Genome-Wide Characterization of Wholly Disordered Proteins in Arabidopsis
Abstract
:1. Introduction
2. Results
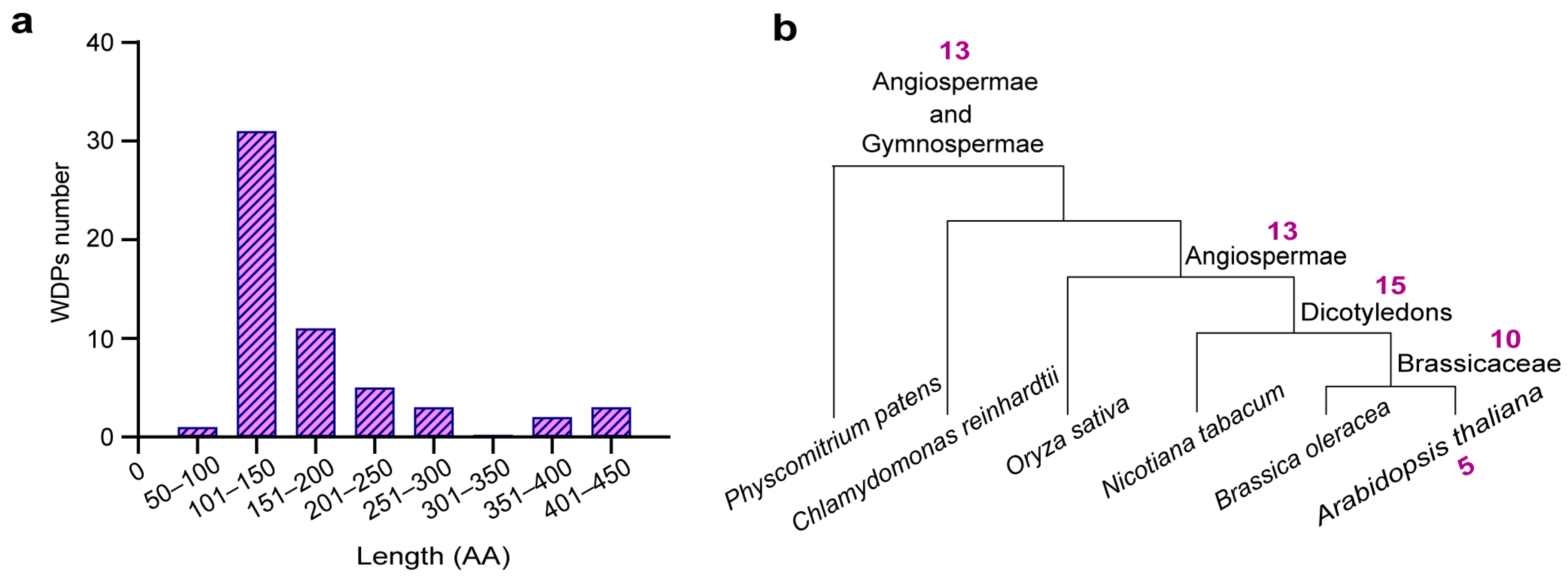
2.1. Identifing WDPs from the Uniprot Proteme
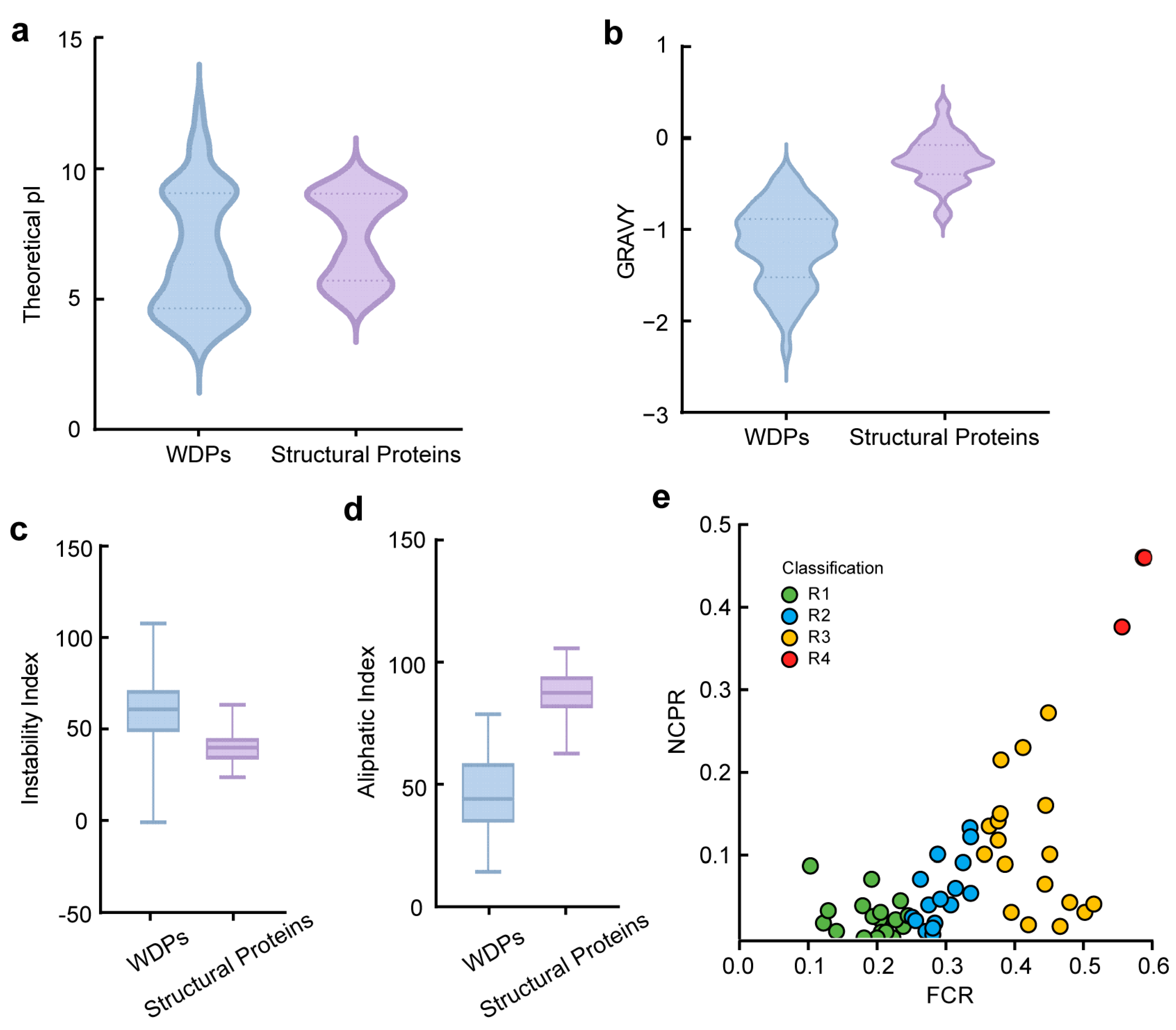
2.2. Comparison of Physicochemical Properties Between WDPs and Structural Proteins
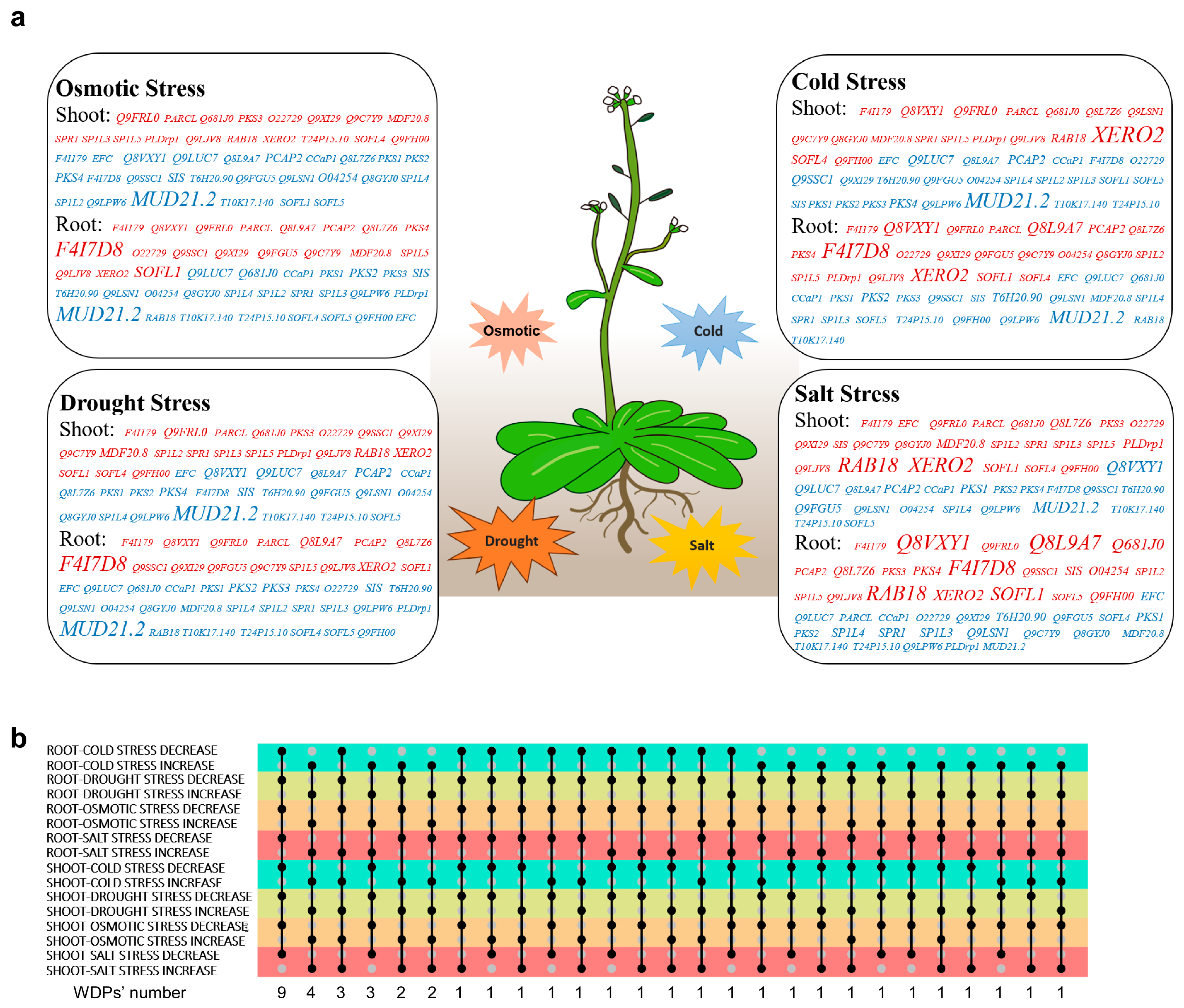
2.3. Expression Profiles of the WDPs in Response to Abiotic Stresses
2.4. Functional Prediction of WDPs
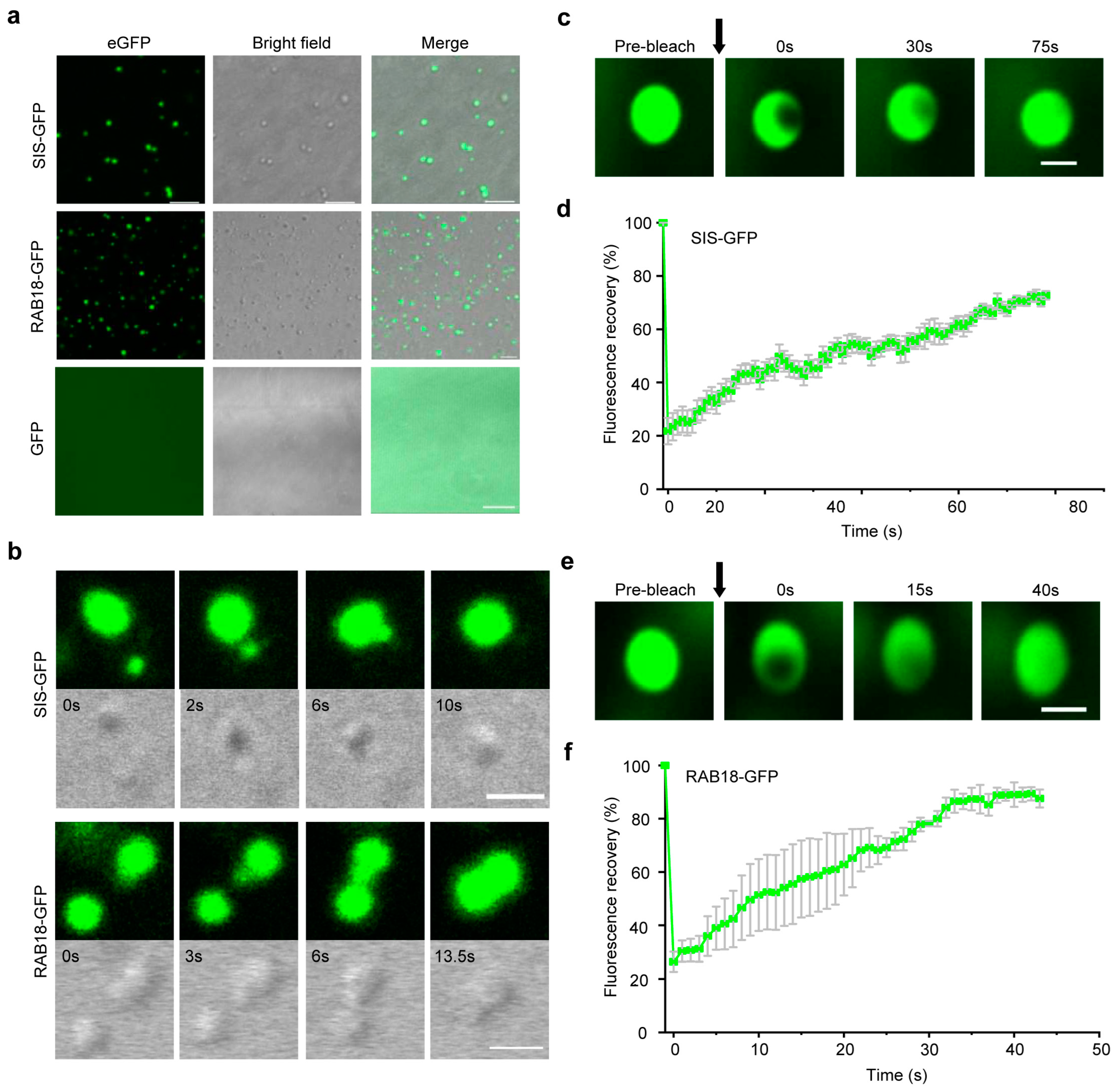
2.5. WDPs Tend to Undergo Liquid-Liquid Phase Separation
3. Discussion
4. Materials and Methods
4.1. Screening of Arabidopsis WDPs
4.2. Classification Method of Arabidopsis WDPs
4.3. Phylogenetic Analysis of Arabidopsis WDPs
4.4. Physicochemical Properties Analysis of WDPs in Arabidopsis
4.5. Calculation of FCR and NCPR Values of WDPs
4.6. Analysis of Expression Patterns and Function Prediction of WDPs
4.7. In Vitro Phase Separation Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lichtenthaler, F.W. 100 Years “Schlüssel-Schloss-Prinzip”: What Made Emil Fischer Use this Analogy? Angew. Chem. Int. Ed. Engl. 1995, 33, 2364–2374. [Google Scholar] [CrossRef]
- Uversky, V.N.; Dunker, A.K. Understanding protein non-folding. Biochim. Biophys. Acta 2010, 1804, 1231–1264. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Natively unfolded proteins: A point where biology waits for physics. Protein. Sci. 2002, 11, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Dyson, H.J.; Wright, P.E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 2005, 6, 197–208. [Google Scholar] [CrossRef]
- Eliezer, D. Biophysical characterization of intrinsically disordered proteins. Curr. Opin. Struct. Biol. 2009, 19, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.R.; Zweckstetter, M.; Huang, J.R.; Blackledge, M. Exploring free-energy landscapes of intrinsically disordered proteins at atomic resolution using NMR spectroscopy. Chem. Rev. 2014, 114, 6632–6660. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Intrinsically unstructured proteins: Re-assessing the protein structure-function paradigm. J. Mol. Biol. 1999, 293, 321–331. [Google Scholar] [CrossRef]
- Nussinov, R.; Liu, Y.; Zhang, W.; Jang, H. Protein conformational ensembles in function: Roles and mechanisms. RSC Chem. Biol. 2023, 4, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Pajkos, M.; Erdős, G.; Dosztányi, Z. The Origin of Discrepancies between Predictions and Annotations in Intrinsically Disordered Proteins. Biomolecules 2023, 13, 1442. [Google Scholar] [CrossRef]
- Bhattarai, A.; Emerson, I.A. Dynamic conformational flexibility and molecular interactions of intrinsically disordered proteins. J. Biosci. 2020, 45, 29. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, X.; Wu, X.; Zhang, M. Formation of biological condensates via phase separation: Characteristics, analytical methods, and physiological implications. J. Biol. Chem. 2019, 294, 14823–14835. [Google Scholar] [CrossRef] [PubMed]
