From Cell Architecture to Mitochondrial Signaling: Role of Intermediate Filaments in Health, Aging, and Disease
Abstract
1. Introduction
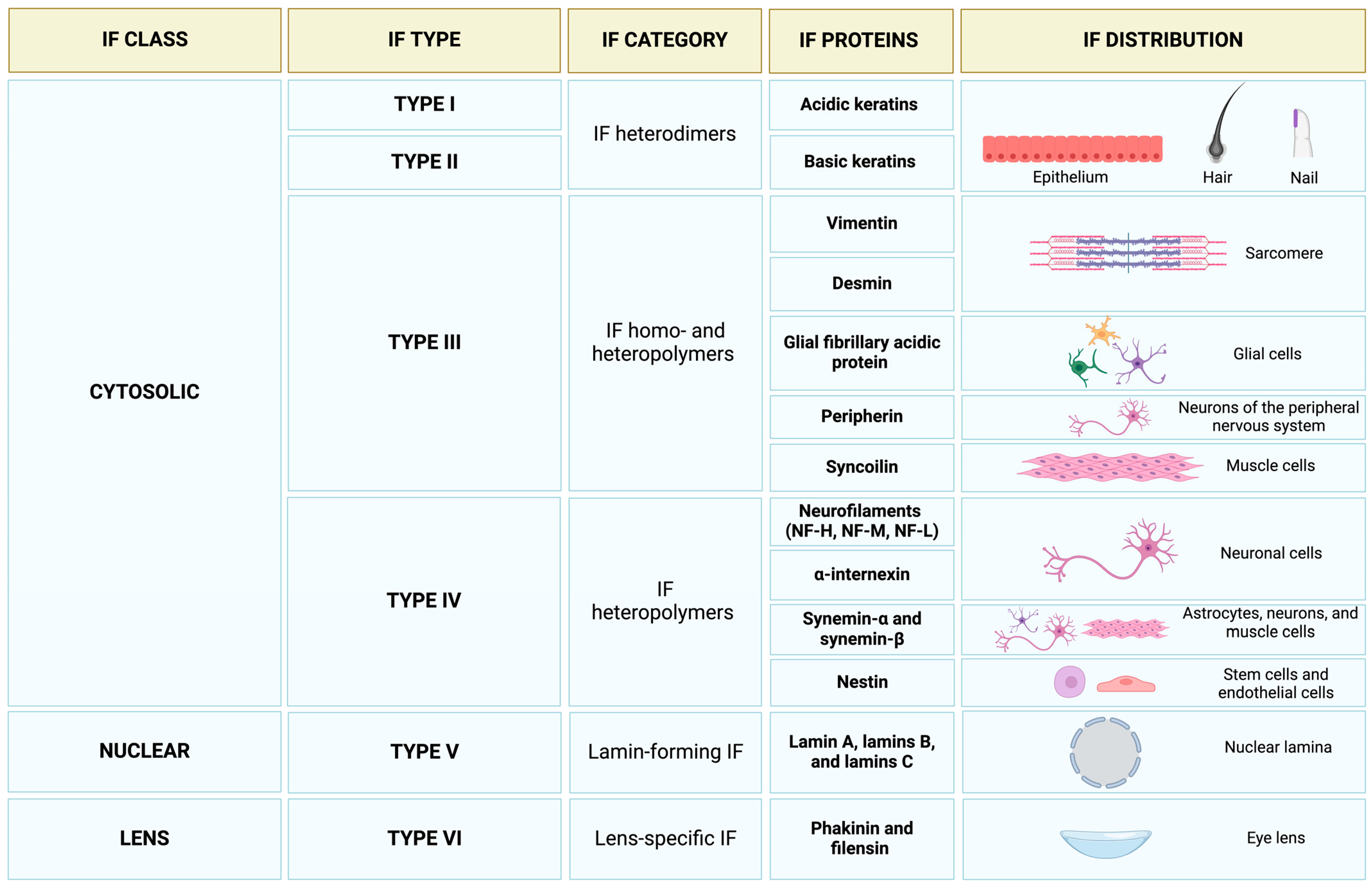
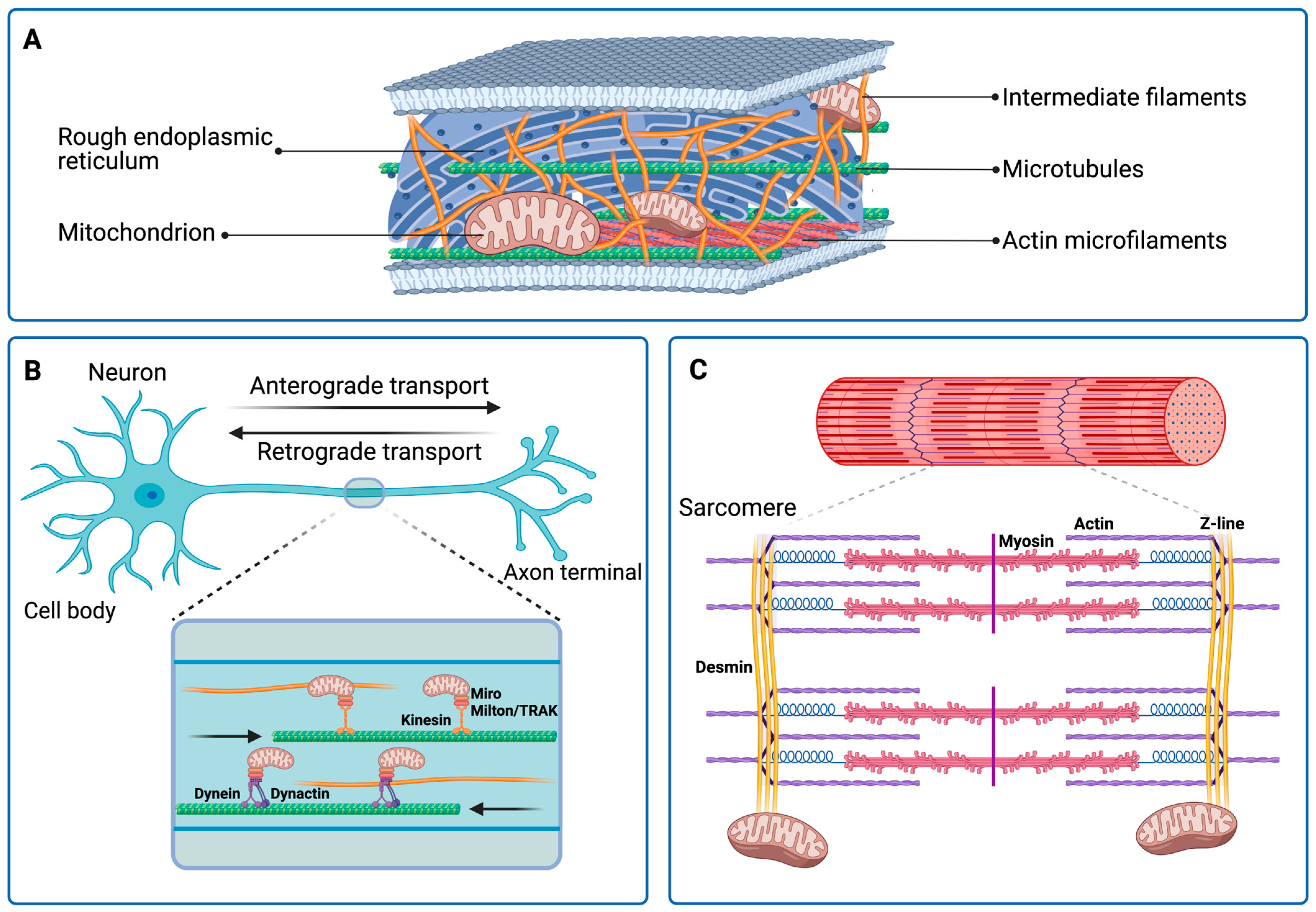
2. Intermediate Filaments in the Economy of the Cytoskeleton
3. Neurofilaments and Neurodegeneration
3.1. Amyotrophic Lateral Sclerosis
3.2. Parkinson’s Disease
3.3. Charcot–Marie–Tooth
4. Intermediate Filaments in Skeletal Muscle Aging: The Role of Vimentin
5. The Cytoskeletal Regulation of Mitochondria
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pegoraro, A.F.; Janmey, P.; Weitz, D.A. Mechanical properties of the cytoskeleton and cells. Cold Spring Harb. Perspect. Biol. 2017, 9, a022038. [Google Scholar] [CrossRef] [PubMed]
- Phillip, J.M.; Wu, P.H.; Gilkes, D.M.; Williams, W.; McGovern, S.; Daya, J.; Chen, J.; Aifuwa, I.; Lee, J.S.H.; Fan, R.; et al. Biophysical and biomolecular determination of cellular age in humans. Nat. Biomed. Eng. 2017, 1, 0093. [Google Scholar] [CrossRef] [PubMed]
- Seawright, J.W.; Sreenivasappa, H.; Gibbs, H.C.; Padgham, S.; Shin, S.Y.; Chaponnier, C.; Yeh, A.T.; Trzeciakowski, J.P.; Woodman, C.R.; Trache, A. Vascular smooth muscle contractile function declines with age in skeletal muscle feed arteries. Front. Physiol. 2018, 9, 856. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiu, H.; Trzeciakowski, J.P.; Sun, Z.; Li, Z.; Hong, Z.; Hill, M.A.; Hunter, W.C.; Vatner, D.E.; Vatner, S.F.; et al. Temporal analysis of vascular smooth muscle cell elasticity and adhesion reveals oscillation waveforms that differ with aging. Aging Cell 2012, 11, 741–750. [Google Scholar] [CrossRef] [PubMed]
- del Campo, L.; Sánchez-López, A.; Salaices, M.; von Kleeck, R.A.; Expósito, E.; González-Gómez, C.; Cussó, L.; Guzmán-Martínez, G.; Ruiz-Cabello, J.; Desco, M.; et al. Vascular smooth muscle cell-specific progerin expression in a mouse model of Hutchinson-Gilford progeria syndrome promotes arterial stiffness: Therapeutic effect of dietary nitrite. Aging Cell 2019, 18, e12936. [Google Scholar] [CrossRef]
- Qiu, H.; Zhu, Y.; Sun, Z.; Trzeciakowski, J.P.; Gansner, M.; Depre, C.; Resuello, R.R.G.; Natividad, F.F.; Hunter, W.C.; Genin, G.M.; et al. Short communication: Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ. Res. 2010, 107, 615–619. [Google Scholar] [CrossRef]
- Herum, K.M.; Choppe, J.; Kumar, A.; Engler, A.J.; McCulloch, A.D. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol. Biol. Cell 2017, 28, 1871–1882. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Manes-Gravina, E.; Spaziani, L.; Landi, F.; Bernabei, R.; Marzetti, E. Bone-muscle crosstalk: Unraveling new therapeutic targets for osteoporosis. Curr. Pharm. Des. 2017, 23, 6256–6263. [Google Scholar] [CrossRef]
- Ferrucci, L.; Baroni, M.; Ranchelli, A.; Lauretani, F.; Maggio, M.; Mecocci, P.; Ruggiero, C. Interaction between bone and muscle in older persons with mobility limitations. Curr. Pharm. Des. 2014, 20, 3178–3197. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Lozanoska-Ochser, B.; Calvani, R.; Landi, F.; Coelho-Júnior, H.J.; Picca, A. Restoring mitochondrial function and muscle satellite cell signaling: Remedies against age-related sarcopenia. Biomolecules 2024, 14, 415. [Google Scholar] [CrossRef]
- Arosio, B.; Picca, A. The biological roots of the sex-frailty paradox. Exp. Gerontol. 2024, 198, 112619. [Google Scholar] [CrossRef] [PubMed]
- Tiede-Lewis, L.A.M.; Xie, Y.; Hulbert, M.A.; Campos, R.; Dallas, M.R.; Dusevich, V.; Bonewald, L.F.; Dallas, S.L. Degeneration of the osteocyte network in the C57BL/6 mouse model of aging. Aging 2017, 9, 2187–2205. [Google Scholar] [CrossRef]
- Tiede-Lewis, L.A.M.; Dallas, S.L. Changes in the osteocyte lacunocanalicular network with aging. Bone 2019, 122, 101–113. [Google Scholar] [CrossRef]
- Glatt, V.; Canalis, E.; Stadmeyer, L.; Bouxsein, M.L. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Miner. Res. 2007, 22, 1197–1207. [Google Scholar] [CrossRef]
- Morrell, A.E.; Robinson, S.T.; Silva, M.J.; Guo, X.E. Mechanosensitive Ca2+ signaling and coordination is diminished in osteocytes of aged mice during ex vivo tibial loading. Connect. Tissue Res. 2020, 61, 389–398. [Google Scholar] [CrossRef]
- González-Bermúdez, B.; Kobayashi, H.; Abarca-Ortega, A.; Córcoles-Lucas, M.; González-Sánchez, M.; De la Fuente, M.; Guinea, G.V.; Elices, M.; Plaza, G.R. Aging is accompanied by T-cell Stiffening and reduced interstitial migration through dysfunctional nuclear organization. Immunology 2022, 167, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Racine, M.L.; Dinenno, F.A. Reduced deformability contributes to impaired deoxygenation-induced ATP release from red blood cells of older adult humans. J. Physiol. 2019, 597, 4503–4519. [Google Scholar] [CrossRef]
- Lawrence, E.J.; Boucher, E.; Mandato, C.A. Mitochondria-cytoskeleton associations in mammalian cytokinesis. Cell Div. 2016, 11, 3. [Google Scholar] [CrossRef]
- Knowles, M.K.; Guenza, M.G.; Capaldi, R.A.; Marcus, A.H. Cytoskeletal-assisted dynamics of the mitochondrial reticulum in living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 14772–14777. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Javadov, S.; Grimm, M.; Margreiter, R.; Ausserlechner, M.J.; Hagenbuchner, J. Crosstalk between mitochondria and cytoskeleton in cardiac cells. Cells 2020, 9, 222. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The role of antioxidants in the interplay between oxidative stress and senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, R.; Chimienti, G.; Picca, A.; Trisolini, L.; Latronico, T.; Liuzzi, G.M.; Pesce, V.; Leeuwenburgh, C.; Lezza, A.M.S. Resveratrol impinges on retrograde communication without inducing mitochondrial biogenesis in aged rat soleus muscle. Exp. Gerontol. 2024, 194, 112485. [Google Scholar] [CrossRef] [PubMed]
