Combining Colchicine and Antiplatelet Therapy to Tackle Atherothrombosis: A Paradigm in Transition?
Abstract
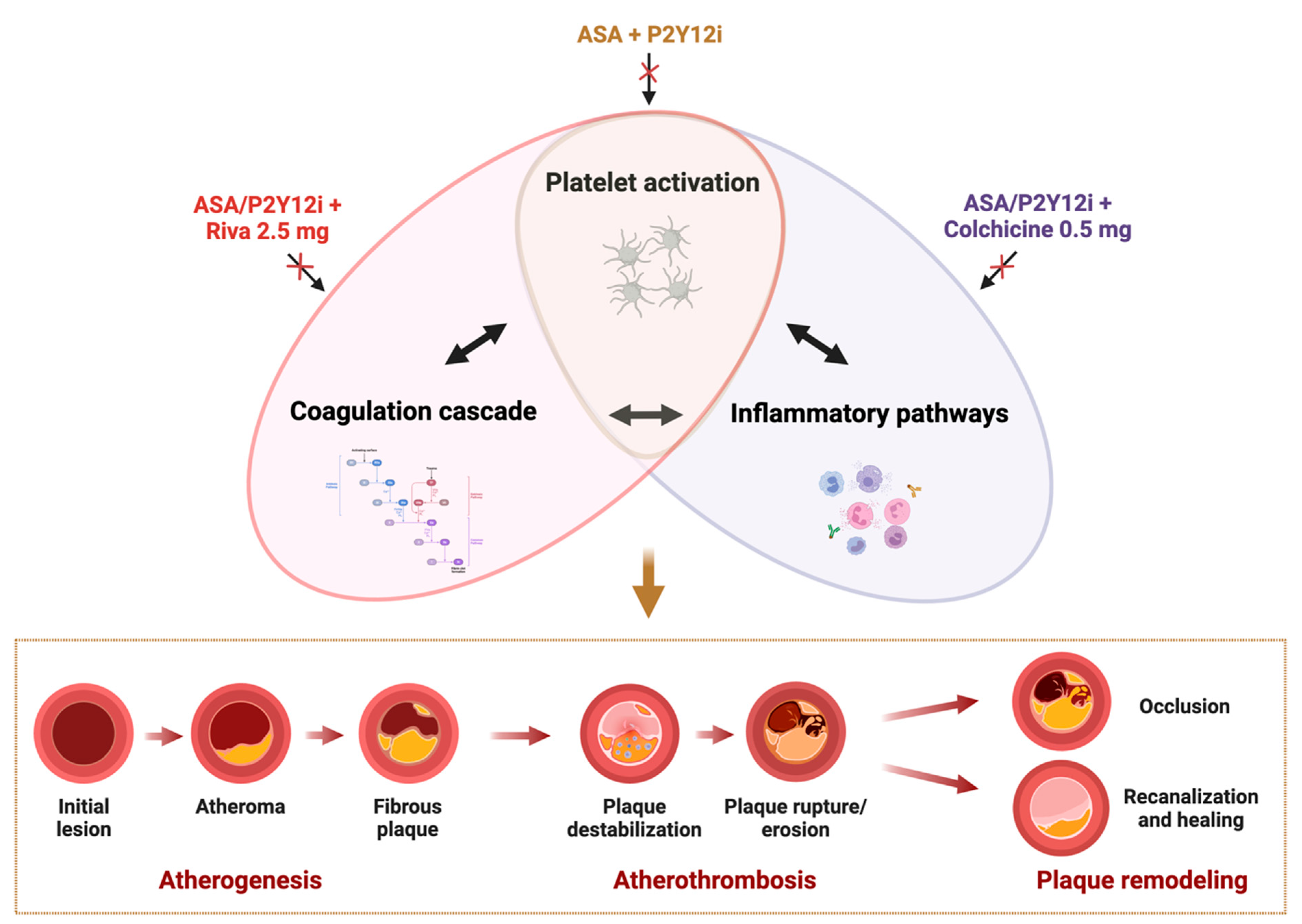
1. Background
2. Interplay Between Coagulation, Platelets, and Inflammation in Atherothrombosis
3. Colchicine: Pharmacokinetics and Pharmacodynamics
4. Colchicine: Mechanism of Action
5. Possible Discomforts and Risks of Colchicine Therapy
6. Antiplatelet Effects of Colchicine
6.1. In Vitro Studies
6.2. In Vivo Studies
7. Current Evidence and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Mortality Database. Available online: https://www.who.int/data/data-collection-tools/who-mortality-database (accessed on 5 November 2024).
