Molecular and Physiological Responses of Plants that Enhance Cold Tolerance
Abstract
1. Introduction
2. Plant Physiological Responses to Low-Temperature Stress
2.1. Impact of Low Temperature on Plant Cell Membrane Stability and Metabolism
2.2. Impact of Low Temperature on ROS Metabolism and Antioxidant Defense in Plants
2.3. Role of Osmotic Regulators in Enhancing Cold Tolerance in Plants
2.4. Regulatory Roles of Plant Hormones During Low-Temperature Stress
2.4.1. Abscisic Acid (ABA)
2.4.2. Auxin (IAA)
2.4.3. Gibberellin (GA)
2.4.4. Cytokinin (CTK)
2.4.5. Ethylene (ETH)
2.4.6. Jasmonic Acid (JA)
3. Molecular Mechanisms of Cold Acclimation
3.1. CBF-Dependent Mechanism
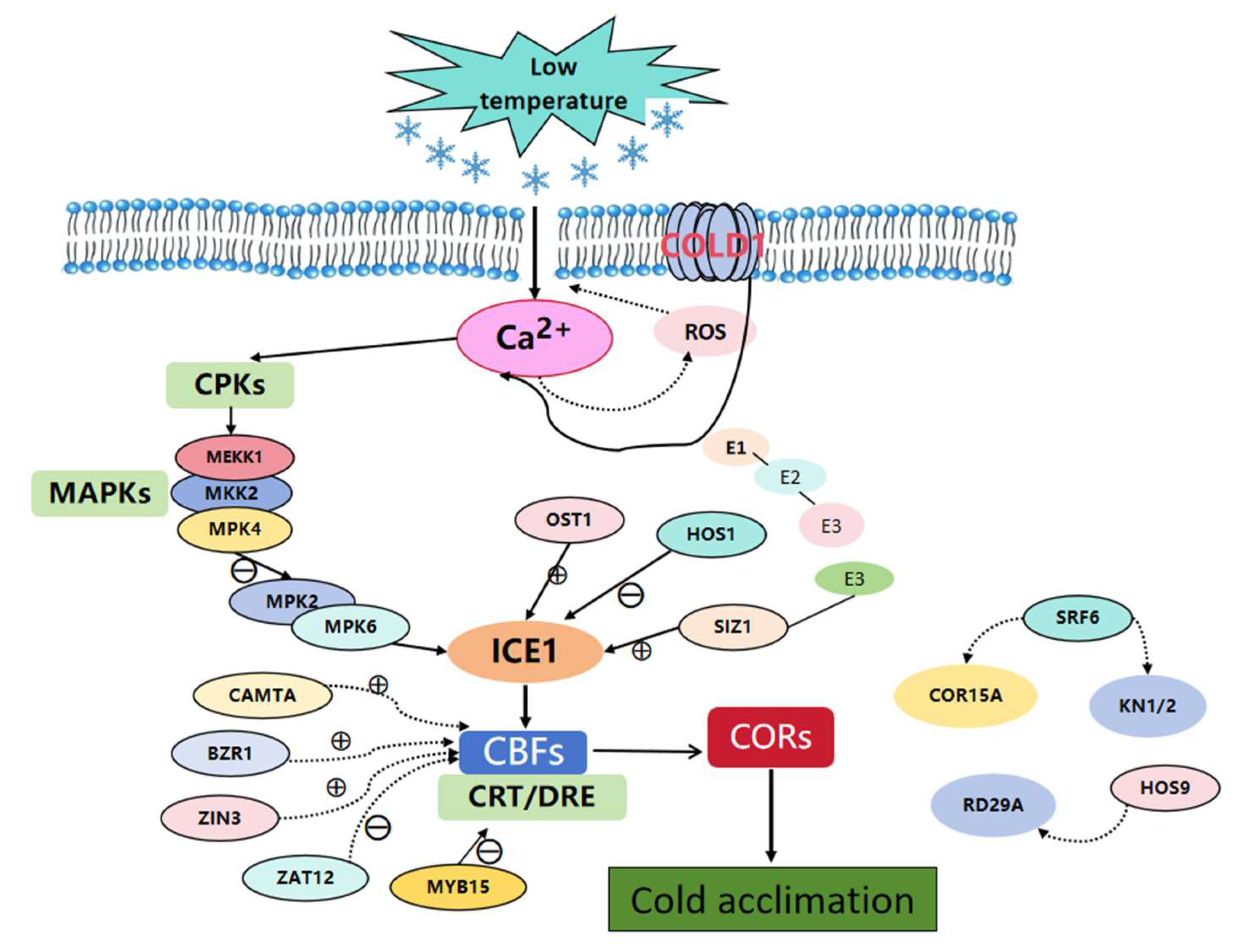
3.1.1. ICE1-CBF-COR Regulatory Pathway
3.1.2. Positive Regulation in CBF Transcriptional Regulation
3.1.3. Negative Regulatory Roles in CBF Transcriptional Regulation
3.2. CBF Independent Mechanisms
3.3. Post-Transcriptional Regulation
3.4. Post-Translational Regulation
3.4.1. Phosphorylation Modification
3.4.2. Ubiquitination and SUMOylation Modifications
3.5. Non-Coding RNA Regulation
3.5.1. Mechanisms of miRNAs in Regulating Plant Tolerance to Low Temperature
3.5.2. Mechanisms of lncRNA in Regulating Plant Tolerance to Low Temperature
3.5.3. Mechanisms of siRNA in Regulating Plant Tolerance to Low Temperature
3.6. Epigenetic Mechanism Responses to Stress
3.6.1. DNA Methylation
3.6.2. Histone Modifications
3.6.3. Chromatin Remodeling
3.6.4. Non-Coding RNA Regulation
4. Research Progress on Improving Plant Cold Resistance by Genetic Engineering
5. Future Directions
5.1. In-Depth Analysis of Molecular Regulatory Mechanisms
5.1.1. Refinement of Epigenetic Regulatory Networks
5.1.2. Precise Regulation of Transcription Factors and Downstream Genes
5.1.3. Role of Post-Translational Modifications of Proteins
5.2. Completion of the Signal Transduction Pathway for Low Temperature Responses
5.2.1. Mechanisms of Signal Molecules
5.2.2. Mechanisms of Low Temperature Sensing by the Cell Membrane
5.3. Integrated Analysis of Multi-Omics
5.3.1. Integration of Transcriptomic, Proteomic, and Metabolomic Data
5.3.2. Spatiotemporal Multi-Omics Research
5.4. Interaction Between Plant Low Temperature Responses and Other Environmental Factors
5.4.1. Synergistic Effects of Low Temperature with Drought, Salt Stress, etc.
5.4.2. Interaction Between Low Temperature and Microorganisms
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Saijo, Y.; Loo, E.P. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kapoor, D.; Gautam, S.; Landi, M.; Kandhol, N.; Araniti, F.; Ramakrishnan, M.; Satish, L.; Singh, V.P.; Sharma, P.; et al. Heavy metal induced regulation of plant biology: Recent insights. Physiol. Plant. 2022, 174, e13688. [Google Scholar] [CrossRef]
- Nowicka, B.; Ciura, J.; Szymańska, R.; Kruk, J. Improving photosynthesis, plant productivity and abiotic stress tolerance-current trends and future perspectives. J. Plant Physiol. 2018, 231, 415–433. [Google Scholar] [CrossRef]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022, 27, 922–935. [Google Scholar] [CrossRef]
- Kim, J.S.; Kidokoro, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulatory networks in plant responses to drought and cold stress. Plant Physiol. 2024, 195, 170–189. [Google Scholar] [CrossRef]
- Nakaminami, K.; Seki, M. RNA Regulation in Plant Cold Stress Response. Adv. Exp. Med. Biol. 2018, 1081, 23–44. [Google Scholar]
- Li, J.; Zhang, Z.; Chong, K.; Xu, Y. Chilling tolerance in rice: Past and present. J. Plant Physiol. 2022, 268, 153576. [Google Scholar] [CrossRef]
- Zhao, M.; Tian, R.; Sun, X.; Zhang, W.H. lncRNA MtCIR2 positively regulates plant-freezing tolerance by modulating CBF/DREB1 gene clusters. Plant Cell Environ. 2023, 46, 2450–2469. [Google Scholar] [CrossRef]
