Feasibility of Genipin to Evaluate Chitosan Rainfastness for Biopesticide Applications
Abstract
:1. Introduction
2. Results
2.1. Chitosan Characterization
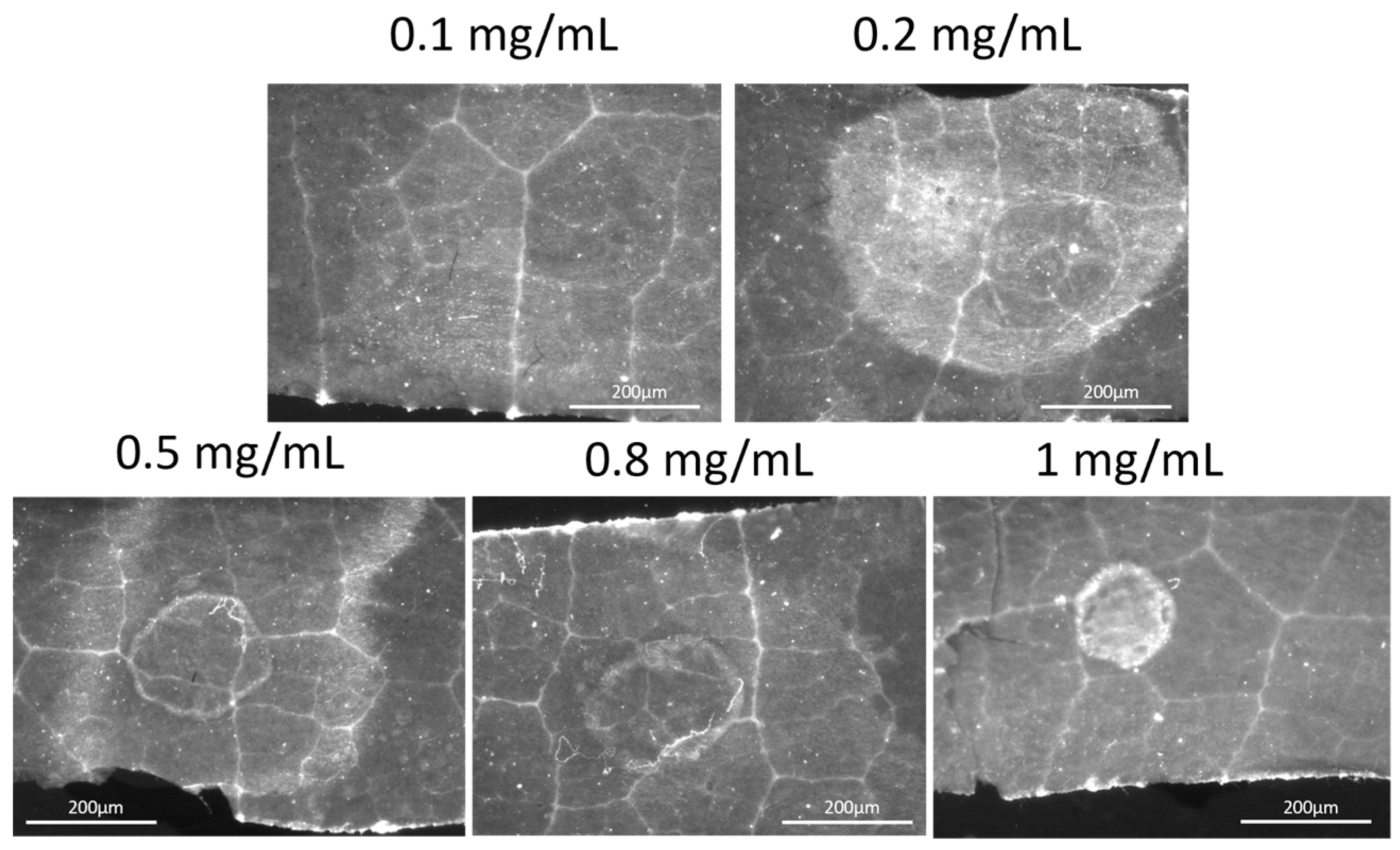
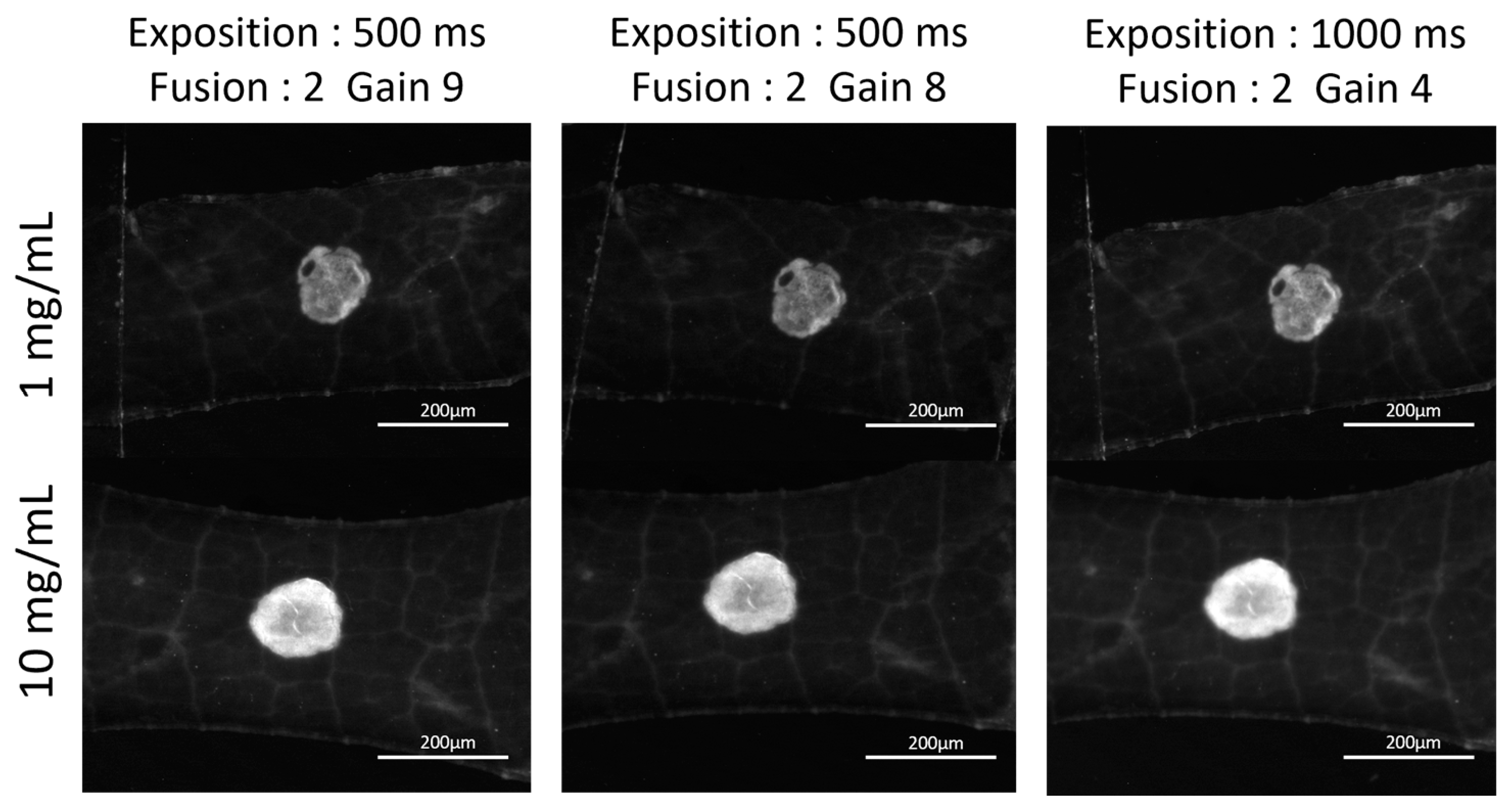
2.2. Feasibility of the Evaluation of the Residual Chitosan Content on a Vine Leaf Using MacroFluo Microscopy
