Complete Polar Lipid Profile of Kefir Beverage by Hydrophilic Interaction Liquid Chromatography with HRMS and Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
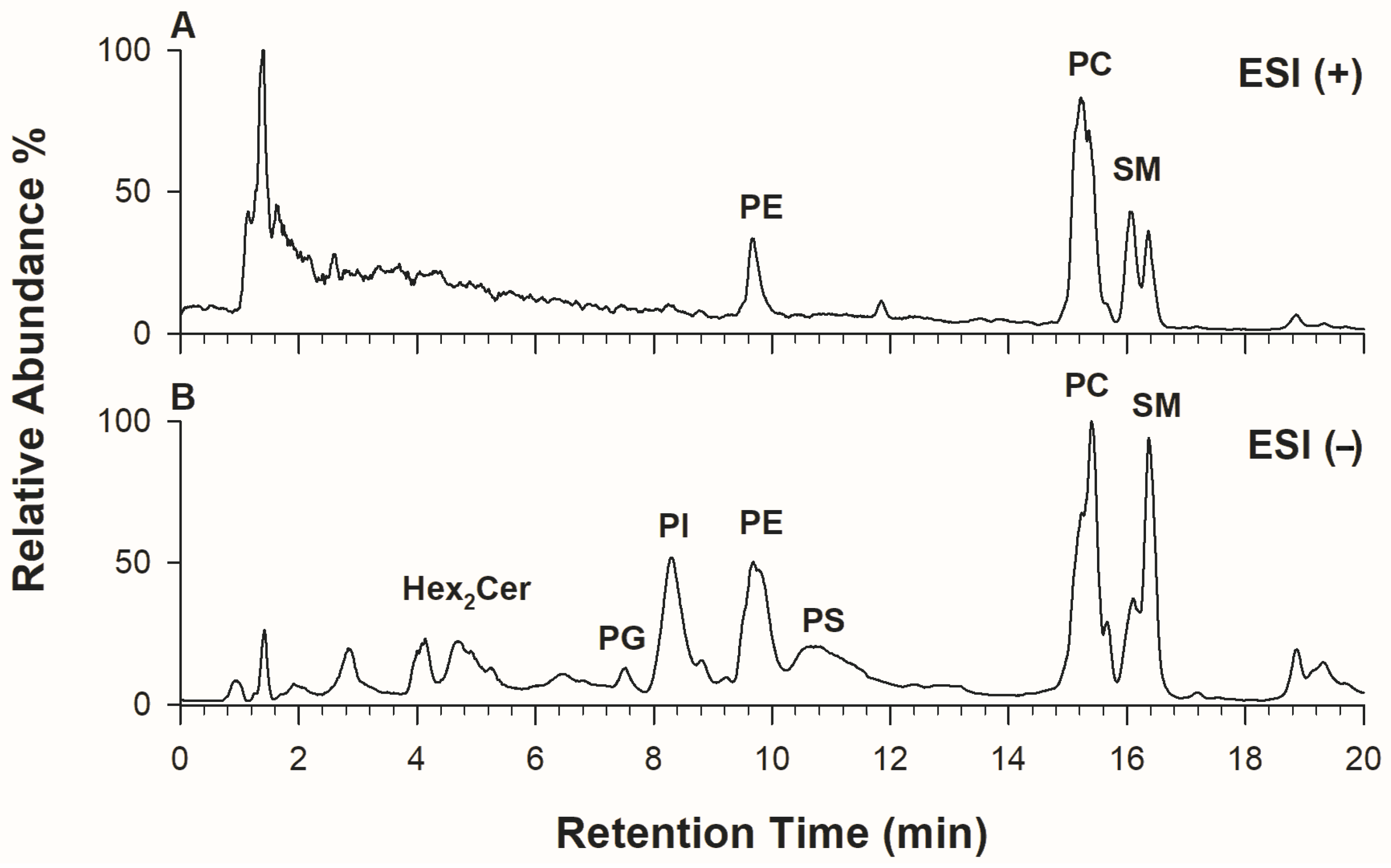
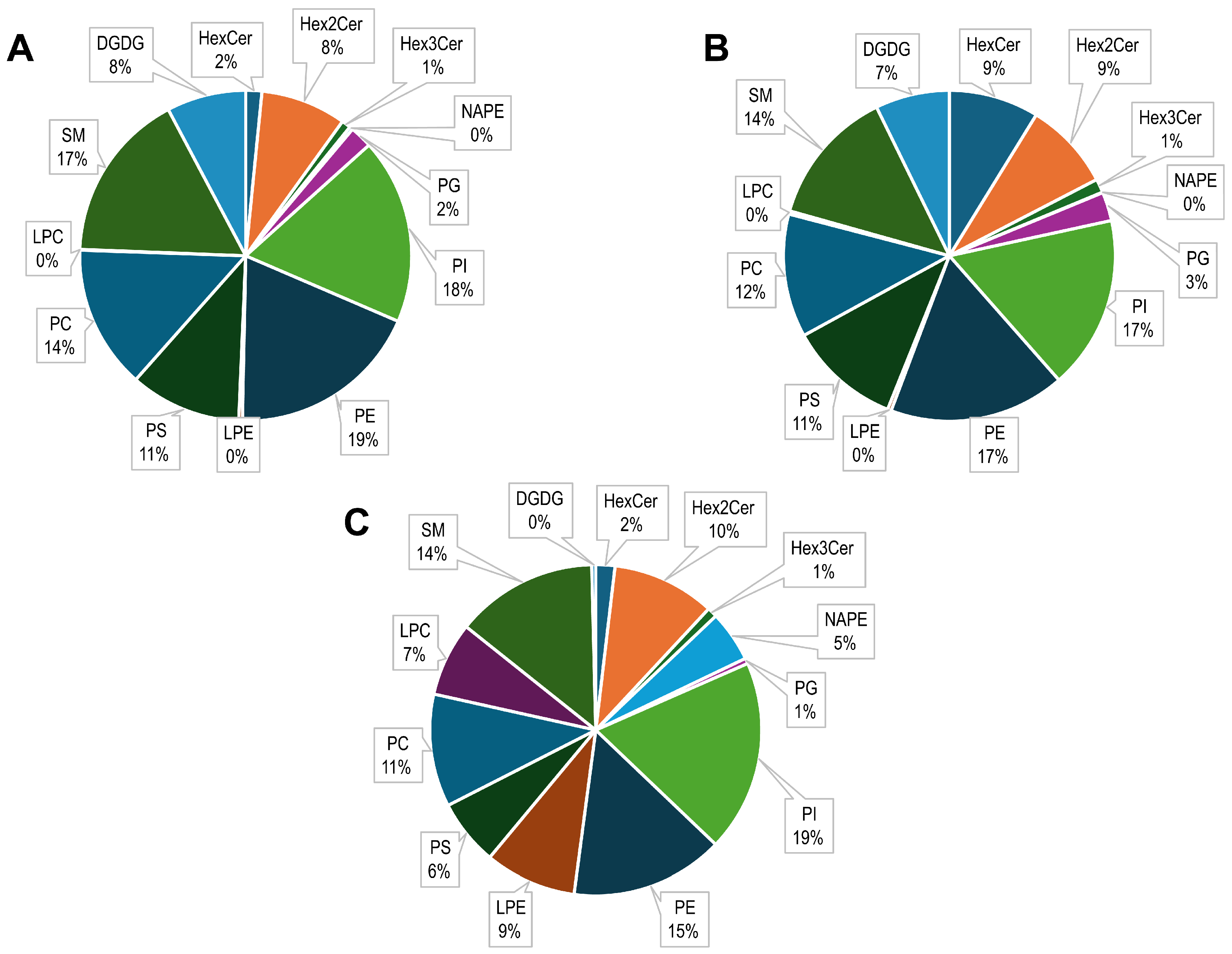
2.1. Phospholipid Analysis of Kefir by HILIC-ESI-FTMS
2.2. Exploring Glycosphingolipids (GSLs) in Kefir by HILIC-ESI-FTMS
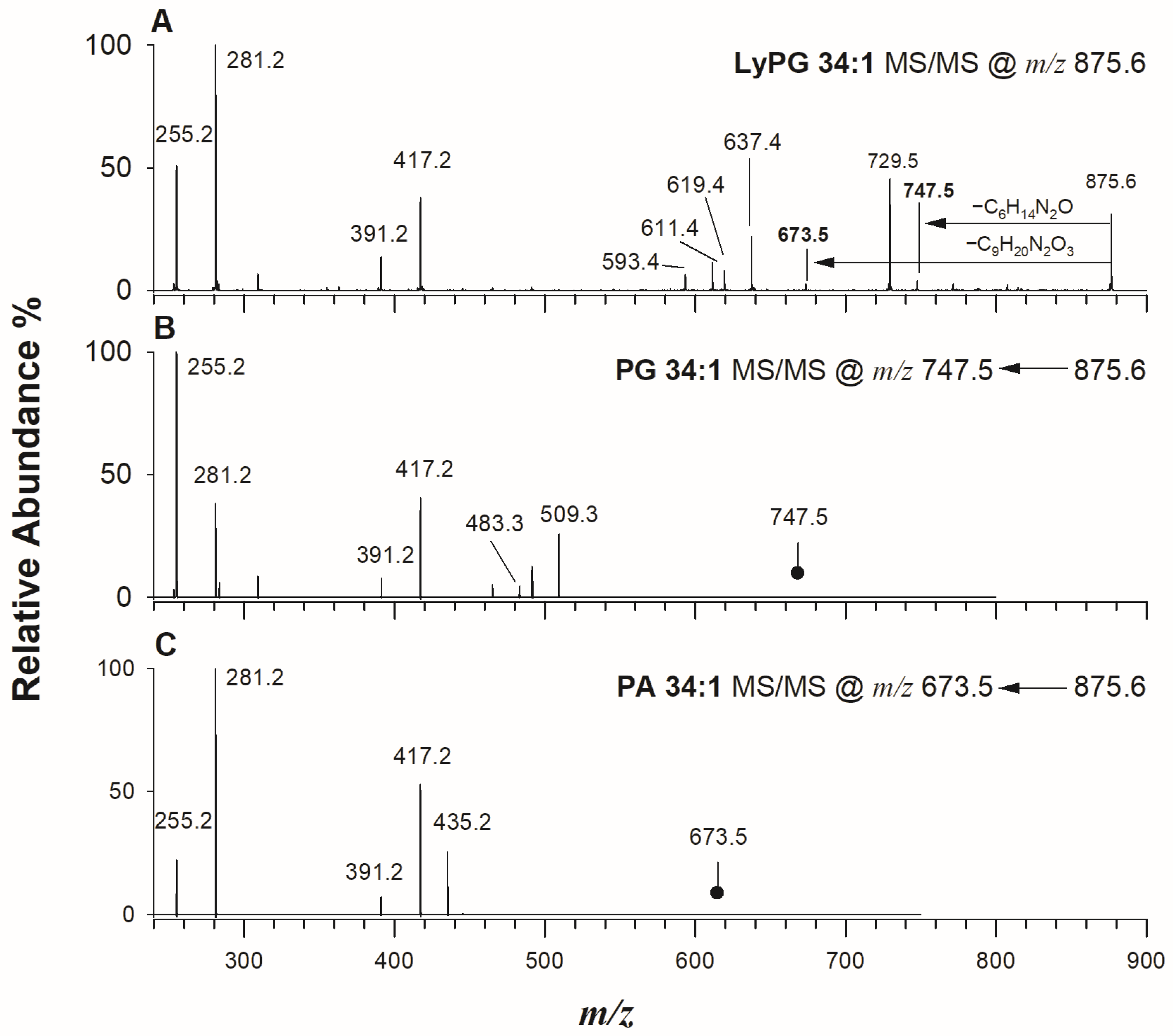
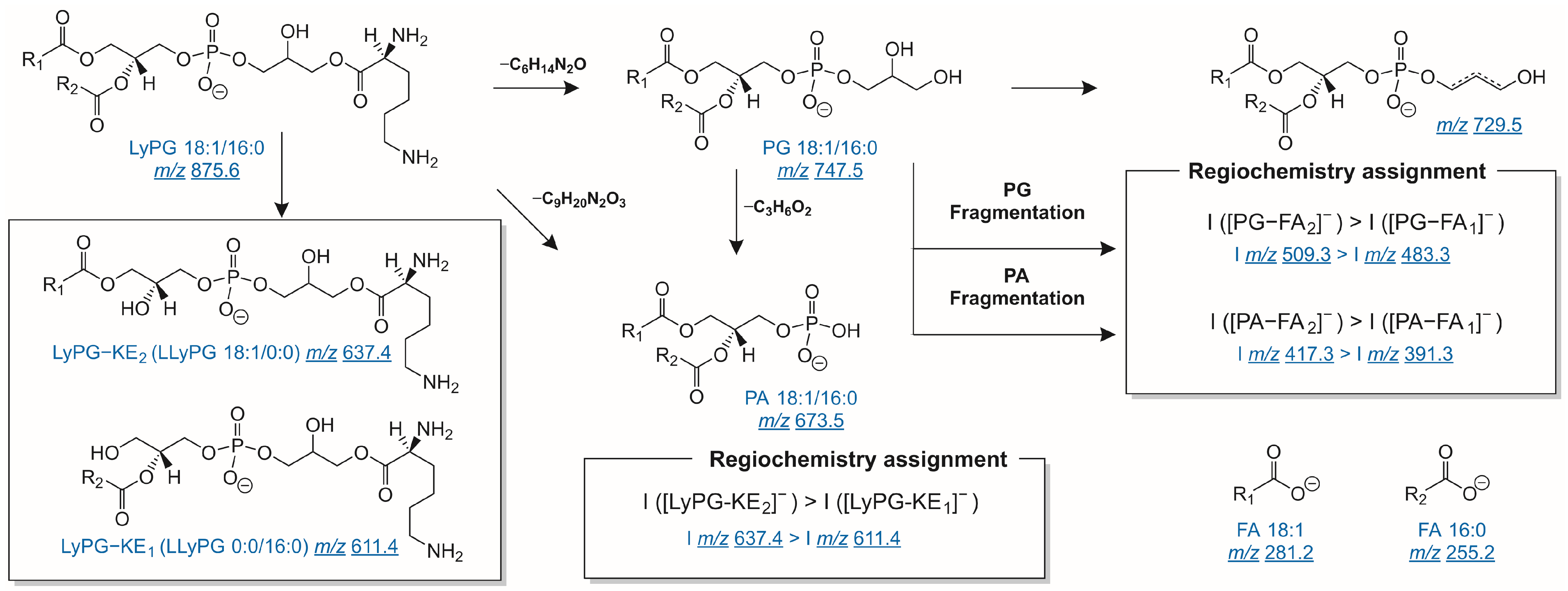
2.3. Lysyl-Phosphatidylglycerols in Kefir Beverage
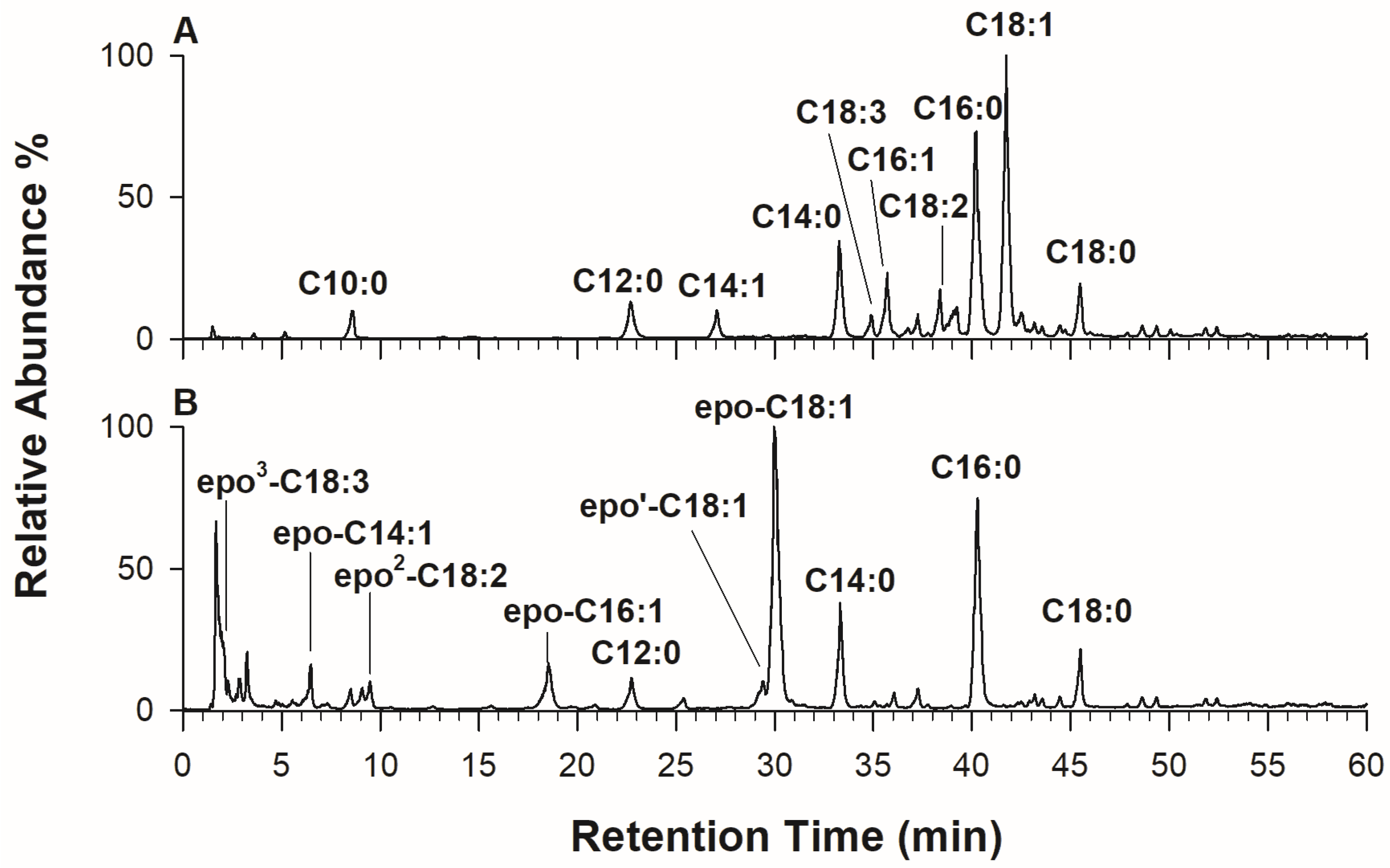
2.4. RPLC-ESI-MS/MS of Linked Fatty Acyl Chains upon Chemical Hydrolysis
3. Materials and Methods
3.1. Chemicals and Commercial Samples
3.2. Sample Preparation
3.2.1. Lipid Extraction
3.2.2. Chemical Hydrolysis of Linked Fatty Acyl Chains
3.2.3. Olefine Epoxidation
3.3. LC-ESI-MS Instrumentation and Operating Conditions
3.4. Preliminary Identification of Lipids by the Online Lipid Calculator
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk Kefir: Nutritional, Microbiological and Health Benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Ogrodowczyk, A.M.; Modzelewska-Kapituła, M. Recent Advances in Fermented Milk and Meat Products—Quality, Nutritional Value and Safety. Animals 2023, 13, 2048. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Yu, B.; Wang, J.; Zhu, M.; Liu, J.; Zheng, Z.; Qian, Z.; Wei, L.; Lv, H.; et al. Fermentation Improves Flavors, Bioactive Substances, and Antioxidant Capacity of Bian-Que Triple-Bean Soup by Lactic Acid Bacteria. Front. Microbiol. 2023, 14, 1152654. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Ahn, K.S.; Kwon, O.K.; Kim, M.J.; Kim, M.K.; Lee, I.Y.; Oh, S.R.; Lee, H.K. Anti-Inflammatory and Anti-Allergic Effects of Kefir in a Mouse Asthma Model. Immunobiology 2007, 212, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.L.; Gaudino Caputo, L.R.; Tavares Carvalho, J.C.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and Healing Activity of Kefir and Kefiran Extract. Int. J. Antimicrob. Agents 2005, 25, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Hertzler, S.R.; Clancy, S.M. Kefir Improves Lactose Digestion and Tolerance in Adults with Lactose Maldigestion. J. Am. Diet. Assoc. 2003, 103, 582–587. [Google Scholar] [CrossRef]