- Bondos, S.E.; Dunker, A.K.; Uversky, V.N. Intrinsically disordered proteins play diverse roles in cell signaling. Cell Commun. Signal. 2022, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Galea, C.A.; High, A.A.; Obenauer, J.C.; Mishra, A.; Park, C.; Punta, M.; Schlessinger, A.; Ma, J.; Rost, B.; Slaughter, C.A.; et al. Large-Scale Analysis of Thermostable, Mammalian Proteins Provides Insights into the Intrinsically Disordered Proteome. J. Proteome Res. 2009, 8, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. The Mysterious Unfoldome: Structureless, Underappreciated, Yet Vital Part of any Given Proteome. J. Biomed. Biotechnol. 2010, 2010, 568068. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, M.; Patel, K.; Gadhe, L.; Sawner, A.S.; Kadu, P.; Datta, D.; Mukherjee, S.; Ray, S.; Navalkar, A.; Maiti, S.; et al. Intermolecular interactions underlie protein/peptide phase separation irrespective of sequence and structure at crowded milieu. Nat. Commun. 2023, 14, 6199. [Google Scholar] [CrossRef]
- Biesaga, M.; Frigole-Vivas, M.; Salvatella, X. Intrinsically disordered proteins and biomolecular condensates as drug targets. Curr. Opin. Chem. Biol. 2021, 62, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, D.M.; Mittasch, M.; Gomes, B.F.; Klein, I.A.; Murcko, M.A. Modulating biomolecular condensates: A novel approach to drug discovery. Nat. Rev. Drug Discov. 2022, 21, 841–862. [Google Scholar] [CrossRef]
- Zhang, F.; Biswas, M.; Massah, S.; Lee, J.; Lingadahalli, S.; Wong, S.; Wells, C.; Foo, J.; Khan, N.; Morin, H.; et al. Dynamic phase separation of the androgen receptor and its coactivators key to regulate gene expression. Nucleic Acids. Res. 2023, 51, 99–116. [Google Scholar] [CrossRef]
- Majewski, J.; Jones, E.M.; Vander, Z.C.; Biernat, J.; Mandelkow, E.; Chi, E.Y. Lipid membrane templated misfolding and self-assembly of intrinsically disordered tau protein. Sci. Rep. 2020, 10, 13324. [Google Scholar] [CrossRef]
- Murray, A.; Yan, L.; Gibson, J.M.; Liu, J.; Eliezer, D.; Lippens, G.; Zhang, F.; Linhardt, R.J.; Zhao, J.; Wang, C. Proline-Rich Region II (PRR2) Plays an Important Role in Tau–Glycan Interaction: An NMR Study. Biomolecules 2022, 12, 1573. [Google Scholar] [CrossRef]
- Hsiao, A. Protein Disorder in Plant Stress Adaptation: From Late Embryogenesis Abundant to Other Intrinsically Disordered Proteins. Int. J. Mol. Sci. 2024, 25, 1178. [Google Scholar] [CrossRef]
- Zavaliev, R.; Mohan, R.; Chen, T.; Dong, X. Formation of NPR1 Condensates Promotes Cell Survival during the Plant Immune Response. Cell 2020, 182, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Brocca, S.; Grandori, R.; Longhi, S.; Uversky, V. Liquid-Liquid Phase Separation by Intrinsically Disordered Protein Regions of Viruses: Roles in Viral Life Cycle and Control of Virus-Host Interactions. Int. J. Mol. Sci. 2020, 21, 9045. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, M.; Hincha, D.K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef]
