Interaction of the Vagus Nerve and Serotonin in the Gut–Brain Axis
Abstract
1. Introduction
2. Vagus Nerve
3. Synthesis and Secretion of Serotonin in the Gut
3.1. 5-HT Synthesis in Intestinal Chromaffin-Affinity Cells (EC Cells)
3.2. Serotonin Synthesis in the Gut Microbiome
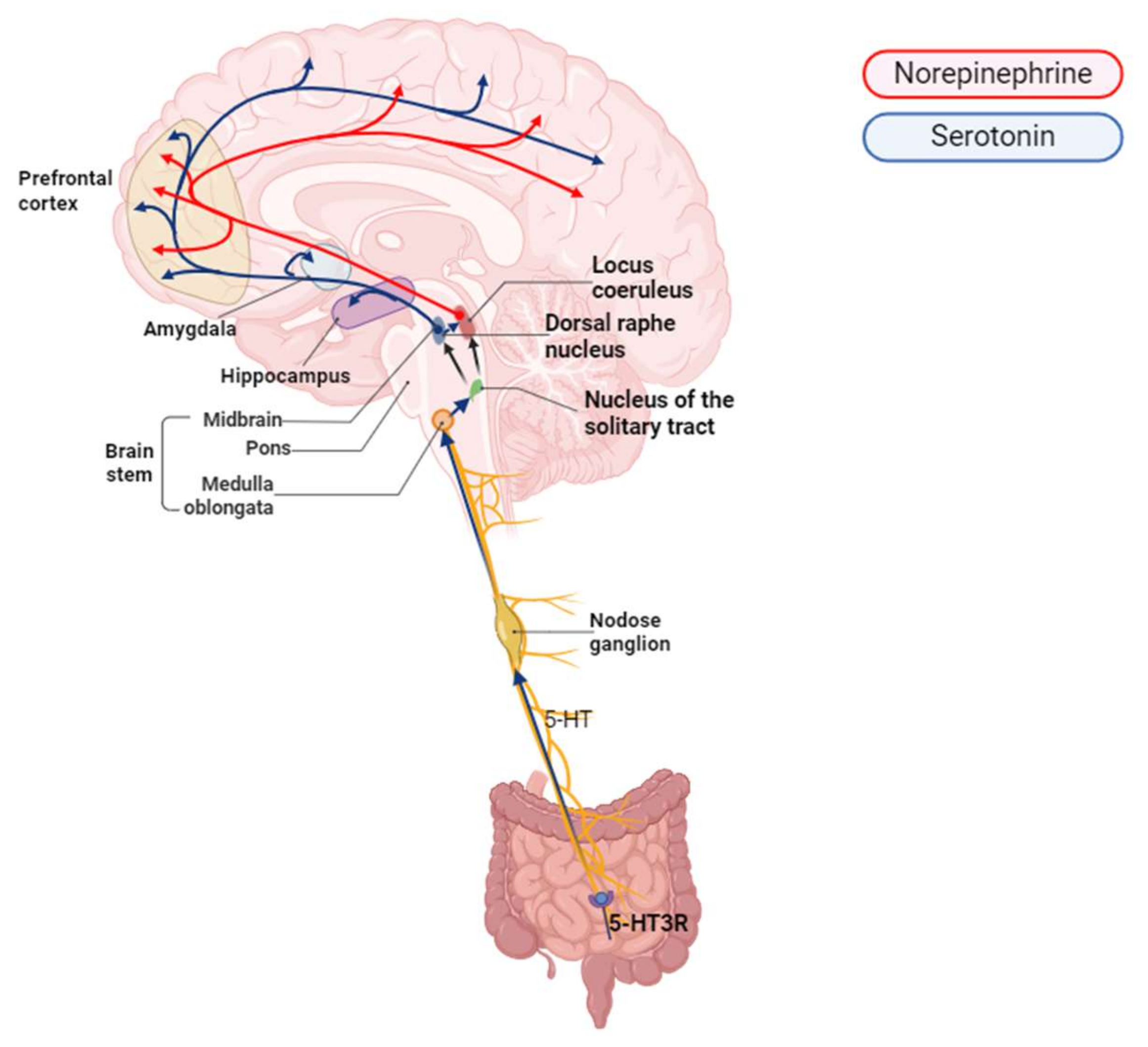
4. Serotonin Produced in the Gut and Delivered to the Afferent Fibers of the Vagus Nerve
5. Signal Processing and Transmission from the NTS to the DRN and Its Connection to the Brain
6. The Role of the NTS in Signaling to the DRN and LC and in Regulating the Release of Serotonin and NE
7. Serotonin and Norepinephrine in Disease
7.1. The Role of Serotonin in MDD and ADS
7.2. The Role of NE in MDD and ADS
7.3. Interaction of Serotonin and Norepinephrine
8. Interspecies Differences in 5-HT Receptor Subtype Expression and Limitations of Analytical Methods
| Receptors | Expression Sites | Receptor Function | References |
|---|---|---|---|
| 5-HT1A | Brainstem, prefrontal cortex, neocortex, hippocampus | Regulates the brain’s mood, anxiety, cognition, sleep, and pain perception. | [109,110,111] |
| 5-HT1B | Cerebral cortex, hippocampus, substantia nigra, brainstem raphe nuclei | Regulates overall serotonin release in the brain by inhibiting serotonin release upon activation. Reduces serotonin release in various brain regions, including the dorsal raphe and raphe median nucleus. | [112,113] |
| 5-HT1F | Neocortex | Activated by a tryptan compound approved for the treatment of migraine. | [114] |
| 5-HT2A | Cortex | Regulates vascular tone, contributes to mood, cognition, and psychedelic effects. Psychedelic substances known for their potent psychoactive effects primarily interact with 5-HTR2A. | [115,116] |
| 5-HT2C | Cortex | Unlike 5-HT2A receptors, 5-HT2C receptors are preferentially distributed in subcortical areas including basal ganglia and related structures | [117] |
| 5-HT3 | Larynx | Forms the only 5-HT receptor subtype that functions as a ligand-gated ion channel (i.e., an ionotropic receptor). | [117] |
| 5-HT4 | Hippocampus | Controls brain physiological functions such as learning and memory, feeding and mood behavior, as well as gastrointestinal transit, couples several G proteins, notably Gs, G13, and in somei cell lines, the q signaling pathway. | [118] |
| 5-HT6 | Prefrontal cortex and insula | Regulates choline function in the brain, modulating GABAergic neurotransmission. | [119] |
9. Discussion
10. Current Challenges and Future Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GBA | Gut–brain axis |
| SCFAs | Short-chain fatty acids |
| NTS | Nucleus tractus solitariu |
| DRN | Dorsal raphe nucleus |
| LC | Locus coeruleus |
| NE | Norepinephrine |
References
- Wachsmuth, H.R.; Weninger, S.N.; Duca, F.A. Role of the gut–brain axis in energy and glucose metabolism. Exp. Mol. Med. 2022, 54, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Baker, G.B.; Dursun, S.M. The Relationship Between the Gut Microbiome-Immune System-Brain Axis and Major Depressive Disorder. Front. Neurol. 2021, 12, 721126. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Kim, Y.-K. Chapter 3—The interactions between gut and brain in psychiatric and neurological disorders. In The Complex Interplay Between Gut-Brain, Gut-Liver, and Liver-Brain Axes; Stasi, C., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 49–65. [Google Scholar] [CrossRef]