- Heart Disease Facts. Available online: https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html#:~:text=In%20the%20United%20States%3A,people%20died%20from%20heart%20disease (accessed on 5 November 2024).
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Viles-Gonzalez, J.F.; Fuster, V.; Badimon, J.J. Atherothrombosis: A widespread disease with unpredictable and life-threatening consequences. Eur. Heart J. 2004, 25, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Pathogenesis of Atherothrombotic Events: From Lumen to Lesion and Beyond. Circulation 2024, 150, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Galli, M.; Collet, J.P.; Kastrati, A.; O’Donoghue, M.L. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention 2022, 17, e1371–e1396. [Google Scholar] [CrossRef]
- Vergallo, R.; Crea, F. Atherosclerotic Plaque Healing. N. Engl. J. Med. 2020, 383, 846–857. [Google Scholar] [CrossRef]
- Galli, M.; Niccoli, G.; De Maria, G.; Brugaletta, S.; Montone, R.A.; Vergallo, R.; Benenati, S.; Magnani, G.; D’Amario, D.; Porto, I.; et al. Coronary microvascular obstruction and dysfunction in patients with acute myocardial infarction. Nat. Rev. Cardiol. 2024, 21, 283–298. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Ueno, M.; Goto, S. Basic principles of platelet biology and clinical implications. Circ. J. 2010, 74, 597–607. [Google Scholar] [CrossRef]
- Galli, M.; Laborante, R.; Andreotti, F.; Vergallo, R.; Montone, R.A.; Iaconelli, A.; Trani, C.; Burzotta, F.; Crea, F.; D’Amario, D. Bleeding Complications in Patients Undergoing Percutaneous Coronary Intervention. Rev. Cardiovasc. Med. 2022, 23, 286. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Foley, J.H.; Conway, E.M. Cross Talk Pathways Between Coagulation and Inflammation. Circ. Res. 2016, 118, 1392–1408. [Google Scholar] [CrossRef]
- Galli, M.; Franchi, F.; Rollini, F.; Ortega-Paz, L.; D’Amario, D.; De Caterina, R.; Mehran, R.; Gibson, C.M.; Angiolillo, D.J. Dual pathway inhibition in patients with atherosclerotic disease: Pharmacodynamic considerations and clinical implications. Expert Rev. Clin. Pharmacol. 2023, 16, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Franchi, F.; Rollini, F.; Been, L.; Jaoude, P.A.; Rivas, A.; Zhou, X.; Jia, S.; Maaliki, N.; Lee, C.H.; et al. Platelet P2Y12 inhibiting therapy in adjunct to vascular dose of rivaroxaban or aspirin: A pharmacodynamic study of dual pathway inhibition vs. dual antiplatelet therapy. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Franchi, F.; Rollini, F.; Been, L.; Jaoude, P.A.; Rivas, A.; Zhou, X.; Jia, S.; Maaliki, N.; Lee, C.H.; et al. Pharmacodynamic Profiles of Dual-Pathway Inhibition with or without Clopidogrel versus Dual Antiplatelet Therapy in Patients with Atherosclerotic Disease. Thromb. Haemost. 2022, 122, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paz, L.; Franchi, F.; Rollini, F.; Galli, M.; Been, L.; Ghanem, G.; Shalhoub, A.; Ossi, T.; Rivas, A.; Zhou, X.; et al. Switching from Dual Antiplatelet Therapy with Aspirin Plus a P2Y12 Inhibitor to Dual Pathway Inhibition with Aspirin Plus Vascular-Dose Rivaroxaban: The Switching Anti-Platelet and Anti-Coagulant Therapy (SWAP-AC) Study. Thromb. Haemost. 2024, 124, 263–273. [Google Scholar] [CrossRef]
- Galli, M.; Capodanno, D.; Benenati, S.; D’Amario, D.; Crea, F.; Andreotti, F.; Angiolillo, D.J. Efficacy and safety of dual-pathway inhibition in patients with cardiovascular disease: A meta-analysis of 49 802 patients from 7 randomized trials. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 519–528. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Biasucci, L.M.; La Rosa, G.; Pedicino, D.; D’Aiello, A.; Galli, M.; Liuzzo, G. Where Does Inflammation Fit? Curr. Cardiol. Rep. 2017, 19, 84. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar]
- D’Amario, D.; Rodolico, D.; Galli, M. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2021, 384, 778. [Google Scholar]