- Maeda, H.; Zhu, X.; Omura, K.; Suzuki, S.; Kitamura, S. Effects of an Exopolysaccharide (Kefiran) on Lipids, Blood Pressure, Blood Glucose, and Constipation. Biofactors 2004, 22, 197–200. [Google Scholar] [CrossRef]
- Hadisaputro, S.; Djokomoeljanto, R.; HNE Soesatyo, M. The Effects of Oral Plain Kefir Supplementation on Proinflammatory Cytokine Properties of the Hyperglycemia Wistar Rats Induced by Streptozotocin. Acta Med. Indones 2012, 44, 100–104. [Google Scholar]
- Steed, H.; Macfarlane, G.T.; Macfarlane, S. Prebiotics, Synbiotics and Inflammatory Bowel Disease. Mol. Nutr. Food Res. 2008, 52, 898–905. [Google Scholar] [CrossRef]
- Eduarda, M.; Soutelino, M.; Rocha, S.; Mársico, E.T.; Esmerino, E.A.; Cristina, A.; Silva, D.O. Innovative Approaches to Kefir Production, Challenges, and Current Remarks. Curr. Opin. Food Sci. 2025, 61, 101252. [Google Scholar]
- Li, S.N.; Tang, S.H.; Ren, R.; Gong, J.X.; Chen, Y.M. Metabolomic Profile of Milk Fermented with Streptococcus Thermophilus Cocultured with Bifidobacterium Animalis Ssp. Lactis, Lactiplantibacillus Plantarum, or Both during Storage. J. Dairy Sci. 2021, 104, 8493–8505. [Google Scholar] [CrossRef]
- Shen, X.; Li, W.; Cai, H.; Guo, S.; Li, M.; Liu, Y.; Sun, Z. Metabolomics Analysis Reveals Differences in Milk Metabolism and Fermentation Rate between Individual Lactococcus Lactis Subsp. Lactis Strains. Food Res. Int. 2022, 162, 111920. [Google Scholar] [CrossRef] [PubMed]
- Ebner, J.; Aşçi Arslan, A.; Fedorova, M.; Hoffmann, R.; Küçükçetin, A.; Pischetsrieder, M. Peptide Profiling of Bovine Kefir Reveals 236 Unique Peptides Released from Caseins during Its Production by Starter Culture or Kefir Grains. J. Proteomics 2015, 117, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Amorim, F.G.; Coitinho, L.B.; Dias, A.T.; Friques, A.G.F.; Monteiro, B.L.; de