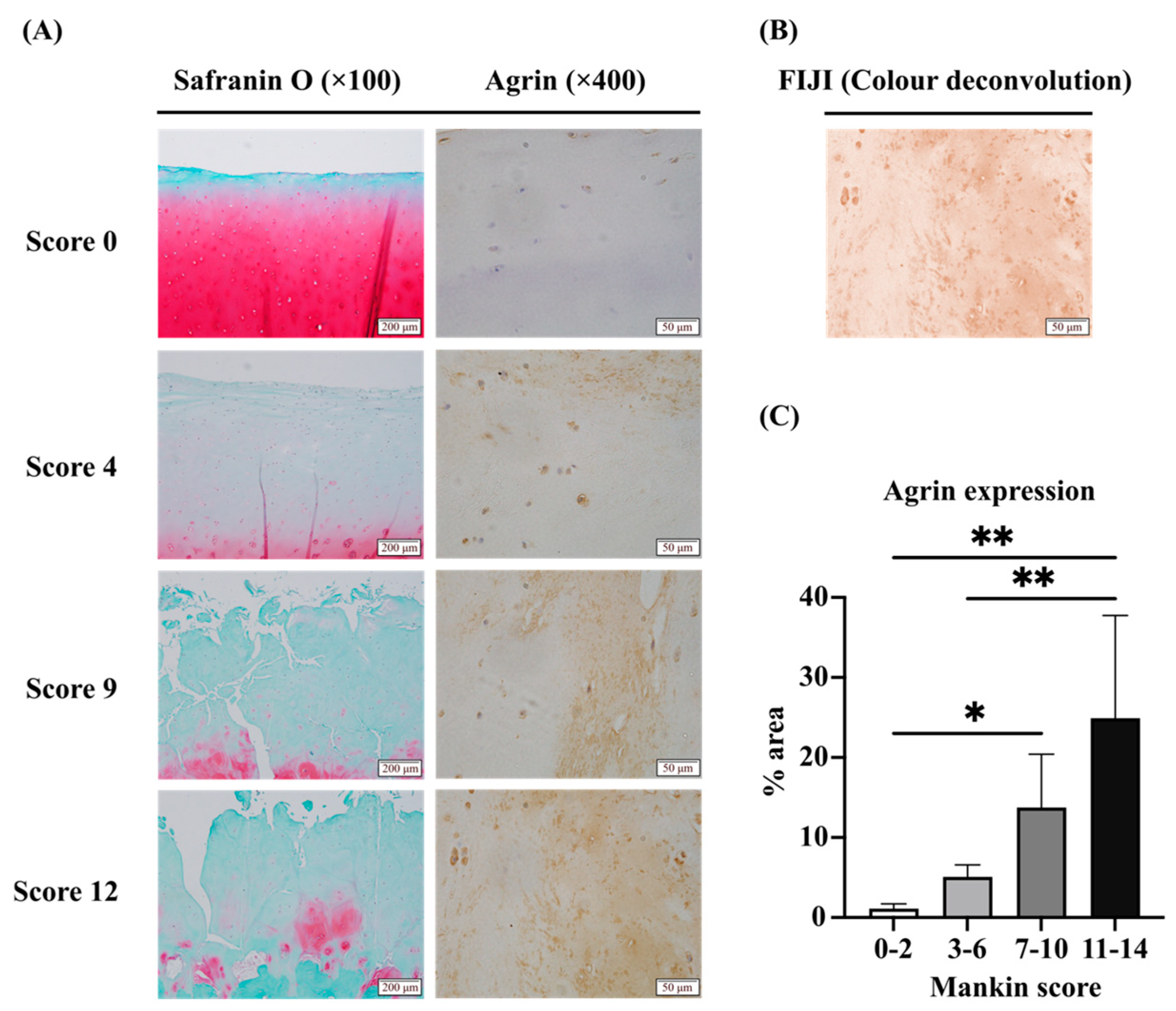
2.1. Expression of LRP4 Increased in the Early Stages of OA and Decreased with Cartilage Degeneration, Whereas Agrin Was Consistently Increased with Cartilage Degeneration in Human Articular Cartilage Tissues
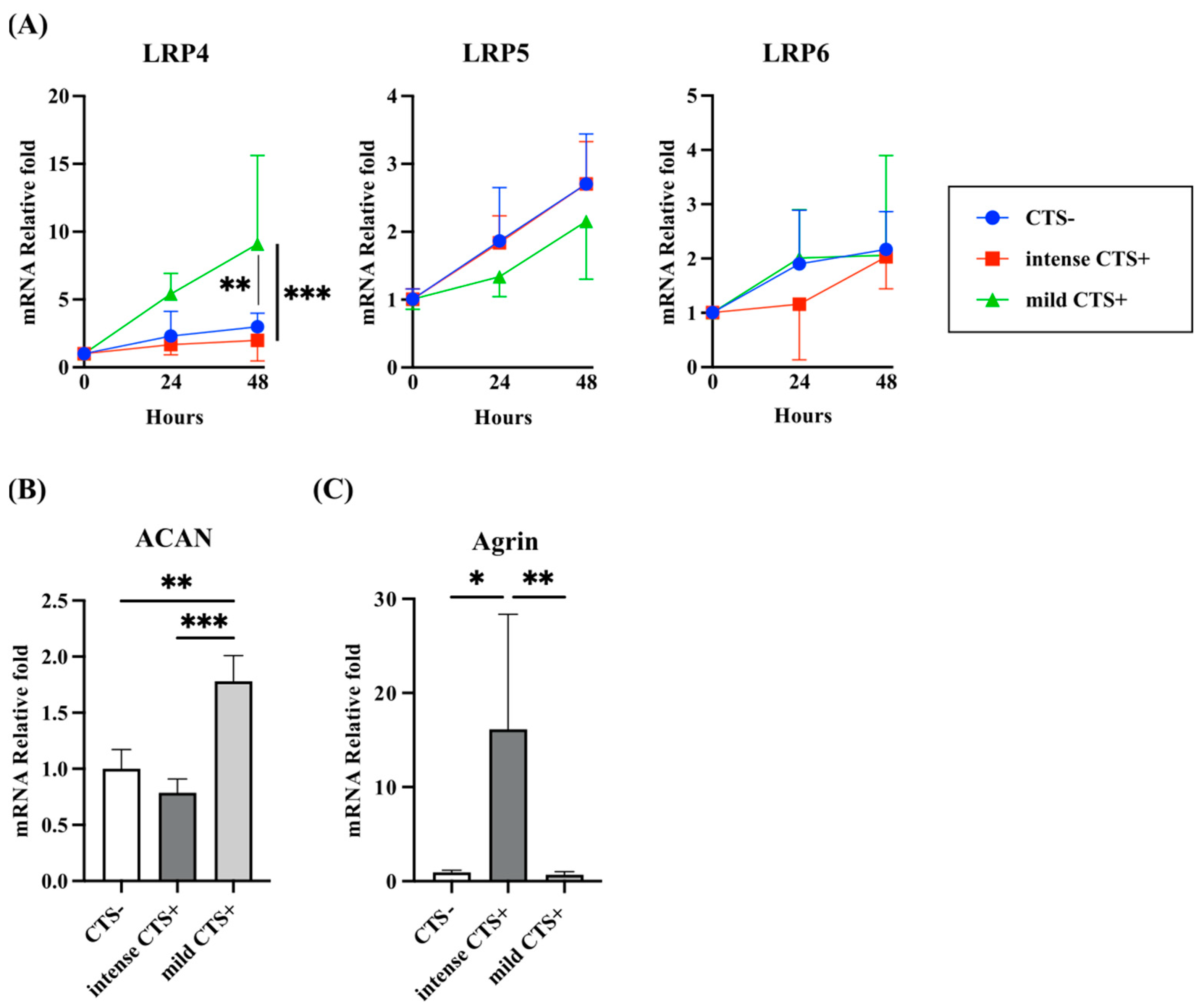
2.2. Expression of LRP4 and Agrin Were Regulated by Mechanical Stress with Cyclic Tensile Strain (CTS) in Human Articular Chondrocytes
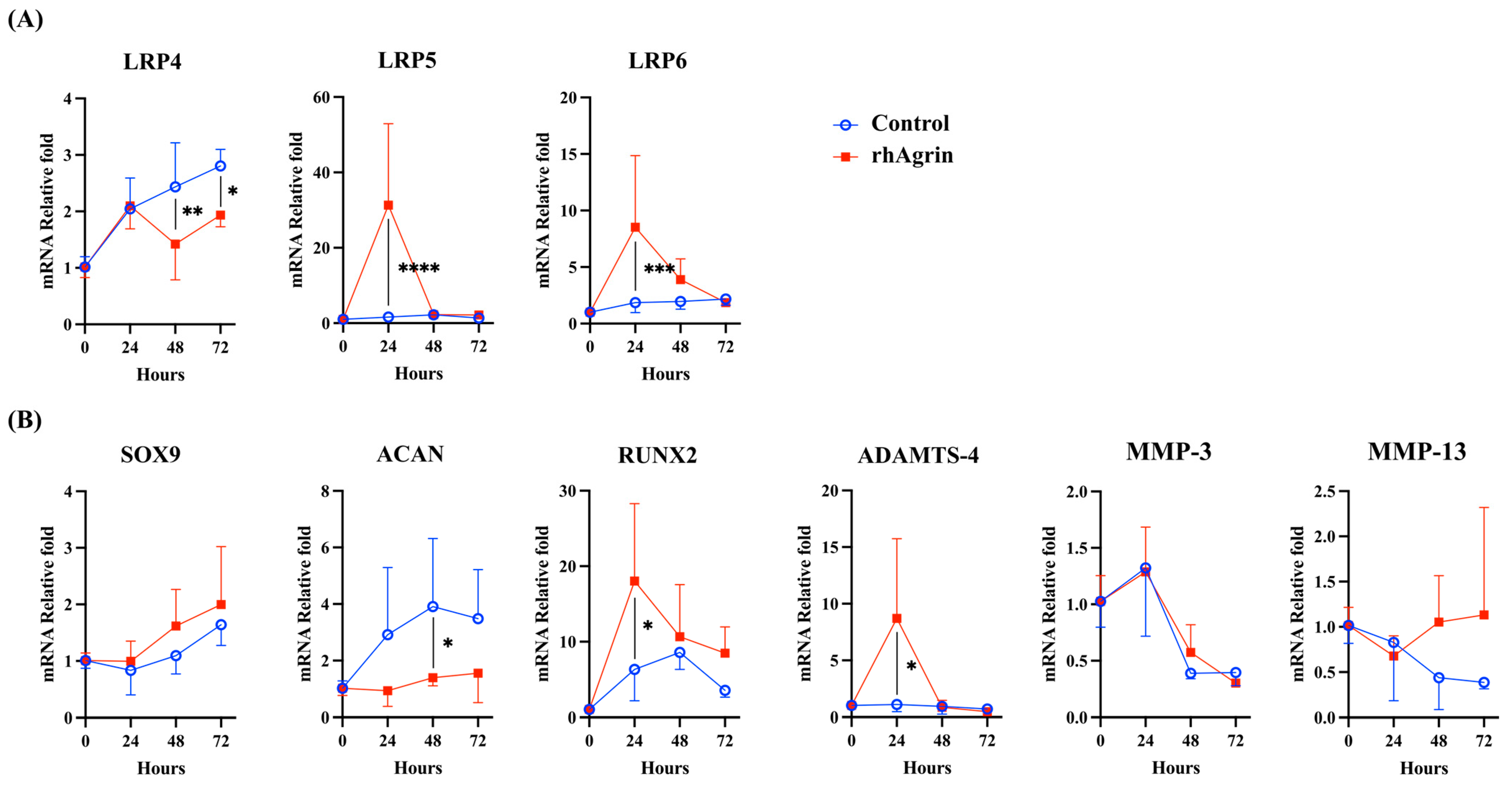
2.3. Agrin Treatment Upregulated the Gene Expression of LRP5/6 and Catabolic Factors in Human Articular Chondrocytes
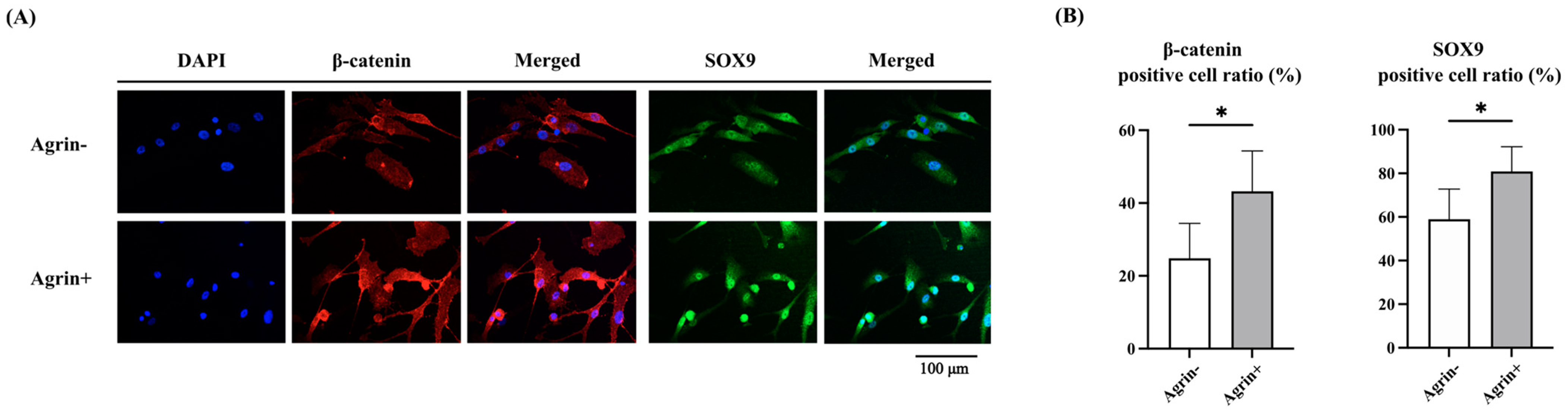
2.4. Agrin Promoted Nuclear Translocation of β-Catenin and SOX9 in Human Articular Chondrocytes
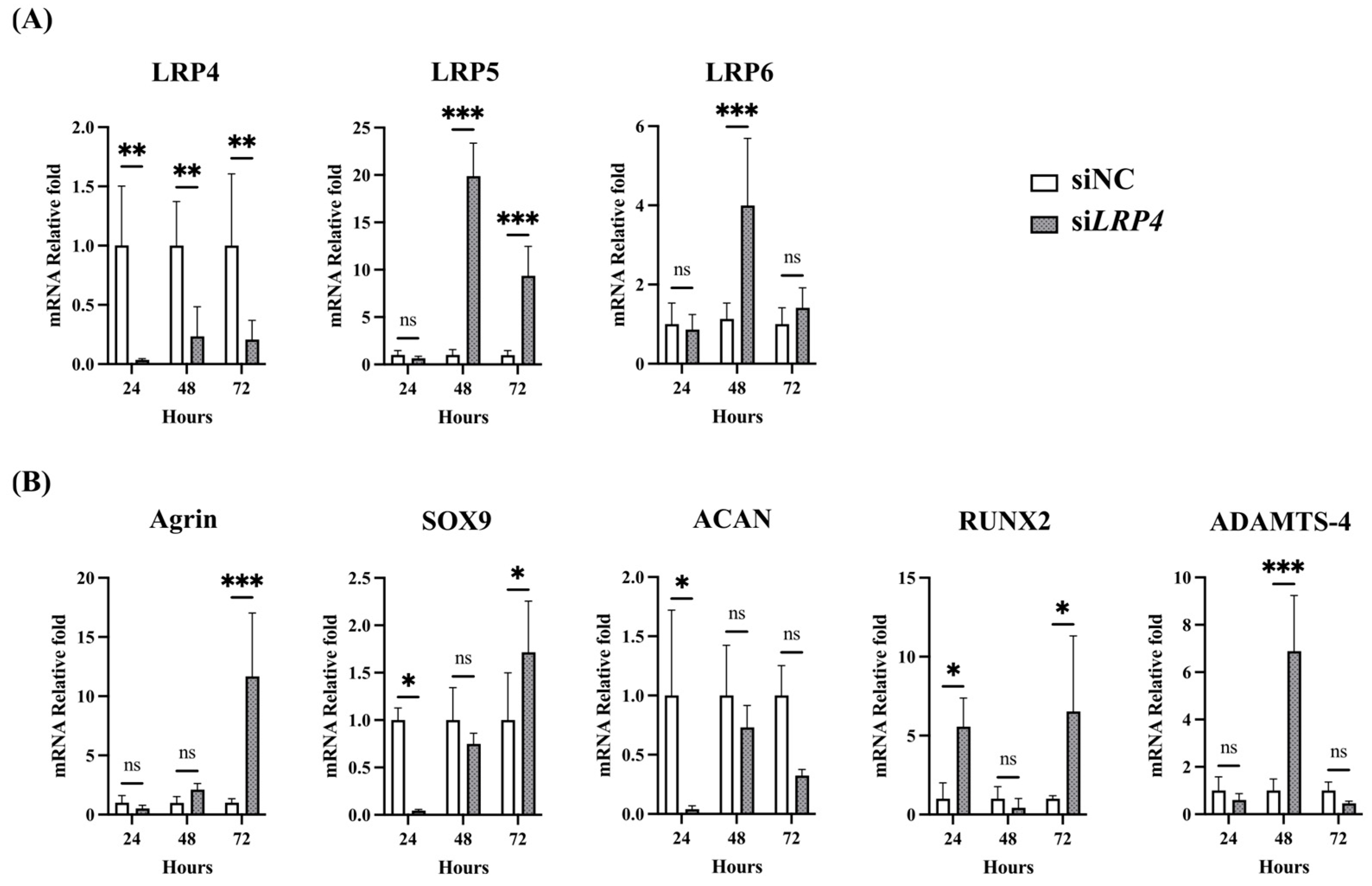
2.5. Effects of Agrin Knockdown on Modulation of Intense CTS-Induced Genes in Human Articular Chondrocytes
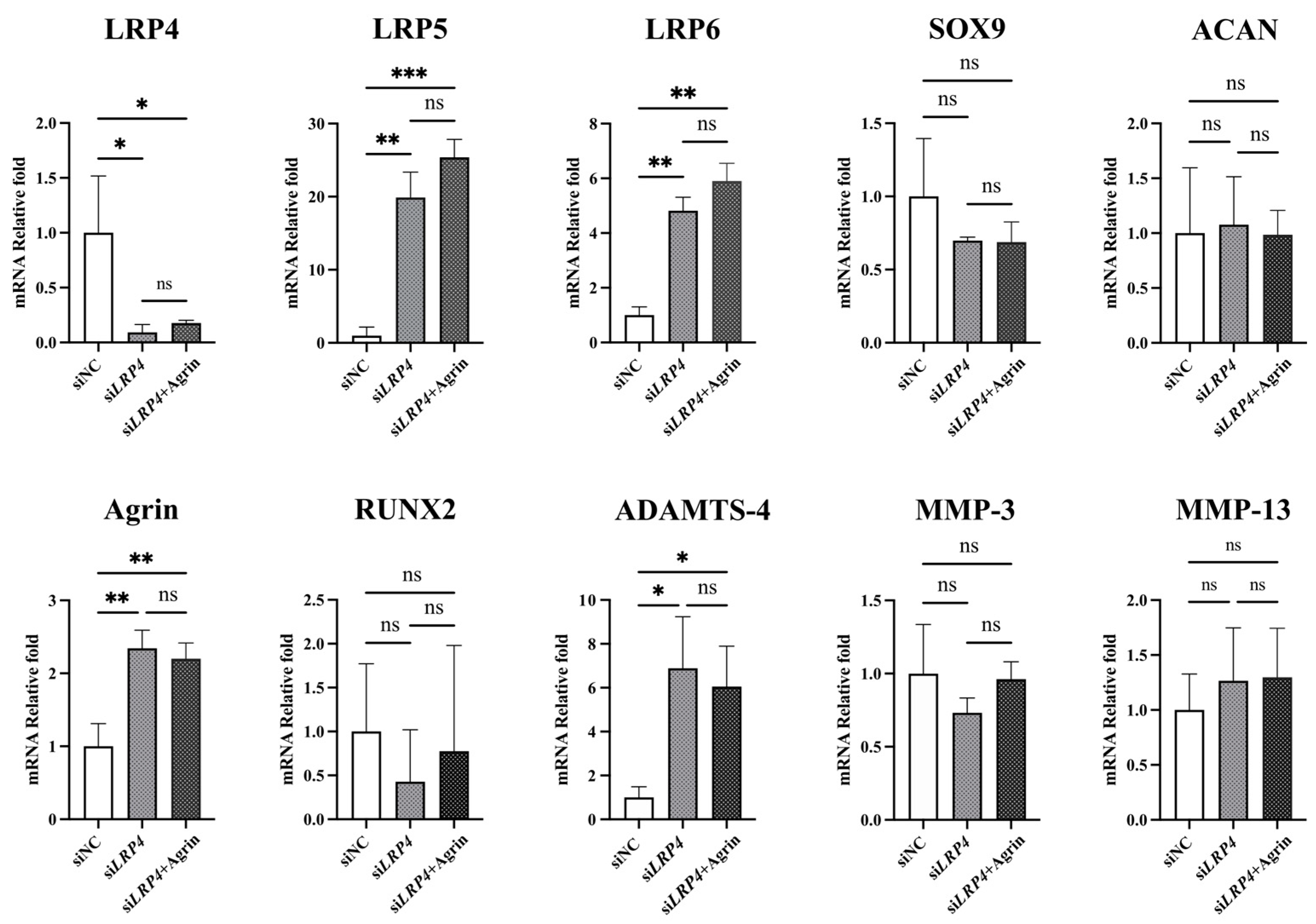
2.6. LRP4 Knockdown Showed a Similar Trend to Agrin After Treatment in Human Articular Chondrocytes
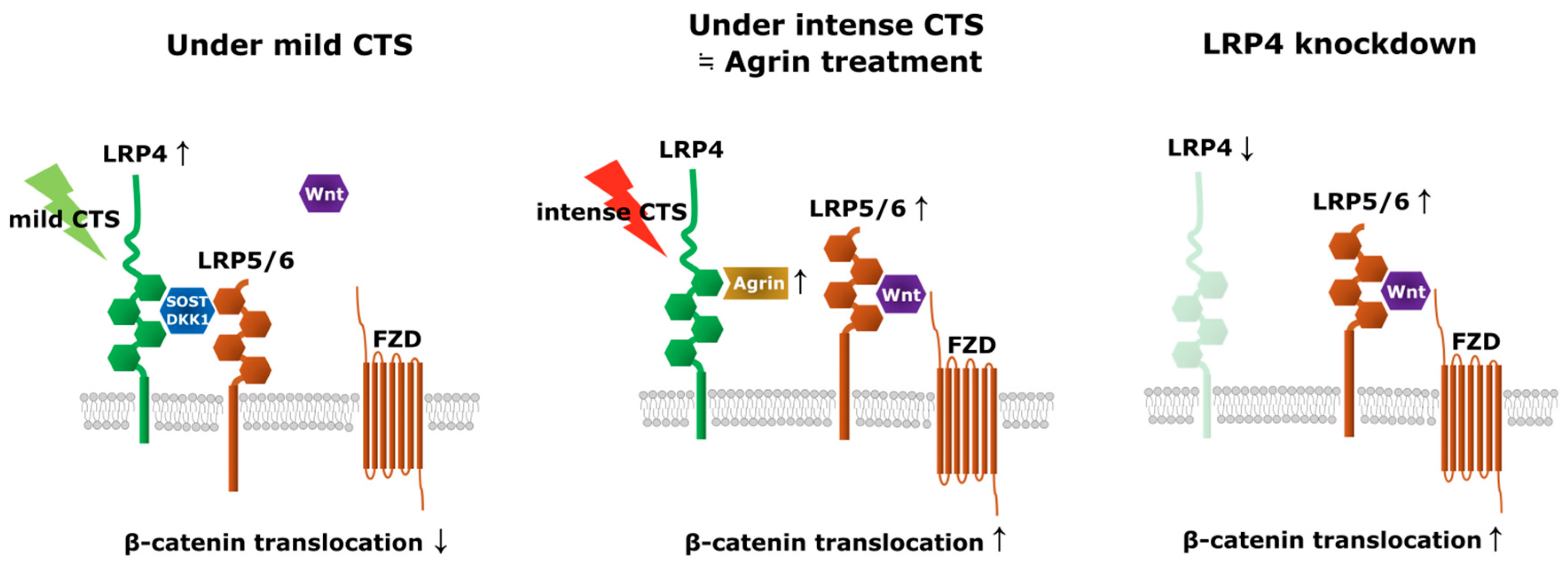
3. Discussion
4. Materials and Methods
4.1. Cells and Cell Culture
4.2. Clinical Samples of Human Articular Cartilage Tissues
4.3. Histological Evaluation of Cartilage Destruction
4.4. Immunohistochemical Evaluation of Articular Cartilage
4.5. CTS on Chondrocytes Cultured in Monolayer
4.6. RT-qPCR Analysis
4.7. Treatment with Reagents
4.8. siRNA Transfection
4.9. Immunocytochemistry