- Aksu, K.; Donmez, A.; Keser, G. Inflammation-induced thrombosis: Mechanisms, disease associations and management. Curr. Pharm. Des. 2012, 18, 1478–1493. [Google Scholar]
- De Caterina, R.; D’Ugo, E.; Libby, P. Inflammation and thrombosis—Testing the hypothesis with anti-inflammatory drug trials. Thromb. Haemost. 2016, 116, 1012–1021. [Google Scholar] [PubMed]
- Camera, M.; Frigerio, M.; Toschi, V.; Brambilla, M.; Rossi, F.; Cottell, D.C.; Maderna, P.; Parolari, A.; Bonzi, R.; De Vincenti, O.; et al. Platelet activation induces cell-surface immunoreactive tissue factor expression, which is modulated differently by antiplatelet drugs. Arter. Thromb. Vasc. Biol. 2003, 23, 1690–1696. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brambilla, M.; Facchinetti, L.; Canzano, P.; Rossetti, L.; Ferri, N.; Balduini, A.; Abbonante, V.; Boselli, D.; De Marco, L.; Di Minno, M.N.; et al. Human megakaryocytes confer tissue factor to a subset of shed platelets to stimulate thrombin generation. Thromb. Haemost. 2015, 114, 579–592. [Google Scholar]
- Brambilla, M.; Rossetti, L.; Zara, C.; Canzano, P.; Giesen, P.L.A.; Tremoli, E.; Camera, M. Do methodological differences account for the current controversy on tissue factor expression in platelets? Platelets 2018, 29, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.R.; Storey, R.F. The role of platelets in inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [PubMed]
- Monroe, D.M.; Hoffman, M.; Roberts, H.R. Platelets and Thrombin Generation. Arter. Thromb. Vasc. Biol. 2002, 22, 1381–1389. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Camera, M.; Toschi, V.; Brambilla, M.; Lettino, M.; Rossetti, L.; Canzano, P.; Di Minno, A.; Tremoli, E. The Role of Tissue Factor in Atherothrombosis and Coronary Artery Disease: Insights into Platelet Tissue Factor. Semin. Thromb. Hemost. 2015, 41, 737–746. [Google Scholar]
- Drake, T.A.; Morrissey, J.H.; Edgington, T.S. Selective cellular expression of tissue factor in human tissues. Implic. Disord. Hemost. Thromb. Am. J. Pathol. 1989, 134, 1087–1097. [Google Scholar]
- Brambilla, M.; Camera, M.; Colnago, D.; Marenzi, G.; De Metrio, M.; Giesen, P.L.; Balduini, A.; Veglia, F.; Gertow, K.; Biglioli, P.; et al. Tissue factor in patients with acute coronary syndromes: Expression in platelets, leukocytes, and platelet-leukocyte aggregates. Arter. Thromb. Vasc. Biol. 2008, 28, 947–953. [Google Scholar] [CrossRef]
- Nerlekar, N.; Beale, A.; Harper, R.W. Colchicine—A short history of an ancient drug. Med. J. Aust. 2014, 201, 687–688. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, D.; Cappetta, D.; Cappannoli, L.; Princi, G.; Migliaro, S.; Diana, G.; Chouchane, K.; Borovac, J.A.; Restivo, A.; Arcudi, A.; et al. Colchicine in ischemic heart disease: The good, the bad and the ugly. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2021, 110, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Rochdi, M.; Sabouraud, A.; Girre, C.; Venet, R.; Scherrmann, J.M. Pharmacokinetics and absolute bioavailability of colchicine after i.v. and oral administration in healthy human volunteers and elderly subjects. Eur. J. Clin. Pharmacol. 1994, 46, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Niel, E.; Scherrmann, J.M. Colchicine today. Jt. Bone Spine 2006, 73, 672–678. [Google Scholar] [CrossRef]
- Chappey, O.N.; Niel, E.; Wautier, J.L.; Hung, P.P.; Dervichian, M.; Cattan, D.; Scherrmann, J.M. Colchicine disposition in human leukocytes after single and multiple oral administration. Clin. Pharmacol. Ther. 1993, 54, 360–367. [Google Scholar] [CrossRef]
- González, L.; Bulnes, J.F.; Orellana, M.P.; Muñoz Venturelli, P.; Martínez Rodriguez, G. The Role of Colchicine in Atherosclerosis: From Bench to Bedside. Pharmaceutics 2022, 14, 1395. [Google Scholar] [CrossRef]
- Slobodnick, A.; Shah, B.; Pillinger, M.H.; Krasnokutsky, S. Colchicine: Old and new. Am. J. Med. 2015, 128, 461–470. [Google Scholar] [CrossRef]