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef]
- Viedma-Poyatos, Á.; González-Jiménez, P.; Langlois, O.; Company-Marín, I.; Spickett, C.M.; Pérez-Sala, D. Protein lipoxidation: Basic concepts and emerging roles. Antioxidants 2021, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Perrot, R.; Berges, R.; Bocquet, A.; Eyer, J. Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol. Neurobiol. 2008, 38, 27–65. [Google Scholar] [CrossRef]
- Didonna, A.; Opal, P. The role of neurofilament aggregation in neurodegeneration: Lessons from rare inherited neurological disorders. Mol. Neurodegener. 2019, 14, 19. [Google Scholar] [CrossRef]
- Verde, F.; Otto, M.; Silani, V. neurofilament light chain as biomarker for amyotrophic lateral sclerosis and frontotemporal dementia. Front. Neurosci. 2021, 15, 679199. [Google Scholar] [CrossRef]
- van Spronsen, M.; Mikhaylova, M.; Lipka, J.; Schlager, M.A.; van den Heuvel, D.J.; Kuijpers, M.; Wulf, P.S.; Keijzer, N.; Demmers, J.; Kapitein, L.C.; et al. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron 2013, 77, 485–502. [Google Scholar] [CrossRef]
- López-Doménech, G.; Higgs, N.F.; Vaccaro, V.; Roš, H.; Arancibia-Cárcamo, I.L.; MacAskill, A.F.; Kittler, J.T. Loss of dendritic complexity precedes neurodegeneration in a mouse model with disrupted mitochondrial distribution in mature dendrites. Cell Rep. 2016, 17, 317–327. [Google Scholar] [CrossRef]
- Sanghvi-Shah, R.; Weber, G.F. Intermediate filaments at the junction of mechanotransduction, migration, and development. Front. Cell Dev. Biol. 2017, 5, 81. [Google Scholar] [CrossRef]
- Schweizer, J.; Bowden, P.E.; Coulombe, P.A.; Langbein, L.; Lane, E.B.; Magin, T.M.; Maltais, L.; Omary, M.B.; Parry, D.A.D.; Rogers, M.A.; et al. New consensus nomenclature for mammalian keratins. J. Cell Biol. 2006, 174, 169–174. [Google Scholar] [CrossRef]
- Dutour-Provenzano, G.; Etienne-Manneville, S. Intermediate filaments. Curr. Biol. 2021, 31, R522–R529. [Google Scholar] [CrossRef]
- Toivola, D.M.; Tao, G.Z.; Habtezion, A.; Liao, J.; Omary, M.B. Cellular integrity plus: Organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 2005, 15, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, H.; Suda, M. Seven kinds of intermediate filament networks in the cytoplasm of polarized cells: Structure and function. Acta Histochem. Cytochem. 2010, 43, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Lépinoux-Chambaud, C.; Eyer, J. Review on intermediate filaments of the nervous system and their pathological alterations. Histochem. Cell Biol. 2013, 140, 13–22. [Google Scholar] [CrossRef]
- Lund, L.M.; Kerr, J.P.; Lupinetti, J.; Zhang, Y.; Russell, M.A.; Bloch, R.J.; Bond, M. Synemin isoforms differentially organize cell junctions and desmin filaments in neonatal cardiomyocytes. FASEB J. 2012, 26, 137–148. [Google Scholar] [CrossRef]
- Michalczyk, K.; Ziman, M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol. Histopathol. 2005, 20, 665–671. [Google Scholar] [CrossRef]
- Duarte, S.; Viedma-Poyatos, Á.; Navarro-Carrasco, E.; Martínez, A.E.; Pajares, M.A.; Pérez-Sala, D. Vimentin filaments interact with the actin cortex in mitosis allowing normal cell division. Nat. Commun. 2019, 10, 4200. [Google Scholar] [CrossRef] [PubMed]
- Jokhadar, Š.Z.; Stojković, B.; Vidak, M.; Sorčan, T.; Liovic, M.; Gouveia, M.; Travasso, R.D.M.; Derganc, J. Cortical stiffness of keratinocytes measured by lateral indentation with optical tweezers. PLoS ONE 2020, 15, e0231606. [Google Scholar] [CrossRef]
- Ndiaye, A.B.; Koenderink, G.H.; Shemesh, M. Intermediate filaments in cellular mechanoresponsiveness: Mediating cytoskeletal crosstalk from membrane to nucleus and back. Front. Cell Dev. Biol. 2022, 10, 882037. [Google Scholar] [CrossRef] [PubMed]
- Stenvall, C.G.A.; Nyström, J.H.; Butler-Hallissey, C.; Jansson, T.; Heikkilä, T.R.H.; Adam, S.A.; Foisner, R.; Goldman, R.D.; Ridge, K.M.; Toivola, D.M. Cytoplasmic keratins couple with and maintain nuclear envelope integrity in colonic epithelial cells. Mol. Biol. Cell 2022, 33, ar121. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Zhou, B.; Bewersdorf, L.; Schwarz, N.; Schacht, G.M.; Boor, P.; Hoeft, K.; Hoffmann, B.; Fuchs, E.; Kramann, R.; et al. Desmoplakin maintains transcellular keratin scaffolding and protects from intestinal injury. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1181–1200. [Google Scholar] [CrossRef]
- Fuchs, E.; Weber, K. Intermediate filaments: Structure, dynamics, function, and disease. Annu. Rev. Biochem. 1994, 63, 345–382. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, K.H.; Kennedy, B.K. When lamins go bad: Nuclear structure and disease. Cell 2013, 152, 1365–1375. [Google Scholar] [CrossRef]
- Graziano, S.; Coll-Bonfill, N.; Teodoro-Castro, B.; Kuppa, S.; Jackson, J.; Shashkova, E.; Mahajan, U.; Vindigni, A.; Antony, E.; Gonzalo, S. Lamin A/C recruits ssdna protective proteins RPA and RAD51 to stalled replication forks to maintain fork stability. J. Biol. Chem. 2021, 297, 101301. [Google Scholar] [CrossRef]
- Georgatos, S.D.; Gounari, F.; Remington, S. The beaded intermediate filaments and their potential functions in eye lens. Bioessays 1994, 16, 413–418. [Google Scholar] [CrossRef]
- Song, S.; Landsbury, A.; Dahm, R.; Liu, Y.; Zhang, Q.; Quinlan, R.A. Functions of the intermediate filament cytoskeleton in the eye lens. J. Clin. Investig. 2009, 119, 1837–1848. [Google Scholar] [CrossRef]
- Viedma-Poyatos, Á.; Pajares, M.A.; Pérez-Sala, D. Type III intermediate filaments as targets and effectors of electrophiles and oxidants. Redox Biol. 2020, 36, 101582. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Sasaki, T.; Chen, Y.; Kumar, A.; Veeranna; Liem, R.K.H.; Eyer, J.; Peterson, A.C.; Julien, J.P.; et al. Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J. Neurosci. 2006, 26, 10006–10019. [Google Scholar] [CrossRef]