- Ahlman, H.; Dahlström, A. Vagal mechanisms controlling serotonin release from the gastrointestinal tract and pyloric motor function. J. Auton. Nerv. Syst. 1983, 9, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Forstenpointner, J.; Maallo, A.M.S.; Elman, I.; Holmes, S.; Freeman, R.; Baron, R.; Borsook, D. The solitary nucleus connectivity to key autonomic regions in humans. Eur. J. Neurosci. 2022, 56, 3938–3966. [Google Scholar] [CrossRef] [PubMed]
- Levinson, S.; Miller, M.; Iftekhar, A.; Justo, M.; Arriola, D.; Wei, W.; Hazany, S.; Avecillas-Chasin, J.M.; Kuhn, T.P.; Horn, A.; et al. A structural connectivity atlas of limbic brainstem nuclei. Front. Neuroimaging 2022, 1, 1009399. [Google Scholar] [CrossRef]
- Everett, B.A.; Tran, P.; Prindle, A. Toward manipulating serotonin signaling via the microbiota-gut-brain axis. Curr. Opin. Biotechnol. 2022, 78, 102826. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Lee, J.; del Carmen Rosas, M.; Chen, J.; Henderson, W.A.; Starkweather, A.R.; Cong, X.S. Associations of neurotransmitters and the gut microbiome with emotional distress in mixed type of irritable bowel syndrome. Sci. Rep. 2022, 12, 1648. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef]
- Jadhav, V.V.; Han, J.; Fasina, Y.; Harrison, S.H. Connecting gut microbiomes and short chain fatty acids with the serotonergic system and behavior in Gallus gallus and other avian species. Front. Physiol. 2022, 13, 1035538. [Google Scholar] [CrossRef]
- Mayer, E.A.; Ryu, H.J.; Bhatt, R.R. The neurobiology of irritable bowel syndrome. Mol. Psychiatry 2023, 28, 1451–1465. [Google Scholar] [CrossRef]
- Pellissier, S.; Dantzer, C.; Canini, F.; Mathieu, N.; Bonaz, B. Psychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndrome. Psychoneuroendocrinology 2010, 35, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Bottari, S.A.; Rodriguez, A.; Williamson, J.B. Influence of Vagus Nerve Stimulation on Mood and Associated Disorders. In Vagus Nerve Stimulation; Frasch, M.G., Porges, E.C., Eds.; Springer: New York, NY, USA, 2024; pp. 131–155. [Google Scholar] [CrossRef]
- Fraga, A.; Mesquita, B.; Esteves-Sousa, D.; Facucho-Oliveira, J.; Albuquerque, M.; Espada-Santos, P.; Cintra, P.; Moutinho, A. The Role of Vagus Nerve Stimulation in Depression: What We Know? Eur. Psychiatry 2022, 65, S566. [Google Scholar] [CrossRef]
- Strodl, E.; Bambling, M.; Parnam, S.; Ritchie, G.; Cramb, S.; Vitetta, L. Probiotics and magnesium orotate for the treatment of major depressive disorder: A randomised double blind controlled trial. Sci. Rep. 2024, 14, 20841. [Google Scholar] [CrossRef] [PubMed]
- Goggins, E.; Mitani, S.; Tanaka, S. Clinical perspectives on vagus nerve stimulation: Present and future. Clin. Sci. 2022, 136, 695–709. [Google Scholar] [CrossRef]
- Asala, S.A.; Bower, A.J. An electron microscope study of vagus nerve composition in the ferret. Anat. Embryol. 1986, 175, 247–253. [Google Scholar] [CrossRef]
- Dolphin, H.; Dukelow, T.; Finucane, C.; Commins, S.; McElwaine, P.; Kennelly, S.P. “The Wandering Nerve Linking Heart and Mind”—The Complementary Role of Transcutaneous Vagus Nerve Stimulation in Modulating Neuro-Cardiovascular and Cognitive Performance. Front. Neurosci. 2022, 16, 897303. [Google Scholar] [CrossRef]