- Lin, R.; Song, J.; Tang, M.; Wang, L.; Yu, J.; Zhou, Y. CALMODULIN6 negatively regulates cold tolerance by attenuating ICE1-dependent stress responses in tomato. Plant Physiol. 2023, 193, 2105–2121. [Google Scholar] [CrossRef]
- Su, C.F.; Wang, Y.C.; Hsieh, T.H.; Lu, C.A.; Tseng, T.H.; Yu, S.M. A novel MYBS3-dependent pathway confers cold tolerance in rice. Plant Physiol. 2010, 153, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Ma, S.L.; Bai, L.P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Knight, H.; Deyholos, M. The sfr6 mutant of Arabidopsis is defective in transcriptional activation via CBF/DREB1 and DREB2 and shows sensitivity to osmotic stress. Plant J. 2003, 34, 395–406. [Google Scholar] [CrossRef]
- Steiner, P.; Buchner, O.; Andosch, A.; Wanner, G.; Neuner, G.; Lütz-Meindl, U. Fusion of Mitochondria to 3-D Networks, Autophagy and Increased Organelle Contacts are Important Subcellular Hallmarks during Cold Stress in Plants. Int. J. Mol. Sci. 2020, 21, 8753. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Huang, Y.X.; Zhou, Z.F.; Zhou, S.; Duan, W.X.; Yang, C.F.; Gao, Y.J.; Zhang, G.M.; Song, X.P.; Zhang, X.Q.; et al. Cold-Induced Physiological and Biochemical Alternations and Proteomic Insight into the Response of Saccharum spontaneum to Low Temperature. Int. J. Mol. Sci. 2022, 23, 14244. [Google Scholar] [CrossRef]
- Zareei, E.; Karami, F.; Gholami, M.; Ershadi, A.; Avestan, S.; Aryal, R.; Gohari, G.; Farooq, M. Physiological and biochemical responses of strawberry crown and leaf tissues to freezing stress. BMC Plant Biol. 2021, 21, 532. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar]
- Manchanda, P.; Chaudhary, P.; Deswal, R. Photosynthesis regulation, cell membrane stabilization and methylglyoxal detoxification seems major altered pathways under cold stress as revealed by integrated multi-omics meta-analysis. Physiol. Mol. Biol. Plants. 2023, 29, 1395–1407. [Google Scholar] [CrossRef]
- Mishra, G.; Mohapatra, S.K.; Rout, G.R. Plant membrane transporters function under abiotic stresses: A review. Planta 2024, 260, 125. [Google Scholar] [CrossRef]
- Herrera, C.M.; Voss, B.J.; Trent, M.S. Homeoviscous Adaptation of the Acinetobacter baumannii Outer Membrane: Alteration of Lipooligosaccharide Structure during Cold Stress. mBio 2021, 12, e0129521. [Google Scholar] [CrossRef]
- Xie, M.; Koch, E.H.W.; van Walree, C.A.; Sobota, A.; Sonnen, A.F.P.; Breukink, E.; Killian, J.A.; Lorent, J.H. Two separate mechanisms are involved in membrane permeabilization during lipid oxidation. Biophys. J. 2023, 122, 4503–4517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, S.Y.; Bai, B.; Chen, Y.J.; Xiang, Z.P.; Chen, C.; Kuang, X.M.; Yang, Y.Z.; Fu, J.; Chen, L.B.; et al. OsKASI-2 is required for the regulation of unsaturation levels of membrane lipids and chilling tolerance in rice. Plant Biotechnol. J. 2024, 22, 2157–2172. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, W.C.; Han, C.; Wang, S.; Bai, M.Y.; Song, C.P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Ali, M.F.; Muday, G.K. Reactive oxygen species are signaling molecules that modulate plant reproduction. Plant Cell Environ. 2024, 47, 1592–1605. [Google Scholar] [CrossRef]
- Ambekar, A.A.; Sivaperumal, P.; Kamala, K.; Kubal, P.; Prakash, C. Effect of temperature changes on antioxidant enzymes and oxidative stress in gastropod Nerita oryzarum collected along India’s first Tarapur Atomic Power Plant site. Environ. Res. 2023, 216, 114334. [Google Scholar] [CrossRef]
- Khanna, K.; Bhardwaj, R.; Alam, P.; Reiter, R.J.; Ahmad, P. Phytomelatonin: A master regulator for plant oxidative stress management. Plant Physiol. Biochem. 2023, 196, 260–269. [Google Scholar] [CrossRef]
- Marta, B.; Szafrańska, K.; Posmyk, M.M. Exogenous Melatonin Improves Antioxidant Defense in Cucumber Seeds (Cucumis sativus L.) Germinated under Chilling Stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar] [CrossRef]