- Liem, R.K.H.; Messing, A. Dysfunctions of neuronal and glial intermediate filaments in disease. J. Clin. Investig. 2009, 119, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Kirkcaldie, M.T.K.; Dwyer, S.T. The third wave: Intermediate filaments in the maturing nervous system. Mol. Cell. Neurosci. 2017, 84, 68–76. [Google Scholar] [CrossRef]
- Bomont, P. The dazzling rise of neurofilaments: Physiological functions and roles as biomarkers. Curr. Opin. Cell Biol. 2021, 68, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Gentil, B.J.; Tibshirani, M.; Durham, H.D. Neurofilament dynamics and involvement in neurological disorders. Cell Tissue Res. 2015, 360, 609–620. [Google Scholar] [CrossRef]
- Kotaich, F.; Caillol, D.; Bomont, P. Neurofilaments in health and Charcot-Marie-Tooth disease. Front. Cell Dev. Biol. 2023, 11, 1275155. [Google Scholar] [CrossRef]
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef] [PubMed]
- Bomont, P. Degradation of the intermediate filament family by gigaxonin. Methods Enzymol. 2016, 569, 215–231. [Google Scholar] [CrossRef]
- Fauré, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef]
- Lachenal, G.; Pernet-Gallay, K.; Chivet, M.; Hemming, F.J.; Belly, A.; Bodon, G.; Blot, B.; Haase, G.; Goldberg, Y.; Sadoul, R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 2011, 46, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Carare, R.O.; Bernardes-Silva, M.; Newman, T.A.; Page, A.M.; Nicoll, J.A.R.; Perry, V.H.; Weller, R.O. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 2008, 34, 131–144. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Lehmann, S.; Otto, M.; Piehl, F.; Ziemssen, T.; Bittner, S.; Sormani, M.P.; Gattringer, T.; Abu-Rumeileh, S.; et al. Neurofilaments as biomarkers in neurological disorders—Towards clinical application. Nat. Rev. Neurol. 2024, 20, 269–287. [Google Scholar] [CrossRef]
- Kuhle, J.; Barro, C.; Andreasson, U.; Derfuss, T.; Lindberg, R.; Sandelius, Å.; Liman, V.; Norgren, N.; Blennow, K.; Zetterberg, H. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 2016, 54, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Truffi, M.; Garofalo, M.; Ricciardi, A.; Cotta Ramusino, M.; Perini, G.; Scaranzin, S.; Gastaldi, M.; Albasini, S.; Costa, A.; Chiavetta, V.; et al. Neurofilament-light chain quantification by Simoa and Ella in plasma from patients with dementia: A comparative study. Sci. Rep. 2023, 13, 4041. [Google Scholar] [CrossRef]
- Meeker, K.L.; Butt, O.H.; Gordon, B.A.; Fagan, A.M.; Schindler, S.E.; Morris, J.C.; Benzinger, T.L.S.; Ances, B.M. Cerebrospinal fluid neurofilament light chain is a marker of aging and white matter damage. Neurobiol. Dis. 2022, 166, 105662. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.A.; Strain, J.F.; Adedokun, A.; Wang, Q.; Preische, O.; Kuhle, J.; Flores, S.; Keefe, S.; Dincer, A.; Ances, B.M.; et al. Serum neurofilament light chain levels are associated with white matter integrity in autosomal dominant Alzheimer’s disease. Neurobiol. Dis. 2020, 142, 104960. [Google Scholar] [CrossRef]
- Jung, Y.; Damoiseaux, J.S. The potential of blood neurofilament light as a marker of neurodegeneration for Alzheimer’s disease. Brain 2024, 147, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Longinetti, E.; Fang, F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr. Opin. Neurol. 2019, 32, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chió, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 2022, 21, 480–493. [Google Scholar] [CrossRef]
- Tosolini, A.P.; Sleigh, J.N.; Surana, S.; Rhymes, E.R.; Cahalan, S.D.; Schiavo, G. BDNF-dependent modulation of axonal transport is selectively impaired in ALS. Acta Neuropathol. Commun. 2022, 10, 121. [Google Scholar] [CrossRef]
- Štetkárová, I.; Ehler, E. Diagnostics of amyotrophic lateral sclerosis: Up to date. Diagnostics 2021, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Sleigh, J.N.; Tosolini, A.P.; Gordon, D.; Devoy, A.; Fratta, P.; Fisher, E.M.C.; Talbot, K.; Schiavo, G. Mice carrying ALS mutant TDP-43, but not mutant FUS, display in vivo defects in axonal transport of signaling endosomes. Cell Rep. 2020, 30, 3655–3662.e2. [Google Scholar] [CrossRef]
- Millecamps, S.; Julien, J.P. Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 161–176. [Google Scholar] [CrossRef]
- Lefebvre-Omar, C.; Liu, E.; Dalle, C.; d’Incamps, B.L.; Bigou, S.; Daube, C.; Karpf, L.; Davenne, M.; Robil, N.; Jost Mousseau, C.; et al. Neurofilament accumulations in amyotrophic lateral sclerosis patients’ motor neurons impair axonal initial segment integrity. Cell. Mol. Life Sci. 2023, 80, 150. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.L.; Singleton, A.B.; Hernandez, D.; Ward, C.M.; Evey, C.; Sapp, P.A.; Hardy, J.; Brown, R.H.; Cleveland, D.W. Mutations in neurofilament genes are not a significant primary cause of non-SOD1-mediated amyotrophic lateral sclerosis. Neurobiol. Dis. 2006, 21, 102–109. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Miller, C.C.J. Neurofilaments and neurological disease. Bioessays 2003, 25, 346–355. [Google Scholar] [CrossRef]
- Figlewicz, D.A.; Rouleau, G.A.; Krizus, A.; Julien, J.P. Polymorphism in the multi-phosphorylation domain of the human neurofilament heavy-subunit-encoding gene. Gene 1993, 132, 297–300. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Andersen, P.M.; Nilsson, P.; Chioza, B.; Andersson, J.L.; Russ, C.; Shaw, C.E.; Powell, J.F.; Leigh, P.N. Deletions of the heavy neurofilament subunit tail in amyotrophic lateral sclerosis. Hum. Mol. Genet. 1999, 8, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Figlewicz, D.A.; Krizus, A.; Martinoli, M.G.; Meininger, V.; Dib, M.; Rouleau, G.A.; Julien, J.P. Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum. Mol. Genet. 1994, 3, 1757–1761. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, J.; Usher, P.; Slade, J.Y.; Ince, P.G.; Curtis, A.; Bushby, K.; Shaw, P.J. Novel insertion in the KSP region of the neurofilament heavy gene in amyotrophic lateral sclerosis (ALS). Neuroreport 1998, 9, 3967–3970. [Google Scholar] [CrossRef]