2.3. Feasibility of Chitosan Evaluation on a Leaf Model Using CLSM
2.3.1. Selection of an Appropriate Leaf Model
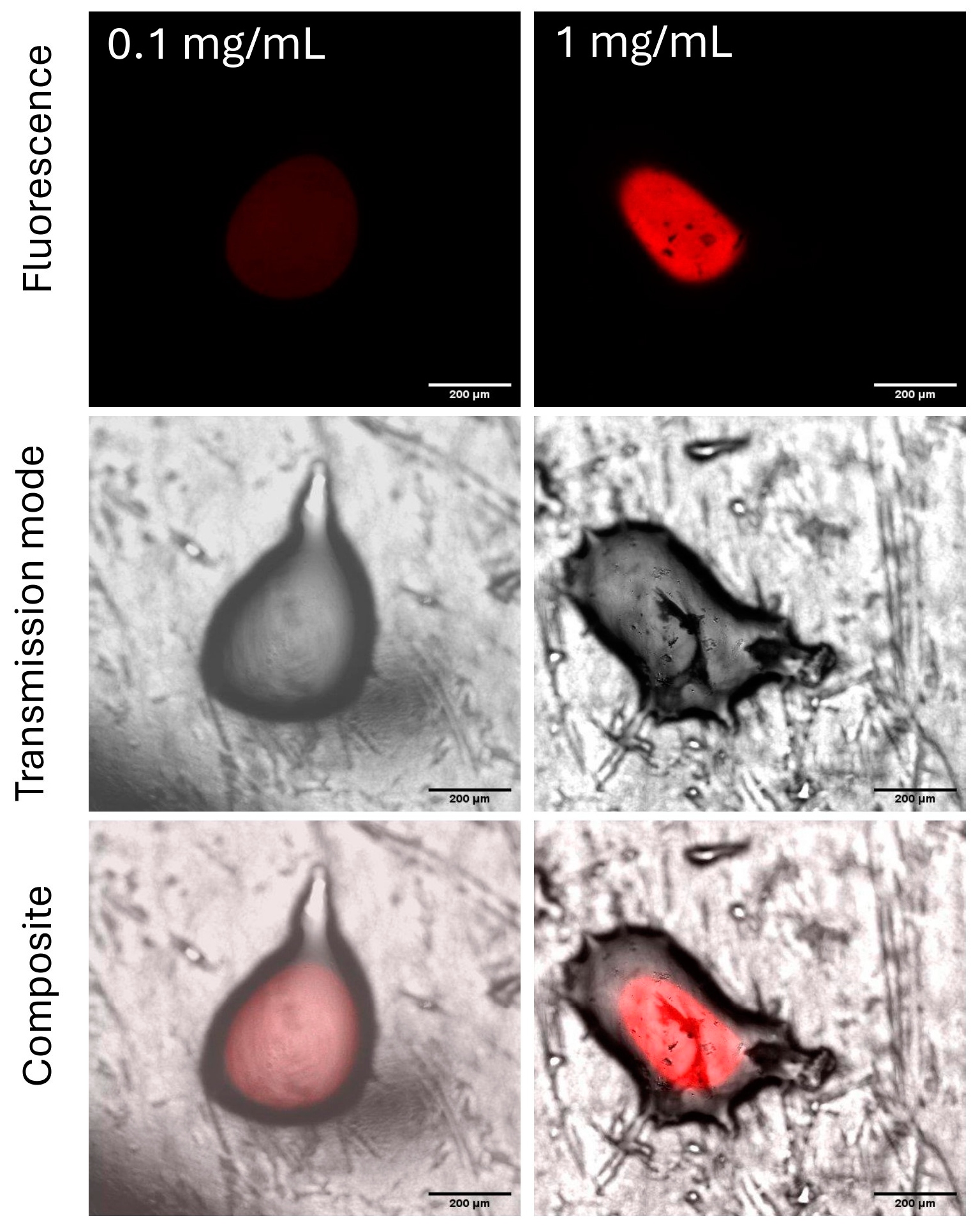
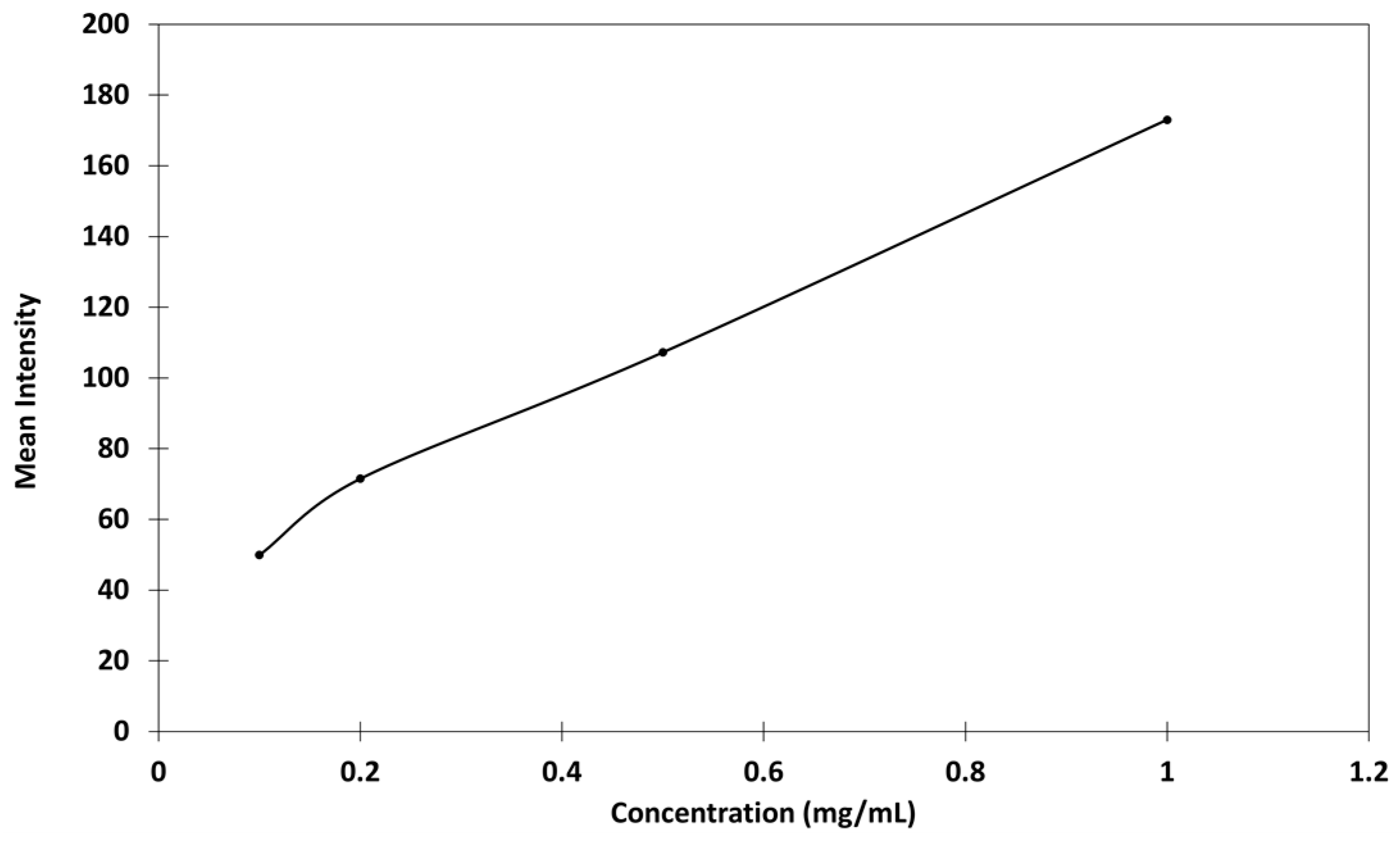
2.3.2. Relationship Between Concentration and Fluorescence of Genipin-Treated Chitosan
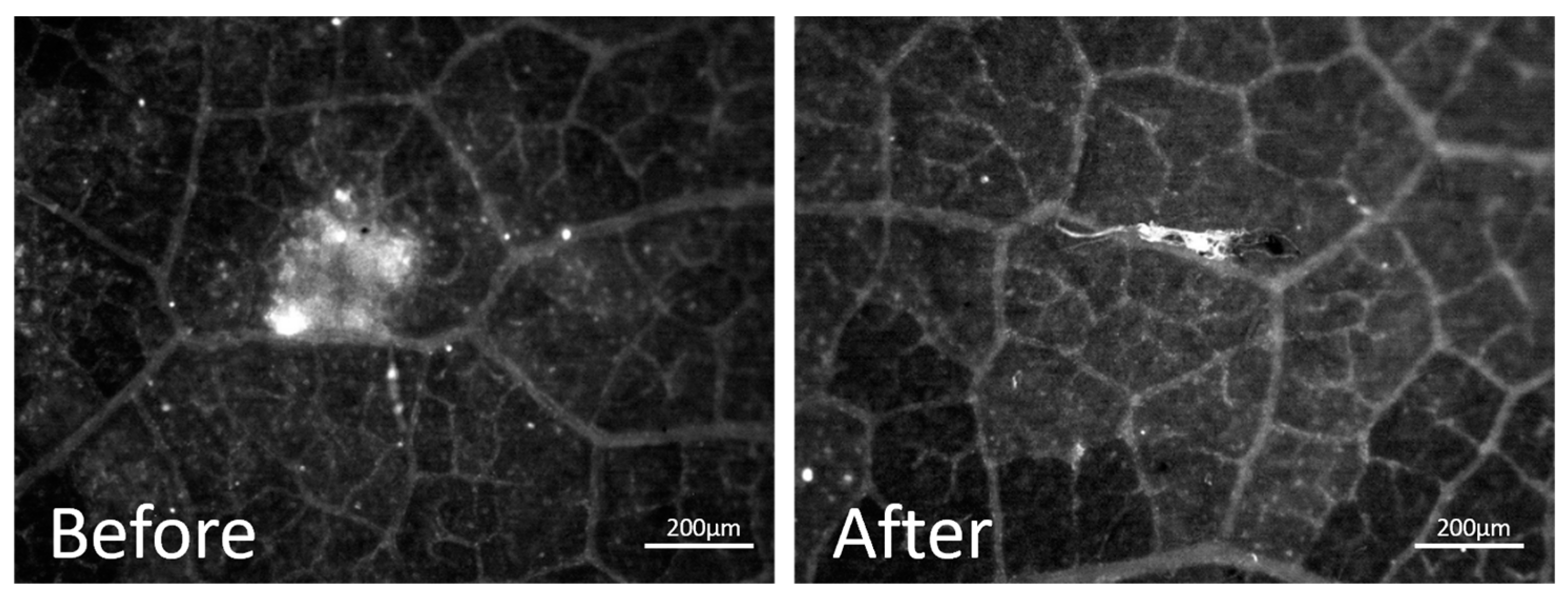
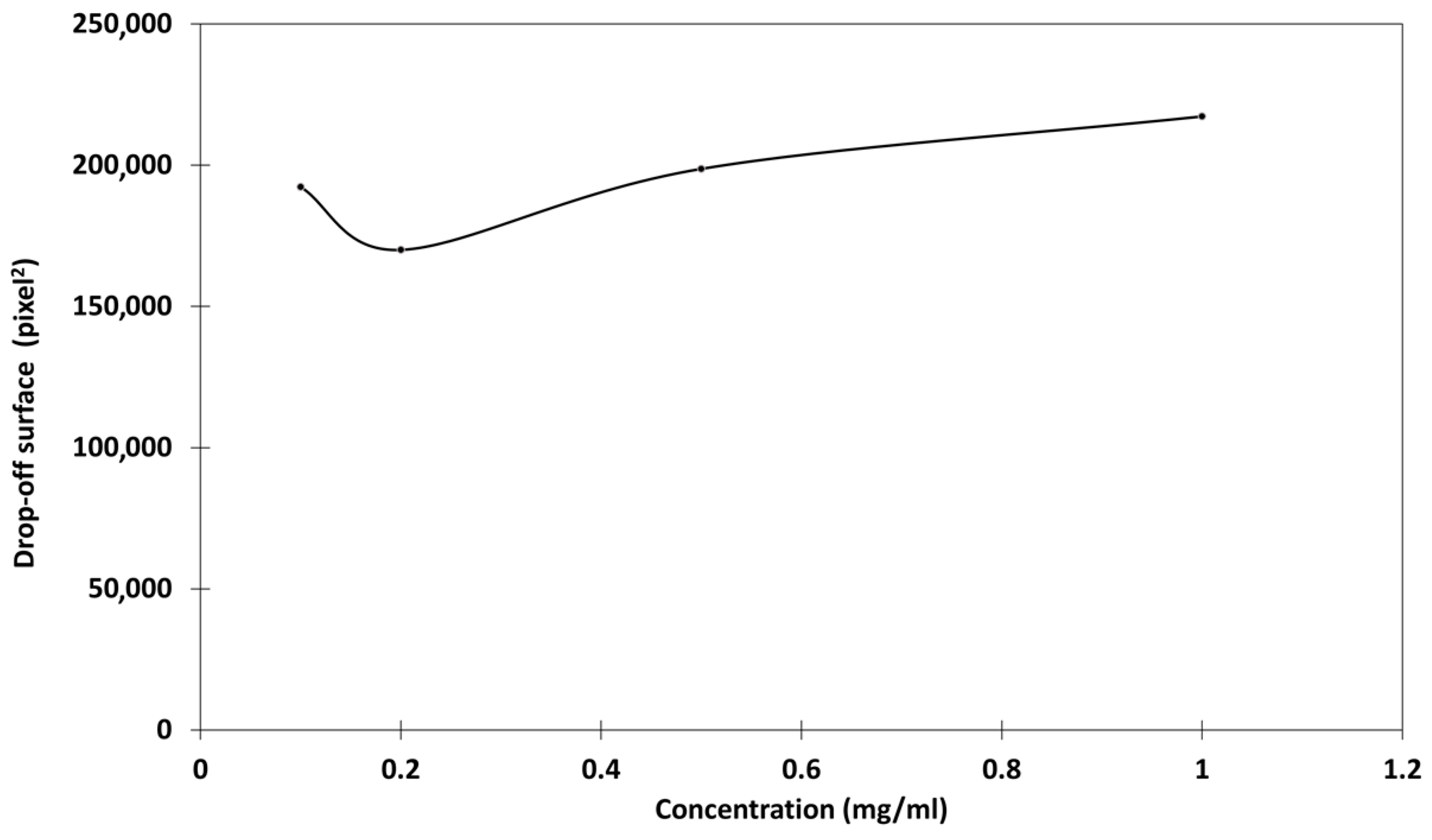
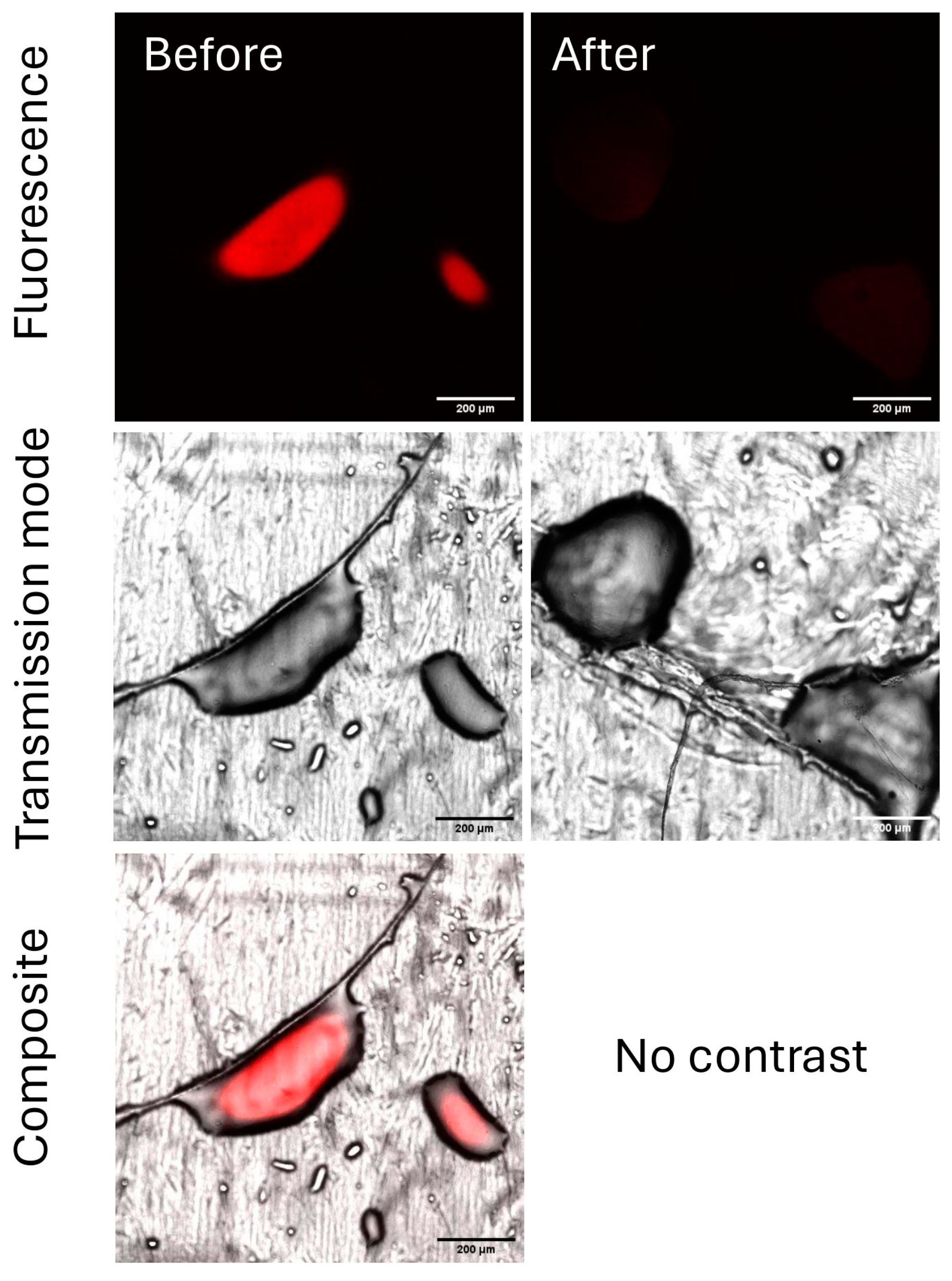