- Wu, Z.X.; Xu, N.W.; Yang, M.; Li, X.L.; Han, J.L.; Lin, X.H.; Yang, Q.; Lv, G.H.; Wang, J. Responses of photosynthesis, antioxidant enzymes, and related gene expression to nicosulfuron stress in sweet maize (Zea mays L.). Environ. Sci. Pollut. Res. Int. 2022, 29, 37248–37265. [Google Scholar] [CrossRef]
- Sodhi, G.K.; Saxena, S. Plant growth-promoting endophyte Nigrospora oryzae mitigates abiotic stress in rice (Oryza sativa L.). FEMS Microbiol. Ecol. 2023, 99, fiad094. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Y.H.; Ai, C.X.; Zhang, H.; Huang, Y.C.; Zou, W.G. Different cold tolerances among three strains of large yellow croaker: Related to antioxidant defense and energy metabolism. Fish. Physiol. Biochem. 2023, 49, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Ge, W.; Zhou, Q.; Zhou, X.; Luo, M.; Zhao, Y.; Wei, B.; Ji, S. Exogenous glutathione alleviates chilling injury in postharvest bell pepper by modulating the ascorbate-glutathione (AsA-GSH) cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Ascorbate peroxidase in fruits and modulation of its activity by reactive species. J. Exp. Bot. 2024, 75, 2716–2732. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wei, Q.; Kong, Y.; Zhu, L.; Tian, W.; Huang, J.; Pan, L.; Jin, Q.; Zhang, J.; Zhu, C. Unearthing the Alleviatory Mechanisms of Brassinolide in Cold Stress in Rice. Life 2022, 12, 833. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous Melatonin Enhances Cold, Salt and Drought Stress Tolerance by Improving Antioxidant Defense in Tea Plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826. [Google Scholar] [CrossRef]
- Guo, Z.; Cai, L.; Liu, C.; Chen, Z.; Guan, S.; Ma, W.; Pan, G. Low-temperature stress affects reactive oxygen species, osmotic adjustment substances, and antioxidants in rice (Oryza sativa L.) at the reproductive stage. Sci Rep. 2022, 12, 6224.31. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Yang, H.; Wang, T.; Yu, X.; Yang, Y.; Wang, C.; Yang, Q.; Wang, X. Enhanced sugar accumulation and regulated plant hormone signalling genes contribute to cold tolerance in hypoploid Saccharum spontaneum. BMC Genom. 2020, 21, 507. [Google Scholar] [CrossRef]
- Li, M.; Yue, T.; Han, J.; Wang, J.; Xiao, H.; Shang, F. Exogenous glucose irrigation alleviates cold stress by regulating soluble sugars, ABA and photosynthesis in melon seedlings. Plant Physiol. Biochem. 2024, 217, 109214. [Google Scholar] [CrossRef]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A unified mechanism in plant development and stress response? Plants 2024, 14, 2. [Google Scholar] [CrossRef]
- Jahed, K.R.; Saini, A.K.; Sherif, S.M. Coping with the cold: Unveiling cryoprotectants, molecular signaling pathways, and strategies for cold stress resilience. Front. Plant Sci. 2023, 14, 1246093. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Luobu, Z.; Zhuoga, D.; Wei, X.; Tang, Y. Advances in plant response to low-temperature stress. Plant Growth Regul. 2024, 104, 1–19. [Google Scholar] [CrossRef]
- Njenga, R.; Boele, J.; Öztürk, Y.; Koch, H.-G. Coping with stress: How bacteria fine-tune protein synthesis and protein transport. J. Biol. Chem. 2023, 299, 105163. [Google Scholar] [CrossRef]
- Peppino Margutti, M.; Vilchez, A.C.; Sosa-Alderete, L.; Agostini, E.; Villasuso, A.L. Lipid signaling and proline catabolism are activated in barley roots (Hordeum vulgare L.) during recovery from cold stress. Plant Physiol. Biochem. 2024, 206, 108208. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, Z.; Chen, J.; Dong, Y.; Qu, K.; Guo, T.; Wang, F.; Liu, A.; Chen, S.; Li, X. Reactive oxygen species signaling in melatonin-mediated plant stress response. Plant Physiol. Biochem. 2024, 207, 108398. [Google Scholar] [CrossRef]
- Sagor, G.H.M.; Inoue, M.; Kusano, T.; Berberich, T. Expression profile of seven polyamine oxidase genes in rice (Oryza sativa) in response to abiotic stresses, phytohormones and polyamines. Physiol. Mol. Biol. Plants 2021, 27, 1353–1359. [Google Scholar] [CrossRef]