- Mukai, H.; Toshimori, M.; Shibata, H.; Kitagawa, M.; Shimakawa, M.; Miyahara, M.; Sunakawa, H.; Ono, Y. PKN associates and phosphorylates the head-rod domain of neurofilament protein. J. Biol. Chem. 1996, 271, 9816–9822. [Google Scholar] [CrossRef] [PubMed]
- Manser, C.; Stevenson, A.; Banner, S.; Davies, J.; Tudor, E.L.; Ono, Y.; Nigel Leigh, P.; McLoughlin, D.M.; Shaw, C.E.; Miller, C.C.J. Deregulation of PKN1 activity disrupts neurofilament organisation and axonal transport. FEBS Lett. 2008, 582, 2303–2308. [Google Scholar] [CrossRef] [PubMed]
- Shea, T.B.; Chan, W.K.H. Regulation of neurofilament dynamics by phosphorylation. Eur. J. Neurosci. 2008, 27, 1893–1901. [Google Scholar] [CrossRef]
- Ackerley, S.; Grierson, A.J.; Banner, S.; Perkinton, M.S.; Brownlees, J.; Byers, H.L.; Ward, M.; Thornhill, P.; Hussain, K.; Waby, J.S.; et al. P38α stress-activated protein kinase phosphorylates neurofilaments and is associated with neurofilament pathology in amyotrophic lateral sclerosis. Mol. Cell. Neurosci. 2004, 26, 354–364. [Google Scholar] [CrossRef]
- Chen, H.; Qian, K.; Du, Z.; Cao, J.; Petersen, A.; Liu, H.; Blackbourn, L.W.; Huang, C.L.; Errigo, A.; Yin, Y.; et al. Modeling ALS with iPSCs reveals that mutant SOD1 misregulates neurofilament balance in motor neurons. Cell Stem Cell 2014, 14, 796–809. [Google Scholar] [CrossRef] [PubMed]
- McMackin, R.; Bede, P.; Ingre, C.; Malaspina, A.; Hardiman, O. Biomarkers in amyotrophic lateral sclerosis: Current status and future prospects. Nat. Rev. Neurol. 2023, 19, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, P.; Huss, A.; Mayer, B.; Grehl, T.; Grosskreutz, J.; Borck, G.; Kuhle, J.; Lulé, D.; Meyer, T.; Oeckl, P.; et al. Diagnostic and prognostic significance of neurofilament light chain NF-L, but not progranulin and S100B, in the course of amyotrophic lateral sclerosis: Data from the German MND-Net. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 112–119. [Google Scholar] [CrossRef]
- Rossi, D.; Volanti, P.; Brambilla, L.; Colletti, T.; Spataro, R.; La Bella, V. CSF neurofilament proteins as diagnostic and prognostic biomarkers for amyotrophic lateral sclerosis. J. Neurol. 2018, 265, 510–521. [Google Scholar] [CrossRef]
- Meyer, T.; Schumann, P.; Weydt, P.; Petri, S.; Koc, Y.; Spittel, S.; Bernsen, S.; Günther, R.; Weishaupt, J.H.; Dreger, M.; et al. Neurofilament light-chain response during therapy with antisense oligonucleotide tofersen in SOD1-related ALS: Treatment experience in clinical practice. Muscle Nerve 2023, 67, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Puentes, F.; Topping, J.; Kuhle, J.; Van Der Star, B.J.; Douiri, A.; Giovannoni, G.; Baker, D.; Amor, S.; Malaspina, A. Immune reactivity to neurofilament proteins in the clinical staging of amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2014, 85, 274–278. [Google Scholar] [CrossRef]
- Singh, T.; Jiao, Y.; Ferrando, L.M.; Yablonska, S.; Li, F.; Horoszko, E.C.; Lacomis, D.; Friedlander, R.M.; Carlisle, D.L. Neuronal mitochondrial dysfunction in sporadic amyotrophic lateral sclerosis is developmentally regulated. Sci. Rep. 2021, 11, 18916. [Google Scholar] [CrossRef]
- Calió, M.L.; Henriques, E.; Siena, A.; Bertoncini, C.R.A.; Gil-Mohapel, J.; Rosenstock, T.R. Mitochondrial dysfunction, neurogenesis, and epigenetics: Putative implications for amyotrophic lateral sclerosis neurodegeneration and treatment. Front. Neurosci. 2020, 14, 679. [Google Scholar] [CrossRef]
- Liu, Y.; Dou, K.; Xue, L.; Li, X.; Xie, A. Neurofilament light as a biomarker for motor decline in Parkinson’s disease. Front. Neurosci. 2022, 16, 959261. [Google Scholar] [CrossRef] [PubMed]
- Bacioglu, M.; Maia, L.F.; Preische, O.; Schelle, J.; Apel, A.; Kaeser, S.A.; Schweighauser, M.; Eninger, T.; Lambert, M.; Pilotto, A.; et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 2016, 91, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Ashton, N.J.; Lupini, A.; Battaglio, B.; Zatti, C.; Trasciatti, C.; Gipponi, S.; Cottini, E.; Grossi, I.; Salvi, A.; et al. Plasma NfL, GFAP, amyloid, and p-Tau species as prognostic biomarkers in Parkinson’s disease. J. Neurol. 2024, 271, 7537–7546. [Google Scholar] [CrossRef]
- Urso, D.; Batzu, L.; Logroscino, G.; Ray Chaudhuri, K.; Pereira, J.B. Neurofilament light predicts worse nonmotor symptoms and depression in Parkinson’s disease. Neurobiol. Dis. 2023, 185, 106237. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Dakna, M.; Kruse, N.; Galasko, D.; Foroud, T.; Zetterberg, H.; Schade, S.; Gera, R.G.; Wang, W.; Gao, F.; et al. Validation of serum neurofilament light chain as a biomarker of Parkinson’s disease progression. Mov. Disord. 2020, 35, 1999–2008. [Google Scholar] [CrossRef]
- Halloway, S.; Desai, P.; Beck, T.; Aggarwal, N.; Agarwal, P.; Evans, D.; Rajan, K.B. Association of neurofilament light with the development and severity of Parkinson disease. Neurology 2022, 98, E2185–E2193. [Google Scholar] [CrossRef]
- Millere, E.; Rots, D.; Simrén, J.; Ashton, N.J.; Kupats, E.; Micule, I.; Priedite, V.; Kurjane, N.; Blennow, K.; Gailite, L.; et al. Plasma neurofilament light chain as a potential biomarker in Charcot-Marie-Tooth disease. Eur. J. Neurol. 2021, 28, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Rossor, A.M.; Kapoor, M.; Wellington, H.; Spaulding, E.; Sleigh, J.N.; Burgess, R.W.; Laura, M.; Zetterberg, H.; Bacha, A.; Wu, X.; et al. A Longitudinal and cross-sectional study of plasma neurofilament light chain concentration in Charcot-Marie-Tooth disease. J. Peripher. Nerv. Syst. 2022, 27, 50–57. [Google Scholar] [CrossRef]