Rezende, L.C.D.; Pereira, T.D.M.C.; Campagnaro, B.P.; De Pauw, E.; Vasquez, E.C.; et al. Identification of New Bioactive Peptides from Kefir Milk through Proteopeptidomics: Bioprospection of Antihypertensive Molecules. Food Chem. 2019, 282, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Nadeem, M.; Imran, M.; Ullah, R.; Tayyab, M.; Khan, F.A.; Al-Asmari, F.; Rahim, M.A.; Rocha, J.M.; Korma, S.A.; et al. Effect of Fat Contents of Buttermilk on Fatty Acid Composition, Lipolysis, Vitamins and Sensory Properties of Cheddar-Type Cheese. Front. Microbiol. 2023, 14, 1209509. [Google Scholar] [CrossRef]
- Lordan, R.; Vidal, N.P.; Huong Pham, T.; Tsoupras, A.; Thomas, R.H.; Zabetakis, I. Yoghurt Fermentation Alters the Composition and Antiplatelet Properties of Milk Polar Lipids. Food Chem. 2020, 332, 127384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, J.; Liu, S.; Sang, Y.; Wang, X. Analysis of the Lipid Composition of Milk Fermented by Streptococcus Thermophilus Using Ultra-High Performance Liquid Chromatography Quadrupole/Orbitrap High Resolution Mass Spectrometry. Food Sci. 2021, 42, 197–205. [Google Scholar] [CrossRef]
- Schifano, E.; Cicalini, I.; Pieragostino, D.; Heipieper, H.J.; Del Boccio, P.; Uccelletti, D. In Vitro and in Vivo Lipidomics as a Tool for Probiotics Evaluation. Appl. Microbiol. Biotechnol. 2020, 104, 8937–8948. [Google Scholar] [CrossRef]
- Qiao, F.; Wang, S.; He, J.; Hung, W.; Ma, X.; Gong, P.; Li, J.; Sun, T.; De Souza, C.; Zhang, L.; et al. Investigating the Role of Membrane Lipid Composition Differences on Spray Drying Survival in Lactobacillus Bulgaricus Using Non-Targeted Lipidomics. Food Chem. 2024, 459, 140336. [Google Scholar] [CrossRef]