- Alcázar, R.; Cuevas, J.C.; Planas, J.; Zarza, X.; Bortolotti, C.; Carrasco, P.; Salinas, J.; Tiburcio, A.F.; Altabella, T. Integration of polyamines in the cold acclimation response. Plant Sci. 2011, 180, 31–38. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Fatima, T.; Handa, A.K.; Mattoo, A.K. Polyamines and Their Biosynthesis/Catabolism Genes Are Differentially Modulated in Response to Heat Versus Cold Stress in Tomato Leaves (Solanum lycopersicum L.). Cells 2020, 9, 1749. [Google Scholar] [CrossRef]
- Cuevas, J.C.; López-Cobollo, R.; Alcázar, R.; Zarza, X.; Koncz, C.; Altabella, T.; Salinas, J.; Tiburcio, A.F.; Ferrando, A. Putrescine as a signal to modulate the indispensable ABA increase under cold stress. Plant Signal Behav. 2009, 4, 219–220. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Zhou, X.; Muhammad, I.; Lan, H.; Xia, C. Recent Advances in the Analysis of Cold Tolerance in Maize. Front. Plant Sci. 2022, 13, 866034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Liu, S.Y.; Ma, J.H.; Wang, X.K.; Haq, S.U.; Meng, Y.C.; Zhang, Y.M.; Chen, R.G. CaDHN4, a Salt and Cold Stress-Responsive Dehydrin Gene from Pepper Decreases Abscisic Acid Sensitivity in Arabidopsis. Int. J. Mol. Sci. 2019, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A. Auxin: A regulator of cold stress response. Physiol. Plant. 2013, 147, 28–35. [Google Scholar] [CrossRef]
- Yang, J.; Lin, S.; Shen, Y.; Ye, J.; Jiang, X.; Li, S.; Jiang, M. Transcriptome analysis of Sesuvium portulacastrum L. uncovers key genes and pathways involved in root formation in response to low-temperature stress. Plant Mol. Biol. 2024, 114, 89. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Prerostova, S.; Černý, M.; Dobrev, P.I.; Motyka, V.; Hluskova, L.; Zupkova, B.; Gaudinova, A.; Knirsch, V.; Janda, T.; Brzobohatý, B.; et al. Light Regulates the Cytokinin-Dependent Cold Stress Responses in Arabidopsis. Front. Plant Sci. 2021, 11, 608711. [Google Scholar] [CrossRef]
- Qian, L.; Yin, S.; Lu, N.; Yue, E.; Yan, J. Full-length transcriptome reveals the pivotal role of ABA and ethylene in the cold stress response of Tetrastigma hemsleyanum. Front. Plant Sci. 2024, 15, 1285879. [Google Scholar] [CrossRef]
- Kumar, D.; Hazra, S.; Datta, R.; Chattopadhyay, S. Transcriptome analysis of Arabidopsis mutants suggests a crosstalk between ABA, ethylene and GSH against combined cold and osmotic stress. Sci. Rep. 2016, 6, 36867. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Molecular Regulation of CBF Signaling in Cold Acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef]
- Xie, Z.; Lin, W.; Yu, G.; Cheng, Q.; Xu, B.; Huang, B. Improved cold tolerance in switchgrass by a novel CCCH-type zinc finger transcription factor gene, PvC3H72, associated with ICE1-CBF-COR regulon and ABA-responsive genes. Biotechnol. Biofuels 2019, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; He, L.; Li, F. Understanding cold stress response mechanisms in plants: An overview. Front. Plant Sci. 2024, 15, 1443317. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ding, Y.; Yang, S. Cold signal transduction and its interplay with phytohormones during cold acclimation. Plant Cell Physiol. 2015, 56, 7–15. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Li, M.; Yang, H.; Fu, D.; Lv, J.; Ding, Y.; Gong, Z.; Shi, Y.; Yang, S. The direct targets of CBFs: In cold stress response and beyond. J. Integr. Plant Biol. 2021, 63, 1874–1887. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Zhang, Z.J.; Xie, S.J. Mutational evidence for the critical role of CBF2 transcription factors in cold acclimation in Arabidopsis. Plant Physiol. 2016, 171, 2744–2759. [Google Scholar] [CrossRef]
- Novillo, F.; Medina, J.; Salinas, J. Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc. Natl. Acad. Sci. USA 2007, 104, 21002–21007. [Google Scholar] [CrossRef]