- Pisciotta, C.; Bai, Y.; Brennan, K.M.; Wu, X.; Grider, T.; Feely, S.; Wang, S.; Moore, S.; Siskind, C.; Gonzalez, M.; et al. Reduced neurofilament expression in cutaneous nerve fibers of patients with CMT2E. Neurology 2015, 85, 228–234. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The epidemiology of Parkinson’s disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. (Schol. Ed.) 2014, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Janelidze, S.; Hall, S.; Magdalinou, N.; Lees, A.J.; Andreasson, U.; Norgren, N.; Linder, J.; Forsgren, L.; Constantinescu, R.; et al. Blood-based NfL: A biomarker for differential diagnosis of Parkinsonian disorder. Neurology 2017, 88, 930–937. [Google Scholar] [CrossRef]
- Parnetti, L.; Gaetani, L.; Eusebi, P.; Paciotti, S.; Hansson, O.; El-Agnaf, O.; Mollenhauer, B.; Blennow, K.; Calabresi, P. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019, 18, 573–586. [Google Scholar] [CrossRef]
- Hall, S.; Öhrfelt, A.; Constantinescu, R.; Andreasson, U.; Surova, Y.; Bostrom, F.; Nilsson, C.; Håkan, W.; Decraemer, H.; Någga, K.; et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or Parkinsonian disorders. Arch. Neurol. 2012, 69, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, W.W.; Waligorska, T.; Shen, J.; Tropea, T.F.; Siderowf, A.; Weintraub, D.; Grossman, M.; Irwin, D.; Wolk, D.A.; Xie, S.X.; et al. Neurofilament light chain as a biomarker for cognitive decline in Parkinson disease. Mov. Disord. 2021, 36, 2945–2950. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.H.; Ma, L.Z.; Liu, J.Y.; Ou, Y.N.; Zhao, B.; Ma, Y.H.; Tan, L. Cerebrospinal fluid neurofilament dynamic profiles predict cognitive progression in individuals with de novo Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 1061096. [Google Scholar] [CrossRef]
- Buhmann, C.; Lezius, S.; Pötter-Nerger, M.; Gerloff, C.; Kuhle, J.; Choe, C.U. Age-adjusted serum neurofilament predicts cognitive decline in Parkinson’s disease (MARK-PD). Mov. Disord. 2022, 37, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Z.; Zhang, C.; Wang, H.; Ma, Y.H.; Shen, X.N.; Wang, J.; Tan, L.; Dong, Q.; Yu, J.T. Serum neurofilament dynamics predicts cognitive progression in de novo Parkinson’s disease. J. Parkinson’s Dis. 2021, 11, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.L.; Pollak, T.A.; Heslegrave, A.; Hye, A.; Batzu, L.; Rota, S.; Trivedi, D.; Nicholson, T.R.; Ffytche, D.; Zetterberg, H.; et al. Plasma neurofilament light and P-Tau181 and risk of psychosis in Parkinson’s disease. J. Parkinson’s Dis. 2022, 12, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Berciano, J.; García, A.; Gallardo, E.; Peeters, K.; Pelayo-Negro, A.L.; Álvarez-Paradelo, S.; Gazulla, J.; Martínez-Tames, M.; Infante, J.; Jordanova, A. Intermediate Charcot-Marie-Tooth disease: An electrophysiological reappraisal and systematic review. J. Neurol. 2017, 264, 1655–1677. [Google Scholar] [CrossRef] [PubMed]
- Morena, J.; Gupta, A.; Hoyle, J.C. Charcot-Marie-Tooth: From molecules to therapy. Int. J. Mol. Sci. 2019, 20, 3419. [Google Scholar] [CrossRef]
- Gentil, B.J.; Minotti, S.; Beange, M.; Baloh, R.H.; Julien, J.; Durham, H.D. Normal role of the low-molecular-weight neurofilament protein in mitochondrial dynamics and disruption in Charcot-Marie-Tooth disease. FASEB J. 2012, 26, 1194–1203. [Google Scholar] [CrossRef]
- Zhao, J.; Brown, K.; Liem, R.K.H. Abnormal neurofilament inclusions and segregations in dorsal root ganglia of a Charcot-Marie-Tooth type 2E mouse model. PLoS ONE 2017, 12, e0180038. [Google Scholar] [CrossRef]
- Yum, S.W.; Zhang, J.; Mo, K.; Li, J.; Scherer, S.S. A novel recessive Nefl mutation causes a severe, early-onset axonal neuropathy. Ann. Neurol. 2009, 66, 759–770. [Google Scholar] [CrossRef]
- Stone, E.J.; Kolb, S.J.; Brown, A. A review and analysis of the clinical literature on Charcot-Marie-Tooth disease caused by mutations in neurofilament protein L. Cytoskeleton 2021, 78, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Brownlees, J.; Ackerley, S.; Grierson, A.J.; Jacobsen, N.J.O.; Shea, K.; Anderton, B.H.; Leigh, P.N.; Shaw, C.E.; Miller, C.C.J. Charcot-Marie-Tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum. Mol. Genet. 2002, 11, 2837–2844. [Google Scholar] [CrossRef]
- Sasaki, T.; Gotow, T.; Shiozaki, M.; Sakaue, F.; Saito, T.; Julien, J.P.; Uchiyama, Y.; Hisanaga, S.I. Aggregate formation and phosphorylation of neurofilament-L Pro22 Charcot-Marie-Tooth disease mutants. Hum. Mol. Genet. 2006, 15, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.T.; Tsolova, K.N.; Watson, A.J.; Khal, S.K.; Green, J.R.; Li, D.; Hu, J.; Soderblom, E.J.; Chi, J.T.; Evans, C.S.; et al. O-GlcNAcylation regulates neurofilament-light assembly and function and is perturbed by Charcot-Marie-Tooth disease mutations. Nat. Commun. 2023, 14, 6558. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Numakura, C.; Saito, K.; Koide, H.; Oka, N.; Honma, A.; Kishikawa, Y.; Hayasaka, K. Neurofilament light chain polypeptide gene mutations in Charcot-Marie-Tooth disease: Nonsense mutation probably causes a recessive phenotype. J. Hum. Genet. 2009, 54, 94–97. [Google Scholar] [CrossRef]
- Horga, A.; Laurà, M.; Jaunmuktane, Z.; Jerath, N.U.; Gonzalez, M.A.; Polke, J.M.; Poh, R.; Blake, J.C.; Liu, Y.T.; Wiethoff, S.; et al. Genetic and clinical characteristics of NEFL-related Charcot-Marie-Tooth disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 575–585. [Google Scholar] [CrossRef]
- Saveri, P.; De Luca, M.; Nisi, V.; Pisciotta, C.; Romano, R.; Piscosquito, G.; Reilly, M.M.; Polke, J.M.; Cavallaro, T.; Maria Fabrizi, G.; et al. Charcot-Marie-Tooth Type 2B: A new phenotype associated with a novel RAB7A mutation and inhibited EGFR degradation. Cells 2020, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Setlere, S.; Grosmane, A.; Kurjane, N.; Gailite, L.; Rots, D.; Blennow, K.; Zetterberg, H.; Kenina, V. Plasma neurofilament light chain level is not a biomarker of Charcot-Marie-Tooth disease progression: Results of 3-year follow-up study. Eur. J. Neurol. 2023, 30, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.E.; Tokuyama, T.; Anzai, T.; Chanthra, N.; Uosaki, H. Sarcomere maturation: Function acquisition, molecular mechanism, and interplay with other organelles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 20210325. [Google Scholar] [CrossRef] [PubMed]