- Simkin, P.A.; Gardner, G.C. Colchicine use in cyclosporine treated transplant recipients: How little is too much? J. Rheumatol. 2000, 27, 1334–1337. [Google Scholar]
- Ben-Chetrit, E.; Bergmann, S.; Sood, R. Mechanism of the anti-inflammatory effect of colchicine in rheumatic diseases: A possible new outlook through microarray analysis. Rheumatology 2006, 45, 274–282. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef]
- Landis, R.C.; Yagnik, D.R.; Florey, O.; Philippidis, P.; Emons, V.; Mason, J.C.; Haskard, D.O. Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis Rheum. 2002, 46, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, S.R.; Conaghan, P.G.; McDermott, M.F. The role of the NLRP3 inflammasome in gout. J. Inflamm. Res. 2011, 4, 39–49. [Google Scholar] [PubMed]
- Cerecedo, D.; Stock, R.; González, S.; Reyes, E.; Mondragón, R. Modification of actin, myosin and tubulin distribution during cytoplasmic granule movements associated with platelet adhesion. Haematologica 2002, 87, 1165–1176. [Google Scholar] [PubMed]
- Menche, D.; Israel, A.; Karpatkin, S. Platelets and microtubules: Effect of colchicine and D2O on platelet aggregation and release induced by calcium ionophore A23187. J. Clin. Investig. 1980, 66, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, G.; Tarallo, R.; Conte, S.; Morello, A.; Pellegrino, G.; Loffredo, F.S.; Calì, G.; De Luca, N.; Golino, P.; Trimarco, B.; et al. Colchicine reduces platelet aggregation by modulating cytoskeleton rearrangement via inhibition of cofilin and LIM domain kinase 1. Vasc. Pharmacol. 2018, 111, 62–70. [Google Scholar] [CrossRef]
- Shah, B.; Allen, N.; Harchandani, B.; Pillinger, M.; Katz, S.; Sedlis, S.P.; Echagarruga, C.; Samuels, S.K.; Morina, P.; Singh, P.; et al. Effect of Colchicine on Platelet-Platelet and Platelet-Leukocyte Interactions: A Pilot Study in Healthy Subjects. Inflammation 2016, 39, 182–189. [Google Scholar] [CrossRef]
- Cimmino, G.; Conte, S.; Morello, A.; Pellegrino, G.; Marra, L.; Calì, G.; Golino, P.; Cirillo, P. Colchicine inhibits the prothrombotic effects of oxLDL in human endothelial cells. Vasc. Pharmacol. 2020, 137, 106822. [Google Scholar] [CrossRef]
- Cirillo, P.; Taglialatela, V.; Pellegrino, G.; Morello, A.; Conte, S.; Di Serafino, L.; Cimmino, G. Effects of colchicine on platelet aggregation in patients on dual antiplatelet therapy with aspirin and clopidogrel. J. Thromb. Thrombolysis 2020, 50, 468–472. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013, 61, 404–410. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.C.; Quinn, S.; Nasis, A.; Hiew, C.; Roberts-Thomson, P.; Adams, H.; Sriamareswaran, R.; Htun, N.M.; Wilson, W.; Stub, D.; et al. Colchicine in Patients with Acute Coronary Syndrome: The Australian COPS Randomized Clinical Trial. Circulation 2020, 142, 1890–1900. [Google Scholar] [CrossRef]
- Deftereos, S.; Giannopoulos, G.; Raisakis, K.; Kossyvakis, C.; Kaoukis, A.; Panagopoulou, V.; Driva, M.; Hahalis, G.; Pyrgakis, V.; Alexopoulos, D.; et al. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J. Am. Coll. Cardiol. 2013, 61, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Jolly, S.S.; d’Entremont, M.-A.; Lee, S.F.; Mian, R.; Tyrwhitt, J.; Kedev, S.; Montalescot, G.; Cornel, J.H.; Stanković, G.; Moreno, R.; et al. Colchicine in Acute Myocardial Infarction. N. Engl. J. Med. 2024, 27, 20. [Google Scholar] [CrossRef] [PubMed]
- Lidar, M.; Scherrmann, J.M.; Shinar, Y.; Chetrit, A.; Niel, E.; Gershoni-Baruch, R.; Langevitz, P.; Livneh, A. Colchicine nonresponsiveness in familial Mediterranean fever: Clinical, genetic, pharmacokinetic, and socioeconomic characterization. Semin. Arthritis Rheum. 2004, 33, 273–282. [Google Scholar] [CrossRef]
- Brambilla, M.; Becchetti, A.; Rovati, G.E.; Cosentino, N.; Conti, M.; Canzano, P.; Giesen, P.L.A.; Loffreda, A.; Bonomi, A.; Cattaneo, M.; et al. Cell Surface Platelet Tissue Factor Expression: Regulation by P2Y(12) and Link to Residual Platelet Reactivity. Arter. Thromb. Vasc. Biol. 2023, 43, 2042–2057. [Google Scholar] [CrossRef]