- Novillo, F.; Alonso, J.M.; Ecker, J.R. CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 3985–3990. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low- temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef]
- Liu, Y.K.; Dang, P.Y.; Liu, L.X. Cold acclimation by the CBF-COR pathway in a changing climate: Lessons from Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 511–519. [Google Scholar] [CrossRef]
- Lee, B.H.; Henderson, D.A.; Zhu, J.K. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 2005, 17, 3155–3175. [Google Scholar] [CrossRef]
- Wu, C.L.; Lin, L.F.; Hsu, H.C.; Huang, L.F.; Hsiao, C.D.; Chou, M.L. Saussurea involucrata (Snow Lotus) ICE1 and ICE2 Orthologues Involved in Regulating Cold Stress Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2021, 22, 10850. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, M.; Lee, J.H.; Lee, H.J.; Park, C.M. The unified ICE-CBF pathway provides a transcriptional feedback control of freezing tolerance during cold acclimation in Arabidopsis. Plant Mol. Biol. 2015, 89, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR Signaling Cascade and Its Regulation in Plants Responding to Cold Stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef]
- Doherty, C.J.; Van Buskirk, H.A.; Myers, S.J.; Thomashow, M.F. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 2009, 21, 972–984. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Cao, K.F.; Wei, Y.Y.; Jiang, S.; Ye, J.F.; Xu, F.; Chen, Y.; Shao, X.F. PpBZR1, a BES/BZR transcription factor, enhances cold stress tolerance by suppressing sucrose degradation in peach fruit. Plant Physiol. Biochem. 2023, 202, 107972. [Google Scholar] [CrossRef]
- Fan, C.; Guo, G.; Yan, H.; Qiu, Z.; Liu, Q.; Zeng, B. Characterization of Brassinazole resistant (BZR) gene family and stress induced expression in Eucalyptus grandis. Physiol. Mol. Biol. Plants 2018, 24, 821–831. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, P.; Yan, Y.; Bao, C.; Li, X.; Wang, L.; Shen, X.; Li, H.; Liu, X.; Niu, C.; et al. An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. New Phytol. 2018, 218, 201–218. [Google Scholar] [CrossRef]
- Zhou, M.Q.; Shen, C.; Wu, L.H.; Tang, K.X.; Lin, J. CBF-dependent signaling pathway: A key responder to low temperature stress in plants. Crit. Rev. Biotechnol. 2011, 31, 186–192. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, S.; Hou, L.; Huang, X.; Zhang, X.; Guo, H.; Yang, S. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 2012, 24, 2578–2595. [Google Scholar] [CrossRef]
- Jung, J.H.; Seo, P.J.; Park, C.M. The E3 ubiquitin ligase HOS1 regulates Arabidopsis flowering by mediating CONSTANS degradation under cold stress. J. Biol. Chem. 2012, 287, 43277–43287. [Google Scholar] [CrossRef]
- Zhu, J.H.; Shi, H.Z.; Lee, B.H. An Arabidopsis homeodomain transcription factor gene, HOS9, mediates cold tolerance through a CBF-independent pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 9873–9878. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.S.; Wang, Z.B.; Yao, S.Q.; Liu, A. The ARF2-ANT-COR15A gene cascade regulates ABA-signaling-mediated resistance of large seeds to drought in Arabidopsis. J. Cell Sci. 2015, 128, 3922–3932. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.H.; Hu, X.; Tang, W.; Zheng, X.; Kim, Y.S.; Lee, B.H.; Zhu, J.K. A putative Arabidopsis nucleoporin, AtNUP160, is critical for RNA export and required for plant tolerance to cold stress. Mol. Cell Biol. 2006, 26, 9533–9543. [Google Scholar] [CrossRef] [PubMed]
- Msanne, J.; Lin, J.; Stone, J.M.; Awada, T. Characterization of abiotic stress-responsive Arabidopsis thaliana RD29A and RD29B genes and evaluation of transgenes. Planta 2011, 234, 97–107. [Google Scholar] [CrossRef]
- Castro, P.H.; Tavares, R.M.; Bejarano, E.R.; Azevedo, H. SUMO, a heavyweight player in plant abiotic stress responses. Cell Mol. Life Sci. 2012, 69, 3269–3283. [Google Scholar] [CrossRef]