- Granger, B.L.; Lazarides, E. Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell 1979, 18, 1053–1063. [Google Scholar] [CrossRef]
- Gard, D.L.; Lazarides, E. The synthesis and distribution of desmin and vimentin during myogenesis in vitro. Cell 1980, 19, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Tokuyasu, K.T.; Maher, P.A.; Singer, S.J. Distributions of vimentin and desmin in developing chick myotubes in vivo. II. Immunoelectron microscopic study. J. Cell Biol. 1985, 100, 1157–1166. [Google Scholar] [CrossRef]
- Solomon, T.; Rajendran, M.; Rostovtseva, T.; Hool, L. How cytoskeletal proteins regulate mitochondrial energetics in cell physiology and diseases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 20210324. [Google Scholar] [CrossRef]
- Fernández Casafuz, A.B.; De Rossi, M.C.; Bruno, L. Mitochondrial cellular organization and shape fluctuations are differentially modulated by cytoskeletal networks. Sci. Rep. 2023, 13, 4065. [Google Scholar] [CrossRef] [PubMed]
- Eibauer, M.; Weber, M.S.; Kronenberg-Tenga, R.; Beales, C.T.; Boujemaa-Paterski, R.; Turgay, Y.; Sivagurunathan, S.; Kraxner, J.; Köster, S.; Goldman, R.D.; et al. Vimentin filaments integrate low-complexity domains in a complex helical structure. Nat. Struct. Mol. Biol. 2024, 31, 939–949. [Google Scholar] [CrossRef]
- Pérez-Sala, D.; Oeste, C.L.; Martínez, A.E.; Carrasco, M.J.; Garzón, B.; Cañada, F.J. Vimentin filament organization and stress sensing depend on its single cysteine residue and zinc binding. Nat. Commun. 2015, 6, 7287. [Google Scholar] [CrossRef] [PubMed]
- Viedma-Poyatos, Á.; de Pablo, Y.; Pekny, M.; Pérez-Sala, D. The cysteine residue of glial fibrillary acidic protein is a critical target for lipoxidation and required for efficient network organization. Free Radic. Biol. Med. 2018, 120, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Moneo-Corcuera, D.; Viedma-Poyatos, Á.; Stamatakis, K.; Pérez-Sala, D. Desmin Reorganization by stimuli inducing oxidative stress and electrophiles: Role of its single cysteine residue. Antioxidants 2023, 12, 1703. [Google Scholar] [CrossRef]
- Quinlan, R.A.; Franke, W.W. Heteropolymer filaments of vimentin and desmin in vascular smooth muscle tissue and cultured baby hamster kidney cells demonstrated by chemical crosslinking. Proc. Natl. Acad. Sci. USA 1982, 79, 3452–3456. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, R.A.; Franke, W.W. Molecular interactions in intermediate-sized filaments revealed by chemical cross-linking. heteropolymers of vimentin and glial filament protein in cultured human glioma cells. Eur. J. Biochem. 1983, 132, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Kaus-Drobek, M.; Mücke, N.; Szczepanowski, R.H.; Wedig, T.; Czarnocki-Cieciura, M.; Polakowska, M.; Herrmann, H.; Wysłouch-Cieszyńska, A.; Dadlez, M. Vimentin S-glutathionylation at Cys328 inhibits filament elongation and induces severing of mature filaments in vitro. FEBS J. 2020, 287, 5304–5322. [Google Scholar] [CrossRef]
- Mónico, A.; Duarte, S.; Pajares, M.A.; Pérez-Sala, D. Vimentin disruption by lipoxidation and electrophiles: Role of the cysteine residue and filament dynamics. Redox Biol. 2019, 23, 101098. [Google Scholar] [CrossRef]
- Rogers, K.R.; Herrmann, H.; Franke, W.W. Characterization of disulfide crosslink formation of human vimentin at the dimer, tetramer, and intermediate filament levels. J. Struct. Biol. 1996, 117, 55–69. [Google Scholar] [CrossRef]
- Day, N.J.; Kelly, S.S.; Lui, L.Y.; Mansfield, T.A.; Gaffrey, M.J.; Trejo, J.B.; Sagendorf, T.J.; Attah, I.K.; Moore, R.J.; Douglas, C.M.; et al. Signatures of cysteine oxidation on muscle structural and contractile proteins are associated with physical performance and muscle function in older adults: Study of muscle, mobility and aging (SOMMA). Aging Cell 2024, 23, e14094. [Google Scholar] [CrossRef] [PubMed]
- Dutka, T.L.; Mollica, J.P.; Lamboley, C.R.; Weerakkody, V.C.; Greening, D.W.; Posterino, G.S.; Murphy, R.M.; Lamb, G.D. S-nitrosylation and S-glutathionylation of Cys134 on troponin I have opposing competitive actions on Ca2+ sensitivity in rat fast-twitch muscle fibers. Am. J. Physiol. Cell Physiol. 2017, 312, C316–C327. [Google Scholar] [CrossRef] [PubMed]
- Giganti, D.; Yan, K.; Badilla, C.L.; Fernandez, J.M.; Alegre-Cebollada, J. Disulfide isomerization reactions in titin immunoglobulin domains enable a mode of protein elasticity. Nat. Commun. 2018, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R.; Ferran, B.; Oh, A.; Croteau, D.; Shao, D.; Han, J.; Pimentel, D.R.; Bachschmid, M.M. Redox regulation via glutaredoxin-1 and protein S-glutathionylation. Antioxid. Redox Signal. 2020, 32, 677–700. [Google Scholar] [CrossRef] [PubMed]
- Alcock, L.J.; Perkins, M.V.; Chalker, J.M. Chemical methods for mapping cysteine oxidation. Chem. Soc. Rev. 2018, 47, 231–268. [Google Scholar] [CrossRef]
- Devarie Baez, N.O.; Reisz, J.A.; Furdui, C.M. Mass spectrometry in studies of protein thiol chemistry and signaling: Opportunities and caveats. Free Radic. Biol. Med. 2015, 80, 191–211. [Google Scholar] [CrossRef]
- Shi, Y.; Carroll, K.S. Activity-based sensing for site-specific proteomic analysis of cysteine oxidation. Acc. Chem. Res. 2020, 53, 20–31. [Google Scholar] [CrossRef]