- Prescott, S.L.; Liberles, S.D. Internal senses of the vagus nerve. Neuron 2022, 110, 579–599. [Google Scholar] [CrossRef]
- Cirillo, G.; Negrete-Diaz, F.; Yucuma, D.; Virtuoso, A.; Korai, S.A.; De Luca, C.; Kaniusas, E.; Papa, M.; Panetsos, F. Vagus Nerve Stimulation: A Personalized Therapeutic Approach for Crohn’s and Other Inflammatory Bowel Diseases. Cells 2022, 11, 4103. [Google Scholar] [CrossRef]
- Chang, R.B.; Strochlic, D.E.; Williams, E.K.; Umans, B.D.; Liberles, S.D. Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell 2015, 161, 622–633. [Google Scholar] [CrossRef]
- Rajendran, P.S.; Hadaya, J.; Khalsa, S.S.; Yu, C.; Chang, R.; Shivkumar, K. The vagus nerve in cardiovascular physiology and pathophysiology: From evolutionary insights to clinical medicine. Semin. Cell Dev. Biol. 2024, 156, 190–200. [Google Scholar] [CrossRef]
- Amouyal, C.; Andreelli, F. Increasing GLP-1 Circulating Levels by Bariatric Surgery or by GLP-1 Receptor Agonists Therapy: Why Are the Clinical Consequences so Different? J. Diabetes Res. 2016, 2016, 5908656. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, D.; Fenselau, H. Vagal pathways for systemic regulation of glucose metabolism. Semin. Cell Dev. Biol. 2024, 156, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Vespa, S.; Dricot, L.; Dumoulin, M.; Iachim, E.; Doguet, P.; Vandewalle, G.; El Tahry, R. How Is the Norepinephrine System Involved in the Antiepileptic Effects of Vagus Nerve Stimulation? Front. Neurosci. 2021, 15, 790943. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.B.; Powley, T.L. Vagal innervation of intestines: Afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res. 2007, 329, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198.e116. [Google Scholar] [CrossRef]
- Fung, T.C.; Vuong, H.E.; Luna, C.D.G.; Pronovost, G.N.; Aleksandrova, A.A.; Riley, N.G.; Vavilina, A.; McGinn, J.; Rendon, T.; Forrest, L.R.; et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 2019, 4, 2064–2073. [Google Scholar] [CrossRef]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef]
- Walther, D.J.; Bader, M. A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 2003, 66, 1673–1680. [Google Scholar] [CrossRef]
- Zhang, X.; Beaulieu, J.M.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 2004, 305, 217. [Google Scholar] [CrossRef]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Gershon, M.D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.B.; Reid, W.D. Serotonin in brain: Functional considerations. Adv. Pharmacol. 1968, 6 Pt B, 97–113. [Google Scholar] [CrossRef]
- de las Casas-Engel, M.; Domínguez-Soto, A.; Sierra-Filardi, E.; Bragado, R.; Nieto, C.; Puig-Kroger, A.; Samaniego, R.; Loza, M.; Corcuera, M.T.; Gómez-Aguado, F.; et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. J. Immunol. 2013, 190, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.; Rayo, I.; de Las Casas-Engel, M.; Izquierdo, E.; Alonso, B.; Béchade, C.; Maroteaux, L.; Vega, M.A.; Corbí, Á.L. Serotonin (5-HT) Shapes the Macrophage Gene Profile through the 5-HT(2B)-Dependent Activation of the Aryl Hydrocarbon Receptor. J. Immunol. 2020, 204, 2808–2817. [Google Scholar] [CrossRef] [PubMed]
- de Las Casas-Engel, M.; Corbí, A.L. Serotonin modulation of macrophage polarization: Inflammation and beyond. Adv. Exp. Med. Biol. 2014, 824, 89–115. [Google Scholar] [CrossRef]