- Sheremet, Y.A.; Yemets, A.I.; Blume, Y.B. Inhibitors of tyrosine kinases and phosphatases as a tool for the investigation of microtubule role in plant cold response. Tsitol Genet. 2012, 46, 3–9. [Google Scholar] [CrossRef]
- Ding, Y.; Li, H.; Zhang, X.; Xie, Q.; Gong, Z.; Yang, S. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev. Cell 2015, 32, 278–289. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef]
- Huang, Y.; Xiong, K.; Wang, A.; Wang, Z.; Cui, Q.; Xie, H.; Yang, T.; Fan, X.; Jiang, W.; Tan, X.; et al. Cold stress causes liver damage by inducing ferroptosis through the p38 MAPK/Drp1 pathway. Cryobiology 2023, 113, 104563. [Google Scholar] [CrossRef]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3- and MPK6-Mediated ICE1 Phosphorylation Negatively Regulates ICE1 Stability and Freezing Tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642.e4. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Song, C.P.; Gong, Z.; Yang, S.; Ding, Y. PUB25 and PUB26 dynamically modulate ICE1 stability via differential ubiquitination during cold stress in Arabidopsis. Plant Cell 2023, 35, 3585–3603. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, G.; Chen, D.; Jiang, L.; Huang, B.; Jiang, P.; Zhang, C.; Qin, X. Effect of vitamin E on energy metabolism indicators and gill tissue structure of crucian carp (Carassius auratus) under cooling stress. Sci. Rep. 2024, 14, 19484. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, J.; Chen, D.; Wang, Y. E3 ubiquitin ligases: Styles, structures and functions. Mol. Biomed. 2021, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Chaikam, V.; Karlson, D.T. Response and transcriptional regulation of rice SUMOylation system during development and stress conditions. BMB Rep. 2010, 43, 103–109. [Google Scholar] [CrossRef]
- Sayed, E.G.; Desoukey, S.F.; Desouky, A.F.; Farag, M.F.; EI-Kholy, R.I.; Azoz, S.N. Synergistic Influence of Arbuscular mycorrhizal Fungi Inoculation with Nanoparticle Foliar Application Enhances Chili (Capsicum annuum L.) Antioxidant Enzymes, Anatomical Characteristics, and Productivity under Cold-Stress Conditions. Plants 2024, 13, 517. [Google Scholar] [CrossRef]
- Imaduwage, I.; Hewadikaram, M. Predicted roles of long non-coding RNAs in abiotic stress tolerance responses of plants. Mol. Hortic. 2024, 4, 20. [Google Scholar] [CrossRef]
- Vakilian, K.A. Machine learning improves our knowledge about miRNA functions towards plant abiotic stresses. Sci. Rep. 2020, 10, 3041. [Google Scholar]
- Bustamante, A.; Marques, M.C.; Sanz-Carbonell, A.; Mulet, J.M.; Gomez, G. Alternative processing of its precursor is related to miR319 decreasing in melon plants exposed to cold. Sci. Rep. 2022, 12, 18512. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, C.; Xue, Y.Y.; Tian, Y.X.; Zhang, H.Q.; Li, N.; Sheng, C.; Jiang, H.F.; Bai, D.M. Small RNA and Degradome Deep Sequencing Reveals the Roles of microRNAs in Peanut (Arachis hypogaea L.) Cold Response. Front. Plant Sci. 2022, 13, 920195. [Google Scholar] [CrossRef]
- Zhu, M.; Dong, Q.; Bing, J.; Songbuerbatu; Zheng, L.; Dorjee, T.; Liu, Q.; Zhou, Y.; Gao, F. Combined lncRNA and mRNA Expression Profiles Identified the lncRNA-miRNA-mRNA Modules Regulating the Cold Stress Response in Ammopiptanthus nanus. Int. J. Mol. Sci. 2023, 24, 6502. [Google Scholar] [CrossRef]
- Engelhard, C.A.; Huang, C.; Khani, S.; Kasparek, P.; Prochazka, J.; Rozman, J.; Reguera, D.P.; Sedlacek, R.; Kornfeld, J.W. Comprehensive Transcriptional Profiling and Mouse Phenotyping Reveals Dispensable Role for Adipose Tissue Selective Long Noncoding RNA Gm15551. Noncoding RNA 2022, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Jha, U.C.; Nayyar, H.; Roychowdhury, R.; Prasad, P.V.V.; Parida, S.K.; Siddique, K.H.M. Non-coding RNAs (ncRNAs) in plant: Master regulators for adapting to extreme temperature conditions. Plant Physiol. Biochem. 2023, 205, 108164. [Google Scholar] [CrossRef] [PubMed]