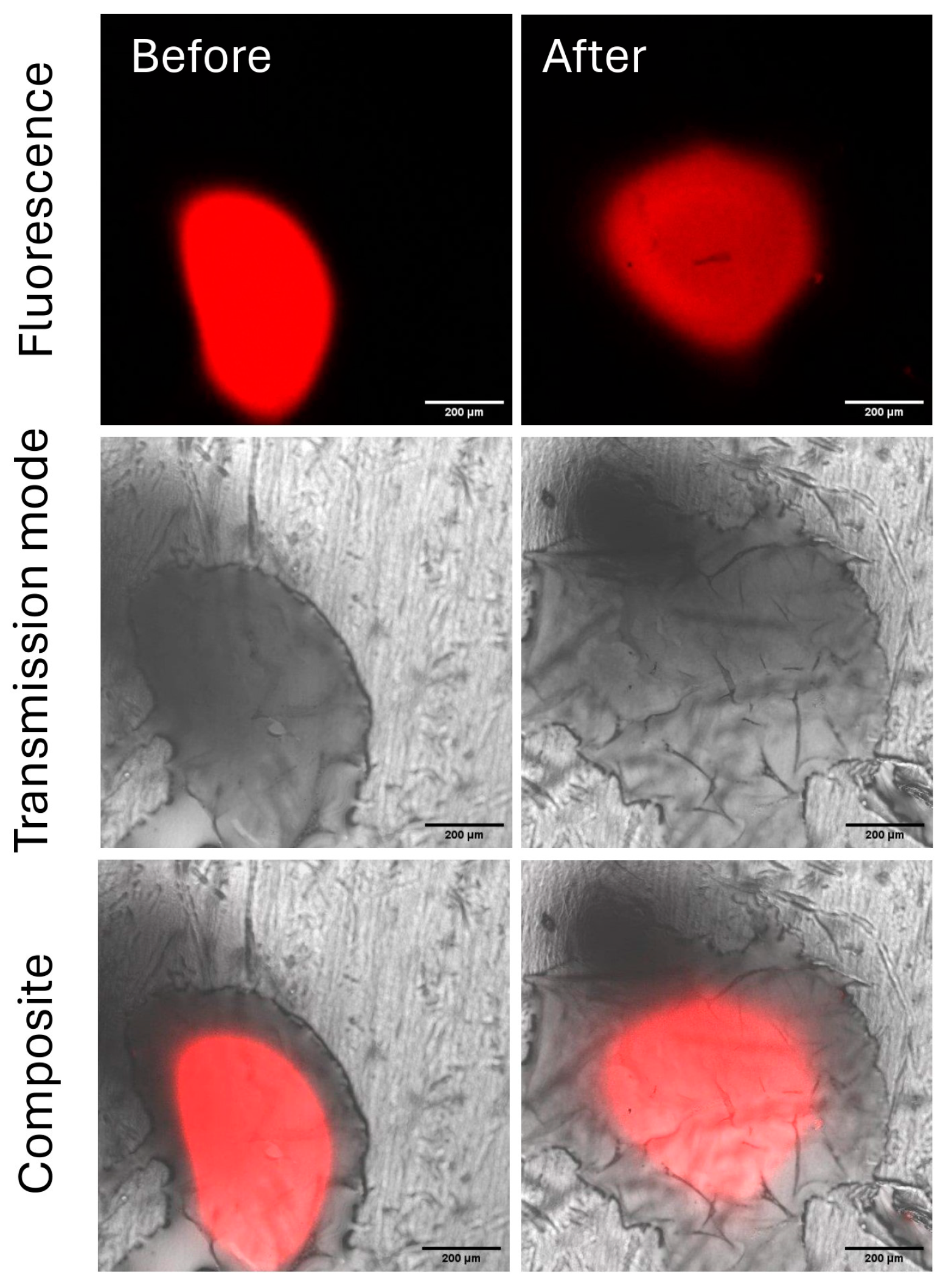
2.3.3. Evaluation of the Residual Chitosan After Rainfastness Test
3. Discussion
3.1. Feasibility of the Evaluation of the Residual Chitosan Content on a Vine Leaf Using MacroFluo Microscopy
3.2. Feasibility of Chitosan Evaluation on a Leaf Model Using CLSM
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Chitosan Characterization