- Ritzhaupt, A.; Ellis, A.; Hosie, K.B.; Shirazi-Beechey, S.P. The characterization of butyrate transport across pig and human colonic luminal membrane. J. Physiol. 1998, 507, 819–830. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., 3rd; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, B.; Tian, P.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Daily intake of Lactobacillus alleviates autistic-like behaviors by ameliorating the 5-hydroxytryptamine metabolic disorder in VPA-treated rats during weaning and sexual maturation. Food Funct. 2021, 12, 2591–2604. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luck, B.; Visuthranukul, C.; Ihekweazu, F.D.; Engevik, A.C.; Shi, Z.; Danhof, H.A.; Chang-Graham, A.L.; Hall, A.; Endres, B.T.; et al. Human-Derived Bifidobacterium dentium Modulates the Mammalian Serotonergic System and Gut-Brain Axis. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 221–248. [Google Scholar] [CrossRef]
- Buey, B.; Forcén, A.; Grasa, L.; Layunta, E.; Mesonero, J.E.; Latorre, E. Gut Microbiota-Derived Short-Chain Fatty Acids: Novel Regulators of Intestinal Serotonin Transporter. Life 2023, 13, 1085. [Google Scholar] [CrossRef]
- Barki, N.; Bolognini, D.; Börjesson, U.; Jenkins, L.; Riddell, J.; Hughes, D.I.; Ulven, T.; Hudson, B.D.; Ulven, E.R.; Dekker, N.; et al. Chemogenetics defines a short-chain fatty acid receptor gut-brain axis. Elife 2022, 11, 73777. [Google Scholar] [CrossRef] [PubMed]
- Lagerström, M.C.; Hellström, A.R.; Gloriam, D.E.; Larsson, T.P.; Schiöth, H.B.; Fredriksson, R. The G protein-coupled receptor subset of the chicken genome. PLoS Comput. Biol. 2006, 2, e54. [Google Scholar] [CrossRef] [PubMed]
- Meslin, C.; Desert, C.; Callebaut, I.; Djari, A.; Klopp, C.; Pitel, F.; Leroux, S.; Martin, P.; Froment, P.; Guilbert, E.; et al. Expanding Duplication of Free Fatty Acid Receptor-2 (GPR43) Genes in the Chicken Genome. Genome Biol. Evol. 2015, 7, 1332–1348. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Wurtman, R.J. Brain serotonin content: Physiological dependence on plasma tryptophan levels. Science 1971, 173, 149–152. [Google Scholar] [CrossRef]
- Peters, J.C. Tryptophan nutrition and metabolism: An overview. Adv. Exp. Med. Biol. 1991, 294, 345–358. [Google Scholar] [CrossRef]
- Wirleitner, B.; Neurauter, G.; Schröcksnadel, K.; Frick, B.; Fuchs, D. Interferon-gamma-induced conversion of tryptophan: Immunologic and neuropsychiatric aspects. Curr. Med. Chem. 2003, 10, 1581–1591. [Google Scholar] [CrossRef]
- Hestad, K.A.; Engedal, K.; Whist, J.E.; Farup, P.G. The Relationships among Tryptophan, Kynurenine, Indoleamine 2,3-Dioxygenase, Depression, and Neuropsychological Performance. Front. Psychol. 2017, 8, 1561. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Liu, Y.; Xiao, N.; Suo, H.; Xie, K.; Yang, C.; Wu, C. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation 2012, 35, 1676–1684. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Lutfalla, G.; Forlenza, M. IL10, A Tale of an Evolutionarily Conserved Cytokine across Vertebrates. Crit. Rev. Immunol. 2016, 36, 99–129. [Google Scholar] [CrossRef]
- Powley, T.L.; Jaffey, D.M.; McAdams, J.; Baronowsky, E.A.; Black, D.; Chesney, L.; Evans, C.; Phillips, R.J. Vagal innervation of the stomach reassessed: Brain-gut connectome uses smart terminals. Ann. N. Y. Acad. Sci. 2019, 1454, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Page, A.J. Altered Vagal Signaling and Its Pathophysiological Roles in Functional Dyspepsia. Front. Neurosci. 2022, 16, 858612. [Google Scholar] [CrossRef] [PubMed]