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dequeker, J.; Luyten, F.P. The history of osteoarthritis-osteoarthrosis. Ann. Rheum. Dis. 2008, 67, 5–10. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Gupta, S.; Hawker, G.A.; Laporte, A.; Croxford, R.; Coyte, P.C. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology 2005, 44, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.-J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Zhou, X.; Jin, M.; Nie, J.; Li, X. Molecular mechanisms of mechanical load-induced osteoarthritis. Int. Orthop. 2021, 45, 1125–1136. [Google Scholar] [CrossRef]
- Mow, V.C.; Ratcliffe, A.; Poole, A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and Structures. Biomaterials 1992, 13, 67–97. [Google Scholar] [CrossRef]
- Poole, A.R. An Introduction to the Pathophysiology of Osteoarthritis. Front. Biosci. 1999, 4, D662–D670. [Google Scholar] [CrossRef] [PubMed]
- Dell’Accio, F.; De Bari, C.; El Tawil, N.M.F.; Barone, F.; Mitsiadis, T.A.; O’Dowd, J.; Pitzalis, C. Activation of WNT and BMP signaling in adult human articular cartilage following mechanical injury. Arthritis Res. Ther. 2006, 8, R139. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Nakasa, T.; Hikata, T.; Asahara, H. Molecular network of cartilage homeostasis and osteoarthritis. Med. Res. Rev. 2008, 28, 464–481. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.S.; Oh, H.; Yang, S.; Park, M. Wnt Signaling in Cartilage Development and Degeneration. BMB Rep. 2008, 41, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Lietman, C.; Wu, B.; Lechner, S.; Shinar, A.; Sehgal, M.; Rossomacha, E.; Datta, P.; Sharma, A.; Gandhi, R.; Kapoor, M.; et al. Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight 2018, 3, e96308. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Xiong, W.C.; Mei, L. LRP4 in neuromuscular junction and bone development and diseases. Bone 2015, 80, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, Y.; Mortier, G.; Boudin, E.; Van Hul, W. WNT Signaling and Bone: Lessons From Skeletal Dysplasias and Disorders. Front. Endocrinol. 2020, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-d.; Ding, X.; Jiang, N.; Zeng, C.; Wu, J.; Cai, X.-Y.; Hettinghouse, A.; Khleborodova, A.; Lei, Z.-N.; Chen, Z.-S.; et al. Digoxin targets low density lipoprotein receptor-related protein 4 and protects against osteoarthritis. Ann. Rheum. Dis. 2022, 81, 544–555. [Google Scholar] [CrossRef]
- Rupp, F.; Hoch, W.; Campanelli, J.T.; Kreiner, T.; Scheller, R.H. Agrin and the Organization of the Neuromuscular Junction. Curr. Opin. Neurobiol. 1992, 2, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Coldefy, A.S.; Hubbard, S.R.; Burden, S.J. Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first immunoglobulin-like domain in muscle-specific kinase (MuSK). J. Biol. Chem. 2011, 286, 40624–40630. [Google Scholar] [CrossRef]
- Hausser, H.J.; Ruegg, M.A.; Brenner, R.E.; Ksiazek, I. Agrin is highly expressed by chondrocytes and is required for normal growth. Histochem. Cell Biol. 2007, 127, 363–374. [Google Scholar] [CrossRef]
- Eldridge, S.; Nalesso, G.; Ismail, H.; Vicente-Greco, K.; Kabouridis, P.; Ramachandran, M.; Niemeier, A.; Herz, J.; Pitzalis, C.; Perretti, M.; et al. Agrin mediates chondrocyte homeostasis and requires both LRP4 and α-dystroglycan to enhance cartilage formation in vitro and in vivo. Ann. Rheum. Dis. 2016, 75, 1228–1235. [Google Scholar] [CrossRef]