- Du, D.; Zhang, Q.; Cheng, T.; Pan, H.; Yang, W.; Sun, L. Genome-wide identification and analysis of late embryogenesis abundant (LEA) genes in Prunus mume. Mol. Biol. Rep. 2013, 40, 1937–1946. [Google Scholar] [CrossRef]
- Cao, J.; Li, X. Identification and phylogenetic analysis of late embryogenesis abundant proteins family in tomato (Solanum lycopersicum). Planta 2015, 241, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.T.; Kato, M.; Lin, Y.; Thurber, K.R.; Hung, I.; Mcknight, S.L.; Tycko, R. Structure of FUS Protein Fibrils and its Relevance to Self-Assembly and Phase Separation of Low-Complexity Domains. Cell 2017, 171, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Artur, M.A.S.; Zhao, T.; Ligterink, W.; Schranz, E.; Hilhorst, H.W.M. Dissecting the Genomic Diversification of Late Embryogenesis Abundant (LEA) Protein Gene Families in Plants. Genome Biol. Evol. 2019, 11, 459–471. [Google Scholar] [CrossRef]
- Sivamani, E.; Bahieldin, A.; Wraith, J.M.; Al-Niemi, T.; Dyer, W.E.; Ho, T.D.; Qu, R. Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci. 2000, 155, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Duan, X.; Wang, B.; Hong, B.; Ho, T.; Wu, R. Expression of a Late Embryogenesis Abundant Protein Gene, HVA1, from Barley Confers Tolerance to Water Deficit and Salt Stress in Transgenic Rice. Plant Physiol. 1996, 110, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Liu, B. A comprehensive review and comparison of existing computational methods for intrinsically disordered protein and region prediction. Brief. Bioinform. 2019, 20, 330–346. [Google Scholar] [CrossRef]
- Necci, M.; Piovesan, D.; Tosatto, S. Critical assessment of protein intrinsic disorder prediction. Nat. Methods 2021, 18, 472–481. [Google Scholar] [CrossRef]
- Theillet, F.X.; Kalmar, L.; Tompa, P.; Han, K.H.; Selenko, P.; Dunker, A.K.; Daughdrill, G.W.; Uversky, V.N. The alphabet of intrinsic disorder: I. Act like a Pro: On the abundance and roles of proline residues in intrinsically disordered proteins. Intrinsically Disord. Proteins 2013, 1, e24360. [Google Scholar] [CrossRef] [PubMed]
- Roesgaard, M.A.; Lundsgaard, J.E.; Newcombe, E.A.; Jacobsen, N.L.; Pesce, F.; Tranchant, E.E.; Lindemose, S.; Prestel, A.; Hartmann-Petersen, R.; Lindorff-Larsen, K.; et al. Deciphering the Alphabet of Disorder-Glu and Asp Act Differently on Local but Not Global Properties. Biomolecules 2022, 12, 1426. [Google Scholar] [CrossRef]
- Quaglia, F.; Mészáros, B.; Salladini, E.; Hatos, A.; Pancsa, R.; Chemes, L.B.; Pajkos, M.; Lazar, T.; Peña-Díaz, S.; Santos, J.; et al. DisProt in 2022: Improved quality and accessibility of protein intrinsic disorder annotation. Nucleic Acids Res. 2022, 50, D480–D487. [Google Scholar] [CrossRef] [PubMed]