- González-Jiménez, P.; Duarte, S.; Martínez, A.E.; Navarro-Carrasco, E.; Lalioti, V.; Pajares, M.A.; Pérez-Sala, D. Vimentin single cysteine residue acts as a tunable sensor for network organization and as a key for actin remodeling in response to oxidants and electrophiles. Redox Biol. 2023, 64, 102756. [Google Scholar] [CrossRef]
- Pekovic, V.; Gibbs-Seymour, I.; Markiewicz, E.; Alzoghaibi, F.; Benham, A.M.; Edwards, R.; Wenhert, M.; von Zglinicki, T.; Hutchison, C.J. Conserved cysteine residues in the mammalian lamin a tail are essential for cellular responses to ROS generation. Aging Cell 2011, 10, 1067–1079. [Google Scholar] [CrossRef]
- Unoki, T.; Akiyama, M.; Kumagai, Y. Nrf2 activation and its coordination with the protective defense systems in response to electrophilic stress. Int. J. Mol. Sci. 2020, 21, 545. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Landi, F.; Coelho-Júnior, H.J.; Picca, A. Mitochondrial quality control processes at the crossroads of cell death and survival: Mechanisms and signaling pathways. Int. J. Mol. Sci. 2024, 25, 7305. [Google Scholar] [CrossRef]
- Picca, A.; Mankowski, R.T.; Burman, J.L.; Donisi, L.; Kim, J.-S.S.; Marzetti, E.; Leeuwenburgh, C. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat. Rev. Cardiol. 2018, 15, 543–554. [Google Scholar] [CrossRef]
- Picca, A.; Faitg, J.; Auwerx, J.; Ferrucci, L.; D’Amico, D. Mitophagy in human health, ageing and disease. Nat. Metab. 2023, 5, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Anesti, V.; Scorrano, L. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim. Biophys. Acta 2006, 1757, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.S.; Holzbaur, E.L. Mitochondrial-cytoskeletal interactions: Dynamic associations that facilitate network function and remodeling. Curr. Opin. Physiol. 2018, 3, 94–100. [Google Scholar] [CrossRef]
- Kurd, D.D.; Saxton, W.M. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in drosophila. Genetics 1996, 144, 1075–1085. [Google Scholar] [CrossRef]
- Pilling, A.D.; Horiuchi, D.; Lively, C.M.; Saxton, W.M. Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell 2006, 17, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Macleod, G.T.; Wellington, A.; Hu, F.; Panchumarthi, S.; Schoenfield, M.; Marin, L.; Charlton, M.P.; Atwood, H.L.; Zinsmaier, K.E. The GTPase DMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron 2005, 47, 379–393. [Google Scholar] [CrossRef]
- Stowers, R.S.; Megeath, L.J.; Górska-Andrzejak, J.; Meinertzhagen, I.A.; Schwarz, T.L. Axonal transport of mitochondria to synapses depends on Milton, a novel Drosophila protein. Neuron 2002, 36, 1063–1077. [Google Scholar] [CrossRef]
- Cardanho-Ramos, C.; Faria-Pereira, A.; Morais, V.A. Orchestrating mitochondria in neurons: Cytoskeleton as the conductor. Cytoskeleton 2020, 77, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Lezi, E.; Swerdlow, R.H. Mitochondria in neurodegeneration. Adv. Exp. Med. Biol. 2012, 942, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Alberti, P.; Semperboni, S.; Cavaletti, G.; Scuteri, A. Neurons: The interplay between cytoskeleton, ion channels/transporters and mitochondria. Cells 2022, 11, 2499. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.D.; Shen, K.; Sapio, M.R.; Glenn, T.D.; Talbot, W.S.; Marlow, F.L. Unique function of kinesin Kif5A in localization of mitochondria in axons. J. Neurosci. 2014, 34, 14717–14732. [Google Scholar] [CrossRef] [PubMed]
- Guillaud, L.; El-Agamy, S.E.; Otsuki, M.; Terenzio, M. Anterograde axonal transport in neuronal homeostasis and disease. Front. Mol. Neurosci. 2020, 13, 556175. [Google Scholar] [CrossRef]
- Brenner, D.; Yilmaz, R.; Müller, K.; Grehl, T.; Petri, S.; Meyer, T.; Grosskreutz, J.; Weydt, P.; Ruf, W.; Neuwirth, C.; et al. Hot-Spot KIF5A mutations cause familial ALS. Brain 2018, 141, 688–697. [Google Scholar] [CrossRef]
- Saez-Atienzar, S.; Dalgard, C.L.; Ding, J.; Chiò, A.; Alba, C.; Hupalo, D.N.; Wilkerson, M.D.; Bowser, R.; Pioro, E.P.; Bedlack, R.; et al. Identification of a pathogenic intronic KIF5A mutation in an ALS-FTD kindred. Neurology 2020, 95, 1015–1018. [Google Scholar] [CrossRef]
- Filosto, M.; Piccinelli, S.C.; Palmieri, I.; Necchini, N.; Valente, M.; Zanella, I.; Biasiotto, G.; Di Lorenzo, D.; Cereda, C.; Padovani, A. A novel mutation in the stalk domain of KIF5A causes a slowly progressive atypical motor syndrome. J. Clin. Med. 2018, 8, 17. [Google Scholar] [CrossRef]
- Nicolas, A.; Kenna, K.; Renton, A.E.; Ticozzi, N.; Faghri, F.; Chia, R.; Dominov, J.A.; Kenna, B.J.; Nalls, M.A.; Keagle, P.; et al. Genome-wide analyses identify KIF5A as a novel ALS Gene. Neuron 2018, 97, 1268–1283.e6. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Xu, H.; Fu, Y.; Qian, T.; Bo, D.; Lu, Y.X.; Xiong, Y.; Wan, J.; Zhang, X.; et al. Dync1h1 Mutation causes proprioceptive sensory neuron loss and impaired retrograde axonal transport of dorsal root ganglion neurons. CNS Neurosci. Ther. 2016, 22, 593–601. [Google Scholar] [CrossRef]
- Chen, X.J.; Levedakou, E.N.; Millen, K.J.; Wollmann, R.L.; Soliven, B.; Popko, B. Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic dynein heavy chain 1 gene. J. Neurosci. 2007, 27, 14515–14524. [Google Scholar] [CrossRef]