- Souza, A.T.P.; Lopes, H.B.; Oliveira, F.S.; Weffort, D.; Freitas, G.P.; Adolpho, L.F.; Fernandes, R.R.; Rosa, A.L.; Beloti, M.M. The extracellular matrix protein Agrin is expressed by osteoblasts and contributes to their differentiation. Cell Tissue Res. 2021, 386, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, R.C.; Mellor, L.F.; Krause, A.R.; Donahue, H.J.; Loboa, E.G. LRP receptors in chondrocytes are modulated by simulated microgravity and cyclic hydrostatic pressure. PLoS ONE 2019, 14, e0223245. [Google Scholar] [CrossRef] [PubMed]
- Asai, N.; Ohkawara, B.; Ito, M.; Masuda, A.; Ishiguro, N.; Ohno, K. LRP4 induces extracellular matrix productions and facilitates chondrocyte differentiation. Biochem. Biophys. Res. Commun. 2014, 451, 302–307. [Google Scholar] [CrossRef]
- Geng, S.; Paul, F.; Kowalczyk, I.; Raimundo, S.; Sporbert, A.; Mamo, T.M.; Hammes, A. Balancing WNT signalling in early forebrain development: The role of LRP4 as a modulator of LRP6 function. Front. Cell Dev. Biol. 2023, 11, 1173688. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gil, N.; Ugartondo, N.; Grinberg, D.; Balcells, S. Wnt Pathway Extracellular Components and Their Essential Roles in Bone Homeostasis. Genes 2022, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Bullock, W.A.; Hoggatt, A.M.; Horan, D.J.; Elmendorf, A.J.; Sato, A.Y.; Bellido, T.; Loots, G.G.; Pavalko, F.M.; Robling, A.G. Lrp4 Mediates Bone Homeostasis and Mechanotransduction through Interaction with Sclerostin In