- Linan-Rico, A.; Ochoa-Cortes, F.; Beyder, A.; Soghomonyan, S.; Zuleta-Alarcon, A.; Coppola, V.; Christofi, F.L. Mechanosensory Signaling in Enterochromaffin Cells and 5-HT Release: Potential Implications for Gut Inflammation. Front. Neurosci. 2016, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Galligan, J.J. 5-HT secretion by enterochromaffin cells is a very touching story. J. Physiol. 2017, 595, 3. [Google Scholar] [CrossRef]
- Paintal, A.S. A method of locating the receptors of visceral afferent fibres. J. Physiol. 1954, 124, 166–172. [Google Scholar] [CrossRef]
- Hillsley, K.; Kirkup, A.J.; Grundy, D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J. Physiol. 1998, 506, 551–561. [Google Scholar] [CrossRef]
- Browning, K.N. Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front. Neurosci. 2015, 9, 413. [Google Scholar] [CrossRef]
- Nemeroff, C.B.; Mayberg, H.S.; Krahl, S.E.; McNamara, J.; Frazer, A.; Henry, T.R.; George, M.S.; Charney, D.S.; Brannan, S.K. VNS therapy in treatment-resistant depression: Clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology 2006, 31, 1345–1355. [Google Scholar] [CrossRef]
- Décarie-Spain, L.; Hayes, A.M.R.; Lauer, L.T.; Kanoski, S.E. The gut-brain axis and cognitive control: A role for the vagus nerve. Semin. Cell Dev. Biol. 2024, 156, 201–209. [Google Scholar] [CrossRef]
- Jordan, D. Vagal control of the heart: Central serotonergic (5-HT) mechanisms. Exp. Physiol. 2005, 90, 175–181. [Google Scholar] [CrossRef]
- Andermann, M.L.; Lowell, B.B. Toward a Wiring Diagram Understanding of Appetite Control. Neuron 2017, 95, 757–778. [Google Scholar] [CrossRef] [PubMed]
- Dockray, G.J. Enteroendocrine cell signalling via the vagus nerve. Curr. Opin. Pharmacol. 2013, 13, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Hyland, N.P.; Cryan, J.F. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev. Biol. 2016, 417, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Roman, C.W.; Sloat, S.R.; Palmiter, R.D. A tale of two circuits: CCKNTS neuron stimulation controls appetite and induces opposing motivational states by projections to distinct brain regions. Neuroscience 2017, 358, 316–324. [Google Scholar] [CrossRef]
- Travagli, R.A. The nucleus tractus solitarius: An integrative centre with ‘task-matching’ capabilities. J. Physiol. 2007, 582, 471. [Google Scholar] [CrossRef]
- Li, Y. Sensory signal transduction in the vagal primary afferent neurons. Curr. Med. Chem. 2007, 14, 2554–2563. [Google Scholar] [CrossRef]
- Ragozzino, F.J.; Peterson, B.A.; Karatsoreos, I.N.; Peters, J.H. Circadian regulation of glutamate release pathways shapes synaptic throughput in the brainstem nucleus of the solitary tract (NTS). J. Physiol. 2023, 601, 1881–1896. [Google Scholar] [CrossRef]
- Rao, V.R.; Finkbeiner, S. NMDA and AMPA receptors: Old channels, new tricks. Trends Neurosci. 2007, 30, 284–291. [Google Scholar] [CrossRef]
- Hankir, M.K.; Seyfried, F.; Miras, A.D.; Cowley, M.A. Brain Feeding Circuits after Roux-en-Y Gastric Bypass. Trends Endocrinol. Metab. 2018, 29, 218–237. [Google Scholar] [CrossRef]