- Bianco, M.; Calvano, C.D.; Losito, I.; Palmisano, F.; Cataldi, T.R. Cataldi Targeted Analysis of Ceramides and Cerebrosides in Yellow Lupin Seeds by Reversed-Phase Liquid Chromatography Coupled to Electrospray Ionization and Multistage Mass Spectrometry. Food Chem. 2020, 324, 126878. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.; Calvano, C.D.; Huseynli, L.; Ventura, G.; Losito, I.; Cataldi, T.R.I. Identification and Quantification of Phospholipids in Strawberry Seeds and Pulp ( Fragaria × ananassa Cv San Andreas) by Liquid Chromatography with Electrospray Ionization and Tandem Mass Spectrometry. J. Mass Spectrom. 2020, 55, e4523. [Google Scholar] [CrossRef]
- Zhou, Q.; Gao, B.; Zhang, X.; Xu, Y.; Shi, H.; Yu, L. Chemical Profiling of Triacylglycerols and Diacylglycerols in Cow Milk Fat by Ultra-Performance Convergence Chromatography Combined with a Quadrupole Time-of-Flight Mass Spectrometry. Food Chem. 2014, 143, 199–204. [Google Scholar] [CrossRef]
- Granafei, S.; Losito, I.; Salivo, S.; Tranchida, P.Q.; Mondello, L.; Palmisano, F.; Cataldi, T.R.I. Occurrence of Oleic and 18:1 Methyl-Branched Acyl Chains in Lipids of Rhodobacter Sphaeroides 2.4.1. Anal. Chim. Acta 2015, 885, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Granafei, S.; Losito, I.; Palmisano, F.; Cataldi, T.R.I. Identification of Isobaric Lyso-Phosphatidylcholines in Lipid Extracts of Gilthead Sea Bream (Sparus aurata) Fillets by Hydrophilic Interaction Liquid Chromatography Coupled to High-Resolution Fourier-Transform Mass Spectrometry. Anal. Bioanal. Chem. 2015, 407, 6391–6404. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Noga, S. Hydrophilic Interaction Liquid Chromatography (HILIC)—A Powerful Separation Technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Sohlenkamp, C.; Galindo-Lagunas, K.A.; Guan, Z.; Vinuesa, P.; Robinson, S.; Thomas-Oates, J.; Raetz, C.R.H.; Geiger, O. The Lipid Lysyl-Phosphatidylglycerol Is Present in Membranes of Rhizobium tropici CIAT899 and Confers Increased Resistance to Polymyxin B Under Acidic Growth Conditions. Mol. Plant Microbe Interact. 2007, 20, 1421–1430. [Google Scholar] [CrossRef]