Vivo. iScience 2019, 20, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, G.Y. The osteocyte as a therapeutic target in the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2014, 6, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Sims, C.; Murray, M.J.; Kuhlmann, P.K.; Fuentes-Antrás, J.; Weatherbee, S.D.; Krumlauf, R. Multiple modes of Lrp4 function in modulation of Wnt/β-catenin signaling during tooth development. Development 2017, 144, 2824–2836. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tian, S.; Fan, L.; Niu, R.; Yan, M.; Chen, S.; Zheng, M.; Zhang, S. Integrated regulation of chondrogenic differentiation in mesenchymal stem cells and differentiation of cancer cells. Cancer Cell Int. 2022, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tan, X.-N.; Hu, S.; Liu, R.-Q.; Peng, L.-H.; Li, Y.-M.; Wu, P. Molecular Mechanisms of Chondrocyte Proliferation and Differentiation. Front. Cell Dev. Biol. 2021, 9, 664168. [Google Scholar] [CrossRef]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef]
- Nagata, K.; Hojo, H.; Chang, S.H.; Okada, H.; Yano, F.; Chijimatsu, R.; Omata, Y.; Mori, D.; Makii, Y.; Kawata, M.; et al. Runx2 and Runx3 differentially regulate articular chondrocytes during surgically induced osteoarthritis development. Nat. Commun. 2022, 13, 6187. [Google Scholar] [CrossRef] [PubMed]
- Saito, T. The superficial zone of articular cartilage. Inflamm. Regen. 2022, 42, 14. [Google Scholar] [CrossRef] [PubMed]