- Sreedevi, P.R.; Suresh, K. Cold atmospheric plasma mediated cell membrane permeation and gene delivery-empirical interventions and pertinence. Adv. Colloid. Interface Sci. 2023, 320, 102989. [Google Scholar] [CrossRef]
- Chung, S.; Kwon, C.; Lee, J.H. Epigenetic control of abiotic stress signaling in plants. Genes Genom. 2022, 44, 267–278. [Google Scholar] [CrossRef]
- Sun, M.; Yang, Z.; Liu, L.; Duan, L. DNA Methylation in Plant Responses and Adaption to Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 6910. [Google Scholar] [CrossRef]
- Song, Y.; Jia, Z.; Hou, Y.; Ma, X.; Li, L.; Jin, X.; An, L. Roles of DNA Methylation in Cold Priming in Tartary Buckwheat. Front. Plant Sci. 2020, 11, 608540. [Google Scholar] [CrossRef]
- Nunez-Vazquez, R.; Desvoyes, B.; Gutierrez, C. Histone variants and modifications during abiotic stress response. Front. Plant Sci. 2022, 13, 984702. [Google Scholar] [CrossRef]
- Sokol, A.; Kwiatkowska, A.; Jerzmanowski, A.; Prymakowska-Bosak, M. Up-regulation of stress-inducible genes in tobacco and Arabidopsis cells in response to abiotic stresses and ABA treatment correlates with dynamic changes in histone H3 and H4 modifications. Planta 2007, 227, 245–254. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Zan, X.; Zhu, J.; Chen, H.; Li, X.; Zhou, Z.; Gao, X.; Chen, R.; Huang, Z.; et al. Overexpression of Rice Histone H1 Gene Reduces Tolerance to Cold and Heat Stress. Plants 2023, 12, 2408. [Google Scholar] [CrossRef]
- Faivre, L.; Kinscher, N.F.; Kuhlmann, A.B.; Xu, X.; Kaufmann, K.; Schubert, D. Cold stress induces rapid gene-specific changes in the levels of H3K4me3 and H3K27me3 in Arabidopsis thaliana. Front. Plant Sci. 2024, 15, 1390144. [Google Scholar] [CrossRef]
- Lim, C.J.; Ali, A.; Park, J.; Shen, M.; Park, K.S.; Baek, D.; Yun, D.J. HOS15-PWR chromatin remodeling complex positively regulates cold stress in Arabidopsis. Plant Signal Behav. 2021, 16, 1893978. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Upadhyay, S.; Bhat, B.; Singh, G.; Bhattacharya, S.; Singh, A. Abiotic stress induced miRNA-TF-gene regulatory network: A structural perspective. Genomics 2020, 112, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cheng, Z.; Dong, S.; Li, Z.; Zou, L.; Zhao, P.; Guo, X.; Bao, Y.; Wang, W.; Peng, M. Global identification of full-length cassava lncRNAs unveils the role of cold-responsive intergenic lncRNA 1 in cold stress response. Plant Cell Environ. 2022, 45, 412–426. [Google Scholar] [CrossRef]
- Thiebaut, F.; Hemerly, A.S.; Ferreira, P.C.G. A role for epigenetic regulation in the adaptation and stress responses of non-model plants. Front. Plant Sci. 2019, 10, 246. [Google Scholar] [CrossRef]
- Xiang, C.; Du, Y.; Han, W.; Guan, B.; Liu, H.; An, Y.; Liu, Y.; Jiang, H.; Chang, J.; Ge, Y. Proper C/N ratio enhances the effect of plant diversity on nitrogen removal and greenhouse effect mitigation in floating constructed wetlands. Environ. Sci. Pollut. Res. Int. 2024, 31, 12036–12051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Z.; Unver, T.; Zhang, B. CRISPR/Cas: A powerful tool for gene function study and crop improvement. J. Adv. Res. 2020, 29, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Zhang, Y.P.; Zhou, J.H.; Wang, L. Mini review roles of the bZIP gene family in rice. Genet. Mol. Res. 2014, 13, 3025–3036. [Google Scholar]
- Berchembrock, Y.V.; Pathak, B.; Maurya, C.; Botelho, F.B.S.; Srivastava, V. Phenotypic and transcriptomic analysis reveals early stress responses in transgenic rice expressing Arabidopsis DREB1a. Plant Direct. 2022, 6, e456. [Google Scholar] [CrossRef]
- Nie, S.; Huang, W.; He, C.; Wu, B.; Duan, H.; Ruan, J.; Zhao, Q.; Fang, Z. Transcription Factor OsMYB2 Triggers Amino Acid Transporter OsANT1 expression to Regulate Rice Growth and Salt Tolerance. Plant Physiol. 2024, 19, kiae559. [Google Scholar] [CrossRef]