- Aspromonte, M.C.; Nugnes, M.V.; Quaglia, F.; Bouharoua, A.; Sagris, V.; Promponas, V.J.; Chasapi, A.; Fichó, E.; Balatti, G.E.; Parisi, G.; et al. DisProt in 2024: Improving function annotation of intrinsically disordered proteins. Nucleic Acids Res. 2024, 52, D434–D441. [Google Scholar] [CrossRef]
- Wells, M.; Tidow, H.; Rutherford, T.J.; Markwick, P.; Jensen, M.R.; Mylonas, E.; Svergun, D.I.; Blackledge, M.; Fersht, A.R. Structure of tumor suppressor p53 and its intrinsically disordered N-terminal transactivation domain. Proc. Natl. Acad. Sci. USA 2008, 105, 5762–5767. [Google Scholar] [CrossRef]
- Uversky, V.N.; Santambrogio, C.; Brocca, S.; Grandori, R. Length-dependent compaction of intrinsically disordered proteins. FEBS Lett. 2012, 586, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.W.; Holehouse, A.S.; Peran, I.; Farag, M.; Incicco, J.J.; Bremer, A.; Grace, C.R.; Soranno, A.; Pappu, R.V.; Mittag, T. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 2020, 367, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P. Intrinsically unstructured proteins evolve by repeat expansion. Bioessays 2003, 25, 847–855. [Google Scholar] [CrossRef]
- Verbiest, M.A.; Delucchi, M.; Bilgin, S.T.; Anisimova, M. Beyond Microsatellite Instability: Intrinsic Disorder as a Potential Link Between Protein Short Tandem Repeats and Cancer. Front. Bioinform. 2021, 1, 685844. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Gamage, D.G.; Gunaratne, A.; Periyannan, G.R.; Russell, T.G. Applicability of Instability Index for in vitro Protein Stability Prediction. Protein Pept. Lett. 2019, 26, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Marzinek, J.K.; Jefferies, D.; Bond, P.J.; Harryson, P.; Wohland, T. The disordered plant dehydrin Lti30 protects the membrane during water-related stress by cross-linking lipids. J. Biol. Chem. 2019, 294, 6468–6482. [Google Scholar] [CrossRef]
- Mouillon, J.M.; Eriksson, S.K.; Harryson, P. Mimicking the plant cell interior under water stress by macromolecular crowding: Disordered dehydrin proteins are highly resistant to structural collapse. Plant Physiol. 2008, 148, 1925–1937. [Google Scholar] [CrossRef]
- Kaur, A.; Pati, P.K.; Pati, A.M.; Nagpal, A.K. Physico-chemical characterization and topological analysis of pathogenesis-related proteins from Arabidopsis thaliana and Oryza sativa using in-silico approaches. PLoS ONE 2020, 15, e0239836. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Chen, J.L.; Zhang, G.Y.; Li, Y.; Duan, H.Z.; Luo, S.Z.; Chen, Y.X. Intrinsically Disordered Protein Condensate-Modified Surface for Mitigation of Biofouling and Foreign Body Response. J. Am. Chem. Soc. 2022, 144, 12147–12157. [Google Scholar] [CrossRef]