- Deacetylation degree
- Molecular weight
- Surface analysis by XPS
- Elemental analysis (C, H, N, O)
4.2.2. Chitosan Solution Preparation
4.2.3. MacroFluo Analyses
- Calibration on vine leaf
- Simulated rain resistance test on vine leaf
4.2.4. Elaboration of Leaf Models
- Carnauba wax model
- Silicone model
- Parafilm® model
4.2.5. Surface Analysis of Leaf Models
4.2.6. Confocal Analyses
- Feasibility test on various chitosan concentrations
- Calibration
- Simulated rain resistance test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loron, A.; Wang, Y.; Atanasova, V.; Richard-Forget, F.; Gardrat, C.; Coma, V. Chitosan for Eco-Friendly Control of Mycotoxinogenic Fusarium Graminearum. Food Hydrocoll. 2023, 134, 108067. [Google Scholar] [CrossRef]
- Stasińska-Jakubas, M.; Hawrylak-Nowak, B. Protective, Biostimulating, and Eliciting Effects of Chitosan and Its Derivatives on Crop Plants. Molecules 2022, 27, 2801. [Google Scholar] [CrossRef] [PubMed]
- Anonymous Le Plan Écophyto, Qu’est-Ce Que c’est ? Available online: https://agriculture.gouv.fr/le-plan-ecophyto-quest-ce-que-cest (accessed on 31 July 2024).