- Coniglio, D.; Calvano, C.D.; Ventura, G.; Losito, I.; Cataldi, T.R.I. Arsenosugar Phospholipids (As-PL) in Edible Marine Algae: An Interplay between Liquid Chromatography with Electrospray Ionization Multistage Mass Spectrometry and Phospholipases A1 and A2 for Regiochemical Assignment. J. Am. Soc. Mass Spectrom. 2020, 31, 1260–1270. [Google Scholar] [CrossRef]
- Coniglio, D.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Positional Assignment of C-C Double Bonds in Fatty Acyl Chains of Intact Arsenosugar Phospholipids Occurring in Seaweed Extracts by Epoxidation Reactions. J. Am. Soc. Mass Spectrom. 2022, 33, 823–831. [Google Scholar] [CrossRef]
- Chen, K.L.; Kuo, T.H.; Hsu, C.C. Mapping Lipid C═C Isomer Profiles of Human Gut Bacteria through a Novel Structural Lipidomics Workflow Assisted by Chemical Epoxidation. Anal. Chem. 2024, 96, 17526–17536. [Google Scholar] [CrossRef] [PubMed]
- Castellaneta, A.; Losito, I.; Leoni, B.; Santamaria, P.; Calvano, C.D.; Cataldi, T.R.I. Glycerophospholipidomics of Five Edible Oleaginous Microgreens. J. Agric. Food Chem. 2022, 70, 2410–2423. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.M.; Martin, J.; Uppuluri, A.; Joyce, L.R.; Wei, Y.; Guan, Z.; Morcos, F.; Palmer, K.L. Lipid Discovery Enabled by Sequence Statistics and Machine Learning. Elife 2024, 13, RP94929. [Google Scholar] [CrossRef] [PubMed]
- Ventura, G.; Bianco, M.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Tandem Mass Spectrometry in Untargeted Lipidomics: A Case Study of Peripheral Blood Mononuclear Cells. Int. J. Mol. Sci. 2024, 25, 12077. [Google Scholar] [CrossRef] [PubMed]
- Calvano, C.D.; Ventura, G.; Sardanelli, A.M.; Losito, I.; Palmisano, F.; Cataldi, T.R.I. Identification of Neutral and Acidic Glycosphingolipids in the Human Dermal Fibroblasts. Anal. Biochem. 2019, 581, 113348. [Google Scholar] [CrossRef]
- Calvano, C.D.; Glaciale, M.; Palmisano, F.; Cataldi, T.R.I. Glycosphingolipidomics of Donkey Milk by Hydrophilic Interaction Liquid Chromatography Coupled to ESI and Multistage MS. Electrophoresis 2018, 39, 1634–1644. [Google Scholar] [CrossRef]