- Campen, A.; Williams, R.M.; Brown, C.J.; Meng, J.; Uversky, V.N.; Dunker, A.K. TOP-IDP-scale: A new amino acid scale measuring propensity for intrinsic disorder. Protein Pept. Lett. 2008, 15, 956–963. [Google Scholar] [CrossRef]
- van der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Pappu, R.V. Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues. Proc. Natl. Acad. Sci. USA 2013, 110, 13392–13397. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Longhi, S.; Grandori, R.; Brocca, S. Relevance of Electrostatic Charges in Compactness, Aggregation, and Phase Separation of Intrinsically Disordered Proteins. Int. J. Mol. Sci. 2020, 21, 6208. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.B.; Sanabria, H.; Smirnova, T.; Bowen, M.E.; Weninger, K.R. Spontaneous Switching among Conformational Ensembles in Intrinsically Disordered Proteins. Biomolecules 2019, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.H.; Crick, S.L.; Vitalis, A.; Chicoine, C.L.; Pappu, R.V. Net charge per residue modulates conformational ensembles of intrinsically disordered proteins. Proc. Natl. Acad. Sci. USA 2010, 107, 8183–8188. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.G.; Teilum, K.; Kragelund, B.B. Behaviour of intrinsically disordered proteins in protein-protein complexes with an emphasis on fuzziness. Cell. Mol. Life Sci. 2017, 74, 3175–3183. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar]
- Henry, C.; John, G.P.; Pan, R.; Bartlett, M.K.; Fletcher, L.R.; Scoffoni, C.; Sack, L. A stomatal safety-efficiency trade-off constrains responses to leaf dehydration. Nat. Commun. 2019, 10, 3398. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chen, Y.; Qian, Y.; Chan, Z. Low Temperature-Induced 30 (LTI30) positively regulates drought stress resistance in Arabidopsis: Effect on abscisic acid sensitivity and hydrogen peroxide accumulation. Front. Plant Sci. 2015, 6, 893. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant. J. 2021, 105, 307–321. [Google Scholar] [CrossRef] [PubMed]
- de Carbonnel, M.; Davis, P.; Roelfsema, M.R.; Inoue, S.; Schepens, I.; Lariguet, P.; Geisler, M.; Shimazaki, K.; Hangarter, R.; Fankhauser, C. The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol. 2010, 152, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.T.; Fan, S.X.; Wang, J.J.; An, Y.; Guo, Z.Q.; Li, K.; Liu, J.X. The plasma membrane-associated transcription factor NAC091 regulates unfolded protein response in Arabidopsis thaliana. Plant Sci. 2023, 334, 111777. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Liu, J.; Jia, A.; Xu, C.; Deng, X.W.; He, G. Natural variation in the transcription factor REPLUMLESS contributes to both disease resistance and plant growth in Arabidopsis. Plant Commun. 2022, 3, 100351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, N.; Wen, W.; Liu, S.; Mo, X.; An, Y.; Zhou, P. Genome-wide identification and analysis of LEA_2 gene family in alfalfa (Medicago sativa L.) Under aluminum stress. Front. Plant Sci. 2022, 13, 976160. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, C.Y.; Qi, Y.; Park, S.; Gibson, S.I. SIS8, a putative mitogen-activated protein kinase kinase kinase, regulates sugar-resistant seedling development in Arabidopsis. Plant. J. 2014, 77, 577–588. [Google Scholar] [CrossRef]