- Mukhtar Ahmed, K.B.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and Its Oligosaccharides, a Promising Option for Sustainable Crop Production- a Review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef]
- Kurniadie, D.; Umiyati, U.; Widianto, R.; Kato-Noguchi, H. Effect of Chitosan Molecules on Paraquat Herbicidal Efficacy under Simulated Rainfall Conditions. Agronomy 2022, 12, 1666. [Google Scholar] [CrossRef]
- Présidence Du Centre Inra Nouvelle-Aquitaine-Poitiers; Présidence de La Chambre Régionale d’Agriculture de Nouvelle-Aquitaine; Présidence Régionale Nouvelle-Aquitaine de L’Acta. Construisons l’avenir Avec Le Biocontrôle. In Proceedings of the Rencontres Régionales de La Recherche, du Développement et de La Formation, Montagne, France, 13 December 2017; p. 60. [Google Scholar]
- Bariuan, J.V.; Reddy, K.N.; Wills, G.D. Glyphosate Injury, Rainfastness, Absorption, and Translocation in Purple Nutsedge (Cyperus Rotundus). Weed Technol. 1999, 13, 112–119. [Google Scholar] [CrossRef]
- Mojumdar, A.; Upadhyay, A.K.; Raina, V.; Ray, L. A Simple and Rapid Colorimetric Method for the Estimation of Chitosan Produced by Microbial Degradation of Chitin Waste. J. Microbiol. Methods 2019, 158, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, J.; Altenbach, H.J.; Malolepszy, T.; Mölleken, H. A New Method for the Quantification of Chitin and Chitosan in Edible Mushrooms. Carbohydr. Res. 2011, 346, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Paik, Y.S.; Lee, C.M.; Cho, M.H.; Hahn, T.R. Physical Stability of the Blue Pigments Formed from Geniposide of Gardenia Fruits: Effects of Ph, Temperature, and Light. J. Agric. Food Chem. 2001, 49, 430–432. [Google Scholar] [CrossRef]
- Ahmed, R.; ul ain Hira, N.; Wang, M.; Iqbal, S.; Yi, J.; Hemar, Y. Genipin, a Natural Blue Colorant Precursor: Source, Extraction, Properties, and Applications. Food Chem. 2024, 434, 137498. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D.A. Mechanism and Kinetics of the Crosslinking Reaction between Biopolymers Containing Primary Amine Groups and Genipin. J. Polym. Sci. Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Peng, C.K. Characterization of Ring-Opening Polymerization of Genipin and PH-Dependent Cross-Linking Reactions between Chitosan and Genipin. J. Polym. Sci. Polym. Chem. 2005, 43, 1985–2000. [Google Scholar] [CrossRef]
- Almog, J.; Cohen, Y.; Azoury, M.; Hahn, T.-R. Genipin--a Novel Fingerprint Reagent with Colorimetric and Fluorogenic Activity. J. Forensic Sci. 2004, 49, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Gonçalves, I.; Nunes, C.; Ferreira, P.; Coimbra, M.A.; Martin, C.; Bras, J. Feasibility of Chitosan Crosslinked with Genipin as Biocoating for Cellulose-Based Materials. Carbohydr. Polym. 2020, 242, 116429. [Google Scholar] [CrossRef] [PubMed]
- Alorbu, C.; Cai, L. Fungal Resistance and Leachability of Genipin-Crosslinked Chitosan Treated Wood. Int. Biodeterior. Biodegrad. 2022, 169, 105378. [Google Scholar] [CrossRef]
- Chen, H.; Ouyang, W.; Lawuyi, B.; Martoni, C.; Prakash, S. Reaction of Chitosan with Genipin and Its Fluorogenic Attributes for Potential Microcapsule Membrane Characterization. J. Biomed. Mater. Res. A 2005, 75, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Bottegoni, C.; Aquili, A.; Gigante, A. Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone. Mar. Drugs 2015, 13, 7314–7338. [Google Scholar] [CrossRef] [PubMed]
- Matienzo, L.J.; Winnacker, S.K. Dry Processes for Surface Modification of a Biopolymer: Chitosan. Macromol. Mater. Eng. 2002, 287, 871–880. [Google Scholar] [CrossRef]
- Brulé, D.; Héloir, M.C.; Roudaire, T.; Villette, J.; Bonnet, S.; Pascal, Y.; Darblade, B.; Crozier, P.; Hugueney, P.; Coma, V.; et al. Increasing Vineyard Sustainability: Innovating a Targeted Chitosan-Derived Biocontrol Solution to Induce Grapevine Resistance against Downy and Powdery Mildews. Front. Plant Sci. 2024, 15, 1360254. [Google Scholar] [CrossRef]
- Lamprecht, A.; Schäfer, U.F.; Lehr, C.M. Visualization and Quantification of Polymer Distribution in Microcapsules by Confocal Laser Scanning Microscopy (CLSM). Int. J. Pharm. 2000, 196, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Depypere, F.; Van Oostveldt, P.; Pieters, J.G.; Dewettinck, K. Quantification of Microparticle Coating Quality by Confocal Laser Scanning Microscopy (CLSM). Eur. J. Pharm. Biopharm. 2009, 73, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Symonds, B.L.; Lindsay, C.I.; Thomson, N.R.; Khutoryanskiy, V.V. Chitosan as a Rainfastness Adjuvant for Agrochemicals. RSC Adv. 2016, 6, 102206–102213. [Google Scholar] [CrossRef]
- Dorr, G.J.; Kempthorne, D.M.; Mayo, L.C.; Forster, W.A.; Zabkiewicz, J.A.; McCue, S.W.; Belward, J.A.; Turner, I.W.; Hanan, J. Towards a Model of Spray-Canopy Interactions: Interception, Shatter, Bounce and Retention of Droplets on Horizontal Leaves. Ecol. Modell. 2014, 290, 94–101. [Google Scholar] [CrossRef]