- Raju, N.C.; Yi, Q.; Nidorf, M.; Fagel, N.D.; Hiralal, R.; Eikelboom, J.W. Effect of colchicine compared with placebo on high sensitivity C-reactive protein in patients with acute coronary syndrome or acute stroke: A pilot randomized controlled trial. J. Thromb. Thrombolysis 2012, 33, 88–94. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, Y.H.; Yun, K.H.; Cho, J.Y.; Gorog, D.A.; Angiolillo, D.J.; Kim, J.W.; Jang, Y. P2Y(12) Inhibitor Monotherapy Combined With Colchicine Following PCI in ACS Patients: The MACT Pilot Study. JACC Cardiovasc. Interv. 2023, 16, 1845–1855. [Google Scholar] [CrossRef]
- Fiolet, A.T.L.; Opstal, T.S.J.; Mosterd, A.; Eikelboom, J.W.; Jolly, S.S.; Keech, A.C.; Kelly, P.; Tong, D.C.; Layland, J.; Nidorf, S.M.; et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: A systematic review and meta-analysis of randomized trials. Eur. Heart J. 2021, 42, 2765–2775. [Google Scholar] [CrossRef]
- Galli, M.; Princi, G.; Crea, F.; D’Amario, D. Colchicine and risk of non-cardiovascular death in patients with coronary artery disease: A pooled analysis underling possible safety concerns. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, e18–e19. [Google Scholar] [CrossRef]
- Opstal, T.S.J.; Nidorf, S.M.; Fiolet, A.T.L.; Eikelboom, J.W.; Mosterd, A.; Bax, W.A.; Budgeon, C.A.; Ronner, E.; Prins, F.J.; Tijssen, J.G.P.; et al. Drivers of mortality in patients with chronic coronary disease in the low-dose colchicine 2 trial. Int. J. Cardiol. 2023, 372, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Mewton, N.; Roubille, F.; Bresson, D.; Prieur, C.; Bouleti, C.; Bochaton, T.; Ivanes, F.; Dubreuil, O.; Biere, L.; Hayek, A.; et al. Effect of Colchicine on Myocardial Injury in Acute Myocardial Infarction. Circulation 2021, 144, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Bouleti, C.; Viscogliosi, S.; Bresson, D.; Leboube, S.; Bochaton, T.; El-Jonhy, N.; Amaz, C.; Prunier, F.; Bidaux, G.; Roubille, F.; et al. Colchicine in acute myocardial infarction: Cardiovascular events at 1-year follow up. Open Heart 2024, 11, e002474. [Google Scholar] [CrossRef]
- Shah, B.; Pillinger, M.; Zhong, H.; Cronstein, B.; Xia, Y.; Lorin, J.D.; Smilowitz, N.R.; Feit, F.; Ratnapala, N.; Keller, N.M.; et al. Effects of Acute Colchicine Administration Prior to Percutaneous Coronary Intervention: COLCHICINE-PCI Randomized Trial. Circ. Cardiovasc. Interv. 2020, 13, e008717. [Google Scholar] [CrossRef]
- Cole, J.; Htun, N.; Lew, R.; Freilich, M.; Quinn, S.; Layland, J. Colchicine to Prevent Periprocedural Myocardial Injury in Percutaneous Coronary Intervention: The COPE-PCI Pilot Trial. Circ. Cardiovasc. Interv. 2021, 14, e009992. [Google Scholar] [CrossRef]
- Yu, M.; Yang, Y.; Dong, S.L.; Zhao, C.; Yang, F.; Yuan, Y.F.; Liao, Y.H.; He, S.L.; Liu, K.; Wei, F.; et al. Effect of Colchicine on Coronary Plaque Stability in Acute Coronary Syndrome as Assessed by Optical Coherence Tomography: The COLOCT Randomized Clinical Trial. Circulation 2024, 150, 981–993. [Google Scholar] [CrossRef]
- Vaidya, K.; Arnott, C.; Martínez, G.J.; Ng, B.; McCormack, S.; Sullivan, D.R.; Celermajer, D.S.; Patel, S. Colchicine Therapy and Plaque Stabilization in Patients with Acute Coronary Syndrome: A CT Coronary Angiography Study. JACC Cardiovasc. Imaging 2018, 11 Pt 2, 305–316. [Google Scholar] [CrossRef]
- Zuriaga, M.A.; Yu, Z.; Matesanz, N.; Truong, B.; Ramos-Neble, B.L.; Asensio-López, M.C.; Uddin, M.M.; Nakao, T.; Niroula, A.; Zorita, V.; et al. Colchicine prevents accelerated atherosclerosis in TET2-mutant clonal haematopoiesis. Eur. Heart J. 2024, 45, 4601–4615. [Google Scholar] [CrossRef]