- Mello-Carpes, P.B.; Izquierdo, I. The Nucleus of the Solitary Tract→Nucleus Paragigantocellularis→Locus Coeruleus→CA1 region of dorsal hippocampus pathway is important for consolidation of object recognition memory. Neurobiol. Learn. Mem. 2013, 100, 56–63. [Google Scholar] [CrossRef]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef] [PubMed]
- López-Terrones, E.; Paz, V.; Campa, L.; Conde-Berriozabal, S.; Masana, M.; Artigas, F.; Riga, M.S. Differential Modulation of Dorsal Raphe Serotonergic Activity in Rat Brain by the Infralimbic and Prelimbic Cortices. Int. J. Mol. Sci. 2023, 24, 4891. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Keller, J.; Grön, G. Dorsal Raphe Nucleus Down-Regulates Medial Prefrontal Cortex during Experience of Flow. Front. Behav. Neurosci. 2016, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Bombardi, C.; Di Giovanni, G. Functional anatomy of 5-HT2A receptors in the amygdala and hippocampal complex: Relevance to memory functions. Exp. Brain Res. 2013, 230, 427–439. [Google Scholar] [CrossRef]
- Chaouloff, F.; Berton, O.; Mormède, P. Serotonin and stress. Neuropsychopharmacology 1999, 21, 28s–32s. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Braun, T.; Voland, P.; Kunz, L.; Prinz, C.; Gratzl, M. Enterochromaffin cells of the human gut: Sensors for spices and odorants. Gastroenterology 2007, 132, 1890–1901. [Google Scholar] [CrossRef]
- Ross, J.A.; Van Bockstaele, E.J. The Locus Coeruleus- Norepinephrine System in Stress and Arousal: Unraveling Historical, Current, and Future Perspectives. Front. Psychiatry 2020, 11, 601519. [Google Scholar] [CrossRef]
- Fuchs, B.A.; Campbell, K.S.; Munson, A.E. Norepinephrine and serotonin content of the murine spleen: Its relationship to lymphocyte beta-adrenergic receptor density and the humoral immune response in vivo and in vitro. Cell. Immunol. 1988, 117, 339–351. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Cechetto, D.F. Central representation of visceral function. Fed. Proc. 1987, 46, 17–23. [Google Scholar] [PubMed]
- Coppen, A. The biochemistry of affective disorders. Br. J. Psychiatry 1967, 113, 1237–1264. [Google Scholar] [CrossRef] [PubMed]
- Bot, M.; Chan, M.K.; Jansen, R.; Lamers, F.; Vogelzangs, N.; Steiner, J.; Leweke, F.M.; Rothermundt, M.; Cooper, J.; Bahn, S.; et al. Serum proteomic profiling of major depressive disorder. Transl. Psychiatry 2015, 5, e599. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C. Physical Activity Modulates Common Neuroplasticity Substrates in Major Depressive and Bipolar Disorder. Neural Plast. 2017, 2017, 7014146. [Google Scholar] [CrossRef]
- Kambeitz, J.P.; Howes, O.D. The serotonin transporter in depression: Meta-analysis of in vivo and post mortem findings and implications for understanding and treating depression. J. Affect. Disord. 2015, 186, 358–366. [Google Scholar] [CrossRef]
- Gryglewski, G.; Lanzenberger, R.; Kranz, G.S.; Cumming, P. Meta-analysis of molecular imaging of serotonin transporters in major depression. J. Cereb. Blood Flow Metab. 2014, 34, 1096–1103. [Google Scholar] [CrossRef]
- Sumner, J.A.; Vrshek-Schallhorn, S.; Mineka, S.; Zinbarg, R.E.; Craske, M.G.; Redei, E.E.; Wolitzky-Taylor, K.; Adam, E.K. Effects of the serotonin transporter polymorphism and history of major depression on overgeneral autobiographical memory. Cogn. Emot. 2014, 28, 947–958. [Google Scholar] [CrossRef][Green Version]