- Bohnsack, M.T.; Sloan, K.E. Modifications in small nuclear RNAs and their roles in spliceosome assembly and function. Biol. Chem. 2018, 399, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Ide, Y.; Tomioka, R.; Ouchi, Y.; Kamiya, T.; Maeshima, M. Transcriptional Induction of Two Genes for CCaPs, Novel Cytosolic Proteins, in Arabidopsis thaliana in the Dark. Plant Cell Physiol. 2007, 48, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Gao, J.; Li, W.; Liu, J.X. CCaP1/CCaP2/CCaP3 interact with plasma membrane H+-ATPases and promote thermo-responsive growth by regulating cell wall modification in Arabidopsis. Plant Commun. 2024, 5, 100880. [Google Scholar] [CrossRef] [PubMed]
- Ostendorp, A.; Ostendorp, S.; Zhou, Y.; Chaudron, Z.; Wolffram, L.; Rombi, K.; von Pein, L.; Falke, S.; Jeffries, C.M.; Svergun, D.I.; et al. Intrinsically disordered plant protein PARCL colocalizes with RNA in phase-separated condensates whose formation can be regulated by mutating the PLD. J. Biol. Chem. 2022, 298, 102631. [Google Scholar] [CrossRef] [PubMed]
- Pignatta, D.; Novitzky, K.; Satyaki, P.; Gehring, M. A variably imprinted epiallele impacts seed development. PLoS Genet. 2018, 14, e1007469. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, D.C.; Lukowitz, W.; Somerville, C.R. Stomatal development and pattern controlled by a MAPKK kinase. Science 2004, 304, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ngwenyama, N.; Liu, Y.; Walker, J.C.; Zhang, S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 2007, 19, 63–73. [Google Scholar] [CrossRef]
- Alex, S.H.; Birthe, B.K. The molecular basis for cellular function of intrinsically disordered protein regions. Nat. Rev. Mol. Cell Biol. 2024, 25, 187–211. [Google Scholar]
- Welin, B.V.; Olson, A.; Nylander, M.; Palva, E.T. Characterization and differential expression of dhn/lea/rab-like genes during cold acclimation and drought stress in Arabidopsis thaliana. Plant Mol. Biol. 1994, 26, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Abzalimov, R.R.; Frimpong, A.K.; Kaltashov, I.A. Detection and characterization of large-scale protein conformational transitions in solution using charge-state distribution analysis in ESI-MS. Methods Mol. Biol. 2012, 896, 365–373. [Google Scholar] [PubMed]
- Harris, B.J.; Clark, J.W.; Schrempf, D.; Szollosi, G.J.; Donoghue, P.; Hetherington, A.M.; Williams, T.A. Divergent evolutionary trajectories of bryophytes and tracheophytes from a complex common ancestor of land plants. Nat. Ecol. Evol. 2022, 6, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Hnisz, D.; Shrinivas, K.; Young, R.A.; Chakraborty, A.K.; Sharp, P.A. A phase separation model predicts key features of transcriptional control. Cell 2017, 3, 13–23. [Google Scholar] [CrossRef]