- Takahata, Y.; Hagino, H.; Kimura, A.; Urushizaki, M.; Yamamoto, S.; Wakamori, K.; Murakami, T.; Hata, K.; Nishimura, R. Regulatory Mechanisms of Prg4 and Gdf5 Expression in Articular Cartilage and Functions in Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 4672. [Google Scholar] [CrossRef]
- Grafe, I.; Alexander, S.; Peterson, J.R.; Snider, T.N.; Levi, B.; Lee, B.; Mishina, Y. TGF-β family signaling in mesenchymal differentiation. Cold Spring Harb. Perspect. Biol. 2018, 10, a022202. [Google Scholar] [CrossRef]
- Lefebvre, V.; De Crombrugghe, B. Toward Understanding SOX9 Function in Chondrocyte Differentiation. Matrix Biol. 1998, 16, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Bastide, P.; Darido, C.; Pannequin, J.; Kist, R.; Robine, S.; Marty-Double, C.; Bibeau, F.; Scherer, G.; Joubert, D.; Hollande, F.; et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J. Cell Biol. 2007, 178, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Ohazama, A.; Johnson, E.B.; Ota, M.S.; Choi, H.J.; Porntaveetus, T.; Oommen, S.; Itoh, N.; Eto, K.; Gritli-Linde, A.; Herz, J.; et al. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS ONE 2008, 3, e4092. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.E.; Barawi, A.; Wang, H.; Roelofs, A.J.; Kaneva, M.; Guan, Z.; Lydon, H.; Thomas, B.L.; Thorup, A.-S.; Fernandez, B.F.; et al. Agrin Induces Long-Term Osteochondral Regeneration by Supporting Repair Morphogenesis. Sci. Transl. Med. 2020, 12, eaax9086. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.T.; Sanes, J.R. Integrins mediate adhesion to agrin and modulate agrin signaling. Development 1997, 124, 3909–3917. [Google Scholar] [CrossRef]