- Deftereos, S.; Giannopoulos, G.; Angelidis, C.; Alexopoulos, N.; Filippatos, G.; Papoutsidakis, N.; Sianos, G.; Goudevenos, J.; Alexopoulos, D.; Pyrgakis, V.; et al. Anti-Inflammatory Treatment with Colchicine in Acute Myocardial Infarction: A Pilot Study. Circulation 2015, 132, 1395–1403. [Google Scholar] [CrossRef]
- Moussa, I.D.; Klein, L.W.; Shah, B.; Mehran, R.; Mack, M.J.; Brilakis, E.S.; Reilly, J.P.; Zoghbi, G.; Holper, E.; Stone, G.W.; et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: An expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). Catheter. Cardiovasc. Interv. 2014, 83, 27–36. [Google Scholar] [CrossRef]
| In Vitro Studies | Study Design | Colchicine Dose | Clinical Setting | Sample Size | Main Findings |
|---|---|---|---|---|---|
| Shah et al., 2016 [48] | Addition of colchicine to PRP (for 30 min) and whole blood (for 5 min) was followed by assessment of platelet activity and adhesion via LTA and flow cytometer | 0.015, 0.15, 1.5, 15, 150, 1500, 15,000 μM | Healthy adults | n = 10 | Addition of colchicine:
|
| Brambilla et al., 2023 [58] | Whole blood was incubated with colchicine and then the expression of platelet-associated TF, P-selectin, and GPIIbIIIa was measured by flow cytometry upon stimulation with ADP | 20 nM, 100 nm, 1 µM, 10 µM, 100 µM | Healthy adults | n = 10 | Colchicine reduced in a concentration-dependent manner the following:
|
| Cirillo et al., 2020 [50] | PRP was pre-incubated with colchicine before being stimulated with ADP or TRAP. PRP not colchicine preincubated served as controls. The level of platelet aggregation was then evaluated by LTA at 30, 60, and 90 min | 10 μM | Patients on DAPT with clopidogrel | n = 35 (28 clopidogrel responders and seven clopidogrel non-responders) | Colchicine:
|
| Shah et al., 2016 [48] | Administration of a 1.8 mg oral colchicine loading dose over one hour. Subsequent blood samples were drawn 2 and 24 h after completion of the loading dose; platelet activity and adhesion were then assessed via LTA, flow cytometer, and fluorescence microscope | 1.8 mg over one hour | Healthy adults | n = 10 | Colchicine
|
| Raju et al., 2012 [59] | Pilot randomized controlled trial comparing the effect of daily colchicine administration with placebo on hs-CRP levels and platelet function by turbidimetric platelet aggregometry | 1 mg/day for 30 days | Patients with ACS or acute ischemic stroke | n = 80 | Colchicine
|
| Lee et al., 2023 [60] | Proof-of-concept pilot trial investigating the feasibility of ticagrelor or prasugrel P2Y12 inhibitor monotherapy combined with colchicine immediately after PCI in patients with ACS | 0.6 mg daily | ACS patients treated with drug-eluting stents | n = 200 | In ACS patients undergoing PCI, discontinuing aspirin therapy and administering low-dose colchicine on the day after PCI in addition to ticagrelor or prasugrel is associated with the following:
|
| Study Design | Outcomes | Colchicine Dose | Sample Size | Follow-Up | Main Findings | |
|---|---|---|---|---|---|---|
| Nidorf et al., 2013 [51] | Randomized, observer-blinded trial. CCS patients were assigned to colchicine or no colchicine | Primary composite: ACS, out of hospital cardiac arrest, or non-cardioembolic stroke Secondary: individual components of the primary outcome and the components of ACS unrelated to stent disease | 0.5 mg/day | Colchicine = 282 Controls = 250 | 3 years |
|
| Nidorf et al., 2020 [52] | Randomized, controlled, double-blind trial. CCS participants were assigned to receive either colchicine or placebo | Primary composite: cardiovascular death, spontaneous (nonprocedural) MI, ischemic stroke, or ischemia-driven coronary revascularization. Secondary composite: cardiovascular death, spontaneous MI, or ischemic stroke | 0.5 mg/day | Colchicine = 2762 Controls = 2760 | 28.6 months |
|