- Wang, N.; Liu, C. Implications of liquid–liquid phase separation in plant chromatin organization and transcriptional control. Curr. Opin. Gemet. Dev. 2019, 4, 59–65. [Google Scholar] [CrossRef]
- Israel, M.L.; Nicolás, E.F.; Itzell, E.H.; Monika, C. Plant Stress Granules: Trends and Beyond. Front. Plant Sci. 2021, 8, 722643. [Google Scholar]
- Wang, H.Y.; Yang, Q.H.; Zhang, D.; Lu, Y.Y.; Wang, Y.C.; Pan, Y.J.; Qiu, Y.P.; Men, Y.F.; Yan, W.; Xiao, Z.N.; et al. A cytoplasmic osmosensing mechanism mediatedby molecular crowding–sensitive DCP5. Science 2024, 11, 510. [Google Scholar]
- Hellinger, R.; Sigurdsson, A.; Wu, W.; Romanova, E.V.; Li, L.; Sweedler, J.V.; Sussmuth, R.D.; Gruber, C.W. Peptidomics. Nat. Rev. Methods Primers 2023, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Holehouse, A.S.; Das, R.K.; Ahad, J.N.; Richardson, M.O.; Pappu, R.V. CIDER: Resources to Analyze Sequence-Ensemble Relationships of Intrinsically Disordered Proteins. Biophys. J. 2017, 112, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. The most important thing is the tail: Multitudinous functionalities of intrinsically disordered protein termini. FEBS Lett. 2013, 587, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Kilian, J.; Whitehead, D.; Horak, J.; Wanke, D.; Weinl, S.; Batistic, O.; D’Angelo, C.; Bornberg-Bauer, E.; Kudla, J.; Harter, K. The AtGenExpress global stress expression data set: Protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2007, 50, 347–363. [Google Scholar] [CrossRef] [PubMed]

| Protein ID | Gene ID | Gene Name | Description |
|---|---|---|---|
| F4I179 | AT1G15840 | / | Hypothetical protein |
| Q9M3G8 | AT4G11430 | / | Hydroxyproline-rich glycoprotein family protein |
| O82760 | AT4G23110 | EFC | Early flowering and curly leaves |
| Q8VXY1 | AT5G28630 | / | Glycine-rich protein |
| Q9FRL0 | AT1G75190 | / | Hypothetical protein |
| Q9LUC7 | AT3G14670 | / | Hypothetical protein |
| Q9C7W1 | AT1G64370 | PARCL | Phloem associated rna chaperone-like |
| Q8L9A7 | AT4G27580 | / | Phosphatidylinositol transfer SFH5-like protein |
| Q681J0 | AT5G54095 | / | Proteoglycan-like protein |
| Q9SCK5 | AT3G49540 | T9C5.130 | Hypothetical protein |
| Q9LU05 | AT5G44610 | PCAP2 | Plasma membrane-associated cation-binding protein 2 |
| A0A1P8ASG6 | AT1G04105 | / | Hypothetical protein |
| Q9SXE9 | AT1G62480 | CCaP1 | Vacuolar calcium-binding protein-like protein |
| Q8L7Z6 | AT3G54680 | / | Proteophosphoglycan-like protein |
| Q5XV49 | AT5G05965 | / | Cell wall RBR3-like protein |
| Q84WZ5 | AT2G39855 | / | Plant/protein |
| Q9SWI1 | AT2G02950 | PKS1 | Protein phytochrome kinase substrate |
| Q9M9T4 | AT1G14280 | PKS2 | |
| Q8GXS8 | AT1G18810 | PKS3 | |
| Q9FYE2 | AT5G04190 | PKS4 | |