- Nairn, J.J.; Forster, W.A.; van Leeuwen, R.M. “Universal” Spray Droplet Adhesion Model-Accounting for Hairy Leaves. Weed Res. 2013, 53, 407–417. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Jiang, W. Analysis of Film-Forming Properties of Chitosan with Different Molecular Weights and Its Adhesion Properties with Different Postharvest Fruit Surfaces. Food Chem. 2022, 395, 133605. [Google Scholar] [CrossRef]
- Tevini, M.; Lichtenthaler, H.K. Lipids and Lipid Polymers in Higher Plants; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9783642666322. [Google Scholar]
- Fernández, V.; Guzmán-Delgado, P.; Graça, J.; Santos, S.; Gil, L. Cuticle Structure in Relation to Chemical Composition: Re-Assessing the Prevailing Model. Front. Plant Sci. 2016, 7, 427. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K.C. The Formation and Function of Plant Cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, R.; Fan, R.; Liu, Z.; Kong, W.; Zhang, P.; Du, F.P. Wettability of Pear Leaves from Three Regions Characterized at Different Stages after Flowering Using the OWRK Method. Pest. Manag. Sci. 2018, 74, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Guo, Z. Superhydrophobic Plant Leaves: The Variation in Surface Morphologies and Wettability during the Vegetation Period. Langmuir 2019, 35, 1047–1053. [Google Scholar] [CrossRef]
- Bi, H.; Kovalchuk, N.; Langridge, P.; Tricker, P.J.; Lopato, S.; Borisjuk, N. The Impact of Drought on Wheat Leaf Cuticle Properties. BMC Plant Biol. 2017, 17, 85. [Google Scholar] [CrossRef]
- Zeisler-Diehl, V.V.; Barthlott, W.; Schreiber, L. Plant Cuticular Waxes: Composition, Function, and Interactions with Microorganisms. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 123–138. [Google Scholar]
- de Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba Wax Uses in Food–A Review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef]
- Wang, F.; Ckurshumova, W.; Liu, J.; Fefer, M.; Pelton, R.H. Preventing the Release of Copper Chlorophyllin from Crop Spray Deposits on Hydrophobic Surfaces. J. Colloid. Interface Sci. 2021, 582, 1149–1157. [Google Scholar] [CrossRef]
- ASTM, E2044-99; (Reapproved 2019) Standard Test Method for Spreading of Liquid. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Fernández, V.; Khayet, M. Evaluation of the Surface Free Energy of Plant Surfaces: Toward Standardizing the Procedure. Front. Plant Sci. 2015, 6, 510. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, J.; Zhang, Z.; Xie, B.; Yang, Z. Performance Matching of Common Pesticides in Banana Plantations on the Surface of Banana Leaves at Different Growth Stages. Pest Manag. Sci. 2023, 79, 5116–5129. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Son, J.H.; Kim, S.-K.; Weller, C.L.; Hanna, M.A. Properties of Chitosan Films as a Function of PH and Solvent Type. J. Food Sci. 2006, 71, E119–E124. [Google Scholar] [CrossRef]
- Chen, P.; Lai, Y.; Kuo, T.; Liu, F. Improvement in the Properties of Chitosan Membranes Using Natural Organic Acid Solutions as Solvents of Chitosan Dissolution. J. Med. Biol. Eng. 2007, 27, 23–28. [Google Scholar]
- Symonds, B.L.; Thomson, N.R.; Lindsay, C.I.; Khutoryanskiy, V.V. Rainfastness of Poly (Vinyl Alcohol) Deposits on Vicia Faba Leaf Surfaces: From Laboratory-Scale Washing to Simulated Rain. ACS Appl. Mater. Interfaces 2016, 8, 14220–14230. [Google Scholar] [CrossRef]
- Zhang, Y.; Adams, M.J.; Zhang, Z.; Vidoni, O.; Leuenberger, B.H.; Achkar, J. Plasticisation of Carnauba Wax with Generally Recognised as Safe (GRAS) Additives. Polymer 2016, 86, 208–219. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]






| DD (%) | Mn (kDa) * | Ð | XPS (Atom %) | Elemental Analysis (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1s | O1s | N1s | Ca2p | Si2p | Cl2p | C | H | N | O | |||
| 93 | 78.7 | 2.15 | 70.89 | 23.05 | 4.96 | 0.78 | 0.23 | 0.09 | 40.70 | 6.85 | 7.54 | 43.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meynaud, S.; Wang, Y.; Huet, G.; Ibarboure, E.; Gardrat, C.; Coma, V. Feasibility of Genipin to Evaluate Chitosan Rainfastness for Biopesticide Applications. Int. J. Mol. Sci. 2025, 26, 1031. https://doi.org/10.3390/ijms26031031
Meynaud S, Wang Y, Huet G, Ibarboure E, Gardrat C, Coma V. Feasibility of Genipin to Evaluate Chitosan Rainfastness for Biopesticide Applications. International Journal of Molecular Sciences. 2025; 26(3):1031. https://doi.org/10.3390/ijms26031031
Chicago/Turabian StyleMeynaud, Solène, Yunhui Wang, Gael Huet, Emmanuel Ibarboure, Christian Gardrat, and Véronique Coma. 2025. "Feasibility of Genipin to Evaluate Chitosan Rainfastness for Biopesticide Applications" International Journal of Molecular Sciences 26, no. 3: 1031. https://doi.org/10.3390/ijms26031031
APA StyleMeynaud, S., Wang, Y., Huet, G., Ibarboure, E., Gardrat, C., & Coma, V. (2025). Feasibility of Genipin to Evaluate Chitosan Rainfastness for Biopesticide Applications. International Journal of Molecular Sciences, 26(3), 1031. https://doi.org/10.3390/ijms26031031