- Schwalbe-Herrmann, M.; Willmann, J.; Leibfritz, D. Separation of Phospholipid Classes by Hydrophilic Interaction Chromatography Detected by Electrospray Ionization Mass Spectrometry. J. Chromatogr. A 2010, 1217, 5179–5183. [Google Scholar] [CrossRef] [PubMed]
- Furse, S.; Torres, A.G.; Koulman, A. Fermentation of Milk into Yoghurt and Cheese Leads to Contrasting Lipid and Glyceride Profiles. Nutrients 2019, 11, 2178. [Google Scholar] [CrossRef]
- Hikmetoglu, M.; Sogut, E.; Sogut, O.; Gokirmakli, C.; Guzel-Seydim, Z.B. Changes in Carbohydrate Profile in Kefir Fermentation. Bioact. Carbohydrates Diet. Fibre 2020, 23, 100220. [Google Scholar] [CrossRef]
- Hsu, F.-F. Complete Structural Characterization of Ceramides as [M−H]− Ions by Multiple-Stage Linear Ion Trap Mass Spectrometry. Biochimie 2016, 130, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.F.; Turk, J. Electrospray Ionization/Tandem Quadrupole Mass Spectrometric Studies on Phosphatidylcholines: The Fragmentation Processes. J. Am. Soc. Mass Spectrom. 2003, 14, 352–363. [Google Scholar] [CrossRef]
- La Torre, C.; Caputo, P.; Cione, E.; Fazio, A. Comparing Nutritional Values and Bioactivity of Kefir from Different Types of Animal Milk. Molecules 2024, 29, 2710. [Google Scholar] [CrossRef]
- Aroua, M.; Ben Haj Koubaier, H.; Bouacida, S.; Ben Saïd, S.; Mahouachi, M.; Salimei, E. Chemical, Physicochemical, Microbiological, Bioactive, and Sensory Characteristics of Cow and Donkey Milk Kefir during Storage. Beverages 2023, 9, 2. [Google Scholar] [CrossRef]
- Ferná Ndez Murga, M.L.; Cabrera, G.M.; Font De Valdez, G.; Disalvo, A.; Seldes, A.M. Influence of Growth Temperature on Cryotolerance and Lipid Composition of Lactobacillus Acidophilus. J. Appl. Microbiol. 2000, 88, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Tatituri, R.V.V.; Wolf, B.J.; Brenner, M.B.; Turk, J.; Hsu, F.F. Characterization of Polar Lipids of Listeria Monocytogenes by HCD and Low-Energy CAD Linear Ion-Trap Mass Spectrometry with Electrospray Ionization. Anal. Bioanal. Chem. 2015, 407, 2519–2528. [Google Scholar] [CrossRef] [PubMed]