- Hu, H.H.; You, J.; Fang, Y.J.; Zhu, X.Y.; Qi, Z.Y.; Xiong, L.Z. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol. Biol. 2008, 67, 169–181. [Google Scholar] [CrossRef]
- Yoshimura, A.; Ito, M.; Mise-Omata, S.; Ando, M. SOCS: Negative regulators of cytokine signaling for immune tolerance. Int. Immunol. 2021, 33, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lin, L.; Zhang, Y.; Sui, N. ZmMYB31, a R2R3-MYB transcription factor in maize, positively regulates the expression of CBF genes and enhances resistance to chilling and oxidative stress. Mol. Biol. Rep. 2019, 46, 3937–3944. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shi, Y.; Liu, J.; Li, Z.; Fu, D.; Wu, S.; Li, M.; Yang, Z.; Shi, Y.; Lai, J.; et al. Natural polymorphism of ZmICE1 contributes to amino acid metabolism that impacts cold tolerance in maize. Nat. Plants 2022, 8, 1176–1190. [Google Scholar] [CrossRef]
- Yang, Y.F.; AI-Baidhani, H.H.J.; Harris, J.; Riboni, M.; Li, Y.; Mazonka, I.; Bazanowa, N.; Chrikova, L.; Hussain, S.S.; Hrmova, M.; et al. DREB/CBF expression in wheat and barley using the stress-inducible promoters of HD-Zip I genes: Impact on plant development, stress tolerance and yield. Plant Biotechnol. J. 2020, 18, 829–844. [Google Scholar] [CrossRef]
- Amalraj, A.; Luang, S.; Kumar, M.Y.; Sornaraj, P.; Eini, O.; Kovalchuk, N.; Bazanova, N.; Li, Y.; Yang, N.N.; Eliby, S.; et al. Change of function of the wheat stress-responsive transcriptional repressor TaRAP2.1L by repressor motif modification. Plant Biotechnol. J. 2016, 14, 820–832. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, X.Y.; Zhang, Z.X. Cold regulated gene LeCOR413PM2 confers cold stress tolerance in tomato plants. Gene 2021, 764, 145097. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Jiang, X.C.; Liu, Q.Y. The HY5 and MYB15 transcription factors positively regulate cold tolerance in tomato via the CBF pathway. Plant Cell Environ. 2020, 43, 2712–2726. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Z.J.; Wu, M.Q. Enhanced brassinosteroid signaling via the overexpression of SlBRI1 positively regulates the chilling stress tolerance of tomato. Plant Sci. 2022, 320, 111281. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Gusain, S.; Joshi, S.; Joshi, R. Sensing, signalling, and regulatory mechanism of cold-stress tolerance in plants. Plant Physiol. Biochem. 2023, 197, 107646. [Google Scholar] [CrossRef]
- Zheng, S.; Su, M.; Wang, L.; Zhang, T.; Wang, J.; Xie, H.; Wu, X.; Haq, S.I.U.; Qiu, Q.S. Small signaling molecules in plant response to cold stress. J. Plant Physiol. 2021, 266, 153534. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, B.; Zhang, H.; Zhang, J.; Cai, J.; Cui, J. Integrative multi-omics analysis of chilling stress in pumpkin (Cucurbita moschata). BMC Genom. 2024, 25, 1042. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Regulatory Networks Underlying Plant Responses and Adaptation to Cold Stress. Annu. Rev. Genet. 2024, 58, 43–65. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Ullah, F.; Zou, J.; Zeng, X. Molecular and Physiological Responses of Plants that Enhance Cold Tolerance. Int. J. Mol. Sci. 2025, 26, 1157. https://doi.org/10.3390/ijms26031157
Zhou L, Ullah F, Zou J, Zeng X. Molecular and Physiological Responses of Plants that Enhance Cold Tolerance. International Journal of Molecular Sciences. 2025; 26(3):1157. https://doi.org/10.3390/ijms26031157
Chicago/Turabian StyleZhou, Lixia, Fazal Ullah, Jixin Zou, and Xianhai Zeng. 2025. "Molecular and Physiological Responses of Plants that Enhance Cold Tolerance" International Journal of Molecular Sciences 26, no. 3: 1157. https://doi.org/10.3390/ijms26031157
APA StyleZhou, L., Ullah, F., Zou, J., & Zeng, X. (2025). Molecular and Physiological Responses of Plants that Enhance Cold Tolerance. International Journal of Molecular Sciences, 26(3), 1157. https://doi.org/10.3390/ijms26031157