- Chung, C.; Massee, M.; Koob, T.J. Human amniotic membrane modulates Wnt/β-catenin and NF-κβ signaling pathways in articular chondrocytes in vitro. Osteoarthr. Cartil. Open 2021, 3, 100211. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and Metabolic Abnormalities in Articular Cartilage from Osteo-Arthritic Human Hips. II. CORRELATION OF MORPHOLOGY WITH BIOCHEMICAL AND METABOLIC DATA. J. Bone Jt. Surg. 1971, 53, 523–537. [Google Scholar] [CrossRef]
- Naruse, K.; Yamada, T.; Sokabe, M. Involvement of SA Channels in Orienting Response of Cultured Endothelial Cells to Cyclic Stretch. Am. J. Physiol. 1998, 274, H1532–H1538. [Google Scholar] [CrossRef]
- Herberhold, C.; Faber, S.; Stammberger, T.; Steinlechner, M.; Putz, R.; Englmeier, K.H.; Reiser, M.; Eckstein, F. In Situ Measurement of Articular Cartilage Deformation in Intact Femoropatellar Joints under Static Loading. J. Biomech. 1999, 32, 1287–1295. [Google Scholar] [CrossRef]
- Madden, R.; Han, S.K.; Herzog, W. Chondrocyte deformation under extreme tissue strain in two regions of the rabbit knee joint. J. Biomech. 2013, 46, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Hotta, Y.; Nishida, K.; Yoshida, A.; Nasu, Y.; Nakahara, R.; Naniwa, S.; Shimizu, N.; Ichikawa, C.; Lin, D.; Fujiwara, T.; et al. Inhibitory Effect of a Tankyrase Inhibitor on Mechanical Stress-Induced Protease Expression in Human Articular Chondrocytes. Int. J. Mol. Sci. 2024, 25, 1443. [Google Scholar] [CrossRef] [PubMed]