- Weedon, M.N.; Hastings, R.; Caswell, R.; Xie, W.; Paszkiewicz, K.; Antoniadi, T.; Williams, M.; King, C.; Greenhalgh, L.; Newbury-Ecob, R.; et al. Exome sequencing identifies a DYNC1H1 mutation in a large pedigree with dominant axonal Charcot-Marie-Tooth disease. Am. J. Hum. Genet. 2011, 89, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, F.; West, P.K.; Brennan, S.; Petrović, B.; Hooshmand, K.; Akkari, P.A.; Keon, M.; Guennewig, B. New perspectives on cytoskeletal dysregulation and mitochondrial mislocalization in amyotrophic lateral sclerosis. Transl. Neurodegener. 2021, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Shaltouki, A.; Gonzalez, A.; Bettencourt da Cruz, A.; Burbulla, L.; St Lawrence, E.; Schüle, B.; Krainc, D.; Palmer, T.; Wang, X. Functional impairment in Miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson’s disease. Cell Stem Cell 2016, 19, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, S.; Sipos, J.; Thau-Habermann, N.; Körner, S.; Rath, K.J.; Dengler, R.; Petri, S. Altered expression of DJ-1 and PINK1 in sporadic ALS and in the SOD1(G93A) ALS mouse model. J. Neuropathol. Exp. Neurol. 2013, 72, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Duan, Y.; Qin, C.; Li, J.C.; Duan, G.; Deng, X.; Ni, J.; Cao, X.; Xiang, K.; Tian, K.; et al. Distinct multilevel misregulations of Parkin and PINK1 revealed in cell and animal models of TDP-43 proteinopathy. Cell Death Dis. 2018, 9, 953. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, J.; Wang, P.; Yang, M.; Chen, X.; Zhu, L.; Liu, J.; Lu, B.; Shen, Y.; Fushimi, K.; et al. PINK1 and Parkin are genetic modifiers for FUS-induced neurodegeneration. Hum. Mol. Genet. 2016, 25, 5059–5068. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, C.; Sharif, B.; Weinreb, A.; Swaim, G.; Hao, H.; Yogev, S.; Watanabe, S.; Hammarlund, M. Polarized localization of kinesin-1 and RIC-7 drives axonal mitochondria anterograde transport. J. Cell Biol. 2024, 223, e202305105. [Google Scholar] [CrossRef] [PubMed]
- Van Steenbergen, V.; Lavoie-Cardinal, F.; Kazwiny, Y.; Decet, M.; Martens, T.; Verstreken, P.; Boesmans, W.; De Koninck, P.; Vanden Berghe, P. Nano-positioning and tubulin conformation contribute to axonal transport regulation of mitochondria along microtubules. Proc. Natl. Acad. Sci. USA 2022, 119, e2203499119. [Google Scholar] [CrossRef]
- Manor, U.; Bartholomew, S.; Golani, G.; Christenson, E.; Kozlov, M.; Higgs, H.; Spudich, J.; Lippincott-Schwartz, J. A Mitochondria-anchored isoform of the actin-nucleating spire protein regulates mitochondrial division. eLife 2015, 4, e08828. [Google Scholar] [CrossRef]
- Friedman, J.R.; Lackner, L.L.; West, M.; DiBenedetto, J.R.; Nunnari, J.; Voeltz, G.K. ER tubules mark sites of mitochondrial division. Science 2011, 334, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Griparic, L.; Shurland, D.L.; Van der Bliek, A.M. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 2001, 12, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.; Haney, M.S.; Couthouis, J.; Morgens, D.W.; Benjamin, A.; Wu, K.; Ousey, J.; Fang, S.; Finer, S.; Bassik, M.C.; et al. Genome-wide synthetic lethal CRISPR screen identifies FIS1 as a genetic interactor of ALS-linked C9ORF72. Brain Res. 2020, 1728, 146601. [Google Scholar] [CrossRef]
- Shen, Q.; Yamano, K.; Head, B.P.; Kawajiri, S.; Cheung, J.T.M.; Wang, C.; Cho, J.H.; Hattori, N.; Youle, R.J.; Van Der Bliek, A.M. Mutations in Fis1 disrupt orderly disposal of defective mitochondria. Mol. Biol. Cell 2014, 25, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Heissler, S.M.; Sellers, J.R. Various themes of myosin regulation. J. Mol. Biol. 2016, 428, 1927–1946. [Google Scholar] [CrossRef]
- Quintero, O.A.; DiVito, M.M.; Adikes, R.C.; Kortan, M.B.; Case, L.B.; Lier, A.J.; Panaretos, N.S.; Slater, S.Q.; Rengarajan, M.; Feliu, M.; et al. Human Myo19 is a novel myosin that associates with mitochondria. Curr. Biol. 2009, 19, 2008–2013. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ma, X.N.; Zhang, H.M.; Ji, H.H.; Ding, H.; Zhang, J.; Luo, D.; Sun, Y.; Li, X.D. Mouse myosin-19 is a plus-end-directed, high-duty ratio molecular motor. J. Biol. Chem. 2014, 289, 18535–18548. [Google Scholar] [CrossRef] [PubMed]
- López-Doménech, G.; Covill-Cooke, C.; Ivankovic, D.; Halff, E.F.; Sheehan, D.F.; Norkett, R.; Birsa, N.; Kittler, J.T. Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution. EMBO J. 2018, 37, 321–336. [Google Scholar] [CrossRef]
- Cipriani, S.; Guerrero-Valero, M.; Tozza, S.; Zhao, E.; Vollmer, V.; Beijer, D.; Danzi, M.; Rivellini, C.; Lazarevic, D.; Pipitone, G.B.; et al. Mutations in MYO9B are associated with Charcot-Marie-Tooth disease type 2 neuropathies and isolated optic atrophy. Eur. J. Neurol. 2023, 30, 511–526. [Google Scholar] [CrossRef]
| Condition | Main Pathological Trait | Circulating Marker(s) | Reference(s) |
|---|---|---|---|
| ALS | Motor neuron disorder | ↑ NF-L, p-NF-H, anti-NF-L | [87,89] |
| PD | Death of dopaminergic neurons in the substantia nigra pars compacta | ↑ GFAP, α-synuclein, NF-L | [92,93,94,95,96,97] |
| CMT1B | Demyelinating peripheral neuropathy | ↑ NF-L | [98,99] |
| CMT1X | Demyelinating peripheral neuropathy | ↑ NF-L | [98,99] |
| CMT2A | Axonal peripheral neuropathy | ↑ NF-L | [98,99] |
| CMT2E | Axonal peripheral neuropathy | ↓ NF-L | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzetti, E.; Di Lorenzo, R.; Calvani, R.; Pesce, V.; Landi, F.; Coelho-Júnior, H.J.; Picca, A. From Cell Architecture to Mitochondrial Signaling: Role of Intermediate Filaments in Health, Aging, and Disease. Int. J. Mol. Sci. 2025, 26, 1100. https://doi.org/10.3390/ijms26031100
Marzetti E, Di Lorenzo R, Calvani R, Pesce V, Landi F, Coelho-Júnior HJ, Picca A. From Cell Architecture to Mitochondrial Signaling: Role of Intermediate Filaments in Health, Aging, and Disease. International Journal of Molecular Sciences. 2025; 26(3):1100. https://doi.org/10.3390/ijms26031100
Chicago/Turabian StyleMarzetti, Emanuele, Rosa Di Lorenzo, Riccardo Calvani, Vito Pesce, Francesco Landi, Hélio José Coelho-Júnior, and Anna Picca. 2025. "From Cell Architecture to Mitochondrial Signaling: Role of Intermediate Filaments in Health, Aging, and Disease" International Journal of Molecular Sciences 26, no. 3: 1100. https://doi.org/10.3390/ijms26031100
APA StyleMarzetti, E., Di Lorenzo, R., Calvani, R., Pesce, V., Landi, F., Coelho-Júnior, H. J., & Picca, A. (2025). From Cell Architecture to Mitochondrial Signaling: Role of Intermediate Filaments in Health, Aging, and Disease. International Journal of Molecular Sciences, 26(3), 1100. https://doi.org/10.3390/ijms26031100