- Joyce, L.R.; Manzer, H.S.; da, C. Mendonça, J.; Villarreal, R.; Nagao, P.E.; Doran, K.S.; Palmer, K.L.; Guan, Z. Identification of a Novel Cationic Glycolipid in Streptococcus Agalactiae That Contributes to Brain Entry and Meningitis. PLoS Biol. 2022, 20, e3001555. [Google Scholar] [CrossRef]
- Hsu, F.-F.; Turk, J. Studies on Phosphatidylglycerol with Triple Quadrupole Tandem Mass Spectrometry with Electrospray Ionization: Fragmentation Processes and Structural Characterization. J. Am. Soc. Mass Spectrom. 2001, 12, 1036–1043. [Google Scholar] [CrossRef]
- Hsu, F.F.; Turk, J. Charge-Driven Fragmentation Processes in Diacyl Glycerophosphatidic Acids upon Low-Energy Collisional Activation. A Mechanistic Proposal. J. Am. Soc. Mass Spectrom. 2000, 11, 797–803. [Google Scholar] [CrossRef]
- Losito, I.; Facchini, L.; Valentini, A.; Cataldi, T.R.I.; Palmisano, F. Fatty Acidomics: Evaluation of the Effects of Thermal Treatments on Commercial Mussels through an Extended Characterization of Their Free Fatty Acids by Liquid Chromatography—Fourier Transform Mass Spectrometry. Food Chem. 2018, 255, 309–322. [Google Scholar] [CrossRef]
- Månsson, H.L. Fatty Acids in Bovine Milk Fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, B.; Yu, Q.; Li, L. Identification of Double Bond Position Isomers in Unsaturated Lipids by M-CPBA Epoxidation and Mass Spectrometry Fragmentation. Anal. Chem. 2019, 91, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Bodkowski, R.; Wierzbicki, H.; Mucha, A.; Cholewińska, P.; Wojnarowski, K.; Patkowska-Sokoła, B. Composition and Fatty Acid Profile of Milk from Cows Fed Diets Supplemented with Raw and N-3 PUFA-Enriched Fish Oil. Sci. Rep. 2024, 14, 10968. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]


| LyPG | Accurate m/z Value | Theoretical m/z Value | D (ppm) | Empirical Formula (M) | Regiochemical Assignment |
|---|---|---|---|---|---|
| 32:0 | 849.597 | 849.598 | 1.8 | C44H87N2O11P | 16:0/16:0 |
| 32:1 | 847.581 | 847.582 | 1.7 | C44H85N2O11P | 16:1/16:0 |
| 34:1 | 875.612 | 875.613 | 0.8 | C46H89N2O11P | 18:1/16:0 |