- Furumatsu, T.; Matsumoto, E.; Kanazawa, T.; Fujii, M.; Lu, Z.; Kajiki, R.; Ozaki, T. Tensile strain increases expression of CCN2 and COL2A1 by activating TGF-β-Smad2/3 pathway in chondrocytic cells. J. Biomech. 2013, 46, 1508–1515. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naniwa, S.; Nishida, K.; Yoshida, A.; Nasu, Y.; Nakahara, R.; Ohtsuki, T.; Hotta, Y.; Shimizu, N.; Ichikawa, C.; Lin, D.; et al. LRP4 and Agrin Are Modulated by Cartilage Degeneration and Involved in β-Catenin Signaling in Human Articular Chondrocytes. Int. J. Mol. Sci. 2025, 26, 1007. https://doi.org/10.3390/ijms26031007
Naniwa S, Nishida K, Yoshida A, Nasu Y, Nakahara R, Ohtsuki T, Hotta Y, Shimizu N, Ichikawa C, Lin D, et al. LRP4 and Agrin Are Modulated by Cartilage Degeneration and Involved in β-Catenin Signaling in Human Articular Chondrocytes. International Journal of Molecular Sciences. 2025; 26(3):1007. https://doi.org/10.3390/ijms26031007
Chicago/Turabian StyleNaniwa, Shuichi, Keiichiro Nishida, Aki Yoshida, Yoshihisa Nasu, Ryuichi Nakahara, Takashi Ohtsuki, Yoshifumi Hotta, Noriyuki Shimizu, Chinatsu Ichikawa, Deting Lin, and et al. 2025. "LRP4 and Agrin Are Modulated by Cartilage Degeneration and Involved in β-Catenin Signaling in Human Articular Chondrocytes" International Journal of Molecular Sciences 26, no. 3: 1007. https://doi.org/10.3390/ijms26031007
APA StyleNaniwa, S., Nishida, K., Yoshida, A., Nasu, Y., Nakahara, R., Ohtsuki, T., Hotta, Y., Shimizu, N., Ichikawa, C., Lin, D., Otsuka, N., & Ozaki, T. (2025). LRP4 and Agrin Are Modulated by Cartilage Degeneration and Involved in β-Catenin Signaling in Human Articular Chondrocytes. International Journal of Molecular Sciences, 26(3), 1007. https://doi.org/10.3390/ijms26031007