| Tardif et al., 2019 [53] | Randomized, double-blind trial involving patients recruited within 30 days after an MI. Patients were randomly assigned to receive either low-dose colchicine or placebo | Primary composite: death from cardiovascular causes, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina leading to coronary revascularization Secondary: consisted of the components of the primary end point; a composite of death from cardiovascular causes, resuscitated cardiac arrest, myocardial infarction, or stroke; and total mortality in time-to-event analyses | 0.5 mg/day | Colchicine = 2366 Placebo = 2379 | 22.6 months |
|
| Tong et al., 2020 [54] | Multicenter, randomized, double-blind, placebo-controlled trial. Patients who presented with ACS and had evidence of coronary artery disease on coronary angiography managed with either PCI or medical therapy were assigned to receive either colchicine or placebo | Primary composite: all-cause mortality, ACS, ischemia-driven (unplanned) urgent revascularization, and non-cardioembolic ischemic stroke in a time to event analysis | 0.5 mg twice daily for the first month, then 0.5 mg daily for 11 months | Colchicine = 396 Placebo = 399 | 12 months |
|
| Deftereos et al., 2013 [55] | Double-blind, prospective, placebo-controlled study. Diabetic patients with contraindication to a drug-eluting stent, undergoing PCI with a BMS, were randomized to colchicine or placebo. Angiography and intravascular ultrasound was performed 6 months after the index PCI | Primary: Angiographic and IVUS restenosis Secondary: angiographic and IVUS parameters of lumen loss and in-stent neointimal hyperplasia | 0.5 mg twice daily | Colchicine = 100 Placebo = 110 | 6 months |
|
| Opstal et al., 2023 [63] | Randomized, parallel, double-blind trial that evaluated the effect of adding colchicine or placebo in patients with chronic coronary disease | Cause-specific mortality data were analyzed, stratified by treatment status | 0.5 mg once daily | Colchicine = 2762 Placebo = 2760 | 29 months |
|
| Jolly et al., 2024 [56] | Multicenter trial with a two-by-two factorial design randomly assigning patients who had myocardial infarction to receive either colchicine or placebo and either spironolactone or placebo. | Primary composite: death from cardiovascular causes, recurrent MI, stroke, or unplanned ischemia-driven coronary revascularization | For the first 90 days: patients weighing >70 kg 0.5 mg twice daily, if <70 kg 0.5 mg daily. After 90 days, 0.5 mg daily for all patients | Colchicine = 3528 Placebo = 3534 | 2.98 years |
|
| Mewton et al., 2021 [65] | Double-blind multicenter trial. Patients admitted for a first episode of STEMI referred for PCI were randomized to receive colchicine or placebo from admission to day 5. Patients underwent a cardiac magnetic resonance at 5 days and at 30 days | Primary: reduction of IS at 5 days. Secondary: LV end-diastolic volume change at 3 months and IS at 3 months | 2 mg loading dose followed by 0.5 mg twice a day for 5 days | Colchicine = 101 Placebo = 91 | 3 months |
|
| Bouleti et al., 2024 [66] | Follow-up analysis of the COVERT-MI study on prespecified secondary clinical endpoints | Primary composite: all-cause death, ACS, heart failure events, ischemic strokes, sustained ventricular arrhythmias, and acute kidney injury | 2 mg loading dose followed by 0.5 mg twice a day for 5 days | Colchicine = 101 Placebo = 91 | 1 year |
|
| Deftereos et al., 2015 [55] | Prospective, double-blinded, placebo-controlled study. Patients presenting with STEMI ≤12 h from pain onset (treated with PCI) were randomly assigned to colchicine or placebo for 5 days. A subset of patients underwent cardiac MRI 6 to 9 days after the index STEMI (MRI subgroup) | Primary: area under the curve of CK-MB fraction concentration over 72 h after admission Secondary: Maximal high-sensitivity troponin T measure during the same time-period. In MRI subgroup, absolute MI volume, determined by LGE, was the primary outcome measure | Loading dose of 2 mg (1.5 mg initially followed by 0.5 mg 1 h later) and continuing with 0.5 mg twice daily | Colchicine = 77 Placebo = 74 MRI subgroup = 60 | 5 days, until 9 days for MRI subgroup |