| Q9XI29 | AT1G15400 | MASS2 | MAPK substrates in the stomatal |
| Q9SSC1 | AT1G80180 | MASS1 | |
| Q3E9A8 | AT5G20100 | MASS3 | |
| Q9LZM9 | AT5G02020 | SIS | Salt induced serine rich |
| Q9STG0 | AT3G46880 | T6H20.90 | Hypothetical protein |
| Q9FGU5 | AT5G59080 | / | Hypothetical protein |
| Q1G3N4 | AT3G55646 | / | Tprxl |
| Q9LSN1 | AT3G17160 | / | Hypothetical protein |
| Q9C7Y9 | AT1G47970 | / | Nucleolin |
| O04254 | AT4G02140 | / | Hypothetical protein |
| Q8GYJ0 | AT4G22320 | BCL7A | BCL-domain homolog |
| Q9FKA5 | AT5G39570 | PLDrp1 | PLD regulated protein |
| Q9LJV8 | AT3G29075 | / | Glycine-rich protein |
| Q9FM74 | AT5G55640 | MDF20.8 | Na-translocating NADH-quinone reductase subunit A |
| Q9LF22 | AT5G15600 | SP1L4 | Protein SPIRAL1-like |
| B3H4F1 | AT1G26355 | SP1L1 | |
| Q9LE54 | AT1G69230 | SP1L2 | |
| Q9SJW3 | AT2G03680 | SPR1 | |
| Q8LGD1 | AT4G23496 | SP1L5 | |
| Q9S7P8 | AT3G02180 | SP1L3 | |
| Q9FL02 | AT5G66780 | MUD21.2 | Late embryogenesis abundant protein |
| P30185 | AT5G66400 | RAB18 | Dehydrin protein family |
| P42758 | AT3G50970 | Xero 2 | Dehydrin protein family |
| Q9M2Q5 | AT3G57930 | T10K17.140 | Rho gtpase-activating gaco-like protein |
| O48526 | AT2G42190 | T24P15.10 | |
| B6IDH8 | AT1G58460 | SOFL6 | Hypothetical protein |
| Q67YG7 | AT1G26210 | SOFL1 | Protein SOB FIVE-LIKE |
| Q9CA45 | AT1G68870 | SOFL2 | Protein SOB FIVE-LIKE |
| F4J6N7 | AT3G30580 | SOFL3 | Hypothetical protein |
| Q9FKQ9 | AT5G38790 | SOFL4 | Hypothetical protein |
| Q8L9K4 | AT4G33800 | SOFL5 | Hypothetical protein |
| Q9LEZ1 | AT1G58460 | SOB5 | Hypothetical protein |
| Q9FH00 | AT5G42290 | / | Transcription activator-like protein |
| Q9LPW6 | AT1G12830 | / | Nucleolin |
| F4I7D8 | AT1G11125 | / | Hypothetical protein |
| O22729 | AT1G61170 | / | Hypothetical protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, W.; Zhao, L.; Yang, H.; Yang, X.; Bai, Y.; Xue, X.; Wang, D.; Han, S. Genome-Wide Characterization of Wholly Disordered Proteins in Arabidopsis. Int. J. Mol. Sci. 2025, 26, 1117. https://doi.org/10.3390/ijms26031117
Long W, Zhao L, Yang H, Yang X, Bai Y, Xue X, Wang D, Han S. Genome-Wide Characterization of Wholly Disordered Proteins in Arabidopsis. International Journal of Molecular Sciences. 2025; 26(3):1117. https://doi.org/10.3390/ijms26031117
Chicago/Turabian StyleLong, Wenfen, Liang Zhao, Huimin Yang, Xinyi Yang, Yulong Bai, Xiuhua Xue, Doudou Wang, and Shengcheng Han. 2025. "Genome-Wide Characterization of Wholly Disordered Proteins in Arabidopsis" International Journal of Molecular Sciences 26, no. 3: 1117. https://doi.org/10.3390/ijms26031117
APA StyleLong, W., Zhao, L., Yang, H., Yang, X., Bai, Y., Xue, X., Wang, D., & Han, S. (2025). Genome-Wide Characterization of Wholly Disordered Proteins in Arabidopsis. International Journal of Molecular Sciences, 26(3), 1117. https://doi.org/10.3390/ijms26031117