| 34:2 | 873.596 | 873.598 | 2.1 | C46H87N2O11P | 18:1/16:1 and 18:2/16:0 |
| 34:3 | 871.580 | 871.582 | 2.4 | C46H85N2O11P | 18:3/16:0 |
| 35:1 | 889.627 | 889.629 | 2.0 | C47H91N2O11P | 19:1/16:0 and 18:1_17:0 |
| 36:1 | 903.644 | 903.645 | 1.0 | C48H93N2O11P | 20:1/16:0 and 18:1/18:0 |
| 36:2 | 901.628 | 901.629 | 1.2 | C48H91N2O11P | 18:1/18:1, 20:1/16:1 and 20:2_16:0 |
| 37:2 | 915.643 | 915.645 | 2.5 | C49H93N2O11P | 19:1/18:1 |
| 38:1 | 931.675 | 931.676 | 0.7 | C50H97N2O11P | 20:0/18:1 |
| 38:2 | 929.660 | 929.660 | 0.2 | C50H95N2O11P | 20:1/18:1 |
| 38:3 | 927.642 | 927.645 | 3.8 | C50H93N2O11P | 20:1/18:2 |
| 40:2 | 957.689 | 957.691 | 2.1 | C52H99N2O11P | 20:1/20:1 and 18:1_22:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura, G.; Bianco, M.; Losito, I.; Cataldi, T.R.I.; Calvano, C.D. Complete Polar Lipid Profile of Kefir Beverage by Hydrophilic Interaction Liquid Chromatography with HRMS and Tandem Mass Spectrometry. Int. J. Mol. Sci. 2025, 26, 1120. https://doi.org/10.3390/ijms26031120
Ventura G, Bianco M, Losito I, Cataldi TRI, Calvano CD. Complete Polar Lipid Profile of Kefir Beverage by Hydrophilic Interaction Liquid Chromatography with HRMS and Tandem Mass Spectrometry. International Journal of Molecular Sciences. 2025; 26(3):1120. https://doi.org/10.3390/ijms26031120
Chicago/Turabian StyleVentura, Giovanni, Mariachiara Bianco, Ilario Losito, Tommaso R. I. Cataldi, and Cosima D. Calvano. 2025. "Complete Polar Lipid Profile of Kefir Beverage by Hydrophilic Interaction Liquid Chromatography with HRMS and Tandem Mass Spectrometry" International Journal of Molecular Sciences 26, no. 3: 1120. https://doi.org/10.3390/ijms26031120
APA StyleVentura, G., Bianco, M., Losito, I., Cataldi, T. R. I., & Calvano, C. D. (2025). Complete Polar Lipid Profile of Kefir Beverage by Hydrophilic Interaction Liquid Chromatography with HRMS and Tandem Mass Spectrometry. International Journal of Molecular Sciences, 26(3), 1120. https://doi.org/10.3390/ijms26031120