|
| Shah et al., 2020 [67] | Randomized, double-blind, placebo-controlled trial. Subjects referred for possible PCI were randomized to acute pre-procedural oral administration of colchicine or placebo | Primary: PCI-related myocardial injury according to the Universal Definition Secondary: Occurrence of 30-day MACEs (earliest occurrence of death from any cause, nonfatal MI, or target vessel revascularization) PCI-related MI as defined by the SCAI (76) | 1.2 mg 1 to 2 h before coronary angiography, followed by colchicine; 0.6 mg 1 h later or immediately pre-procedure | Colchicine = 366 Placebo = 348 Colchicine + PCI = 206 Placebo + PCI = 194 | 30 days |
|
| Cole et al., 2021 [68] | Randomized pilot trial. Patients undergoing PCI for stable angina or NSTEMI were randomized to oral colchicine or placebo, 6 to 24 h pre-procedure | Primary: periprocedural myocardial infarction | 1 mg followed by 0.5 mg 1 h later | Colchicine = 36 Placebo = 39 | 24 h |
|
| Yu et al., 2024 [69] | Prospective, single-center, randomized, double-blind clinical trial. Patients with ACS with lipid-rich plaque detected by optical coherence tomography were included. The subjects were randomly assigned to receive either colchicine or placebo | Primary: Change in the minimal fibrous cap thickness from baseline to the 12-month follow-up | 0.5 mg once daily | Colchicine = 52 Placebo = 52 | 12 months |
|
| Vaidya et al., 2018 [70] | Prospective non-randomized observational study. Patients with recent ACS (<1 month), received either colchicine plus OMT or OMT alone | Primary: change in LAPV, a marker of plaque instability on CCTA and robust predictor of adverse cardiovascular events. Secondary: changes in other CCTA measures and in hs-CRP | 0.5 mg daily | Colchicine = 40 Placebo = 40 | 12.6 months |
|
| Zuriaga et al., 2024 [71] | TET2-mutant clonal hematopoiesis was modeled in mice using bone marrow transplants in Ldlr−/− mice, treated with colchicine or placebo. In humans, data from two large biobanks were analyzed to assess if colchicine reduces the link between TET2 mutations and myocardial infarction | In mice: starting with 0.05 mg/kg/day for the first week, and transitioning to 0.1 mg/kg/day for the second week, and 0.2 mg/kg/day for the remaining 6 weeks | Humans Colchicine = 3849 Non colchicine users: 433,387 | - | Mouse Model
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, S.; Camera, M.; Brambilla, M.; Sarto, G.; Spadafora, L.; Bernardi, M.; Iaconelli, A.; D’Amario, D.; Biondi-Zoccai, G.; Celia, A.I.; et al. Combining Colchicine and Antiplatelet Therapy to Tackle Atherothrombosis: A Paradigm in Transition? Int. J. Mol. Sci. 2025, 26, 1136. https://doi.org/10.3390/ijms26031136
Giordano S, Camera M, Brambilla M, Sarto G, Spadafora L, Bernardi M, Iaconelli A, D’Amario D, Biondi-Zoccai G, Celia AI, et al. Combining Colchicine and Antiplatelet Therapy to Tackle Atherothrombosis: A Paradigm in Transition? International Journal of Molecular Sciences. 2025; 26(3):1136. https://doi.org/10.3390/ijms26031136
Chicago/Turabian StyleGiordano, Salvatore, Marina Camera, Marta Brambilla, Gianmarco Sarto, Luigi Spadafora, Marco Bernardi, Antonio Iaconelli, Domenico D’Amario, Giuseppe Biondi-Zoccai, Alessandra Ida Celia, and et al. 2025. "Combining Colchicine and Antiplatelet Therapy to Tackle Atherothrombosis: A Paradigm in Transition?" International Journal of Molecular Sciences 26, no. 3: 1136. https://doi.org/10.3390/ijms26031136
APA StyleGiordano, S., Camera, M., Brambilla, M., Sarto, G., Spadafora, L., Bernardi, M., Iaconelli, A., D’Amario, D., Biondi-Zoccai, G., Celia, A. I., Tremoli, E., Frati, G., Angiolillo, D. J., Sciarretta, S., & Galli, M. (2025). Combining Colchicine and Antiplatelet Therapy to Tackle Atherothrombosis: A Paradigm in Transition? International Journal of Molecular Sciences, 26(3), 1136. https://doi.org/10.3390/ijms26031136