- Moriguchi, S.; Yamada, M.; Takano, H.; Nagashima, T.; Takahata, K.; Yokokawa, K.; Ito, T.; Ishii, T.; Kimura, Y.; Zhang, M.R.; et al. Norepinephrine Transporter in Major Depressive Disorder: A PET Study. Am. J. Psychiatry 2017, 174, 36–41. [Google Scholar] [CrossRef]
- Klimek, V.; Stockmeier, C.; Overholser, J.; Meltzer, H.Y.; Kalka, S.; Dilley, G.; Ordway, G.A. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J. Neurosci. 1997, 17, 8451–8458. [Google Scholar] [CrossRef]
- Maletic, V.; Eramo, A.; Gwin, K.; Offord, S.J.; Duffy, R.A. The Role of Norepinephrine and Its α-Adrenergic Receptors in the Pathophysiology and Treatment of Major Depressive Disorder and Schizophrenia: A Systematic Review. Front. Psychiatry 2017, 8, 42. [Google Scholar] [CrossRef]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cui, M.; Cao, J.L.; Han, M.H. The Role of Beta-Adrenergic Receptors in Depression and Resilience. Biomedicines 2022, 10, 2378. [Google Scholar] [CrossRef] [PubMed]
- Sulser, F. Serotonin-norepinephrine receptor interactions in the brain: Implications for the pharmacology and pathophysiology of affective disorders. J. Clin. Psychiatry 1987, 48, 12–18. [Google Scholar] [PubMed]
- Gillespie, D.D.; Manier, D.H.; Sanders-Bush, E.; Sulser, F. The serotonin/norepinephrine-link in brain. II. Role of serotonin in the regulation of beta adrenoceptors in the low agonist affinity conformation. J. Pharmacol. Exp. Ther. 1988, 244, 154–159. [Google Scholar]
- Stockmeier, C.A.; Martino, A.M.; Kellar, K.J. A strong influence of serotonin axons on beta-adrenergic receptors in rat brain. Science 1985, 230, 323–325. [Google Scholar] [CrossRef]
- Guiard, B.P.; El Mansari, M.; Merali, Z.; Blier, P. Functional interactions between dopamine, serotonin and norepinephrine neurons: An in-vivo electrophysiological study in rats with monoaminergic lesions. Int. J. Neuropsychopharmacol. 2008, 11, 625–639. [Google Scholar] [CrossRef]
- Blier, P. Crosstalk between the norepinephrine and serotonin systems and its role in the antidepressant response. J. Psychiatry Neurosci. 2001, 26, S3–S10. [Google Scholar]
- Blier, P.; Szabo, S.T. Potential mechanisms of action of atypical antipsychotic medications in treatment-resistant depression and anxiety. J. Clin. Psychiatry 2005, 66 (Suppl. S8), 30–40. [Google Scholar]
- Lucki, I.; O’Leary, O.F. Distinguishing roles for norepinephrine and serotonin in the behavioral effects of antidepressant drugs. J. Clin. Psychiatry 2004, 65, 11–24. [Google Scholar]
- Harrington, M.A.; Zhong, P.; Garlow, S.J.; Ciaranello, R.D. Molecular biology of serotonin receptors. J. Clin. Psychiatry 1992, 53, 8–27. [Google Scholar]
- Cowen, P.J. Serotonin receptor subtypes: Implications for psychopharmacology. Br. J. Psychiatry Suppl. 1991, 12, 7–14. [Google Scholar] [CrossRef]
- Varnäs, K.; Halldin, C.; Hall, H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum. Brain Mapp. 2004, 22, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Marin, P.; Bécamel, C.; Chaumont-Dubel, S.; Vandermoere, F.; Bockaert, J.; Claeysen, S. Chapter 5—Classification and signaling characteristics of 5-HT receptors: Toward the concept of 5-HT receptosomes. In Handbook of Behavioral Neuroscience; Müller, C.P., Cunningham, K.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 91–120. [Google Scholar]
- Morabito, M.V.; Ulbricht, R.J.; O’Neil, R.T.; Airey, D.C.; Lu, P.; Zhang, B.; Wang, L.; Emeson, R.B. High-throughput multiplexed transcript analysis yields enhanced resolution of 5-hydroxytryptamine 2C receptor mRNA editing profiles. Mol. Pharmacol. 2010, 77, 895–902. [Google Scholar] [CrossRef]
- Schmidt, A.W.; Peroutka, S.J. 5-Hydroxytryptamine receptor “families”. FASEB J. 1989, 3, 2242–2249. [Google Scholar] [CrossRef]
- Hirst, W.D.; Abrahamsen, B.; Blaney, F.E.; Calver, A.R.; Aloj, L.; Price, G.W.; Medhurst, A.D. Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol. Pharmacol. 2003, 64, 1295–1308. [Google Scholar] [CrossRef]
- Pazos, A.; Probst, A.; Palacios, J. Serotonin receptors in the human brain—III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience 1987, 21, 97–122. [Google Scholar] [CrossRef]
- de Almeida, J.; Mengod, G. Serotonin 1A receptors in human and monkey prefrontal cortex are mainly expressed in pyramidal neurons and in a GABAergic interneuron subpopulation: Implications for schizophrenia and its treatment. J. Neurochem. 2008, 107, 488–496. [Google Scholar] [CrossRef]
- Aznavour, N.; Zimmer, L. [18F] MPPF as a tool for the in vivo imaging of 5-HT1A receptors in animal and human brain. Neuropharmacology 2007, 52, 695–707. [Google Scholar] [CrossRef]
- Sari, Y. Serotonin1B receptors: From protein to physiological function and behavior. Neurosci. Biobehav. Rev. 2004, 28, 565–582. [Google Scholar] [CrossRef]
- Adell, A.; Celada, P.; Artigas, F. The role of 5-HT1B receptors in the regulation of serotonin cell firing and release in the rat brain. J. Neurochem. 2001, 79, 172–182. [Google Scholar] [CrossRef]
- Ramadan, N.; Skljarevski, V.; Phebus, L.; Johnson, K. 5-HT1F receptor agonists in acute migraine treatment: A hypothesis. Cephalalgia 2003, 23, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Jakab, R.L.; Goldman-Rakic, P.S. 5-Hydroxytryptamine 2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc. Natl. Acad. Sci. USA 1998, 95, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef] [PubMed]
- Marek, G.J. CHAPTER 2.2—Electrophysiology of Serotonin Receptors. In Handbook of Behavioral Neuroscience; Müller, C.P., Jacobs, B.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 21, pp. 163–182. [Google Scholar]
- Bockaert, J.; Claeysen, S.; Compan, V.; Dumuis, A. 5-HT4 receptors, a place in the sun: Act two. Curr. Opin. Pharmacol. 2011, 11, 87–93. [Google Scholar] [CrossRef]
- Dawson, L.A. The central role of 5-HT6 receptors in modulating brain neurochemistry. Int. Rev. Neurobiol. 2011, 96, 1–26. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.K.; Oh, J.S. Interaction of the Vagus Nerve and Serotonin in the Gut–Brain Axis. Int. J. Mol. Sci. 2025, 26, 1160. https://doi.org/10.3390/ijms26031160
Hwang YK, Oh JS. Interaction of the Vagus Nerve and Serotonin in the Gut–Brain Axis. International Journal of Molecular Sciences. 2025; 26(3):1160. https://doi.org/10.3390/ijms26031160
Chicago/Turabian StyleHwang, Young Keun, and Jae Sang Oh. 2025. "Interaction of the Vagus Nerve and Serotonin in the Gut–Brain Axis" International Journal of Molecular Sciences 26, no. 3: 1160. https://doi.org/10.3390/ijms26031160
APA StyleHwang, Y. K., & Oh, J. S. (2025). Interaction of the Vagus Nerve and Serotonin in the Gut–Brain Axis. International Journal of Molecular Sciences, 26(3), 1160. https://doi.org/10.3390/ijms26031160






